1. INTRODUCTION
Over the recent years, alternative cropping systems have been proposed to challenge the negative environmental impacts of conventional field cropping caused by intensive mechanical soil disturbance and use of synthetic pesticides and fertilizers (Carlson et Stockweel, 2013; DeLonge et al., 2016; FAO et ITPS, 2015; Magdoff, 2007; Oerke, 2006; Pimentel et al., 1995; Triplett et Dick, 2008). Direct seeding (DS) systems have been put forward to reduce mechanical tillage and the incidence of soil erosion. DS systems allow reducing carbon and nitrate leaching from soils or emissions to the atmosphere, and maintaining soil organic carbon (SOC) and soil functions (Kassam et al., 2019; Pimentel et al., 1995; Triplett et Dick, 2008; Yu et al., 2020). The use of glyphosate-based herbicides (GBH) in combination with glyphosate resistant (GR) seeds has promoted the adoption of DS systems on a larger scale (Derpsch, 1998; Derpsch et al., 2010). During 1998 and 2008, DS area have increased by 71.6% in soybean production in the United States of America (Yu et al., 2020). Although DS systems aim at maintaining SOC and soil functions, they tend to provoke surface soil compaction, in turn limiting water infiltration, seed germination and the development of crop plants (Triplett et Dick, 2008). Reduced water infiltration into the soil can result in water limitation for crops, which can influence their physiological activities and their gas exchange potential (Domec et al., 2009; Driesen et al., 2020). Stomata present on leaves constitute the main sites for CO2 assimilation by the plants (Tanaka et al., 2010; Zeiger et al., 1987). Stomata also play an important role in plant transpiration, since for the uptake of CO2 corresponds to water release, i.e., a significant trade-off for the metabolic management of the plant (Driesen et al., 2020; Krober et Bruelheide, 2014). Along with climate change, the occurrence of drier periods will be more frequent and will have a considerable influence on the water content of soils during key periods of crop plants growth.
We conducted a two years field study aiming at determining how the implementation of cover crops (CC) may influence the gas exchange potential of glyphosate-tolerant soybean in DS systems. Direct seeding on cover crops (DSCC) imply implementing catch crop between field crop production periods or intercropping during the field crop production periods (Hartwig et Ammon, 2002; Woolford et Jarvis, 2017). The addition of CC may bring agronomic benefits such as limiting surface soil compaction, increasing soil porosity and water infiltration and limiting soil water evaporation (Amsili et Kaye, 2021; Hartwig et Ammon, 2002; Liu et al., 2005; Robertson et al., 2014; Wagg et al., 2021). C3 plants such as soybean are sensitive to abiotic factor such as temperature, air humidity, light intensity in turn influencing the gas exchange potential that contributes to water management and photosynthesis of the plant (Driesen et al., 2020; Roche, 2015). In the coming years, climate change will have strong repercussion on the temperature and air humidity that will cause more frequent and longer drought period (Seager et al., 2015; Zhao et al., 2017). It has been reported that the increase of vapor pressure deficit (Vpd) has a negative impact on field crop production (Seager et al., 2015; Zhao et al., 2017). Vpd represents the difference between the amount of water vapor that air can contain at saturation and the actual vapor pressure in the air. Vpd can thus be considered as a direct measurement of the atmospheric desiccation power, an important factor influencing plants productivity and sensitivity to other abiotic stressors (Grossiord et al., 2020; Ocheltree et al., 2014). Because Vpd is highly dependent upon temperature, it will increase with