1. Introduction
Contamination by pathogenic bacteria is among the most frequent motives for the prohibition of access to rivers worldwide (Garcia-Armisen and Servais 2007; Mitch et al. 2010; Muniz et al. 2020). Adequate river management is crucial, given that high levels of bacterial contamination may represent a risk to the health of bathers, non-contact recreational users, and the consumers of raw or undercooked food that has come into contact with the river water (Lee et al. 2016; Seo et al. 2019). These pathogenic bacteria include coliforms and enterococci, which can cause serious illnesses, such as gastroenteritis and diarrhea. The positive correlation between the density of these enteric bacteria and the occurrence of gastrointestinal diseases has been used as an indicator of the presence of pathogenic microorganisms in aquatic environments, especially in waters used for recreational purposes (WHO 2003). Unplanned urban growth may contribute to the proliferation of these microorganisms due to the absence of adequate municipal services, in particular public sanitation services and sewage treatment facilities (Bek et al. 2018). In this context, contamination may occur through both specific sources, such as untreated sewage, and more general input from urban activities, industrial effluents, dry weather washout from storm sewers, and associated sewer overflows (Garcia-Armisen and Servais 2007; Bohra et al. 2012; Kindiki et al. 2018).
Understanding the use of water and its potential relationship with human activities is fundamental to the development of strategies for local water management policies (Petrucio et al. 2005). Domestic effluents and other residues may cause substantial changes in the quality of the water of an aquatic environment in terms of hydrological indicators and its trophic state (Dodds 2006). The analysis of pathogenic bacteria, hydrological variables, and the trophic state of the system is essential for the evaluation of water quality and the proposal of effective management measures.
Given its enormous size, Brazil presents considerable regional diversity in economic conditions and sanitation infrastructure, with only 40% of the sewage generated nationally being treated adequately (von Sperling 2016). The Brazilian National Environment Council (CONAMA) regulates the criteria used to classify water resources and establishes standards for the evaluation of aquatic environments and freshwater resources. In particular, the concentrations of thermotolerant coliforms and enterococci, and other hydrological variables are used as indicators of water quality, to determine the aptness of aquatic resources for different uses, including drinking water for humans and livestock, the preservation of aquatic environments and their biota, recreational activities, irrigation, aquaculture and fisheries, and navigation.
With an estimated population of 128,914 inhabitants (IBGE 2020), the town of Bragança, located in the North region of Brazil, lacks an adequate basic sanitation system due to the lack of investments in infrastructure, municipal services, and territorial planning, that is, the town lacks any sewage collection system, regular garbage collection or drinking water and sewage treatment plants. While the town’s population grows 2.1% per annum, the municipality does not have any housing program to support the underprivileged sectors of the local population, which tends to occupy areas of environmental preservation through unregulated settlement (Pereira et al. 2021). As a result, local water resources, including the Cereja River (which runs through much of the more densely-populated sectors of Bragança), are being impacted increasingly (Gorayeb et al. 2009; Guimarães et al. 2009).
Given these problems, this present study focuses on the Cereja River, a tributary of the Caeté estuary, and a Permanent Preservation Area (PPA) according to the Federal law number 12,651/2012 (Brazil 2012). This law prohibits the occupation of river margins to ensure the “preservation of water resources, the landscape, geological stability and biodiversity, in order to guarantee the gene flow of the fauna and flora, the protection of the soil, and the welfare of local human populations” (Article 3, item II). In recent decades, however, the water of the Cereja River has been compromised profoundly by the illegal occupation of its margins and the discharge of raw sewage directly into the river (Sousa 2015).
The present study tested the hypothesis that the quality of the water of the Cereja River has worsened significantly over recent years due to unplanned urban growth. To achieve this, the study assessed spatial and temporal changes in the quality of the water of the Cereja River, comparing data from 2013–2014 with those available for 2018–2019. To evaluate water quality for direct or indirect use, we employed the current Brazilian legislation, used international classification criteria for water quality and examined the Master Plan of the municipality to verify the effects of unplanned urban growth. We also adopted the DPSWR (Driver-Pressure-State-Welfare-Response) socioecological framework to identify potential measures for the management of environmental and anthropogenic problems (Cooper 2013). It is hoped that the results of the study can provide important guidelines for further research in equivalent fluvial environments under similar anthropogenic pressures.
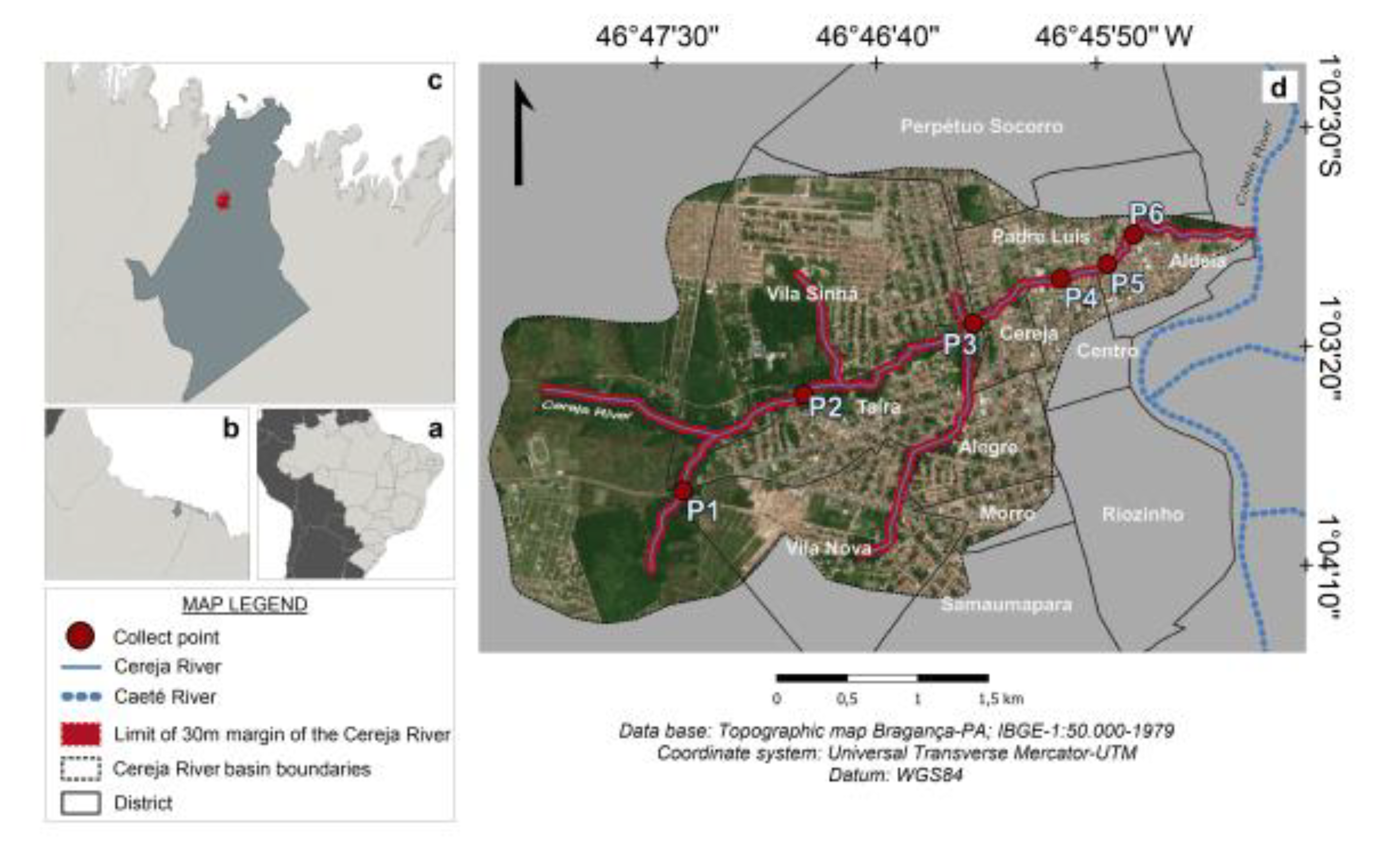
2. Study Area
The Cereja River is located in Bragança, a town in the Northeast mesoregion of the Brazilian state of Pará, which has approximately 128,914 inhabitants (IBGE 2020). The source of this river is located outside the urban zone of Bragança, and it crosses the town from West to East, flowing through the neighborhoods of Vila Sinhá, Taíra, Alegre, Padre Luiz, Centro, and Aldeia (Silva 2000; Santos et al. 2014). The waters of the Cereja River run through 11 of the 12 official neighborhoods that are located partially within its drainage basin, which has an area of approximately 10 km
2; (
Figure 1). The principal course of the river is approximately 5 km long, with a mean depth of 1.4 m (Santos et al. 2013).
