Submitted:
06 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Choice of matrix
2.2. Sample preparation and control
2.3. Irradiation mode and optical emission measurements
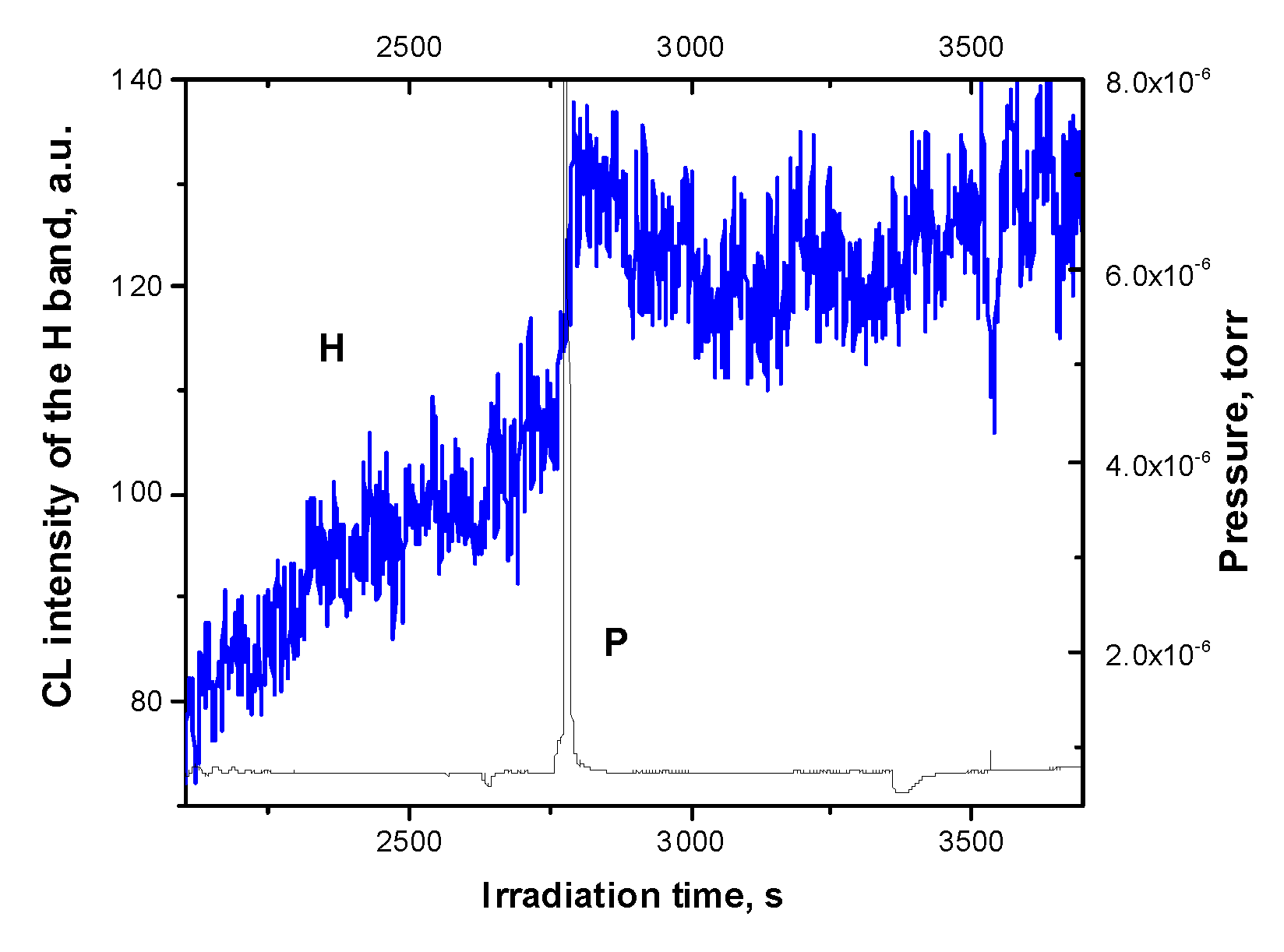
3. Results and Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Clark, R.N.; Carlson, R.; Grundy, W.; Noll , K. Observed Ices in the Solar System. In The Science of Solar System Ices; Gudipati, M.S., Castillo-Rogez, J., Eds.; Astrophysics and Space Science Library: Springer: New York, 2013; Volume 356, pp. 3–46. [Google Scholar] [CrossRef]
- Boogert, A.C.A.; Gerakines, P.A.; Whittet, D.C.B. Observation of the Icy Universe. Annual Review of Astronomy and Astrophysics 2015, 53, 541–581. [Google Scholar] [CrossRef]
- Guzmán-Marmolejo, A.; Segura, A. Methane in the Solar System. Boletín de la Sociedad Geológica Mexicana 2015, 67, 377–385. [Google Scholar] [CrossRef]
- Lacy, J.H.; Carr, J.S.; J, I.E.N.; Baas, F.; Achtermann, J.M.; Arens, J.F. Discovery of interstellar methane - Observations of gaseous and solid CH4 absorption toward young stars in molecular clouds. Astrophys. J. 1991, 376, 556–560. [Google Scholar] [CrossRef]
- Cruikshank, D.P.; Brown, R.H.; Calvin, W.M.; Roush, T.L.; Bartholomew, M.J. Ices on the satellites of Jupiter, Saturn, and Uranus. In Solar System Ices; Schmitt, B., de Bergh, C., Festou, M., Eds.; Kluwer Academic: Netherlands, 1998; pp. 579–606. [Google Scholar]
- Gibb, E.; Mumma, M.; Russo, N.D.; DiSanti, M.; Magee-Sauer, K. Methane in Oort cloud comets. Icarus 2003, 165, 391–406. [Google Scholar] [CrossRef]
- berg, K.I. Photochemistry and astrochemistry: Photochemical pathways to interstellar complex organic molecules. Chem. Rev. 2016, 116, 9631–9663. [Google Scholar]
- Kobayashi, K.; Geppert, W.D.; Carrasco, N.; Holm, N.G.; Mousis, O.; Palumbo, M.E.; Waite, J.H.; Watanabe, N.; Ziurys, L.M.; Bera, P.P.; et al. Laboratory Studies of Methane and Its Relationship to Prebiotic Chemistry. Astrobiology 2017, 17, 786–812. [Google Scholar] [CrossRef]
- Allodi, M.A.; Baragiola, R.A.; Baratta, G.A.; Barucci, M.A.; Blake, G.A.; Boduch, P.; Brucato, J.R.; Contreras, C.; Cuylle, S.H.; Fulvio, D.; et al. Complementary and Emerging Techniques for Astrophysical Ices Processed in the Laboratory. Space Sci. Rev. 2013, 180, 101–175. [Google Scholar] [CrossRef]
- Foti, G.; Calcagno, L.; Sheng, K.L.; Strazzulla, G. Micrometre-sized polymer layers synthesized by MeV ions impinging on frozen methane. Nature 1984, 310, 126–128. [Google Scholar] [CrossRef]
- Kaiser, R.I.; Roessler, K. Theoretical and laboratory study on the interaction of cosmic-ray particles with interstellar ices. III. Suprathermal chemistry-induced formation of hydrocarbon molecules in solid methane (CH4), ethylene (C2H4) and acetylene (C2H2). Astrophys. J. 1998, 503, 959–975. [Google Scholar] [CrossRef]
- Brunetto, R.; Barucci, M.A.; Dotto, E.; Strazzulla, G. Ion Irradiation of Frozen Methanol, Methane, and Benzene: Linking to the Colors of Centaurs and Trans-Neptunian Objects. Astrophys. J. 2006, 644, 646–650. [Google Scholar] [CrossRef]
- E Palumbo, M.; A Baratta, G.; Fulvio, D.; Garozzo, M.; Gomis, O.; Leto, G.; Spinella, F.; Strazzulla, G. Ion irradiation of astrophysical ices. J. Physics: Conf. Ser. 2008, 101, 012002. [Google Scholar] [CrossRef]
- Ennis, C.; Yuan, H.; Sibener, S.J.; Kaiser, R.I. On the chemical processing of hydrocarbon surfaces by fast oxygen ions. Phys. Chem. Chem. Phys. 2011, 13, 17870–17884. [Google Scholar] [CrossRef] [PubMed]
- de Barros, A.L.F.; Bordalo, V.; Duarte, E.S.; da Silveira, E.F.; Domaracka, A.; Rothard, H.; Boduch, P. Cosmic ray impact on astrophysical ices: laboratory studies on heavy ion irradiation of methane. Astron. Astrophys. 2011, 531, A160. [Google Scholar] [CrossRef]
- Mejίa, C.F.; de Barros, A.L.F.; Bordalo, V.; da Silveira, E.F.; Boduch, P.; Domaracka, A.; Rothard, H. Cosmic ray–ice interaction studied by radiolysis of 15 K methane ice with MeV O, Fe and Zn ions. Monthly Notices of the Royal Astronomical Society 2013, 433, 2368–2379. [Google Scholar] [CrossRef]
- Boduch, P.; Dartois, E.; de Barros, A.L.F.; da Silveira, E.F.; Domaracka, A.; Lv, X.-Y.; Palumbo, M.E.; Pilling, S.; Rothard, H.; Duarte, E.S.; et al. Radiation effects in astrophysical ices. J. Physics: Conf. Ser. 2015, 629. [Google Scholar] [CrossRef]