global warming and thus raise questions regarding field crops water management. This study aims at determining if the use of CC in row crops may represent a clue for reducing soybean sensitivity to variation of Vpd and to drought periods. To our knowledge, few studies have yet reported the influence of CC on stomatal conductance while Vpd values are raising. Bernier Brillon et al. (2022) observed that higher Vpd values seemed to have a particular influence on soybean stomatal conductance. This 1-year study also pointed out the potential mitigation effect of CCs on crop plants on experiencing higher Vpd values (Bernier Brillon et al., 2022). In addition, the present study also aims at integrating physiological activity data by complementing them with leaf drought tolerance traits data (ex. foliar size and vein architecture).
2. Materials and methods
2.1. Description of the experimental design
Experiments were conducted in 2019 and 2020 in an open field located at the Grain Research Center (CEROM) in St-Mathieu-de-Beloeil (Quebec, Canada, (45.5828 N, -73.2374 W). The experimental plots were established in 2018 on a humic Gleysol (AAFC, 1998) with a heavy clay texture (average and standard deviation percentage of clay: 72.625 ±0.916 %, loam: 27.375 ±0.916 % and sand: 0%). The 0-20 cm horizon has a soil mineral content of 12.87 ±2.51 mg kg
-1 for P, 313.50 ±20.84 mg kg
-1 for K, 2943.42 ±219.62 mg kg
-1 for C, 803.17 ±48.27 mg kg
-1 for Mg, 1056.71 ±19.32 mg kg
-1 for Al, 11.00 ±0.47 mg kg
-1 for Cu, 217.54 ±13.92 mg kg
-1 for Fe, 24.92 ±5.72 mg kg
-1 for Mn, 2.33 ±0.18 mg kg
-1 for Zn and 47.60 ±3.06 mg kg
-1 for Na. The size of each plot was 9 m x 20 m and each treatment was replicated four times. The experimental design relied on two Direct Seeding practices (DS and DSCC) with glyphosate tolerant soybean (Altitude R2
®). In each plot, soybean was seeded on previous year corn residues at a rate of 90.81 kg ha
-1, May 18th 2019 and May 26th 2020. Autumn rye (200 kg ha
-1) was also sown before the corn harvest as catch crops in those plots. No cover crops were sown in the DS plots. Herbicide treatment (Roundup WheaterMax
® with glyphosate a.i. at 540 g L
-1) has been applied twice at rates of 902 g ha
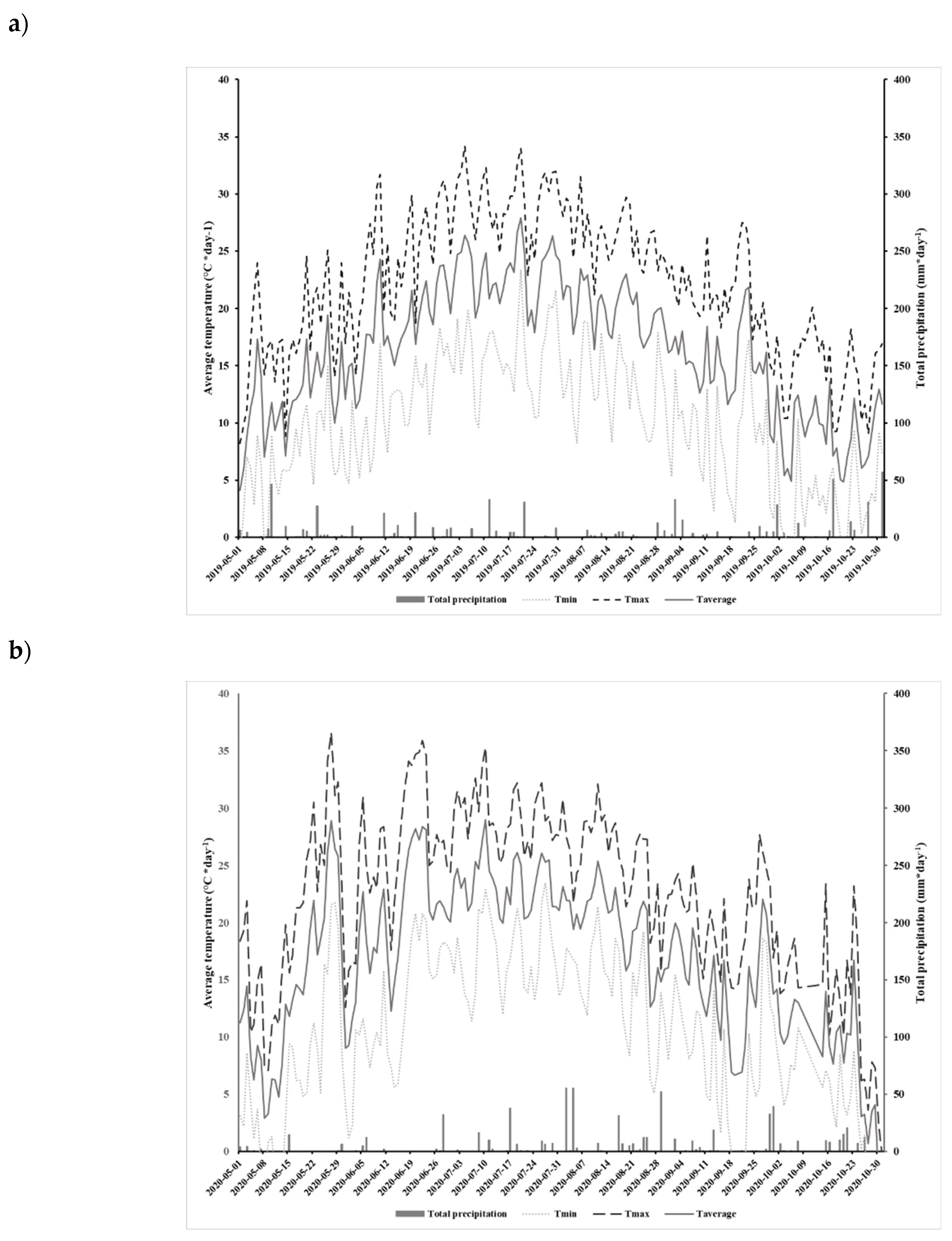
-1 of glyphosate in DS and DSCC plots. The first application was done pre-sowing May 18th 2019 and June 2nd 2020. The second application was done post-emergence June 24th 2019 and July 3rd 2020 at V2 soybean growth stage. Soybean was harvested October 15th 2019 and October 31th 2020. The field meteorological data including total daily precipitation (mm) and minimum, maximum and average daily temperatures were recorded for the 2019 and 2020 production period with a weather station located on the CEROM main building (
Figure 1a and
1b).
2.2. Sampling and measurements
Stomatal conductance and stomatal traits
Stomatal conductance (Gs expressed as mmol m-2 s-1) was measured with a steady-state diffusion porometer (SC-1 Leaf porometer, Decacon Devices®) using one leaflet from three different plants similarly arranged and with initially the same growth stage (V2). All plants and leaves were identified the V2 growth stage in order to follow the same plants and leaflets throughout the study period (from the V2 to the R2-R3 growth stages). The stomatal conductance was measured around midday on abaxial foliar surfaces during five fields sampling campaigns (48h and 7, 14, 21 and 28 days following the second GBH application). Leaves temperature and air relative humidity were also recorded using a portable psychrometer (REED instrument©, model#8706) at the leaf surface. The corresponding Vpd at the leaf surface was calculated according to the August-Roche Magnus formula, where Vpd = 6.109417.625*T/T+243.04 (Alduchov et Eskridge, 1996; Murray, 1967).