The study region has a humid equatorial climate, characterized by high levels of rainfall between January and July, with annual rainfall of over 2,000 mm (Moraes et al. 2005). During the rainy season, the level of the Cereja River rises to 1.5 m above the mean levels of its bed, which often causes flooding and other impacts (Silva Filho and Nunes 2017).
Uncontrolled urban growth and the unplanned occupation of the margins of the Cereja River have led to the widespread and uncontrolled discharge of domestic effluents and solid waste into the river (Gorayeb et al. 2011; Santos et al. 2014). According to the data provided by the Brazilian Institute of Geography and Statistics (IBGE 2020), the population of the municipality of Bragança is currently growing at a rate of approximately 2.1% per annum. This exacerbates the long-standing socio-environmental problems associated with the lack of basic sanitation.
Figure S1 shows the loss of vegetation associated with the erosion in the margins and the deposition of sediment on the riverbed and presence of solid waste.
3. Methods
Data on rainfall and population growth were obtained from public institutions, while hydrological and microbiological data were obtained during fieldwork. A total of six sampling points were selected in the different neighborhoods of Bragança city, where samples were collected during the rainy and dry seasons of 2013–2014 and 2018–2019. High-resolution satellite images from GoogleEarth (2012 and 2019) were used to verify unplanned occupation of land.
3.1. Rainfall Data
Rainfall data were obtained from (INMET - National Institute of Meteorology, Tracuateua meteorological station) for the study period, and for 1982–2019 (historical means). The Tracuateua station is 17 km west of Bragança, at 36 m above sea level. The monitoring of rainfall patterns is essential for the understanding of natural oscillations in hydrological variables and their possible influence on water quality and microbiological contamination.
3.2. Field Survey
The samples were collected during two distinct periods (2013–2014 and 2018–2019) to assess changes in the quality of the water of the Cereja River over time. Seven campaigns were undertaken, four during the dry season (November 2013; August 2014; November 2018; July 2019) and three during the rainy season (April 2014; June 2018; April 2019), with data being collected at six fixed points (supplementary material -
Table S1). A total of 126 samples were collected (42 samples for the measurement of pH, turbidity, and chlorophyll-a, 42 samples for the measurement of dissolved oxygen concentrations, and 42 samples for the evaluation of microbiological variables). Surface water samples were collected near the margin of the river at six points using a Niskin bottle. Once collected, the samples were stored in 250 mL polyethylene containers or glass flasks for laboratory analysis, following the APHA (American Public Health Association) protocol (APHA 2004).
3.3. Laboratory Procedures
The water samples were vacuum-filtered through glass-fiber filters (Millipore GF/F 0.7 mm, 47 mm) and freeze-dried for the analysis of chlorophyll-a. The pH was determined by a HANNA pH meter (HI2211), and a HANNA turbidimeter (HI 93703) was used to measure turbidity. The concentrations of dissolved oxygen (DO) were obtained, according to the method described by Winkler, as modified by (Grasshoff et al. 1983). The chlorophyll-a was extracted from the filters with 90% acetone v.v. and the concentration was determined by spectrophotometry, following (Parsons and Strickland 1963; UNESCO 1996).
The TSI (Trophic State Index) was used to calculate the level of eutrophication of the Cereja River based on its biological productivity. This index was developed by Carlson (1977) and modified by Lamparelli (2004). The water quality was classified in six categories: ultraoligotrophic (TSI ≤ 47), oligotrophic (TSI = 47 < TSI ≤ 52), mesotrophic (52 < TSI ≤ 59), eutrophic (59 < TSI ≤ 63), supereutrophic (63 < TSI ≤ 67), and hypertrophic (TSI > 67).
The method used to determine thermotolerant coliform levels was based on the inoculation of samples in both sodium lauryl sulphate (presumptive test) and brilliant green broths (confirmatory test) with posterior incubation for 48 h at 35±2°C. The positive samples were then inoculated in Escherichia coli (EC) selective broth for 24–48 h at 45±0.2°C. In both procedures, the presence of bacteria was confirmed by the cloudiness of the water and the formation of gas in the Durham tubes (APHA 2004). The Enterococcus sp. levels were analyzed by membrane filtration using the membrane-Enterococcus Indoxyl-ß-D-Glucoside Agar (mEI) method (EPA, 2006). This consists of the direct counting of the bacteria in a selective medium (mEI agar). All the detected colonies greater than or equal to 0.5 mm in diameter were counted for further enumeration.
The quality of the water was evaluated based on the CONAMA resolutions (CONAMA, 2000; 2005), Bricker (2003) and Lamparelli (2004). Freshwater is classified according to the type of use by CONAMA resolutions as shown in supplementary material (supplementary material -
Table S2).
3.4. Unplanned Occupation
The mapping of areas of unplanned occupation on the margins of the Cereja River basin consisted of four phases: (i) the capture of high-resolution satellite images from Google Earth; (ii) the assemblage of image mosaics for 2012 and 2019; (iii) creation of the interpretation key for the visual classification of the images, and (iv) formulation of the database and geoprocessing using the software QGIS 3.16.0 © (
Figure S2).
The total population of the Cereja River basin area was calculated by multiplying the number of properties by the size (individuals) of the mean household in the North region of Brazil, i.e., 4.3 people per household, following IBGE data from 2010 (IBGE 2020). “Unplanned” occupation was defined as any property located within the 30-meter buffer surrounding the margins of the Cereja River, which is considered to be a Permanent Protection Area (PPA) under the Federal law 12,651/2012 (Brazil 2012).
3.5. Sewage Production per Capita
The production of sewage by the inhabitants of each neighborhood bordering the Cereja River was estimated for 2012 and 2019 following the method of von Sperling (2007). Each individual produces 150 liters of sewage per day in urban areas and is responsible for releasing 100–400 billion thermotolerant coliforms into the environment.
3.6. Statistical Analysis
For the statistical analyses, the data were first evaluated for normality using the Anderson-Darling test (Stephens 1974), which has a high statistical power to determine whether a sample was extracted from a population with a specific distribution (Pettitt 1977). Levene’s F test (Levene 1960) with a 95% Bonferroni confidence interval was applied to verify the homogeneity of variances. The parametric one-way Analysis of Variance (ANOVA) and paired t-test were applied to the variables that complied with the assumptions of normality and homogeneity of variances. Otherwise, the nonparametric Welch ANOVA (Field 2009) was used. The paired t-test was used to compare mean values between years (Ross and Wilson 2017). Spearman correlation coefficients were used to examine the influence of rainfall levels on the variables analyzed. All statistical tests were run in the IBM SPSS software v. 25.
Principal Component Analyses (PCAs) was run in R Studio v 1.2.5, based on a correlation matrix generated by the FactorMineR package (Lê et al. 2008). The plots were created successively and edited in the Factoextra package (Kassambara and Mundt 2020). For a better visualization of the data, three sectors were established for the spatial PCA, based on the number of households present in 2019 (see Results): sector I (< 30 households, sampling points P1 and P2), sector II (30–50 households, P3), and sector III (>50 households, P4–P6).