- Vasconcelos, F.A.; Pilling, S.; Rocha, W.R.M.; Rothard, H.; Boduch, P.; Ding, J.J. Ion irradiation of pure and amorphous CH4 ice relevant for astrophysical environments. Phys. Chem. Chem. Phys. 2017, 19, 12845–12856. [Google Scholar] [CrossRef]
- Mejía, C.; de Barros, A.L.F.; Rothard, H.; Boduch, P.; da Silveira, E.F. Radiolysis of Ices by Cosmic-Rays: CH4 and H2O Ices Mixtures Irradiated by 40 MeV 58Ni11+ Ions. Astrophys. J. 2020, 894, 132. [Google Scholar] [CrossRef]
- Huang, T.; Hamill, W.H. Characteristic energy loss, luminescence and luminescence excitation spectra of methane and other alkane solids under low-energy electron impact. J. Phys. Chem. 1974, 78, 2077–2080. [Google Scholar] [CrossRef]
- Bennett, C.J.; Jamieson, C.S.; Osamura, Y.; Kaiser, R.I. Laboratory Studies on the Irradiation of Methane in Interstellar, Cometary, and Solar System Ices. Astrophys. J. 2006, 653, 792–811. [Google Scholar] [CrossRef]
- Barberio, M.; Vasta, R.; Barone, P.; Manicò, G.; Xu, F. Experimental and Theoretical Study on the Ethane and Acetylene Formation from Electron Irradiation of Methane Ices. World J. Condens. Matter Phys. 2013, 03, 14–20. [Google Scholar] [CrossRef]
- Huels, M.; Parenteau, L.; Bass, A.; Sanche, L. Small steps on the slippery road to life: Molecular synthesis in astrophysical ices initiated by low energy electron impact. International Journal of Mass Spectrometry 2008, 277, 256–261. [Google Scholar] [CrossRef]
- Jones, B.M.; Kaiser, R.I. Application of Reflectron Time-of-Flight Mass Spectroscopy in the Analysis of Astrophysically Relevant Ices Exposed to Ionization Radiation: Methane (CH4) and D4-Methane (CD4) as a Case Study. J. Phys. Chem. Lett. 2013, 4, 1965–1971. [Google Scholar] [CrossRef]
- Materese, Ch.K.; Cruikshank, D.P.; Sandford, S.A.; Imanaka, H. and Nuevo, M. Ice chemistry on outer Solar System bodies: Electron radiolysis of N2-, CH4-, and CO-containing ices. Astrophys. J. 2015, 812, 150. [Google Scholar] [CrossRef]
- Abplanalp, M.J.; Jones, B.M.; Kaiser, R.I. Untangling the methane chemistry in interstellar and solar system ices toward ionizing radiation: a combined infrared and reflectron time-of-flight analysis. Phys. Chem. Chem. Phys. 2017, 20, 5435–5468. [Google Scholar] [CrossRef]
- Savchenko, E.; Khyzhniy, I.; Uyutnov, S.; Bludov, M.; Barabashov, A.; Gumenchuk, G.; Bondybey, V. Radiation effects in nitrogen and methane “ices”. Nucl. Instruments Methods Phys. Res. Sect. B: Beam Interactions Mater. Atoms 2018, 435, 38–42. [Google Scholar] [CrossRef]
- Savchenko, E.; Kirichek, O.; Lawson, C.; Khyzhniy, I.; Uyutnov, S.; Bludov, M. Relaxation processes in solid methane pre-irradiated with an electron beam. Nucl. Instrum. Meth. B, 2018; 433, 23–27. [Google Scholar] [CrossRef]
- Savchenko, E.; Khyzhniy, I.; Uyutnov, S.; Bludov, M.; Gumenchuk, G.; Bondybey, V. Effects induced by electron beam in methane ices. Nucl. Instrum. Meth. B , 2019; 460, 244–258. [Google Scholar] [CrossRef]
- Hodyss, R.; Johnson, P.V.; Stern, J.V.; Goguen, J.D.; Kanik, I. Photochemistry of methane-water ices. Icarus 2009, 200, 338–342. [Google Scholar] [CrossRef]
- Hodyss, R.; Howard, H.R.; Johnson, P.V.; Goguen, J.D.; Kanik, I. Formation of radical species in photolyzed CH4:N2 ices. Icarus 2011, 214, 748–753. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Wu, C.Y.R.; Chou, S.-L.; Lin, M.-Y.; Lu, H.-C.; Lo, J.-I.; Cheng, B.-M. SPECTRA AND PHOTOLYSIS OF PURE NITROGEN AND METHANE DISPERSED IN SOLID NITROGEN WITH VACUUM-ULTRAVIOLET LIGHT. Astrophys. J. 2012, 746, 175. [Google Scholar] [CrossRef]
- Bossa, J.-B.; Paardekooper, D.M.; Isokoski, K.; Linnartz, H. Methane ice photochemistry and kinetic study using laser desorption time-of-flight mass spectrometry at 20 K. Phys. Chem. Chem. Phys. 2015, 17, 17346–17354. [Google Scholar] [CrossRef]
- Dupuy, R.; Bertin, M.; Féraud, G.; Michaut, X.; Jeseck, P.; Doronin, M.; Philippe, L.; Romanzin, C.; Fillion, J.-H. Spectrally-resolved UV photodesorption of CH4in pure and layered ices. Astron. Astrophys. 2017, 603, A61. [Google Scholar] [CrossRef]
- Krim, L.; Jonusas, M. VUV Photolysis of CH4–H2O mixture in methane-rich ices: Formation of large complex organic molecules in astronomical environments. Low Temp. Phys. 2019, 45, 606–614. [Google Scholar] [CrossRef]
- Scott, T.L.; Carpenter, J.M.; Miller, M.E. The development of solid methane neutron moderators at the intense pulsed neutron source facility of Argonne National Laboratory Preprint ANL/IPNS/CP-98533 (1999).