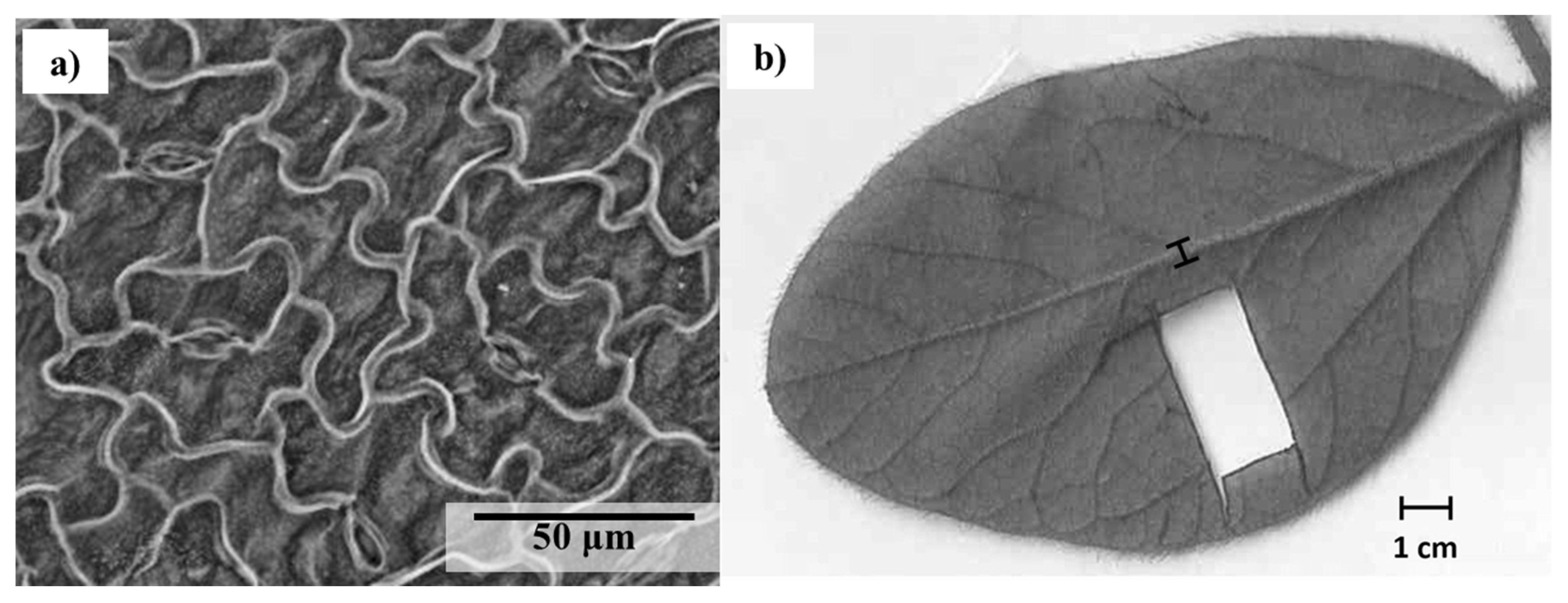
After measuring the stomatal conductance 28 days after the second GBH application, each identified leaf (R2-R3 growth stage) was collected in order to calculate the stomatal size, density and index. On each leaf, three locations on one leaflet were observed for the stomatal density (StoDen) calculation with a scanning electron microscope (Hitachi S-3400N) at a magnification of 400x (
Figure 2a). Pictures of those observations with the SEM were taken and the stomatal sizes (StoSize), width (StoWidth) and length (StoLength) were measured with ImageJ
© software (NIH). The stomatal index (StoIndex) was calculated by multiplying the stomatal density by the stomatal size (Kim
et al., 2021).
2.3. Foliar trait
One of the leaflets from each leaf collected in the field for stomatal trait measurements (28 days after second GBH application) were stored in a decolorizing solution (70% aqueous ethanol solution). Once decolored, these leaves were dipped in a safranin solution (4% v/v) until we obtained a sufficient staining of the foliar veins. This vein staining increased color contrast and allow better accuracy of leaf trait measurements. The colored leaflets were then scanned and the measurements were made using an imagery software (ImageJ© software).
In this study, distance between veins has been used to obtain a proxy of the venation density (Uhl et Mosbrugger, 1999). An average of 11 measurements has been taken between secondary vein for each leaflet. The midrib width and the leaves size of those leaflet have also been measured with the imagery software (ImageJ
© software) (
Figure 2b)
2.4. Statistical analyses
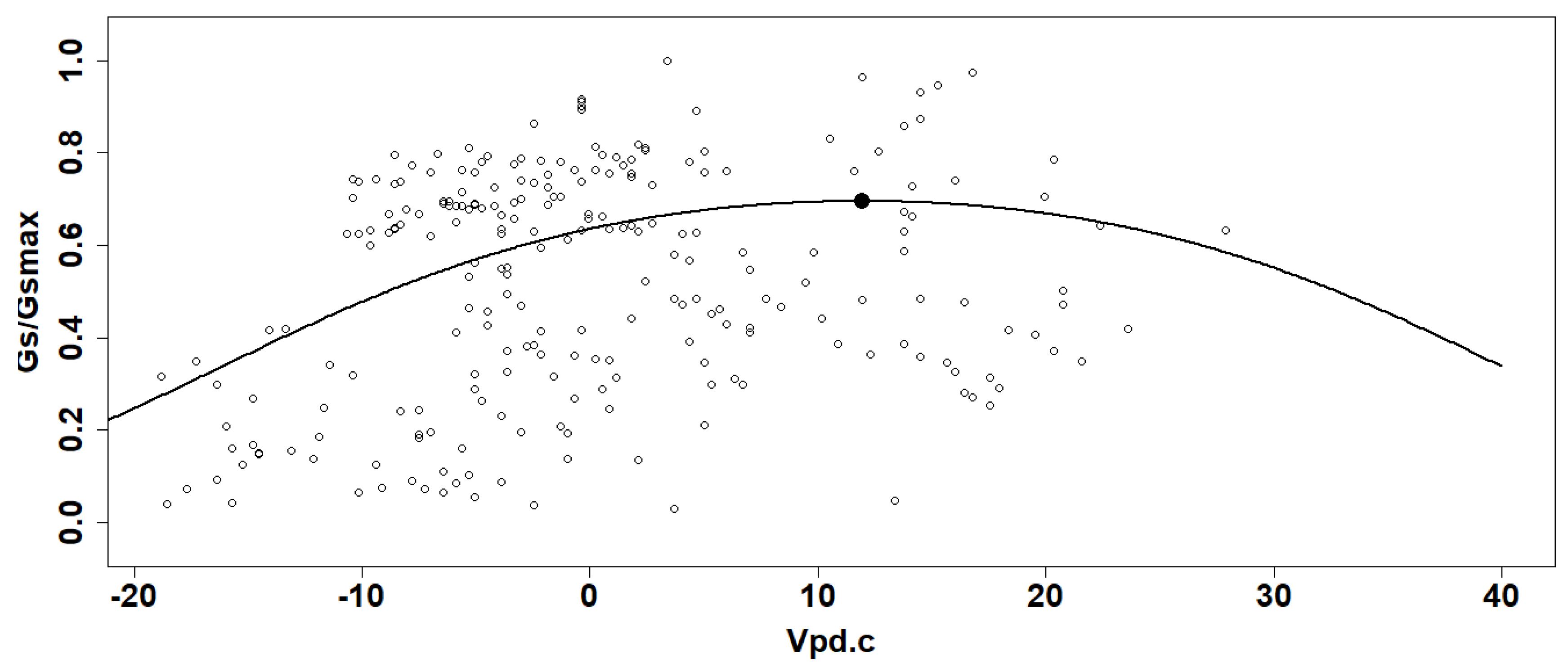
The Gs/Gsmax values from all plants and for both sampled years were used to obtain a generalized linear model (GLM) in function of the corresponding Vpd with beta distribution using a
logit link function) (
Figure 3) (Bernier Brillon
et al., 2022; Krober et Bruelheide, 2014). The inflexion point was calculated for each curve and considered as the optimal condition for gas exchange (Bernier Brillon
et al., 2022; Krober et Bruelheide, 2014). The Vpd values for these optimum points were calculated to determine if there is a difference in plant sensitivity to Vpd between DS systems with or without CC.