4. Results
4.1. Rainfall Levels
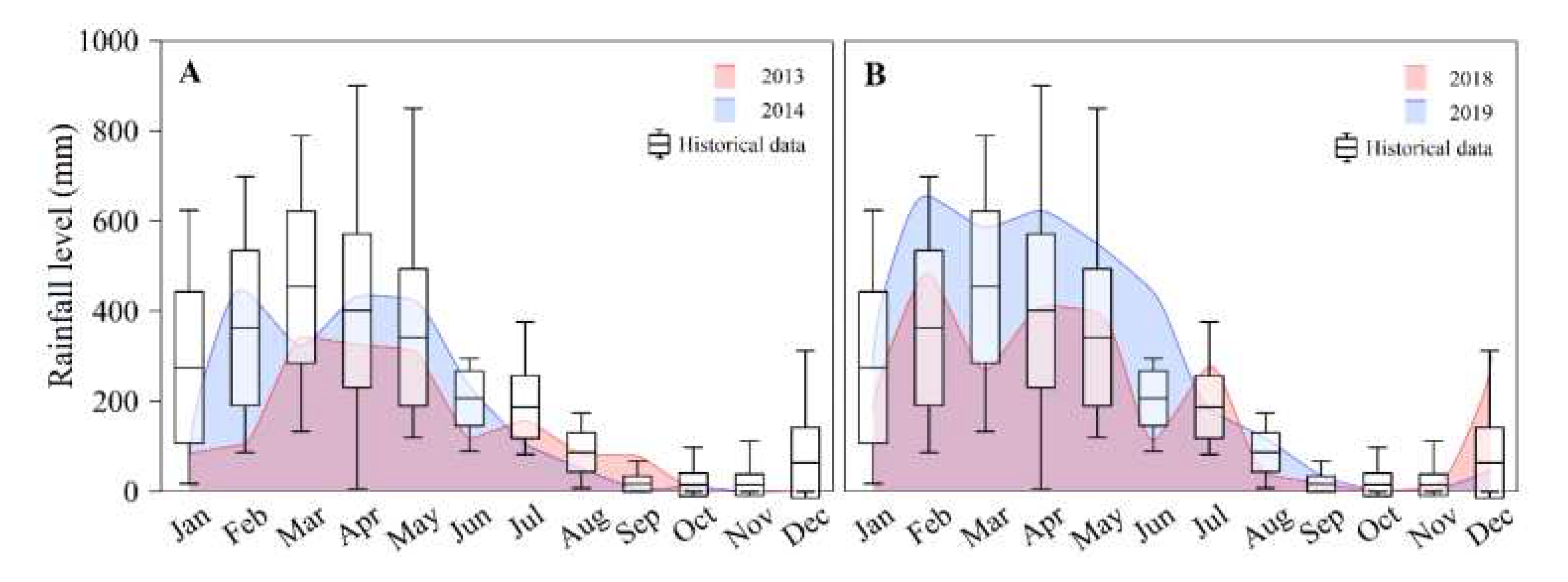
The historical mean annual rainfall (1982–2019) was 2,330 mm (
Figure 2). The driest year of the present study was 2013 (1,612 mm), while the rainiest was 2019 (3,512 mm), which was 151% higher than the historical mean (T = 2.36; p = 0.015). In 2013, the rains were more intense between March and May (~300 mm per month), whereas in 2019, the rainiest months were from February to June (440–660 mm per month). Two distinct seasons (rainy and dry) were identified (F = 80.51; p = 0.000,
Table 1), with April 2014 (total rainfall = 432 mm), April 2018 (410 mm), June 2018 (115 mm), and July 2019 (182 mm) corresponding to the rainy season, while November 2013 (1 mm), August 2014 (50 mm), and November 2018 (1 mm) to the dry season (
Figure 2).
4.2. Hydrological Variables
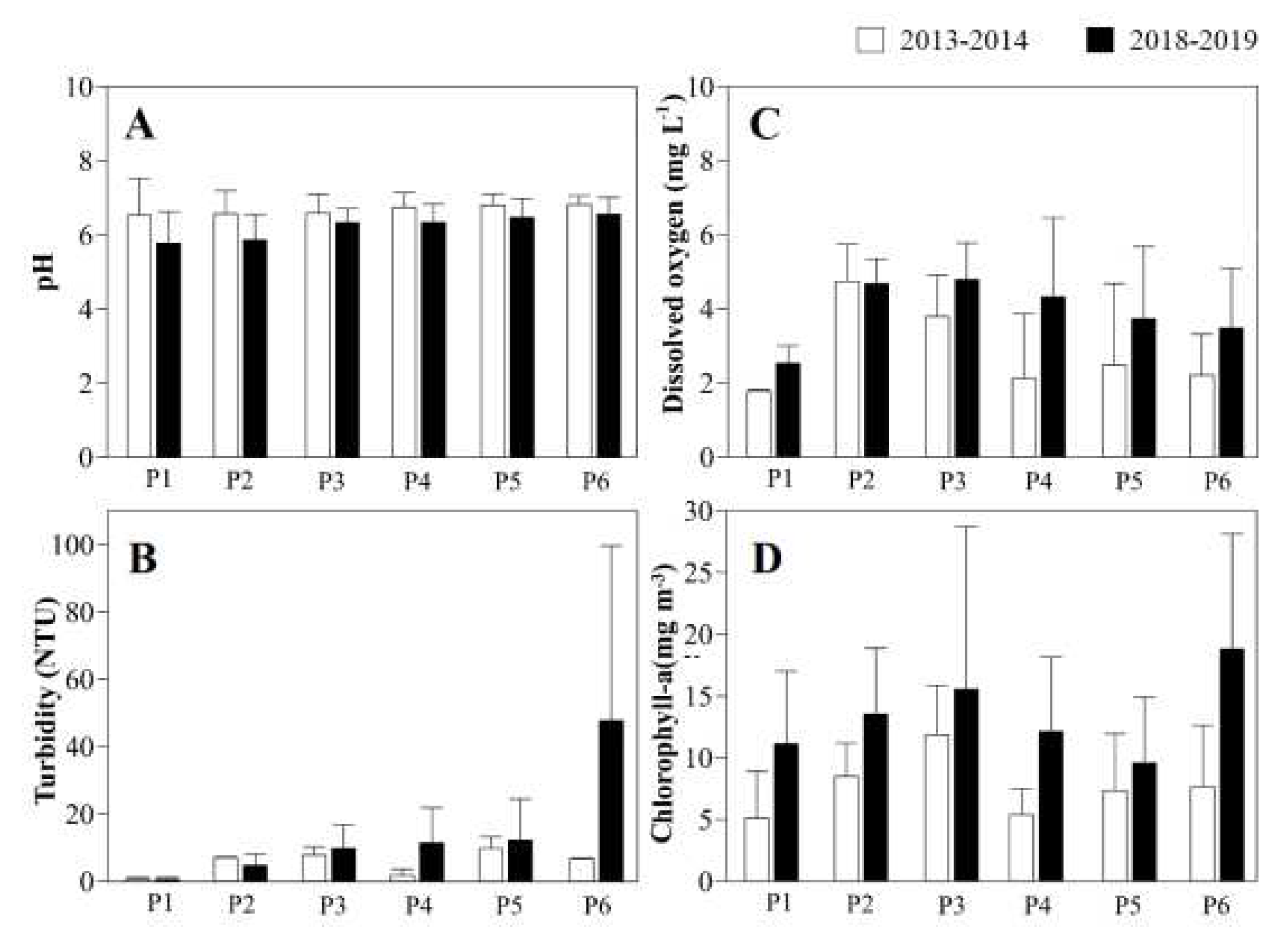
The mean pH (
Figure 3A) was significantly higher in 2013–2014 in comparison with 2018–2019 (t = -4.73; p = 0.000,
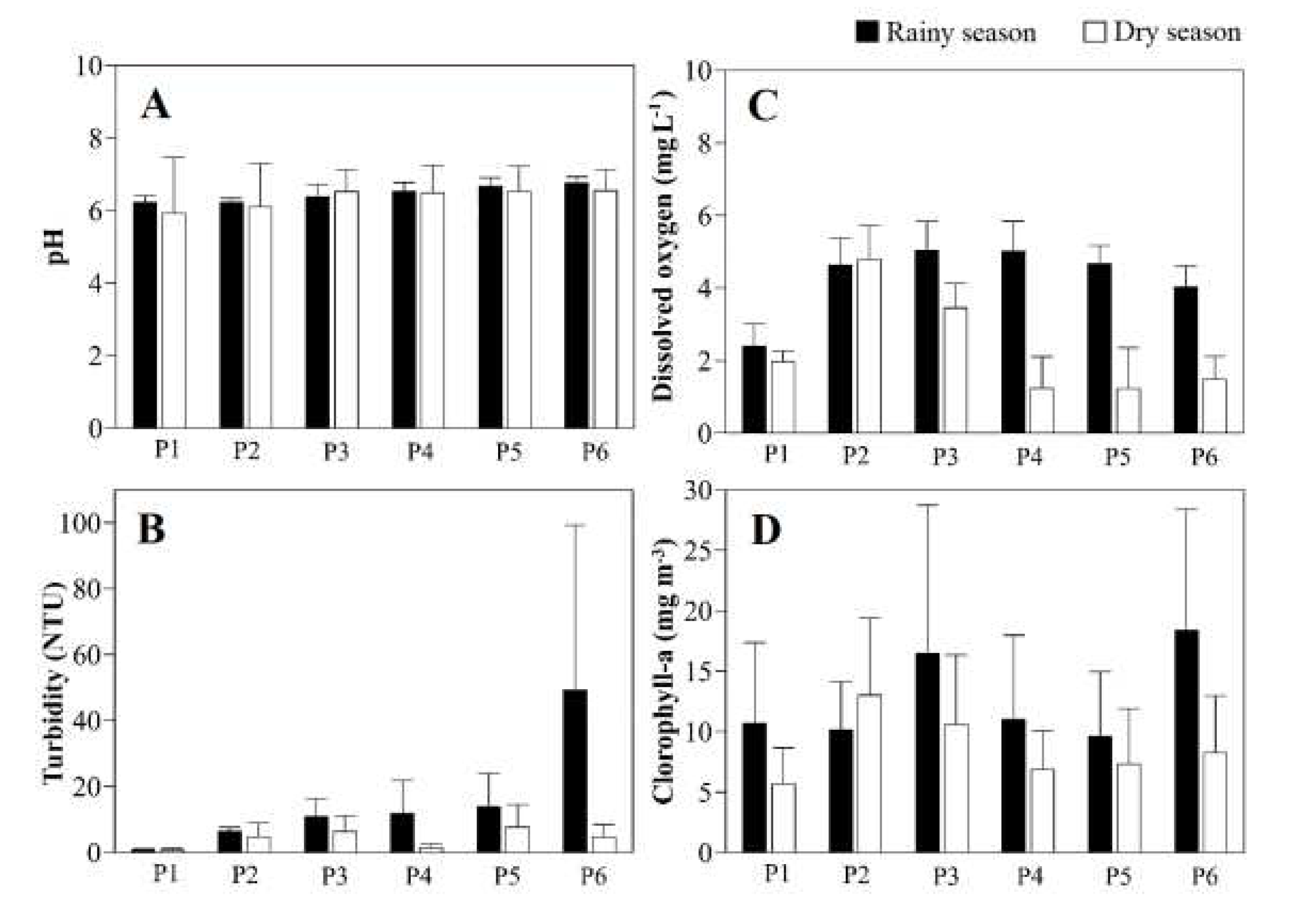
Table 1) with a peak mean of 6.8±0.3 at P5, with lower values at P1 (5.8±0.8). Seasonally, the highest mean pH was recorded during the rainy season (P6, 6.8±0.2) and the lowest in the dry season (P1, 5.9±1.6;
Figure 4A). Overall, 88% of the samples complied with CONAMA standards (Table 2).