- Carpenter, J.M. Thermally activated release of stored chemical energy in cryogenic media. Nature 1987, 330, 358–360. [Google Scholar] [CrossRef]
- Kulagin, E.; Kulikov, S.; Melikhov, V.; Shabalin, E. Radiation effects in cold moderator materials: Experimental study of accumulation and release of chemical energy. Nucl. Instruments Methods Phys. Res. Sect. B: Beam Interactions Mater. Atoms 2004, 215, 181–186. [Google Scholar] [CrossRef]
- Feng, Q.; Kawai, T.; Zhong, B.; Wei, J.; Loong, C.-K.; Liang, T. The Solution of Cold Neutron Source using Solid Methane Moderator for the CPHS. Phys. Procedia 2012, 26, 49–54. [Google Scholar] [CrossRef]
- Kirichek, O.; Lawson, C.R.; Jenkins, D.M.; Ridley, C.J.T.; Haynes, D.J. Solid methane in neutron radiation: Cryogenic moderators and cometary cryovolcanism. Cryogenics 2017, 88, 101–105. [Google Scholar] [CrossRef]
- Kirichek, O.; Savchenko, E.; Lawson, C.; Khyzhniy, I.; Jenkins, D.; Uyutnov, S.; Bludov, M.; Haynes, D. Recombination of radiation defects in solid methane: neutron sources and cryo-volcanism on celestial bodies. 2018, 969, 012006. Journal of Physics: Conference Series 2018, 969, 012006. [Google Scholar] [CrossRef]
- Kirichek, O.; Lawson, C.; Draper, G.; Jenkins, D.; Haynes, D.; Lilley, S. Solid methane moderators: Thermodynamics and chemistry. J. Neutron Res. 2020, 22, 281–286. [Google Scholar] [CrossRef]
- Khizhny, I.V.; Uyutnov, S.A.; Bludov, M.A.; Savchenko, E.V. Explosive desorption of solid methane particles induced by an electron beam. Low Temp. Phys. 2018, 44, 1223–1225. [Google Scholar] [CrossRef]
- Bludov, M.; Khyzhniy, I.; Savchenko, E.; Sugakov, V.; Uyutnov, S. Self-oscillations in solid methane irradiated by electrons. Nucl. Phys. At. Energy 2020, 21, 312–322. [Google Scholar] [CrossRef]
- Sugakov, V.I. Lectures in Synergetics, World Scientific Series on Nonlinear Science; World Scientific: Singapore, 1998; Volume 33. [Google Scholar]