Figure 3 shows an example of the fitted curves from the GLM as a function of Vpd centered value for each system and for the years 2019 and 2020. Since the logit function has only one rising point of inflection, the optimal gas exchange points were calculated from the second derivative for each curve. The confidence interval (95% CI) was calculated for those points to take into consideration the interval on the values for the stomatal conductance (the y-axis interval) and the Vpd (the x-axis interval).
An ANOVA analysis was carried out to assess whether there is a significant difference (p ≤ 0.05) in Gs values between years for the stomatal traits, foliar traits, dry biomass production, different cultivation systems. Also, a Chi square test analysis was also carried out to evaluate the influence of both the year of production and the combination of year-agricultural management.
3. RESULTS
3.1. Stomatal conductance and vapor pressure deficit
Combining DS and DSCC, significant differences in Gs between years are observed (p = 0.0006) where the means ± SE values were 635.15 ± 23.80 mmol m-2s-1 for 2019 and 516.14 ± 24.51 mmol m-2s-1 in 2020. On the opposite, the mean ± SE values of Vpd are significantly higher (p < 0.0001) in 2020 (58.68 ± 0.84 hPa) than in 2019 (51.33 ±0.75 hPa).
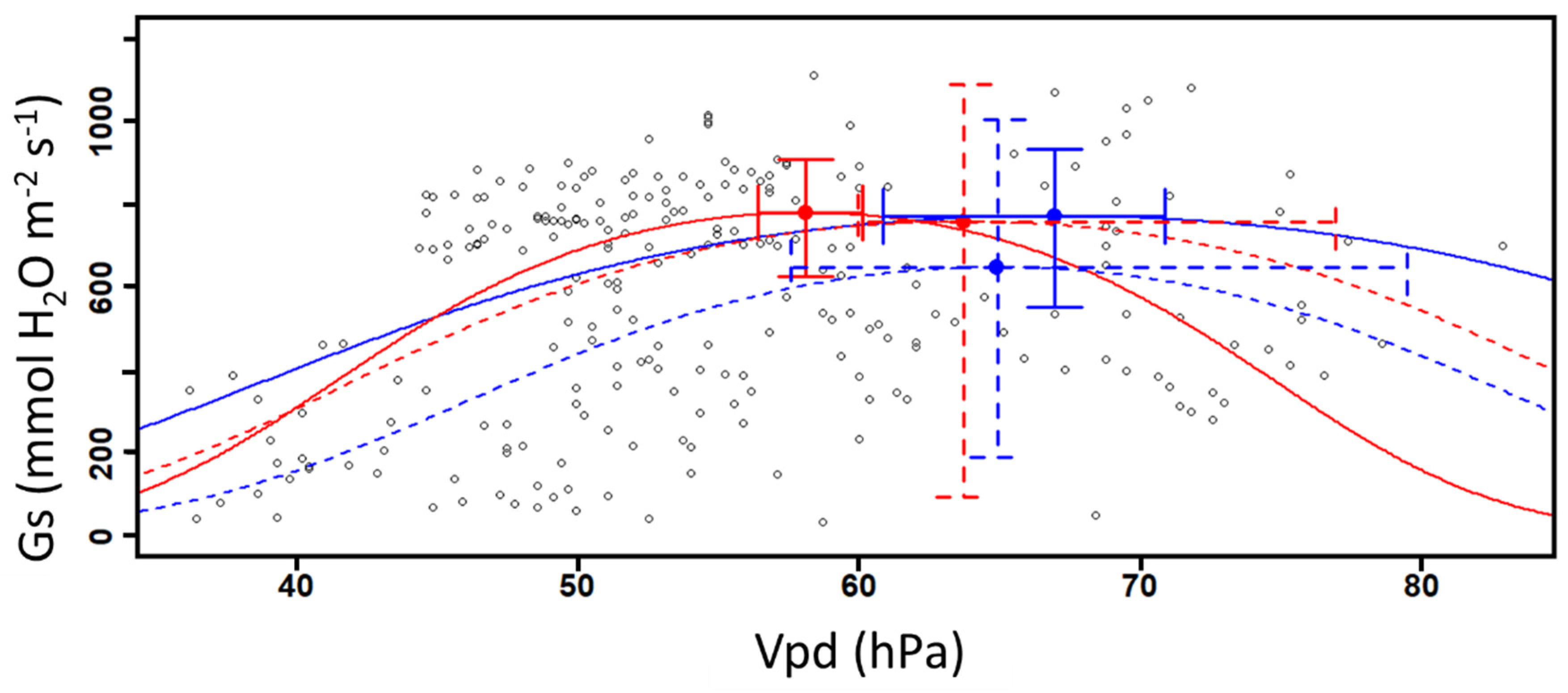
By modelling the relation between Gs and the raising values of Vpd, we observe that the calculated inflexion points from Gs values in 2019 is similar between DS and DSCC plots (
Figure 4 and
Table 1). However, we observe significant difference in 2019 between DSCC and DS Vpd values based on the 95% confidence interval (
Figure 4 and
Table 1) where DSCC have similar Gs values at higher Vpd values. No difference is observed on the calculated inflexion points from Gs values and on Vpd values in 2020 between DSCC and DS. The large variation around the inflexion points values in 2020 do not allow to observe difference with 2019.