The waters of the Cereja River were more turbid in 2018–2019 (
Figure 3B), with the peak mean turbidity being recorded at P6 (47.8±51.8 NTU), while the least turbid water was recorded in 2013–2014, with the minimum value being obtained at P1 (0.7±0.5 NTU). Seasonally, the most turbid water was recorded in the rainy season (F = 4.72; p = 0.04,
Table 1) with a peak mean being recorded at P6 (49.3±50.1 NTU, F = 9.34; p = 0.000,
Table 1), while the least turbid water was recorded in the dry season, with the lowest values being recorded at P1 (0.7 ± 0.6 NTU,
Figure 4B). Overall, 98% of the samples complied with CONAMA standards on turbidity (supplementary material -
Table S3).
The lowest mean dissolved oxygen concentrations (
Figure 3C) were recorded in 2013–2014 (T = 4.59: p = 0.001,
Table 1), with the lowest value being recorded at P1 (1.8±0.0 mg L-1). The highest values were recorded in 2018–2019, in particular at P3 (4.8 ± 1.0 mg L-1). Seasonally (
Figure 4C), the most oxygenated water was recorded during the rainy season (F = 22.75; p = 0.000,
Table 1), with a peak at P4 (5.0±0.9 mg L-1, F = 10.54; p = 0.000), while the lowest concentration was recorded in the dry season at P4 (1.2 ± 0.9 mg L-1). Only 17% of the water samples had dissolved oxygen concentrations consistent with CONAMA’s water use class 2, while 21% were considered hypoxic and 60% indicative of conditions of oxygen stress in the biological community (
Table S3).
The mean chlorophyll-a concentrations peaked at 18.8 ± 9.3 mg m-3 (at P6) in 2018–2019 (t = 2.68; p = 0.008,
Table 1), and were the lowest in 2013–2014, reaching 5.1±3.8 mg.m-3 at P1 (
Figure 3D). The water was significantly richer in chlorophyll-a during the rainy season (F = 4.37; p = 0.043,
Table 1), primarily at P6 (18.4±10.0 mg m-3;
Figure 4D), while the lowest values were recorded in the dry season, reaching 5.7±3.0 mg m-3 at P1. Just over half (57%) of the samples had chlorophyll-a concentrations consistent with CONAMA’s water use class 1 (
Table S3).
4.3. Microbiological Analysis
Over 90% of the water samples presented thermotolerant coliform levels of 1,100 MPN 100 mL-1 or more, which is the threshold recommended by CONAMA for most types of water use (supplementary material-
Tables S4 and S5). Site P1 was the only sector with appropriate conditions for most types of water use during the dry season, when values did not exceed 240 MPN 100 mL-1. The other sectors were impacted intensely by sewage outfalls, however, with up to 90.5% of the samples from the most contaminated points having coliform concentrations of 1,100 MPN 100 mL-1 or more.
As the Enterococcus levels were only analyzed in 2018–2019, it was not possible to compare years (
Table S4). However, the findings did show that the quality of the water at P1 was relatively good (0–101 MPN 100mL-1) in comparison with the other points, peaking at P5 and P6 (generally ~500 MPN 100 mL-1). As CONAMA does not provide a value for this bacterium, the bacterial water quality criteria were adopted according with USEPA (1986). According to these criteria, 93% of the water samples analyzed were unappropriated for recreational contact (
Table S5), and water use would only be permitted at P1 during the periods with the lowest rainfall levels.
4.4. Trophic State Index (TSI)
The water of the Cereja River had a significantly higher trophic level in 2018–2019 (t = 5.17; p = 0.000,
Table S3), peaking at 71.4 ±5.1 (hypertrophic) at P6, with the lowest mean (66.4±6.6 – super-eutrophic) being recorded at P1 (Figure 5A). In 2013–2014, the lowest values were recorded at P1 (59.0±8.2 – eutrophic) and the highest, at P3 (68.1±2.8 – hypertrophic). The highest mean values were recorded during the rainy season (Figure 5B), peaking at P6 (70.8±6.1 – hypertrophic) and reaching their lowest level at P1 (64.7±9.0 – super-eutrophic). During the dry season, the highest values were recorded at P2 (68.5±4.3) and the lowest, at P1 (61.2±4.6). Overall, 78% of the samples were classified as eutrophic, super-eutrophic or hypertrophic (
Table S3).
4.5. Unplanned Occupation
The number of unplanned constructions identified with the PPA increased 18% between 2012 and 2019, with the highest growth rates being recorded in Vila Nova (P1, 53.8%) and Vila Sinhá (P2, 36.4%), while the most populous sectors were Padre Luiz (P4) and Aldeia (P5, P6). In 2019, a total of 318 constructions were recorded on the margins of the Cereja River, within the PPA, with an estimated 1,369 inhabitants (supplementary material -
Table S6). The vegetation cover in the PPA was 0.37 km
2; in 2012 and 0.34 km
2; in 2019, with approximately 4% of this area being lost due to unplanned occupation (
Table S6).
Within the Cereja River PPA, in 2019, the greatest volume of domestic effluents was recorded in Padre Luiz (P4, 60.6 m3 day-1), Aldeia (P5, P6, 35.5 m3 day-1), Cereja (27.1 m3 day-1), and Taíra (P3, 25.8 m3 day-1), with a total of 151.6 m3 day-1. The quantity of effluent recorded in 2019 was 18% greater than in 2012. This resulted in the highest thermotolerant coliform concentrations being recorded in these neighborhoods in 2019 (
Table S6), that is, 1.62 MPN 100 mL-1 (x1014) in Padre Luiz, 0.95 MPN 100 mL-1 (x1014) in Aldeia, 0.79 MPN 100 mL-1 (x1014) in Cereja, and 0.69 MPN 100 mL-1 (x1014) in Taíra. Given the increase in unplanned occupation in Vila Nova (P1), an increase of 54% was recorded in the volume of domestic effluents at this point in 2019.