- Brown, W.; Foti, G.; Lanzerotti, L.; Bower, J.; Johnson, R. Delayed emission of hydrogen from ion bombardment of solid methane. Nucl. Instruments Methods Phys. Res. Sect. B: Beam Interactions Mater. Atoms 1987, 19-20, 899–902. [Google Scholar] [CrossRef]
- Kaiser, R.I.; Eich, G.; Gabrysch, A.; Roessler, K. Theoretical and laboratory studies on the interaction of cosmic-ray particles with interstellar ices. II. Formation of atomic and molecular hydrogen in frozen organic molecules. Astrophys. J. 1997, 484, 487–498. [Google Scholar] [CrossRef]
- d’Hendecourt, L.; Allamandola, L.; Baas, F.; Greenberg, J. Interstellar grain explosions - Molecule cycling between gas and dust. Astronomy & Astrophysics 1982, 109, L12–L14. [Google Scholar]
- Roberts, J.F.; Rawlings, J.M.C.; Viti, S.; Williams, D.A. Desorption from interstellar ices. Mon. Not. R. Astron. Soc. 2007, 382, 733–742. [Google Scholar] [CrossRef]
- Vasyunin, A.I.; Herbst, E. Reactive desorption and radiative association as possible drivers of complex molecule formation in the cold interstellar medium. Astrophys. J. 2013, 769. [Google Scholar] [CrossRef]
- Wakelam, V.; Dartois, E.; Chabot, M.; Spezzano, S.; Navarro-Almaida, D.; Loison, J.-C.; Fuente, A. Efficiency of non-thermal desorptions in cold-core conditions: Testing the sputtering of grain mantles induced by cosmic rays. Astronomy & Astrophysics 2021, 652, A63. [Google Scholar]
- Zhu, C.; Bergantini, A.; Singh, S.K.; Abplanalp, M.J.; Kaiser, R.I. Rapid Radical–Radical Induced Explosive Desorption of Ice-coated Interstellar Nanoparticles. Astrophys. J. 2021, 920, 73. [Google Scholar] [CrossRef]
- Khyzhniy, I.V.; Uyutnov, S.A.; Bludov, M.A.; Savchenko, E.V.; Bondybey, V.E. Electron-induced delayed desorption of solid argon doped with methane. Low Temp. Phys. 2019, 45, 721–726. [Google Scholar] [CrossRef]