3.2. Stomatal traits
Stomatal traits analysis (
Table 2) shows that StoDen is significantly higher in DSCC plots compared to DS ones in both 2019 (
p = 0.0581) and 2020 (
p = 0.0247). StoLength, StoWidth and StoSize have higher values in DS plots in 2019 when compared to DSCC but no significant difference between the system is observed in 2020. In the case of StoIndex, no significant difference between DS and DSCC is observed for either year. However, different results on the stomatal traits are observed between both years where StoDen and StoIndex values for both systems are lower in 2020 compared to 2019. StoWidth and StoSize are significantly higher in DS plots in 2019 than in 2020 whereas no significant differences on those traits are observed in DSCC plots between 2019 and 2020.
3.3. Foliar traits
No difference on the leaf size values between DSCC and DS is observed in 2019 and 2020 (
Table 3). Also, the average leaf sizes are similar between years. Concerning the midrib width, significant differences exist between DSCC and DS in 2019 but not in 2020 (
Table 3). In 2019, the midrib width values are higher in DSCC compared to DS plots. Also, significant smallest DistVein values are observed in DSCC for the year 2019 and 2020.
4. DISCUSSION
The observed correlation between Vpd and Gs confirms that Vpd influences the physiological activity of plants, which is consistent with other publications (Bernier Brillon
et al., 2022; Driesen
et al., 2020; Ocheltree
et al., 2014). Here, a positive relation between Vpd and Gs is observed until the gases exchange potential reaches an inflexion point defined as an optimal point (
Figure 4). Once this value is reached, Gs values decrease along with higher Vpd values. Higher Vpd values promote the ascension of water in the xylem, enhancing water accumulation in the sub-stomatal cavities and its exit through plant transpiration (Driesen
et al., 2020; Sinclair
et al., 2017). The decreasing Gs values observed in this study can be explained by the fact that plants close their stomata in order to limit excessive water loss when their ambient environment becomes drier (higher Vpd values) (Bernier Brillon
et al., 2022; Driesen
et al., 2020; Krober et Bruelheide, 2014). Here, DSCC plots in 2019 appear less sensitive to Vpd while plants maintain the physiological activities and stomata opening during a less favorable periods (i.e., drought and hydric stress episodes). Gs values can be up to 29% higher in DSCC plots compared to DS ones for the same Vpd values in 2019 (
Figure 4 and
Table 1).
Interestingly, we are able to observe differences in morphological traits of plants grown in DS and DSCC plots (
Table 2 and
Table 3). This observation corroborates other research, which proposed that a difference in physiological activity and gas exchange can be explained by different leaf morphological traits. Those differences in leaf can be explained by morphological plasticity to optimize plant performance according to growth conditions (Carins Murphy
et al., 2014; Franks
et al., 2009; Puglielli
et al., 2017; Xiong
et al., 2017). These morphological differences can occur in stomata and other foliar traits such as the main vein structure (Carins Murphy
et al., 2014; Scoffoni
et al., 2011). Stomatal activity could be explained by the stomata density considering that it can be positively correlated with stomatal conductance (Chen
et al., 2016; Driesen
et al., 2020; Gaskell et Pearce, 1983; Qi et Torii, 2018; Roche, 2015; Tanaka
et al., 2010; Tanaka et Shiraiwa, 2009; Tanaka
et al., 2008). StoDen is a good drought proxy and is an indicator of the strategy that plants adopt to develop their stomata while reducing stressful conditions (Bernier Brillon
et al., 2023; Bernier Brillon
et al., 2022; Driesen
et al., 2020; Tanaka et Shiraiwa, 2009). These strategies closely influence the number and size of stomata, which in combination represent the potential for foliar epidermal cells surface allocation for gas exchange and optimal stomatal conductance potential (Franks
et al., 2009). In this study, the epidermal cells surface allocation for gas exchange is represented by the StoIndex value, which shows no significant differences between agricultural managements and years (Tableau 2). We observe that in 2019, StoDen of soybean growing in DSCC plots is higher than that of plants growing in DS plots (
Table 2). This could explain the differences in stomatal behaviour and gas exchange where DSCC StoDen values are significantly different from those for plants growing in DS plots in 2020 (
p value = 0.0247) (
Table 2). Moreover, the stomata of plants growing in DSCC plots were significantly smaller than those in DS plots (
Table 2). This was expected considering that it has been largely demonstrated that a negative relationship generally exists between stomatal size and number of stomata (Franks
et al., 2009). However, smaller and more numerous stomata allows soybean to quickly adapt their stomatal aperture for optimal conductance or closing them in order to avoid excessive transpiration (Aasama
et al., 2001; Bernier Brillon
et al., 2022; Franks
et al., 2009). This can be a significant short-term advantage, especially for crops that have to react quickly without allocated epidermal cell for the development of new stomata. For short-lived crops like soybean, plants tends to optimise resource acquisition by minimizing construction cost (Correia et Ascensão, 2017; Puglielli
et al., 2017). In the case of DSCC plants in 2019, a higher StoDen allowed physiological plasticity, which allows to maintain gas exchange in a context where Vpd values were higher.
In addition, the different stomatal traits between agricultural managements can also be explained by the morphological differences of the foliar veins, i.e., another indicator of drought tolerance of the plants (Scoffoni et al., 2011). In our case, it was observed that soybean growing in DSCC plots in 2019 had a significantly wider midrib and a significantly lower DistVein, which represents a higher venation density. Higher venation density can be an indicator of willingness and resilience of the plants growing in plots with CC during high Vpd or drought episode (Carins Murphy et al., 2014). A more elaborated venation may be linked to a better water management (Uhl et Mosbrugger, 1999). Scoffoni et al. (2011) have proposed that large midrib and small distance between secondary veins allows a more important number of stomata which is also consistent with our observations. Higher major vein density would thus have lower hydraulic vulnerability allowing a larger number of stomata (Scoffoni et al., 2011). Also, the presence of CC can have a positive influence on soil functions, which can explain the willingness that facilitate phenologic plasticity of plants in DSCC plots. It has been shown that CC can increase the number and diversity of root systems in the field which can improve soil porosity, aggregation and fertility (Amsili et Kaye, 2021; Liu et al., 2005). These soil functions can facilitate the accessibility and the uptake of water by crop plants, in turn favoring gas exchange and transpiration with less restriction.