4.6. Integration of the Water Quality Indicators
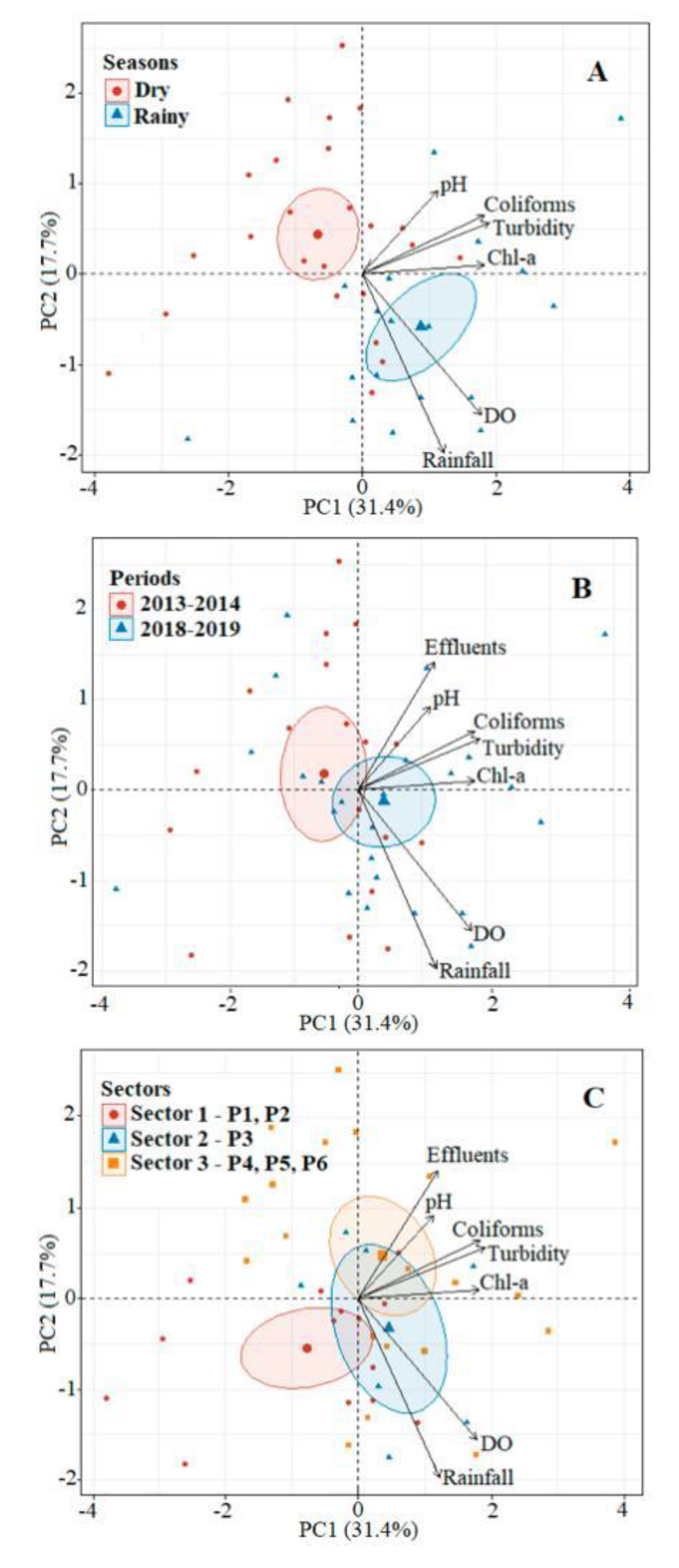
The first two axes of the Principal Components Analysis (PC1 and PC2) explained 49.1% of the variability in the dataset, with 31.4% and 17.7%, respectively (
Figure 6). Axis PC1 was positively related with turbidity (0.67), dissolved oxygen (0.62), chlorophyll-a (0.64), and coliform concentrations (0.64). Effluents (0.50) and pH (0.81) also presented a strong positive correlation with all of these variables in quadrant I. By contrast, PC2 presented a strong correlation with dissolved oxygen concentrations (-0.55) and rainfall (-0.69), clustering in quadrant II.
Seasonally, the variables were grouped with the rainy season (
Figure 6A), indicating a slightly acidic water, which was relatively turbid, well-oxygenated, and rich in chlorophyll-a. A similar pattern was recorded between years, with all the variables grouped with the 2018–2019 (
Figure 6B) due to a longer rainy season. Coliform levels and effluent production were also higher in the second study period, due to population growth over time. Spatially (
Figure 6C), the most urbanized area (sector III, P4-P6) clustered with the highest values of pH, turbidity, chlorophyll-a, coliforms, and effluents. This indicates that these neighborhoods maintain the highest values of these variables between seasons and among years.
5. Discussion
5.1. Natural Conditions, Anthropogenic Activities, and Management Concerns
Oscillations in rainfall levels are one of the principal factors that determine seasonal shifts in the physicochemical and hydrobiological parameters of riverine systems (Zeiringer 2018).
Among the studied variables, the pH is one of the most important for the evaluation of water quality, because it indicates specific conditions and alterations (Ríos-Villamizar et al. 2014; Zebo et al. 2016). In the present study, the water was slightly acidic with lower pH values being recorded during the second period (2018–2019), as a consequence of the more prolonged rainy season, which resulted in a major increase in the input of freshwater in comparison with 2013–2014. The discharge of sewage into a river also tends to reduce its pH (Barakat et al. 2016 and Zebo et al. 2017), and the increased production of effluents recorded in 2019 (18% greater than 2012) may also have contributed to the decrease in pH values during the 2018–2019 period. The presence of an area of better-preserved habitats at the headwaters of the Cereja River may also have favored the lower pH values recorded in sector 1 (P1 and P2), due to the higher concentration of plant-based organic matter in the water, derived from the riparian vegetation and forest fragments upstream of P1, which contributes higher concentrations of humic acid, as reported by Duncan and Fernandes (2010) and Ríos-Villamizar et al. (2014) in other Amazonian rivers.
Turbidity typically depends on the amount of particulate material suspended in the water and it is also considered to be an important indicator of water quality (Alcantara et al. 2009; Marinho et al. 2021; Nittrouer et al. 2021). The turbidity of river water depends on natural conditions (e.g., rainfall patterns, river flow levels) and anthropogenic activities (e.g., effluent discharge, deforestation, mining). In the present study area, the water was relatively more turbid during the first half of the year, in particular in 2018–2019, due to the prolonged rainy season, although no systematic correlation was found between turbidity and precipitation. Even so, in Amazonian river systems, the increase in the water level during the rainy season and the related growth in fluvial discharge is known to intensify the re-suspension of sediments from the bottom to water column (Nittrouer and DeMaster 1996; Asp et al. 2018). Changes in land use associated with urbanization may also contribute to an increase in turbidity (Arnold and Toran 2018), and in the most urbanized sectors of the study area (sectors 2 and 3), the unregulated occupation of the margins of the river have resulted in shifts in the riparian vegetation, which has contributed to the erosion of the margins of Cereja River, and a further increase in turbidity value in these sectors. Turbidity may also be influenced by urban runoff (Díaz-Torres et al. 2021). In the present study, the high levels of effluent discharge into the most urbanized sector of the river contributed to the highest turbidity being recorded in this sector, supported by their positive correlation (rs = 0.404, p < 0.008, n = 56). In fact, excessive turbidity, in terms of the CONAMA criterion, was only recorded in sector P6. The input of the turbid waters from the Caeté estuary may also have contributed to these values, however, given the role of the extensive local mangroves and precipitation patterns in the sedimentary dynamics of an estuary controlled by meso-macrotides (Asp et al. 2018).
Dissolved oxygen is also considered to be a good indicator of water quality (Rabalais and Harper 1992; Diaz-Torres et al. 2021). Under natural conditions, the waters of Amazonian rivers are well oxygenated, due to their high hydrodynamic energy, in particular during the rainy season (Gagne-Maynard et al. 2017). This results in a combination of high water levels and more intense river flow, which increases the efficiency of the exchange between the atmosphere and surface waters. In the Cereja River, the highest values were recorded during the rainy season, when the water level is at its highest and the river flow is most intense, with a positive correlation being recorded between dissolved oxygen concentrations and precipitation (rs = 0.489, p < 0.001, n = 56). Additionally, the local municipal authorities often dredge the river to clean it up during the raining, a process that contributes to an increase in fluvial discharge due to the greater depth of the river, which further enhances the interaction between the water and the atmosphere.