- Savchenko, E.; Khyzhniy, I.; Uyutnov, S.; Bludov, M.; Bondybey, V. Explosive desorption from solid methane and methane-containing argon matrices. Nucl. Instruments Methods Phys. Res. Sect. B: Beam Interactions Mater. Atoms 2020, 469, 37–41. [Google Scholar] [CrossRef]
- Savchenko, E.; Khyzhniy, I.; Uyutnov, S.; Bludov, M.; Bondybey, V. Nonstationary processes in matrix-isolated methane probed by optical and current emission spectroscopy. J. Mol. Struct. 2020, 1221, 128803. [Google Scholar] [CrossRef]
- Eberlein, J.; Creuzburg, M. Dissociation of CH4 in a krypton matrix: VUV spectra of atomic C and of the radical CH. Mol. Phys. 1999, 96, 451–456. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Chen, H.-F.; Camacho, C.; Witek, H.A.; Hsu, S.-C.; Lin, M.-Y.; Chou, S.-L.; Ogilvie, J.F.; Cheng, B.-M. Formation and identification of interstellar molecule linear C5H from photolysis of methane dispersed in solid neon. Astrophys. J. 2009, 701, 8–11. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Lo, J.-I.; Lu, H.-C.; Chou, S.-L.; Peng, Y.-C.; Cheng, B.-M.; Ogilvie, J.F. Vacuum-Ultraviolet Photolysis of Methane at 3 K: Synthesis of Carbon Clusters up to C20. J. Phys. Chem. A 2014, 118, 3438–3449. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, E.; Khyzhniy, I.; Uyutnov, S.; Bludov, M.; Bondybey, V. Explosive desorption induced by radical–radical interaction in methane-doped Ar matrices. Nucl. Instruments Methods Phys. Res. Sect. B: Beam Interactions Mater. Atoms 2023, 536, 113–118. [Google Scholar] [CrossRef]
- Sharp, T. Potential-energy curves for molecular hydrogen and its ions. At. Data Nucl. Data Tables 1970, 2, 119–169. [Google Scholar] [CrossRef]
- Song, K.S.; Williams, R.T. Self-Trapped Excitons; Springer-Verlag: Berlin, Germany, 1996. [Google Scholar]
- Smirnov, B.M.; Yatsenko, A.S. Properties of dimers. Phys. Usp 1996, 39, 211–230. [Google Scholar] [CrossRef]