Other factors resulting from the choice of a given agronomic management can influence the activity of stomata such as the use of herbicides in field crop (Albrecht et al., 2014; Gomes et al., 2014; Kim et al., 2021; Krenchinski et al., 2017; Smedbol et al., 2019; Zobiole et al., 2010). With the increase in the number of weeds resistant to GBH, producers are inclined to use heavier doses of the same herbicide or to combine different types of herbicides in order to efficiently control weeds (Gerhards et Schappert, 2020; Lemessa et Wakjira, 2015; Osipitan et al., 2019). Moreover, this phenomenon of herbicide resistance is likely to be exacerbated with climate change, which is not without impact on cash crops (Fernando et al., 2016). It has been shown that GBH can have a negative impact on the activity and development of stomata in glyphosate tolerant soybean (Albrecht et al., 2014; Gomes et al., 2014; Krenchinski et al., 2017; Smedbol et al., 2019; Zobiole et al., 2010). Glyphosate tolerant soybeans are not resistant to aminomethylphosphonic acid (Kanissery et al.), the main degradation metabolite of glyphosate in soils (Gomes et al., 2014; Reddy et al., 2004; Zobiole et al., 2010). Among the impacts considered, some authors have observed that stomatal conductance was lower following exposure of glyphosate tolerant soybeans to GBH (Bernier Brillon et al., 2023; Bernier Brillon et al., 2022; Smedbol et al., 2019; Zobiole et al., 2010). Other studies have also shown that the use of herbicides other than GBH could also affect stomatal activity and development (Anastasov, 2010a, 2010b; Chen et al., 2016; Semerdjieva et al., 2015). The response of certain crops such as soybean to herbicide applications can therefore induce stresses and decrease the crop tolerance to other disturbances such as water stress induced during a period of water limitation (Petter et al., 2016). In this context, the use of CC can be beneficial considering that on one hand the presence of CC seems to positively influence plants to develop morphological traits making them more tolerant to drought and that on the other hand the use of CC seems to be effective in limiting the presence of weeds in the fields (Gerhards et Schappert, 2020; Osipitan et al., 2019).
5. CONCLUSION
This study suggests that CC contribute to maintain gas exchange potential in a context of soybean exposed to higher Vpd values. The implementation of CC would thus favour a higher resilience to potential combined stress of drought and GBH application by increasing crop plasticity in GR soybean field crops. At similar Vpd values, the stomatal conductance of plants growing in DSCC plots was significantly higher than that of plants growing in DS plots. This can be explained by a higher tolerance under conditions that can cause water limitation to plants. This tolerance is expressed by a more elaborate venation and higher StoDen in plants growing in DSCC plots. Through the response of the plants and their development strategy, the benefits of CC on crops could be observed in the short term in this study, Finally, CC seem to represent, in part, a sustainable solution to fight against drought and future climate changes. CC also seem to be a promising alternative to minimize the reduction of gas exchange of soybean triggered by herbicides spraying during a drought period.
Acknowledgments
We wish to acknowledge the implication of the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) and Le Centre de recherche sur les grains (CEROM) on this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aasama, K., Sober, A. et Rahi, M. (2001). Leaf anatomical characteristics associated with shoot hydraulic conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Australian Journal of Plant Physiology, 28, 765-774. [CrossRef]
- Albrecht, A.J.P., Albrecht, L.P., Krenchinski, F.H., Victoria Filho, R., Placido, H.F. et Barroso, A.A.M. (2014). Behavior of RR soybeans subjected to different formulations and rates of glyphosate in the reproductive period. Planta Daninha, 32(4), 851-859. [CrossRef]
- Alduchov, O.A. et Eskridge, R.E. (1996). Improved Magnus form approximation of saturation vapor pressure. Journal of Applied Meteorology, 35, 601-609. [CrossRef]
- Amsili, J.P. et Kaye, J.P. (2021). Root traits of cover crops and carbon inputs in an organic grain rotation. Renewable Agriculture and Food Systems, 36, 182-191. [CrossRef]
- Anastasov, H. (2010a). Influence of imazamox on some anatomic indices in the leaves of sunflower plant (Helianthus annuus L.). General and Applied Plant Physiology, 36(1-2), 64-68.
- Anastasov, H. (2010b). Influence of oxyfluorfen on some anatomic indices in the leaves of Virginia tobacco plant (Nicotiana Tabacum L.). Biotechnology & Biotechnological Equipment, 24, 33-35. [CrossRef]
- Bernier Brillon, J., Lucotte, M., Tremblay, G., Smedbol, E. et Paquet, S. (2023). Impacts of glyphosate-based herbicide on leaf stomatal density and biomass production of transgenic soybean (Glycine max [L.] Merr.) and corn (Zea mays L.). Acta Physiologiae Plantarum 45(68), 1-12. [CrossRef]
- Bernier Brillon, J., Moingt, M. et Lucotte, M. (2022). Direct seeding under cover crops: A solution to optimize the potential for adaptation of transgenic field crops to water stress in a context of glyphosate exposure. Journal of Agricultural and Crop Research, 10(5), 85-97. [CrossRef]
- Carins Murphy, M.R., Jordan, G.J. et Brodribb, T.J. (2014). Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant, Cell and Environment 37, 124-131. [CrossRef]
- Carlson, S. et Stockweel, R. (2013). Research priorities for advancing adoption of cover crops in agriculture-intensive regions. Journal of Agriculture, Food Systems, and Community Development, 3(4), 125-129. [CrossRef]
- Chen, Z., Chen, H., Zou, Y. et Wen, Y. (2016). Stomatal behaviors reflect enantioselective phytotoxicity of chiral herbicide dichlorprop in Arabidopsis thaliana. Science of the Total Environment (562), 73-80. [CrossRef]
- Correia, O. et Ascensão, L. (2017). Summer semi-deciduous species of the Mediterranean landscape: A winning strategy of Cistus species to face the predicted changes of the Mediterranean climate. Plant biodiversity: Monitoring, assessment and conservation, 195-217. [CrossRef]
- DeLonge, M.S., Miles, A. et Carlisle, L. (2016). Investing in the transition to sustainable agriculture. Environmental Science & Policy 55, 266-273. [CrossRef]
- Derpsch, R. (1998). No-tillage cultivation and future research needs: Historical review of no-tillage cultivations of crops. JIRCAS Working Report(13 ), 1-18.