Unfortunately, the intense discharge of sewage into the river contributed to a reduction in the dissolved oxygen concentrations, which results in conditions of hypoxia (0–2 mg L-1) or oxygen stress for the biological community (2–5 mg L-1). This is the result of the consumption of oxygen by bacterial decomposition, which is a response to the high concentrations of organic matter present in the environment (Bricker 2003; Sanchez et al. 2007; Mocuba 2010). While no correlation was found between dissolved oxygen concentration and the production of effluents, there was a significant correlation between dissolved oxygen and thermotolerant coliform concentrations (rs = 0.338, p < 0.028, n = 56).
The discharge of untreated sewage contributes to eutrophication (Dodds 2006; Wu et al. 2017; Tang et al. 2020) and the density of phytoplankton is considered to be an important indicator of eutrophic environments (Yusuf 2020). In the study area, the discharge of sewage into the Cereja River appears to have contributed to an increase in the chlorophyll-a concentrations in the most urbanized sector through the proliferation of microalgae, which is reflected in a longitudinal gradient, with the lowest chlorophyll-a values being recorded in sector P1 and the highest in P6 (15% higher than P1, on average). The TSI, which is based on biological productivity, once again indicates that the highest trophic levels were recorded in the most urbanized sector in 2018–2019, reflecting the negative influence of population growth and the related increase in effluent discharge on the quality of the water of the Cereja River.
Effluent discharge also may affect human health due to presence of microorganisms (Tryland et al. 2014; Nishiyama et al. 2015; Medeiros et al. 2017). The microbiological data indicate that the quality of the water of the Cereja River was already compromised in 2013–2014, and only worsened in 2018–2019. With the exception of sector P1, all the water samples exceeded the recommended limits for thermotolerant coliforms, whether measured or estimated, and Enterococcus sp. due to the large number of sewage outfalls within the study area. The discharge of untreated sewage into aquatic environments is strictly prohibited in Brazil by CONAMA (resolution 357/2005) and in Pará by state law number 5,887/1995 (Pará 1995). However, based on the CONAMA and USEPA (1986) criteria, the water of the Cereja River is inadequate for any type of human use, given that more than 90% of the water samples tested were contaminated with pathogenic bacteria. Previous studies have also shown that Cereja River is responsible for a considerable proportion of the sewage discharged into the Caeté estuary, given that the concentrations of thermotolerant coliforms at the confluence of these two bodies of water exceed 1,100 MPN per 100 mL-1 (Monteiro et al. 2016; Pereira et al. 2021). Unfortunately, this is a common scenario in many rivers located in the vicinity of urban areas that have inadequate public sanitation or completely lack any infrastructure, resulting in an increase in the levels of human pathogens (Agbogu et al. 2006; Tryland et al. 2014).
5.2. Application of the DPSWR, Management Actions, and Recommendations
Rapid population growth associated with a lack of urban planning and inadequate investment in public sanitation are considered to be the principal drivers responsible for the discharge of large amounts of untreated sewage into urban rivers and the illegal occupation of their margins (supplementary material -
Figure S3). Considering the eight neighborhoods located on the margins of the Cereja River, the number of residences located illegally within the PPA increased by 18% between 2012 (261 households) and 2019 (318 households), while the discharge of effluents grew from 168 m3 day-1 to 205 m3 day-1, an increase of about 18.1%.
As shown in results, with the exception of sector P1, all the water samples exceeded the recommended limits for thermotolerant coliforms and Enterococcus sp. due to the large number of sewage outfalls within the study area and as a consequence local population may be affected by gastroenteritis and diarrhea. High rainfall levels tend to exacerbate this situation. During the rainy season, the Cereja River floods, and its water reaches the level of the sewage outfalls located on higher ground, while also lixiviating other substances into the soil, which leads to a further increase in the concentration of pathogenic bacteria. This reinforces the negative effects of the illegal occupation of river margins, and increases the discharge of sewage, which reached 205 m3 per day in 2019.
Unplanned urbanization and urban growth also affect land use and cover (Kuhlmann et al. 2014; Das and Paul 2020) and lead to the erosion of the river margins, for example, which results in the siltation of the riverbed, as recorded in the present study, where illegal occupation is also associated with deforestation. While the urban development of river margins is strictly prohibited in Brazil by federal law 12,651/2012 (Brazil 2012), failures to enforce this legislation were obvious, and 57 new residences were added to the PPA between 2012 and 2019. In the most urbanized sectors of the study area, population growth was relatively low because the margins are already almost completely occupied, whereas in the least urbanized sectors, there was a considerable increase in the number of residences – 53.8% in sector P1 and 36.4% in P2 – which contributed to a decline in the quality of the water at the source of the Cereja River, based on all the different indices, in 2018–2019 in comparison with 2013–2014.
Overall, then, effluent discharge and illegal occupation have affected the welfare of local populations due to the risks to both human health and environmental equilibrium. In particular, coliform bacteria can cause serious illnesses such as gastroenteritis and diarrhea through contact with water polluted by untreated sewage (Lee et al. 2016; Seo et al. 2019).
Despite being an area protected by Federal law, preservation measures on the Cereja River are limited to annual, but irregular dredging, and warning signs on the margins of the river, announcing the prohibition of unplanned occupation of the river margins. These measures are clearly inadequate, however, and some level of protection could be achieved if the measures proposed initially in the 2006–2016 municipal master plan (and transferred to the 2016–2026 master plan) were implemented systematically.
The findings of the present study lead us to recommend the following measures (responses) – the establishment of an effective public sanitation system that prevents the disposal of solid waste, including plastics, paper, and metal, leftover food, and human and animal excrement, directly onto the rivers that traverse the urban sector of the town of Bragança, to inhibit the possible spread of disease, and prevent the risk of injuries, visual pollution, and the migration of floating debris on the Cereja River and Caeté Estuary.
In addition to this recommendation, education programs and more effective monitoring will be essential to prevent the unplanned occupation of the river margins. Appropriate planning of land use will also be essential to improve the local landscape and avoid potential conflicts.
6. Conclusions
Rapid population growth, associated with a lack of urban planning, illegal and unplanned occupation, and reduced investment in infrastructure or services, have caused intense pressure on the Cereja River in recent years. The increase in the number of households found on the margins has contributed to the river’s intense trophic condition, low concentrations of dissolved oxygen, high concentrations of pathogenic bacteria, and loss of vegetation cover. These processes affect both human welfare and the health of the environment. The negative effects of population growth and the related increase in effluent discharge on the quality of the water of the Cereja River are observed clearly in 2018–2019, in particular during the rainy season.
Overall, the water quality of the Cereja River, including next to its source, already exceeds sanitary standards. The discharge of untreated sewage into aquatic environments is strictly prohibited in Brazil by federal, state, and municipal resolutions, but few responses were apparent from the local authorities to minimize or resolve the existing problems. The water of the Cereja River is inadequate for any human use, and its bacteriologic contamination is one of the principal factors determining the reduced quality of the water of the Caeté estuary. The findings of the present study indicate clearly that contamination by pathogenic bacteria has worsened over the past decade and may be having an impact on public health. Overall, the DPSWR model proved to be an excellent analytical tool for the evaluation of specific local scenarios, through the integrated analysis of socio-environmental issues. It clearly has potential applications for other rivers in the Amazon region, which have a broadly similar configuration of drivers and responses as that observed in the present study. The scenario observed in the Cereja River can be extrapolated to other rivers located within urban areas in the Amazon region that have relatively large populations.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Acknowledgments
This study was financed by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, through a Universal projects (483913/2012-0, 431295/2016-6). Pereira LCC (314037/2021-7) and Costa (314040/2021-8) would like to thank CNPq for research grants, and Costa (88881.736742/2022-01) would also like to thank CAPES for research grants. We are also indebted to Stephen Ferrari for his careful revision of the English text.
References
- Agbogu VN, Umoh VJ, Okuofu CA, Smith SI, Ameh JB. Study of the bacteriological and physicochemical indicators of pollution of surface waters in Zaria, Nigeria. Afr J Biotechnol 2006, 5, 732–737. [Google Scholar]
- Alcântara E, Novo E, Stech J. , Lorenzzetti J, Barbosa C, Assireu A, Souza A. The turbidity behavior in an Amazon floodplain. Hydrol Earth Syst Sci Discuss 2009, 6, 3947–3992. [Google Scholar]
- APHA - American Public Health Association. 2004, Standard Methods for Examination of Water and Wastewater Washington, DC.