- Heays, A.N.; Bosman, A.D.; van Dishoeck, E.F. Photodissociation and photoionisation of atoms and molecules of astrophysical interest. Astron. Astrophys. 2017, 602, A105. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Testing (NIST): Gaithersburg, MD, USA, 2013. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer, 2015. [Google Scholar]
- Hallam, H.E. Vibrational Spectroscopy of Trapped Species; John Wiley & Sons: London, UK, 1973. [Google Scholar]
- Chamberland, A.; Belzile, R.; Cabana, A. Infrared spectra and structure of methane – noble gas mixed crystals: the influence of temperature and methane concentration on the v3 vibration band of methane. Can. J. Chem. 1970, 48, 1129–1139. [Google Scholar] [CrossRef]
- Ferradini, C.; Jay-Gerin, J.-P. Excess Electrons in Dielectric Media; CRC Press: Boca Raton, Ann Arbor, Boston, London, 1991. [Google Scholar]
- Lo, J.-I.; Lin, M.-Y.; Peng, Y.-C.; Chou, S.-L.; Lu, H.-C.; Cheng, B.-M.; Ogilvie, J.F. Far-ultraviolet photolysis of solid methane. Mon. Not. R. Astron. Soc. 2015, 451, 159–166. [Google Scholar] [CrossRef]
- Kraas, M.; Gürtler, P. Emission and excitation spectra of rare gas hydride trimers in rare gas matrices. Chem. Phys. Lett. 1990, 174, 396–400. [Google Scholar] [CrossRef]
- Doronin, Y.S.; Vakula, V.L.; Kamarchuk, G.V.; Tkachenko, A.A.; Khyzhniy, I.V.; Uyutnov, S.A.; Bludov, M.A.; Savchenko, E.V. Desorption of excited H* atoms from free clusters Ar/CH4 and solid Ar doped with CH4. Low Temp. Phys. 2021, 47, 1058–1064. [Google Scholar] [CrossRef]
- Zimmerer, G. Electronic sputtering from rare-gas solids. Nucl. Instruments Methods Phys. Res. Sect. B: Beam Interactions Mater. Atoms 1994, 91, 601–613. [Google Scholar] [CrossRef]
- Gumenchuk, G.B.; Khyzhniy, I.V.; Bludov, M.A.; Uyutnov, S.A.; Belov, A.G.; Savchenko, E.V.; Ponomaryov, A.N.; Bondybey, V.E. Optically stimulated desorption of “hot” excimers from pre-irradiated Ar solids. Low Temp. Phys. 2008, 34, 241–244. [Google Scholar] [CrossRef]
- Ogurtsov, A.; Savchenko, E.; Becker, J.; Runne, M.; Zimmerer, G. Radiative relaxation of optically generated intrinsic charged centers in solid Ar. J. Lumin- 1998, 76-77, 478–481. [Google Scholar] [CrossRef]