- Derpsch, R., Friedrich, T., Kassam, A. et Hongwen, L. (2010). Current status of adoption of no-till farming in the world and some of its main benefits. International Journal of Agricultural and Biological Engineering, 3(1), 1-25. [CrossRef]
- Domec, J.-C., Noormets, A., Gavazzi, M.J., Bogg, J.L., King, J.S., Sun, G.E. et Treasure, E.A. (2009). Decoupling the influence of leaf and root hydraulic conductances on stomatal conductance and its sensitivity to vapour pressure deficit as soil dries in a drained loblolly pine plantation. Plant, Cell and Environment 32, 980-991. [CrossRef]
- Driesen, E., Van den Ende, W., De Proft, M. et Saeys, W. (2020). Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 10(1975), 1-28. [CrossRef]
- FAO et ITPS. (2015). Status of the world’s soil ressources. (Intergovernmental Technical Panel on Soils: Technical summary). Rome, Italy : Food and Agricultue Organization of the United Nations Récupéré de www.fao.org/publications.
- Fernando, N., Manalil, S., Chauhan, B.S., Florentine, S.K. et Seneweera, S. (2016). Glyphosate Resistance of C3 and C4 Weeds under Rising Atmospheric CO2. Frontiers in Plant Science, 7(Article 910), 1-11. [CrossRef]
- Franks, P.J., Drake, P.L. et J., B.D. (2009). Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: An analysis using Eucalyptus globulus. Plant, Cell and Environment 32, 1737-1748. [CrossRef]
- Gaskell, M.L. et Pearce, R.B. (1983). Stomatal frequency and stomatal resistance of maize hybrids differing in photosynthetic capability. Crop Science, 23(1), 176-177. [CrossRef]
- Gerhards, R. et Schappert, A. (2020). Advancing cover cropping in temperate integrated weed management. Pest Manag Sci, 76, 42-46. [CrossRef]
- Gomes, M.P., Smedbol, E., Chalifour, A., Hénault-Ethier, L., Labrecque, M., Lepage, L., Lucotte, M. et Juneau, P. (2014). Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. Journal of Experimental Botany, 65(17), 4691-4703. [CrossRef]
- Grossiord, C., Buckley, T.N., Novick, K.A., Poulter, B., Sperry, J.S. et McDowell, N.G. (2020). Plant responses to rising vapor pressure deficit. New Phytologist 226, 1550-1566. [CrossRef]
- Hartwig, N.L. et Ammon, H.U. (2002). Cover Crops and Living Mulches. Weed Science, 50, 688-699. [CrossRef]
- J.W., F., Zalcmann, A.T. et Talalay, P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry, 56, 5-51. [CrossRef]
- Kanissery, R., Gairhe, B., Kadyampakeni, D., Batuman, O. et Alferez, F. (2019). Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants, 8(499), 1-11. [CrossRef]
- Kassam, A., Friedrich, T. et Derpsch, R. (2019). Global spread of Conservation Agriculture. International Journal of Environmental Studies, 76(1), 29-51. [CrossRef]
- Kim, L., Balani, S., Edelberg, M. et Macke, N. (2021). Effects of various environmental factors on stomatal density, area, and potential conductance index. Journal of Emerging Investigators, 4, 1-8.
- Krenchinski, F.H., Saloma Cesco, V.J., Zobiole, L.H.S., Albrecht, L.P., Rodrigues, D.M., Albrecht, A.J.P. et Portz, R.L. (2017). Glyphosate affects chlorophyll, photosynthesis and water use of four Intacta RR2 soybean cultivars. Acta Physiol Plant 39(63), 1-13. [CrossRef]
- Krober, W. et Bruelheide, H. (2014). Transpiration and stomatal control: A cross-species study of leaf traits in 39 evergreen and deciduous broadleaved subtropical tree species. Trees, 28, 901-914. [CrossRef]
- Lemessa, F. et Wakjira, M. (2015). Cover crops as a means of ecological weed management in agroecosystems. Journal of Crop Science and Biotechnology, 18(2), 133-145. [CrossRef]
- Liu, A., Ma, B.L. et Bomke, A.A. (2005). Effects of cover crops on soil aggregate stability, total organic carbon, and polysaccharides. Soil Science Society of America Journal, 69, 2041-2048. [CrossRef]
- Magdoff, F. (2007). Ecological agriculture: Principles, practices, and constraints. Renewable Agriculture and Food Systems, 22(2), 109-117. [CrossRef]
- Murray, F.W. (1967). On the computation of saturation vapor pressure. Journal of Applied Meteorology, 6, 203-204. [CrossRef]
- Ocheltree, T.W., Nippert, J.B. et Prasad, P.V.V. (2014). Stomatal responses to changes in vapor pressure deficit reflect tissue-specific differences in hydraulic conductance. Plant, Cell and Environment 37, 132-139. [CrossRef]
- Oerke, E.-C. (2006). Crop losses to pests. Journal of Agricultural Science 144, 31-43. [CrossRef]
- Osipitan, O.A., Dille, J.A., Assefa, Y., Radicetti, E., Ayeni, A. et Knezevic, S.Z. (2019). Impact of cover crop management on level of weed suppression: A meta-analysis. Crop Science, 59, 833-842. [CrossRef]
- Petter, F.A., Zuffo, A.M., de Alcântara Neto, F., Pereira Pacheco, L., de Almeida, F.A., Ribeiro Andrade, F. et Zuffo Júnior, J.M. (2016). Effect of glyphosate and water stress on plant morphology and nutrient accumulation in soybean. Australian Journal of Crop Science, 10(2), 251-257.