- Arnold E, Toran L. Effects of Bank Vegetation and Incision on Erosion Rates in an Urban Stream. Water 2018, 10, 482.
- Asp NE, Gomes VJC, Schettini CAF, Souza-Filho PWM, Siegle E, Ogston AS, Nittrouer CA, Silva JNS, Nascimento WR, Souza SR, Pereira LCC, Queiroz, MC. Sediment dynamics of a tropical tide-dominated estuary: Turbidity maximum, mangroves and the role of the Amazon River sediment load. Estuar Coast Shelf Sci 2018, 214, 10–24. [Google Scholar] [CrossRef]
- Bek MA, Azmy N, Elkafrawy S. The effect of unplanned growth of urban areas on heat island phenomena. Ain Shams Eng J 2018, 9, 3169–3177. [Google Scholar] [CrossRef]
- Bohra DL, Modasiya VB, Kumar C. The distribution of coliform bacteria in wastewater. Microbiol Res 2012, 3, 5–7. [Google Scholar]
- Bragança. 2015, Complementary Law no 006 on Master plan.
- Brazil. 2012, Law No 12651 on the Protection of Native Forests.
- Bricker SB, Ferreira JG, Simas T. An integrated methodology for assessment of estuarine trophic status. Ecol Model 2003, 169, 39–60. [Google Scholar] [CrossRef]
- CONAMA - National Council of the Environment. 2000, Resolution 274/2000. Available via http://www2mmagovbr/port/conama/legiabrecfm?codlegi=272. Cited 15 Nov 2022.
- CONAMA - Conselho Nacional do Meio Ambiente. 2005, Resolution 357/2005. Available via http://www2mmagovbr/port/conama/legiabrecfm?codlegi=459. Cited 15 Nov 2022.
- Cooper, P. Socio-ecological accounting: DPSWR, a modified DPSIR framework, and its application to marine ecosystems. Ecol Econ 2013, 94, 106–115. [Google Scholar] [CrossRef]
- Das S, Paul S. Estimation of Land Surface Temperature of Cooch Behar Municipality to study Urban Heat Island using Landsat 8 Image. Indian J Spat Sci 2020, 11, 69–75. [Google Scholar]
- Díaz-Torres O, Anda J, Lugo-Melchor OY, Pacheco A, Orozco-Nunnelly DA, Shear H, Senés-Guerrero C, Gradilla-Hernández MS. 2021, Rapid Changes in the Phytoplankton Community of a Subtropical, Shallow, Hypereutrophic Lake During the Rainy Season. Front Microbiol 12. [CrossRef]
- Dodds WK. 2006, Eutrophication and trophic state in rivers and streams. Limnol Oceanogr 51(1, part 2): 671-680.
- Duncan WP, Fernandes MN. Physicochemical characterization of the white, black, and clearwater rivers of the Amazon Basin and its implications on the distribution of freshwater stingrays (Chondrichthyes, Potamotrygonidae). Pan-Am J Aquat Sci 2010, 5, 454–464. [Google Scholar]
- EPA - Environmental Protection Agency. 2006, Method 1600: enterococci in water by membrane filtration using membrane-Enterococcus indoxyl-beta-D-glucoside agar (mEI) Washington, DC.
- Field A. 2009, Discovering Statistics Using SPSS 3rd Edition, Sage Publications Ltd, London.
- Gagne-Maynard WC, Ward ND, Keil RG, Sawakuchi HO, Cunha AC, Neu V, Brito DC, Silva LDF, Diniz JEM, Matos VA, Kampel M, Krusche AV, Richey JE. Evaluation of Primary Production in the Lower Amazon River Based on a Dissolved Oxygen Stable Isotopic Mass Balance. Front Mar Sci 2017, 4, 26. [Google Scholar]
- Garcia-Armisen T, Servais P. Respective contributions of point and non-point sources of E coli and enterococci in a large urbanized watershed (the Seine river, France). J Environ Manage 2007, 82, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Gorayeb A, Lomabardo MA, Pereira LCC (2011) Natural conditions and environmental impacts in a coastal Hydrographic Basin in the Brazilian Amazon. J Coast Res SI64: 1340-1344.
- Gorayeb A, Lombardo MA, Pereira LCC. Environmental conditions in urban area of the Caeté River Hydrographic Basin in Oriental Brazilian Amazon. J Integr Coast Zone Manag 2009, 9, 59–70. [Google Scholar]
- Grasshoff K, Emrhardt M, Kremling K. 1983, Methods of Seawater Analysis New York: Verlag Chemie.
- Guimarães DO, Pereira LCC, Monteiro MC, Gorayeb A, Costa RM. Effects of urban development on the Cereja River and Caeté estuary (Amazon coast, Brazil). J Coast Res, 2009, SI56, 1219-1223.
- IBGE - Brazilian Institute of Geography and Statistics (2020) Bragança-Pará Census. Available via https://wwwibgegovbr/cidades-e-estados/pa/bragancahtml?.Cited 15 Nov 2022.
- INMET - National Institute of Meteorology. 2020, Monitoring of automatic stations. Available via http://wwwinmetgovbr/sonabra/maps/automaticasphp. Cited 15 Nov 2022. 2022.
- Kassambara A, Mundt F. 2020, Factoextra: Extract and Visualize the Results of Multivariate Data Analyses Advance online publication https://rdrrio/cran/factoextra/.
- Kindiki S, Kollenberg M, Siamba D, Sifuna A, Wekesa C. Analysis of fecal coliform levels at watering points along the upper reaches of river Isiukhu in Kakamega Country. Kenya J Adv Microbiol 2018, 10, 1–7. [Google Scholar]
- Kuhlmann ML, Imbimbo HRV, Ogura LL, Villani JP, Starzynski R, Robim MJ. Effects of human activities on rivers located in protected areas of the Atlantic Forest. Acta Limnol Bras 2014, 26, 60–72. [Google Scholar] [CrossRef]
- Lamparelli, MC. 2004, Degree of trophy in water bodies in the state of São Paulo: Evaluation of Monitoring methods. Doctoral Thesis, in Science, Universidade de São Paulo.
- Lee HJ, Park HK, Lee JH, Park AR, Cheon SU. Coliform pollution status of Nakdong river and tributaries. J Korean Soc Water Environ 2016, 32, 271–280. [Google Scholar] [CrossRef]
- Levene H. 1960, Robust Tests for the equality of variance In: Olkin, I et al (Eds), Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling, Stanford University Press, Palo Alto, California, pp 278-292.
- Lopes FWA, Magalhães Jr AP, von Sperling E. Recreational water quality use in Brazilian freshwaters: Helth Risks, Methodological and Operational Limitations. Hygeia 2013, 9, 28–47. [Google Scholar]
- Marinho RR, Harmel T, Martinez J-M, Filizola Junior NP. Spatiotemporal Dynamics of Suspended Sediments in the Negro River, Amazon Basin, from In Situ and Sentinel-2 Remote Sensing Data ISPRS. Int J Geo-In 2021, 10, 86. [Google Scholar]
- Medeiros AC, Faial KRF, Faial KCF, Lopes IDS, Lima MO, Guimarães RM, Mendonça NM. Quality index of the surface water of Amazonian rivers in industrial areas in Pará, Brazil. Mar Pollut Bull 2017, 123, 156–164. [Google Scholar] [CrossRef]
- Medeiros AC, Lima MO, Guimarães RM. Assessment of the quality of water for consumption by river-bank communities in areas exposed to urban and industrial pollutants in the municipalities of Abaetetuba and Barcarena in the state of Pará, Brazil. Cien Saude Colet 2016, 21, 695–708. [Google Scholar]
- Medeiros AC, Faial KRF, Faial KCF, Lopes IDS, Lima MO, Guimarães, RM, Mendonça, NM. Quality index of the surface water of Amazonian rivers in industrial areas in Pará, Brazil. Mar Pollut Bull 2017, 123, 156–164. [Google Scholar] [CrossRef]
- Mitch AA, Gasner KC, Mitch WA. Fecal coliform accumulation within a river subject to seasonally-disinfected wastewater discharges water research. Water Res 2010, 44, 4776–4782. [Google Scholar] [CrossRef] [PubMed]
- Mocuba, J. 2010, Dissolved Oxygen and Biochemical Oxygen Demand in the waters close to the Quelimane sewage discharge. Master Thesis, in Chemical Oceanography, University of Bergen, Norway. [CrossRef]
- Monteiro, MC, Jiménez, JA, Pereira, LCC. Natural and human controls of water quality of an Amazon estuary (Caeté-PA, Brazil). Ocean Coast Manag 2016, 124, 42–52. [Google Scholar] [CrossRef]
- Moraes BC, Costa JMN, Costa ACL, Costa MH. Spatial and temporal variation of precipitation in the state of Pará. Acta Amaz 2005, 5, 207–214. [Google Scholar]
- Muniz JN, Duarte KG, Braga FHR, Lima NS, Silva DF, Firmo WCA, Batista MRV, Silva FMAM, Miranda RCM, Silva MRC. Limnological Quality: Seasonality Assessment and Potential for Contamination of the Pindaré River Watershed, Pre-Amazon Region, Brazil. Water 2020, 12, 851. [Google Scholar] [CrossRef]
- Nishiyama M, Iguchi A, Suzuki Y. Identification of Enterococcus faecium and Enterococcus faecalis as vanC-type Vancomycin-Resistant Enterococci (VRE) from sewage and river water in the provincial city of Miyazaki. Japan J Environ Sci Health A 2015, 50, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Nittrouer CA, DeMaster DJ, Kuehl SA, Figueiredo Jr AG, Sternberg RW, Ercilio CF, Silveira OM, Allison MA, Kineke GC, Ogston AS, Souza Filho PWM, Asp NE, Nowacki DJ, Fricke AT. Amazon Sediment Transport and Accumulation Along the Continuum of Mixed Fluvial and Marine Processes. Ann Rev Mar Sci 2021, 13, 501–536. [Google Scholar] [CrossRef]
- Nittrouer CA, DeMaster DJ. The Amazon shelf setting tropical, energetic, and influenced by a large river. Cont Shelf Res 1996, 16, 553–574. [Google Scholar] [CrossRef]
- Pará. 1995, Law no 5,887 on the State Environmental Policy, Belém 9-05-1995.