- Lavrov, B.P.; Melnikov, A.S.; Käning, M.; Röpcke, J. uv continuum emission and diagnostics of hydrogen-containing nonequilibrium plasmas. Phys. Rev. E 1999, 59, 3526–3543. [Google Scholar] [CrossRef]
- Coletti, F.; Debever, J.; Zimmerer, G. Electron stimulated desorption of solid argon via exciton creation. J. de Phys. Lettres 1984, 45, 467–473. [Google Scholar] [CrossRef]
- Fugol', I. Free and self-trapped excitons in cryocrystals: kinetics and relaxation processes. Adv. Phys. 1988, 37, 1–35. [Google Scholar] [CrossRef]
- Beyer, M.; Lammers, A.; Savchenko, E.V.; Niedner-Schatteburg, G.; Bondybey, V.E. Proton solvated by noble-gas atoms: simplest case of a solvated ion. Phys. Chem. Chem. Phys. 1999, 1, 2213–2221. [Google Scholar] [CrossRef]
- IdBarkach, T.; Chabot, M.; Béroff, K.; Della Negra, S.; Lesrel, J.; Geslin, F.; Le Padellec, A.; Mahajan, T.; Díaz-Tendero, S. Breakdown curves of CH2(+), CH3(+), and CH4(+) molecules I. Construction and application to electron collisions and UV photodissociation. Astron. Astrophys. 2019, 628, A75. [Google Scholar] [CrossRef]
- Eberlein, J.; Creuzburg, M. Mobility of atomic hydrogen in solid krypton and xenon. J. Chem. Phys. 1997, 106, 2188–2194. [Google Scholar] [CrossRef]
- Vaskonen, K.; Eloranta, J.; Kiljunen, T.; Kunttu, H. Thermal mobility of atomic hydrogen in solid argon and krypton matrices. J. Chem. Phys. 1999, 110, 2122–2128. [Google Scholar] [CrossRef]
- Kiljunen, T.; Eloranta, J.; Kunttu, H. Ab initio and molecular-dynamics studies on rare gas hydrides: Potential-energy curves, isotropic hyperfine properties, and matrix cage trapping of atomic hydrogen. J. Chem. Phys. 1999, 110, 11814–11822. [Google Scholar] [CrossRef]
- Feldman, V.I.; Sukhov, F.F.; Orlov, A.Y. Hydrogen atoms in solid xenon: Trapping site structure, distribution, and stability as revealed by EPR studies in monoisotopic and isotopically enriched xenon matrices. J. Chem. Phys. 2008, 128, 214511. [Google Scholar] [CrossRef] [PubMed]