- Pimentel, D., Harvey, C., Resosudarmo, P., Sinclair, K., Kuiz, D., McNair, M., Crist, S., Shpritz, L., Fitton, L., Saffouri, R. et Blair, R. (1995). Environmental and economic cost of soil erosion and conservation benefit. Science, 267(1117-1123). [CrossRef]
- Puglielli, G., Catoni, R., Spoletini, A., Varone, L. et Gratani, L. (2017). Short- term physiological plasticity: Trade- off between drought and recovery responses in three Mediterranean Cistus species. Ecology and Evolution, 7, 10880-10889. [CrossRef]
- Qi, X. et Torii, K.U. (2018). Hormonal and environmental signals guiding stomatal development. BMC Biology 16(21), 1-11. [CrossRef]
- Reddy, K.N., Rimando, A.M. et Duke, S.O. (2004). Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. Journal of Agricultural and Food Chemistry, 52, 5139-5143. [CrossRef]
- Robertson, G.P., Gross, K.L., Hamilton, S.K., Landis, D.A., Schmidt, T.M., Snapp, S.S. et Swinton, S.M. (2014). Farming for ecosystem services: An ecological approach to production agriculture. BioScience, 64(5), 404-415. [CrossRef]
- Roche, D. (2015). Stomatal conductance is essential for higher yield potential of C3 crops. Critical Reviews in Plant Sciences, 34, 429-453. [CrossRef]
- Scoffoni, C., Rawls, M., McKown, A., Cochard, H. et Lawren Sack, L. (2011). Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiology 156, 832-843. [CrossRef]
- Seager, R., Hooks, A., Parkwilliams, A., Cook, B., Nakamura, J. et Henderson, N. (2015). Climatology, variability, and trends in the U.S. vapor pressure deficit, an important fire-related meteorological quantity. Journal of Applied Meteorology and Climatology, 54, 1121-1141. [CrossRef]
- Semerdjieva, I., Kalinova, S., Yanev, M. et Yankova-Tsvetkova, E. (2015). Anatomical Changes in Tobacco Leaf after Treatment with Isoxaflutol. International Journal of Current Research in Biosciences and Plant Biology, 2(7), 51-56.
- Sinclair, T.R., Devi, J., Shekoofa, A., Choudhary, S., Sadok, W., Vadez, V., Riar, M. et Rufty, T. (2017). Limited-transpiration response to high vapor pressure deficit in crop species. Plant Science 260, 109-118. [CrossRef]
- Smedbol, É., Lucotte, M., Maccario, S., Gomes, M.P., Paquet, S., Moingt, M., Lucero, L., Mercier, C., Perez Sobarzo, M.R. et Blouin, M.-A. (2019). Glyphosate and aminomethylphosphonic acid content in glyphosate-resistant soybean leaves, stems, and roots and associated phytotoxicity following a single glyphosate-based herbicide application. Journal of Agricultural and Food Chemistry, 67, 6133-6142. [CrossRef]
- Tanaka, Y., Fujii, K. et Shiraiwa, T. (2010). Variability of leaf morphology and stomatal conductance in soybean [Glycine max (L.) Merr.] cultivars. Crop Science, 50, 2525-2532. [CrossRef]
- Tanaka, Y. et Shiraiwa, T. (2009). Stem growth habit affects leaf morphology and gas exchange traits in soybean. Annals of Botany 104, 1293-1299. [CrossRef]
- Tanaka, Y., Shiraiwa, T., Nakajima, A., Sato, J. et Nakazaki, T. (2008). Leaf gas exchange activity in soybean as related to leaf traits and stem growth habit. Crop Sicence, 48, 1925-1932. [CrossRef]
- Triplett, G.B. et Dick, W.A. (2008). No-tillage crop production: A revolution in agriculture! Agronomy Journal, 100, 153-166. [CrossRef]
- Uhl, D. et Mosbrugger, V. (1999). Leaf venation density as a climate and environmental proxy: A critical review and new data. Palaeogeography, Palaeoclimatology, Palaeoecology, 149, 15-26. [CrossRef]
- Wagg, C., van Erk, A., Fava, E., Comeau, L.-P., Mitterboeck, T.F., Goyer, C., Li, S., McKenzie-Gopsill, A. et Mills, A. (2021). Full-season cover crops and their traits that promote agroecosystem services. Agriculture 2021, 11, 830, 11(830), 1-26. [CrossRef]
- Xiong, D., Flexas, J., Yu, T., Peng, S. et Huang, J. (2017). Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytologist, 213, 572-583. [CrossRef]
- Yu, Z., Lu, C., Hennessy, D.A., Feng, H. et Tian, H. (2020). Impacts of tillage practices on soil carbon stocks in the US corn-soybean cropping system during 1998 to 2016. Environmental Research Letters, 15, 1-13. [CrossRef]
- Zeiger, E., Farquhar, G.D. et Cowan, I.R. (1987). Stomatal Function The evolution of stomata. California, United States of America : Stanford University Press.
- Zhao, C., Liu, B., Piao, S., Xuhui Wang, X., Lobell, D.B., Huang, Y., Huang, M., Yao, Y., Bassu, S., Ciais, P., Durand, J.-L., Elliott, J., Ewert, F., Janssen, I.A., Li, T., Lin, E., Liu, Q., Martre, P., Müller, C., Peng, S., Peñuelas, J., Ruane, A.C., Daniel Wallach, D., Wang, T., Wu, D., Liu, Z., Zhu, Y., Zhu, Z. et Asseng, S. (2017). Temperature increase reduces global yields of major crops in four independent estimates. PNAS, 114(35), 9326-9331. [CrossRef]
- Zobiole, L.H.S., Kremer, R.J., de Oliveira Jr, R.S. et Constantin, J. (2010). Glyphosate affects photosynthesis in first and second generation of glyphosate-resistant soybeans. Plant Soil, 336, 251-265. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).