- Parsons TR, Strickland JDH. Discussion of spectrophotometric determination of marine plant pigments with revised equations of ascertaining chlorophyll-a and carotenoids. J Mar Res 1963, 21, 155–163. [Google Scholar]
- Pereira LCC, Sousa NSS, Rodrigues LMS, Monteiro MC, Silva SRS, Oliveira ARG, Dias ABB, Costa RM. Effects of the lack of basic public sanitation on the water quality of the Caeté River estuary in northern Brazil. Ecohydrol Hydrobiol 2021, 21, 299–314. [Google Scholar] [CrossRef]
- Petrucio MM, Medeiros AO, Rosa CA, Barbosa FAR. Trophic State and Microorganisms Community of Major Sub-Basins of the Middle Rio Doce Basin, Southeast Brazil. Braz Arch Biol Technol 2005, 48, 625–633. [Google Scholar] [CrossRef]
- Pettitt, A. Testing the Normality of Several Independent Samples Using the Anderson-Darling Statistic. J R Stat Soc Series C (Applied Statistics) 1977, 26, 156–161. [Google Scholar] [CrossRef]
- Rabalais NN, Harper Jr DE. 1992, Studies of benthic biota in area affected by moderate and severe hypoxia In: National Oceanic and Atmospheric Administration, Coastal Ocean Program Office, Nutrient Enhanced Coastal Ocean Productivity, Proceedings of a Workshop Louisiana Universities Marine Consortium, October 1991 Sea Grant Program, Texas A & M University, Galveston, TX, TAMU-SG-92–109 pp 150–153. 19 October.
- Ríos-Villamizar EA, Piedade MTF, Costa J G, Adeney JM, Junk WJ. Chemistry of different Amazonian water types for river classification: a preliminary review. WIT Trans Ecol Environ 2013, 178, 17–28. [Google Scholar]
- Ross A, Willson VL. 2017, Paired Samples T-Test In: Basic and Advanced Statistical Tests SensePublishers, Rotterdam Advance online publication. https://doi.org/10.1007/978-94-6351-086-8_4. [CrossRef]
- Sanchez E, Colmenarejo MF, Vicente J, Rubio A, Garcia MG, Travieso L, Borja R. Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol Indic 2007, 7, 315–328. [Google Scholar] [CrossRef]
- Santos MRS, Moreira AM, Santos MNS. 2013, Cereja occupation area in Bragança - Pará: a socio-environmental analysis In: IV ConGeA, Salvador, Brazil.
- Santos MRS, Moreira AM, Santos MNS. 2014, Socio-environmental analysis of the residents of the urban PPA of Rio Cereja, Bragança-Pará In: 3rd National Seminar on Treatment of Permanent Preservation Areas in Urban Areas and Environmental Restrictions on Land Installment, Experiences of interventions in urban APPs: technologies, urban regulation, intervention plans and projects, Belém, Brazil. t.
- Seo M, Lee L, Kim Y. Relationship between Coliform Bacteria and Water Quality Factors at Weir Stations in the Nakdong River, South Korea. Water 2019, 11, 1171. [Google Scholar] [CrossRef]
- Silva Filho CP, Nunes ZMP. 2017, Environmental integrity index of the Cereja river - Bragança - PA In: 8th International Solid Waste Forum, Proceedings of the 8th International Solid Waste Forum Venturini Institute: Curitiba, Brazil, p 1-10. C.
- Silva, MC. Estuaries-Criteria for an environmental assessment. Rer Bras de Recur Hidr 2000, 5, 25–35. [Google Scholar]
- 61. Sousa SSN. 2015, Influence of domestic sewage disposal on the water quality of the Caeté River and the Cereja River (Bragança-Pará). Master Thesis, in Environmental Biology, Universidade Federal do Pará.
- Stephens MA. EDF statistics for goodness-of-fit and some comparisons J Am Stat Assoc 1974, 69, 730–737.
- Tang X, Li R, Han D, Scholz M. Response of Eutrophication Development to Variations in Nutrients and Hydrological Regime: A Case Study in the Changjiang River (Yangtze) Basin. Water 2020, 12, 1634. [Google Scholar] [CrossRef]
- Tryland I, Myrmel M, Østensvik Ø, Wennberg AC, Robertson LJ. Impact of rainfall on the hygienic quality of blue mussels and water in urban areas in the Inner Oslofjord, Norway. Mar Pollut Bull 2014, 85, 42–49. [Google Scholar] [CrossRef]
- UNESCO. 1996, Monograph on Oceanographic Methodology I Determination of Photosynthetic Pigments in Sea Water United Nations Education, Science, and Culture Organization, Paris.
- USEPA - US Environmental Protection Agency. 1986, Bacteriological Ambient Water Quality Criteria for Marine and Fresh Recreational Waters EPA440/5-84-002, 24p.
- von Sperling M. 2007, Eutrophication of water bodies DESA - UFMG.
- von Sperling M. 2016, Urban wastewater treatment in Brazil Technical Note Nº IDB-TN-970 Department of Sanitary and Environmental Engineering Federal University of Minas Gerais Brazil Inter-American Development Bank Felipe Herrera Library.
- WHO - World Health Organization. 2003, Guidelines for safe recreational water environments - coastal and fresh waters Geneva, Switzerland, 1: 253p.
- Wu N, Dong X, Liu Y, Wang C, Baattrup-Pedersen A, Riis T. Using river microalgae as indicators for freshwater biomonitoring: Review of published research and future directions. Ecol Indic 2017, 81, 124–131. [Google Scholar] [CrossRef]
- Yusuf, ZH. Phytoplankton as bioindicators of water quality in Nasarawa reservoir, Katsina State Nigeria. Acta Limnol Bras 2020, 32, e4. [Google Scholar] [CrossRef]
- Zebo F, Bin S, Dong-Dong X, Liao-Yuan Y. Study on pH value and its variation characteristics of the main rivers into Dianchi lake under the anthropogenic and natural processes, Yunnan, China. J Inform Optimization Sci 2017, 38, 1197–1210. [Google Scholar] [CrossRef]
- Zeiringer B, Seliger C, Greimel F, Schmutz S. River Hydrology, Flow Alteration, and Environmental Flow In: Schmutz S, Sendzimir J (Eds), Riverine Ecosystem Management Aquat Ecol Series, v 8 Springer, Cham 2018. [CrossRef]
- Zhao Y, Song Y, Cui J, Gan S, Yang X, Wu R, Guo P. Assessment of Water Quality Evolution in the Pearl River Estuary (South Guangzhou) from 2008 to 2017. Water 2020, 12, 59. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).