- Ozerov, G.K.; Bezrukov, D.S.; Buchachenko, A.A. Computational study of the stable atomic trapping sites in Ar lattice. Low Temp. Phys. 2019, 45, 301–309. [Google Scholar] [CrossRef]
- Sheludiakov, S.; Ahokas, J.; Järvinen, J.; Lehtonen, L.; Vasiliev, S.; Dmitriev, Y.A.; Lee, D.M.; Khmelenko, V.V. Electron spin resonance study of atomic hydrogen stabilized in solid neon below 1 K. Phys. Rev. B 2018, 97, 104108. [Google Scholar] [CrossRef]
- Sawada, K.; Fujimoto, T. Effective ionization and dissociation rate coefficients of molecular hydrogen in plasma. J. Appl. Phys. 1995, 78, 2913–2924. [Google Scholar] [CrossRef]
- Massey, H. Negative Ion. In Negative Ions; Cambridge University Press: Cambridge, 1976. [Google Scholar]
- Chang, Y.; Yang, J.; Chen, Z.; Zhang, Z.; Yu, Y.; Li, Q.; He, Z.; Zhang, W.; Wu, G.; Ingle, R.A.; et al. Ultraviolet photochemistry of ethane: implications for the atmospheric chemistry of the gas giants. Chem. Sci. 2020, 11, 5089–5097. [Google Scholar] [CrossRef]
- Gao, H. Molecular photodissociation in the vacuum ultraviolet region: implications for astrochemistry and planetary atmospheric chemistry. Mol. Phys. 2020, 119. [Google Scholar] [CrossRef]
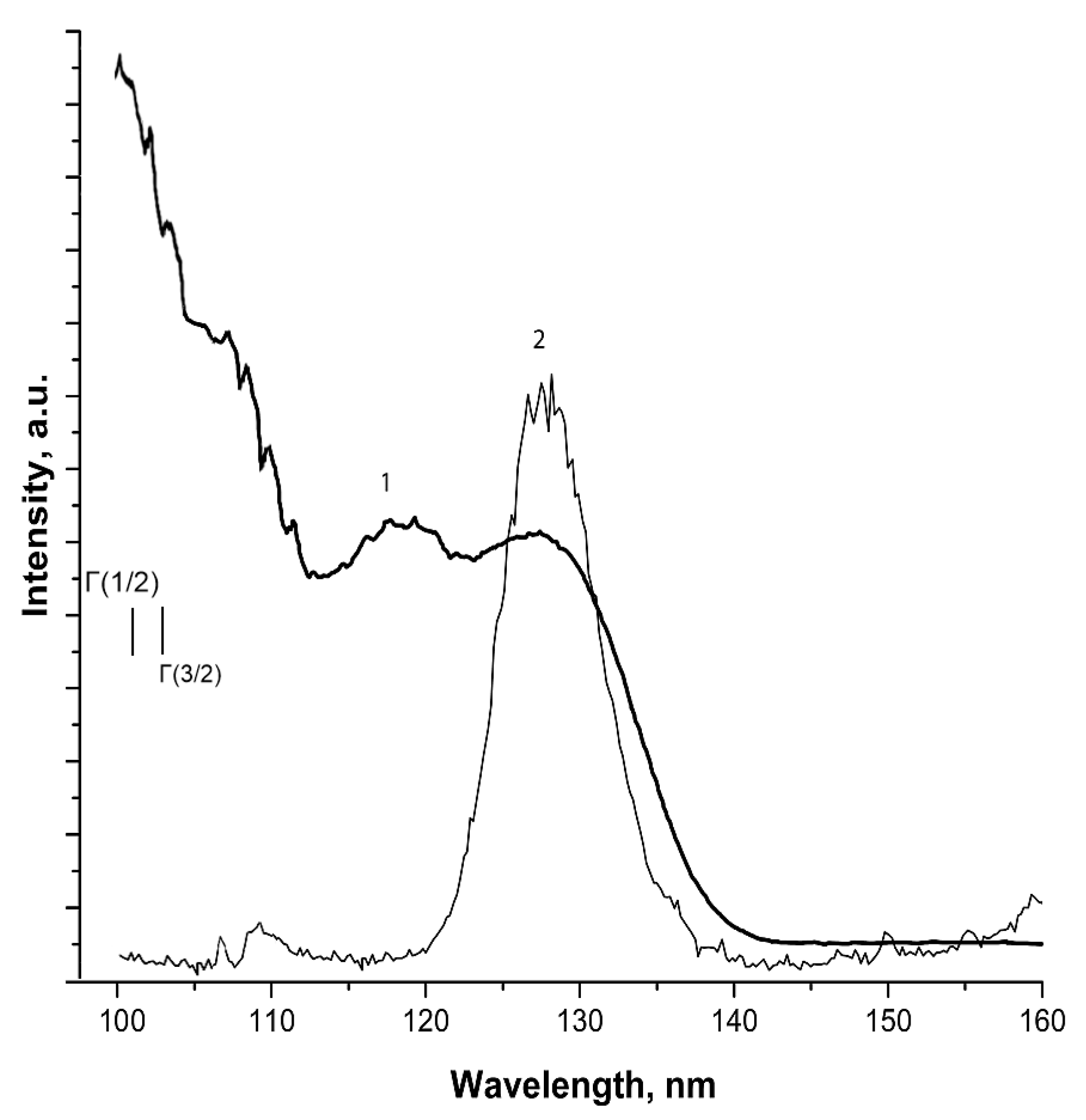
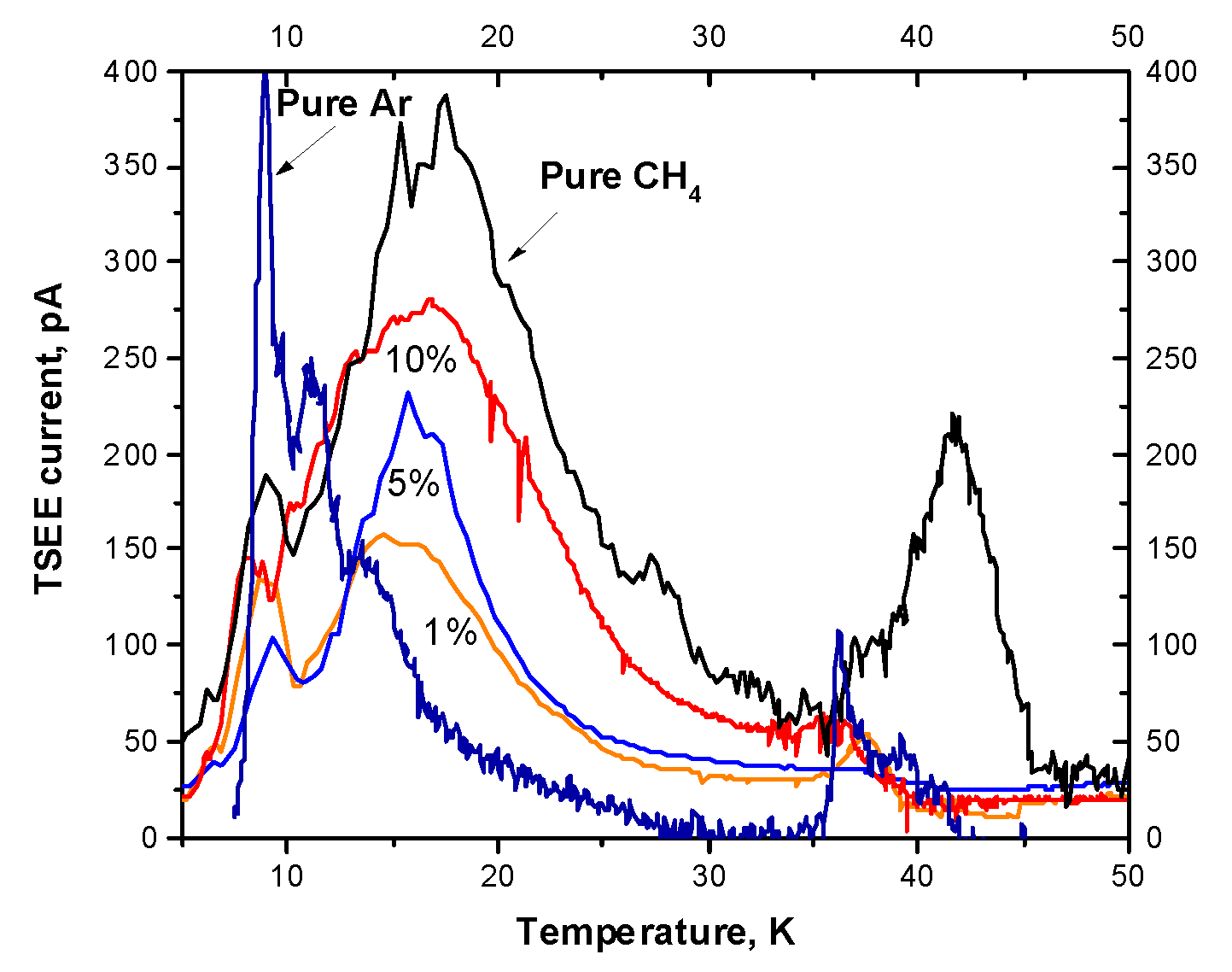
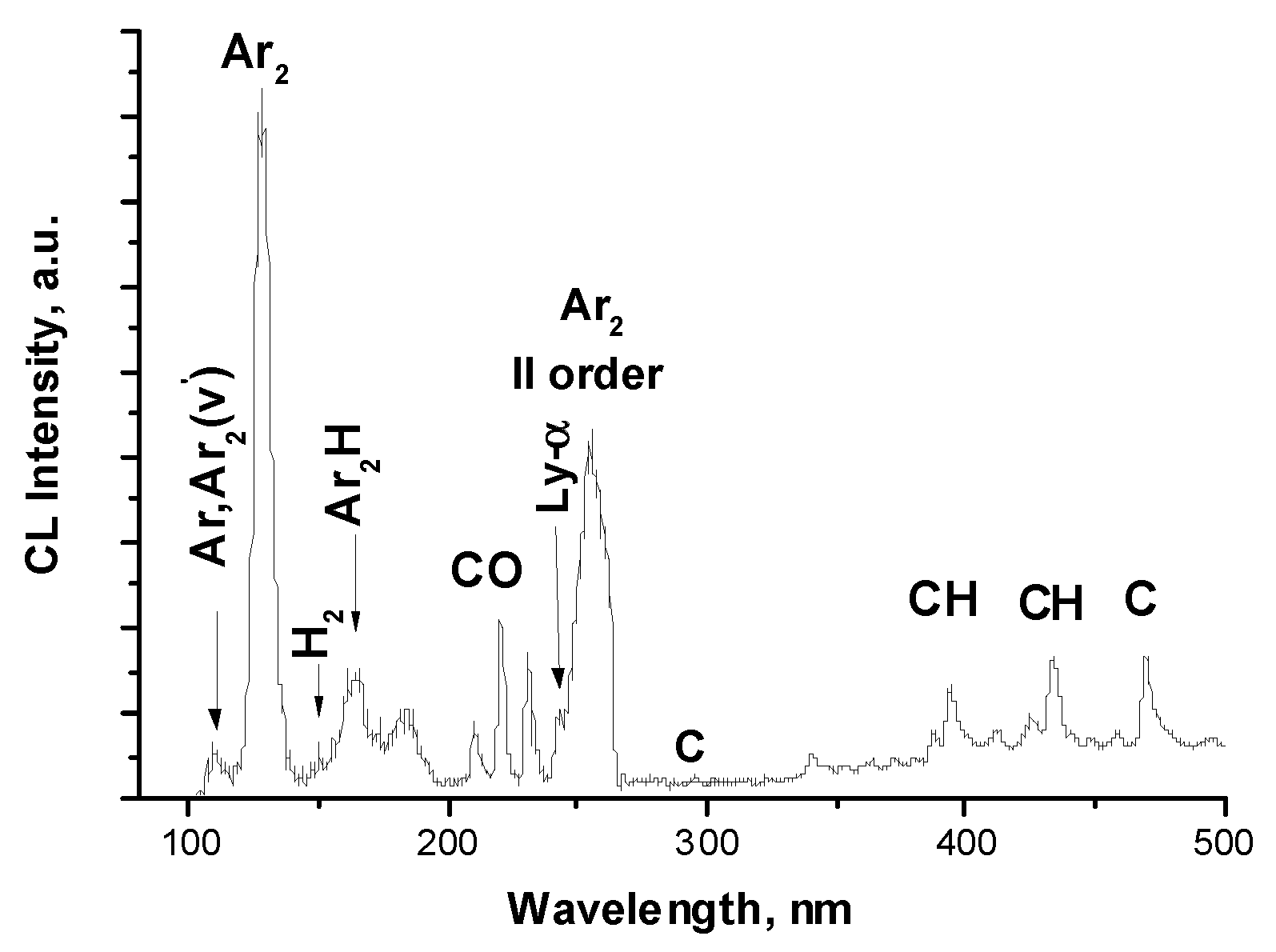
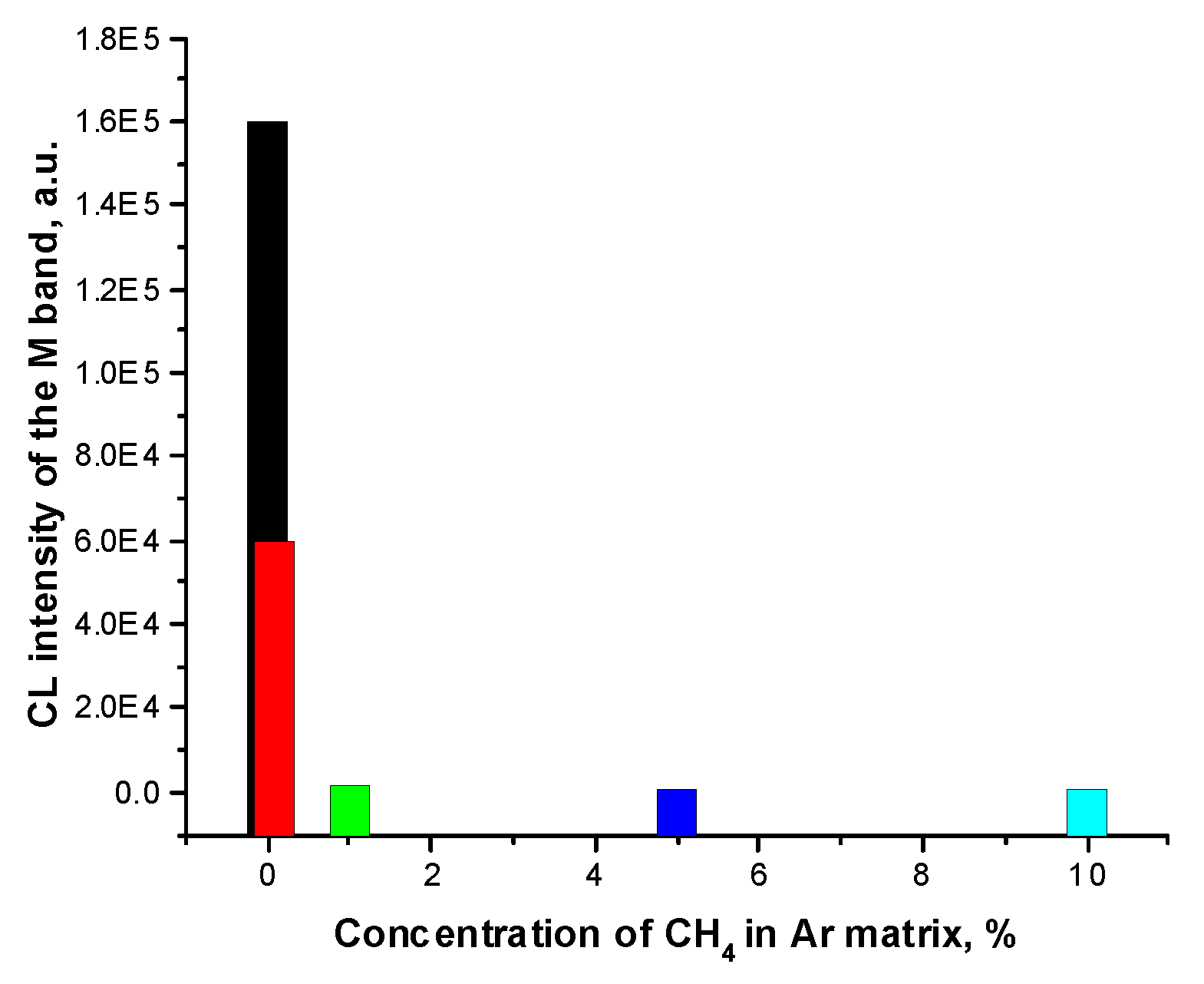
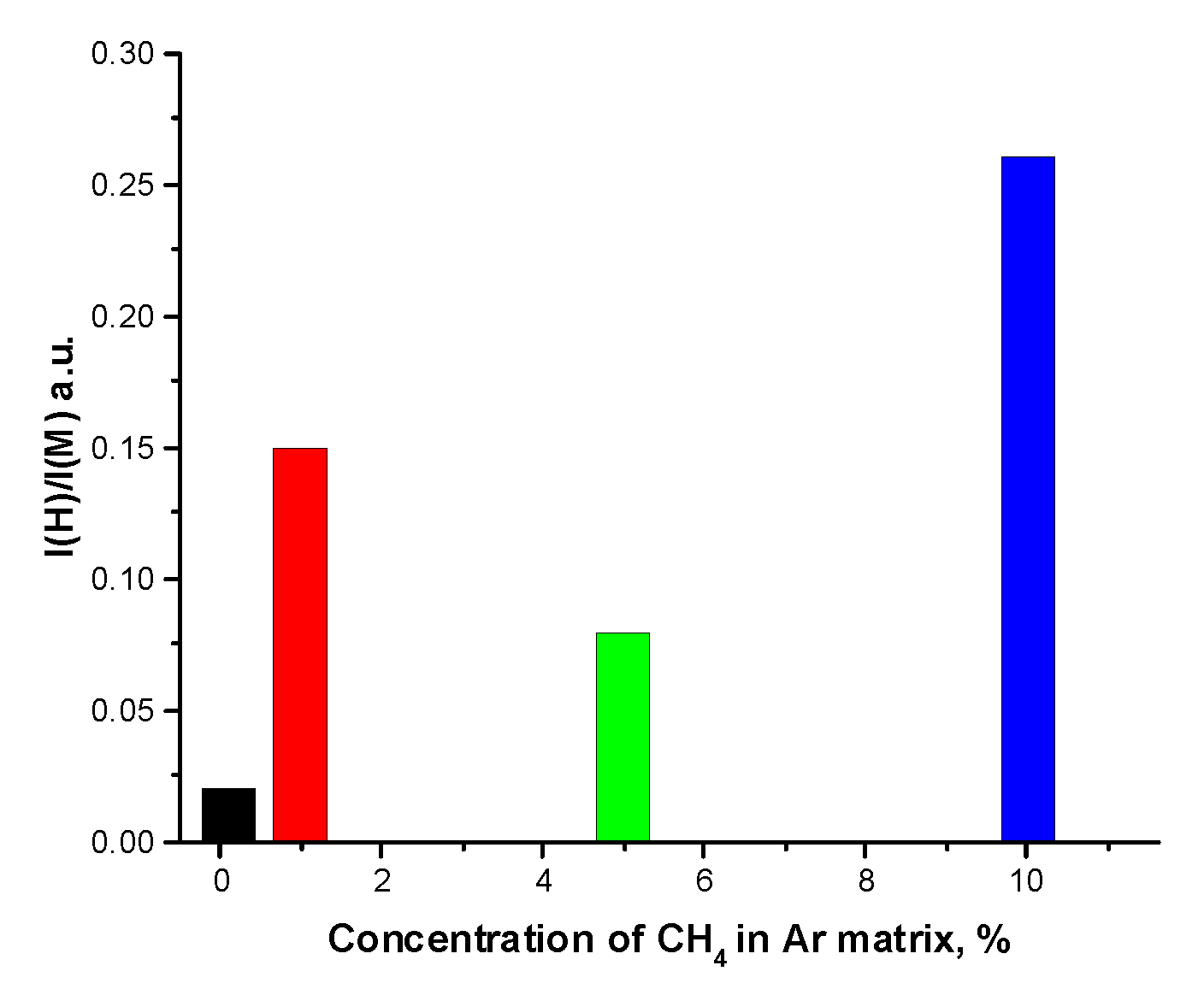
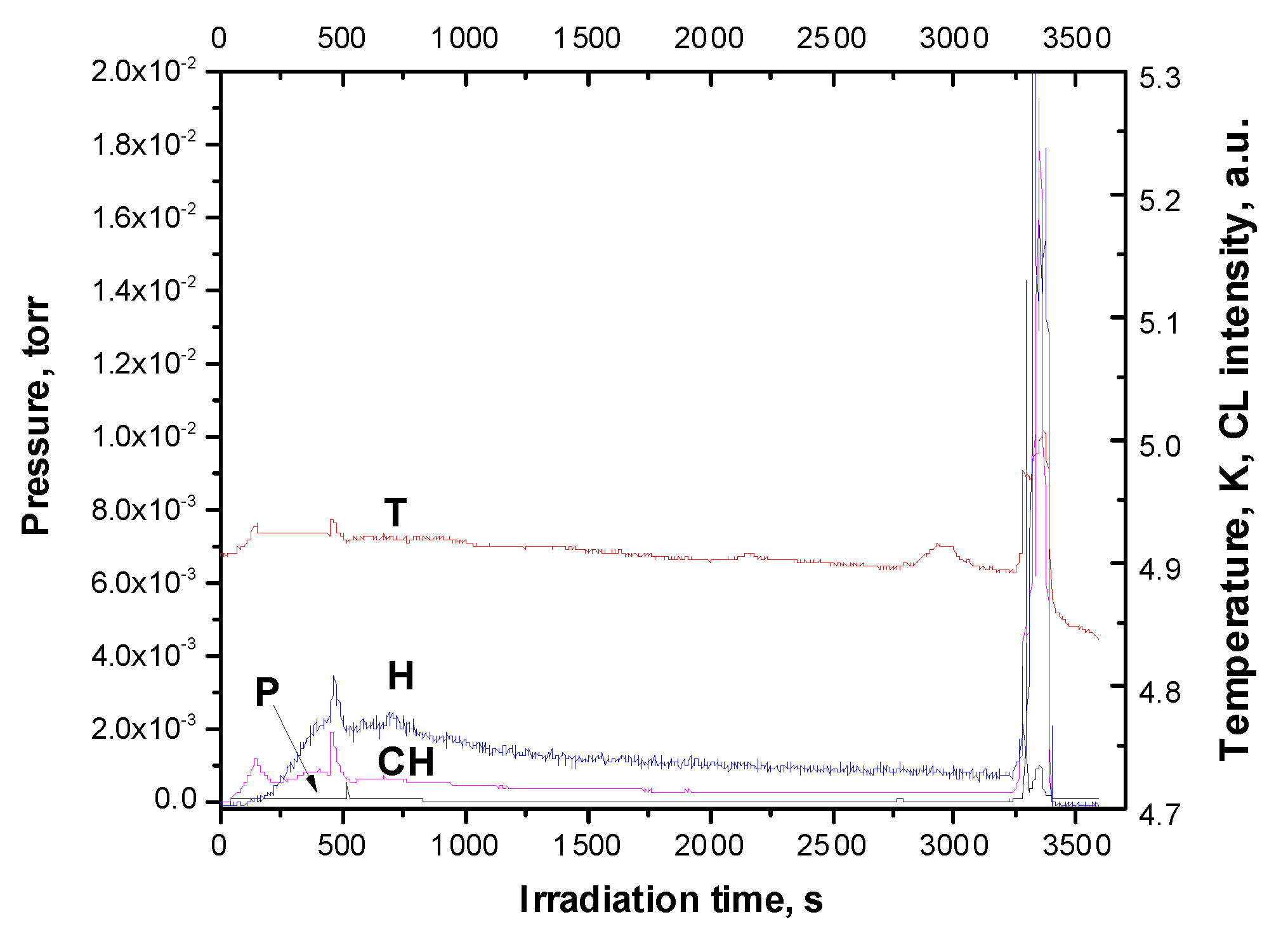
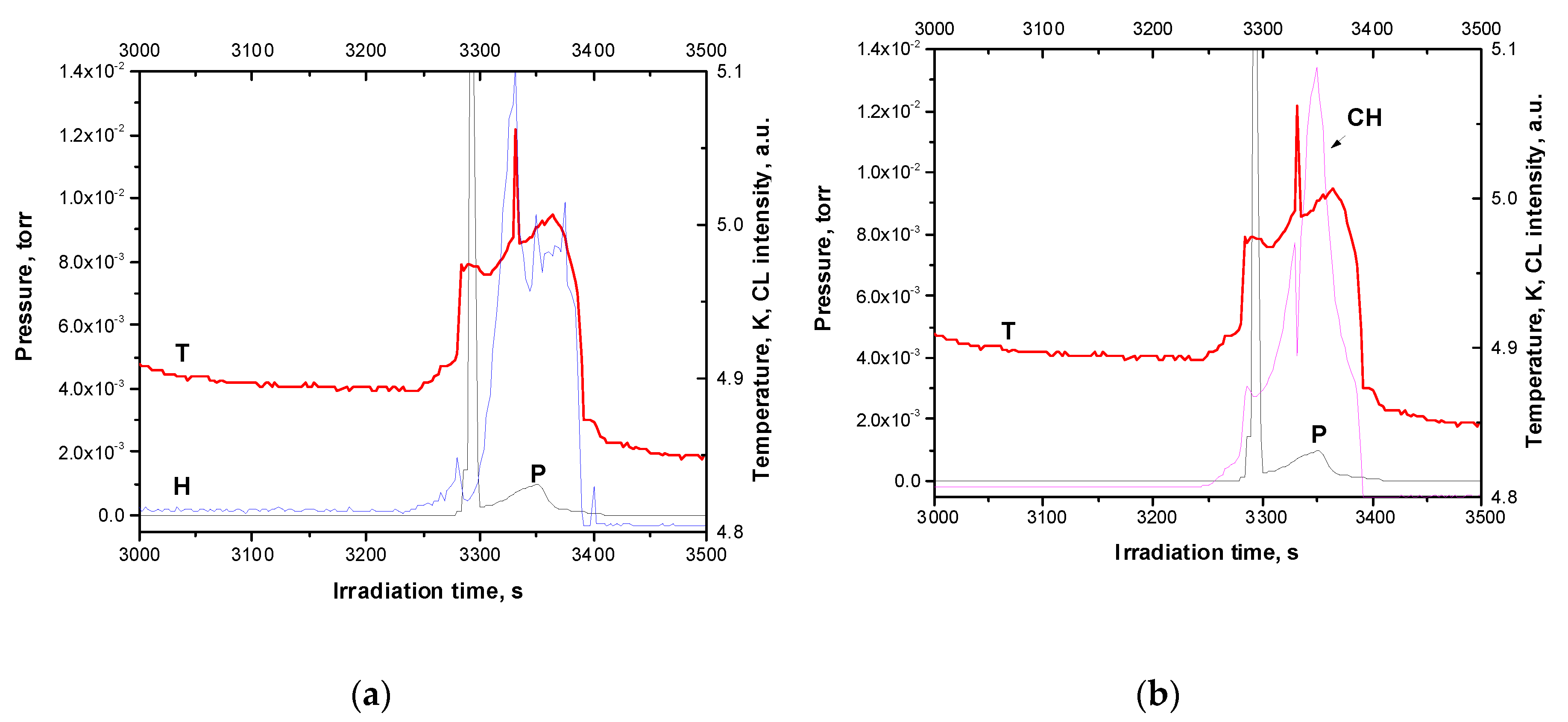
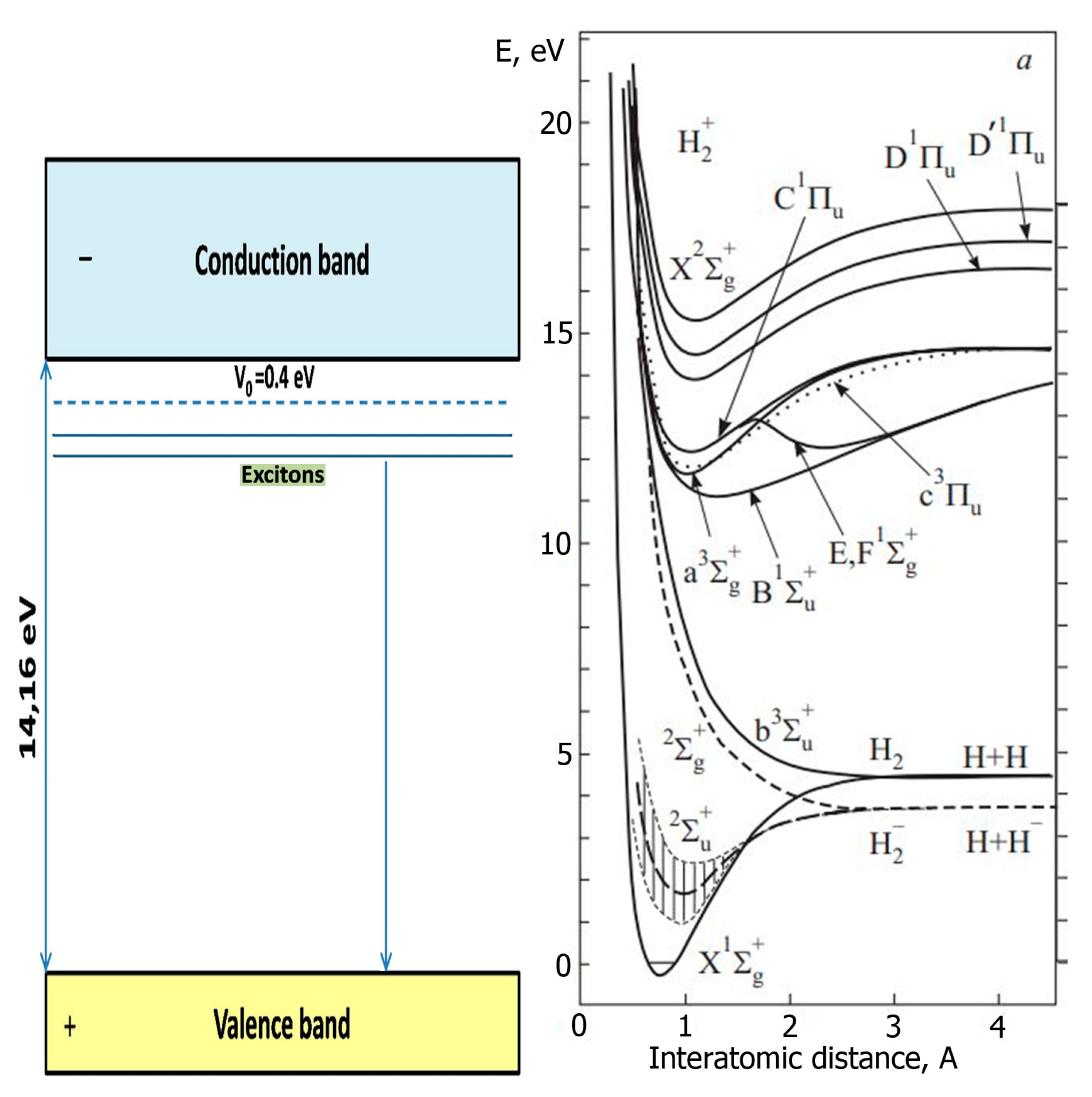
| Species | Ionization potential, eV | Electron affinity, eV |
|---|---|---|
| CH4 | 12.61 | unstable |
| CH3 | 9.84 | +0.09 |
| CH2 | 10.35 | +0.65 |
| CH | 10.64 | +1.24 |
| H | 13.59 | +0.75 |
| H2 | 15.43 | unstable |
| C | 11.26 | 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).