Submitted:
07 August 2023
Posted:
08 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. In Vitro Evidence Anti-Aging
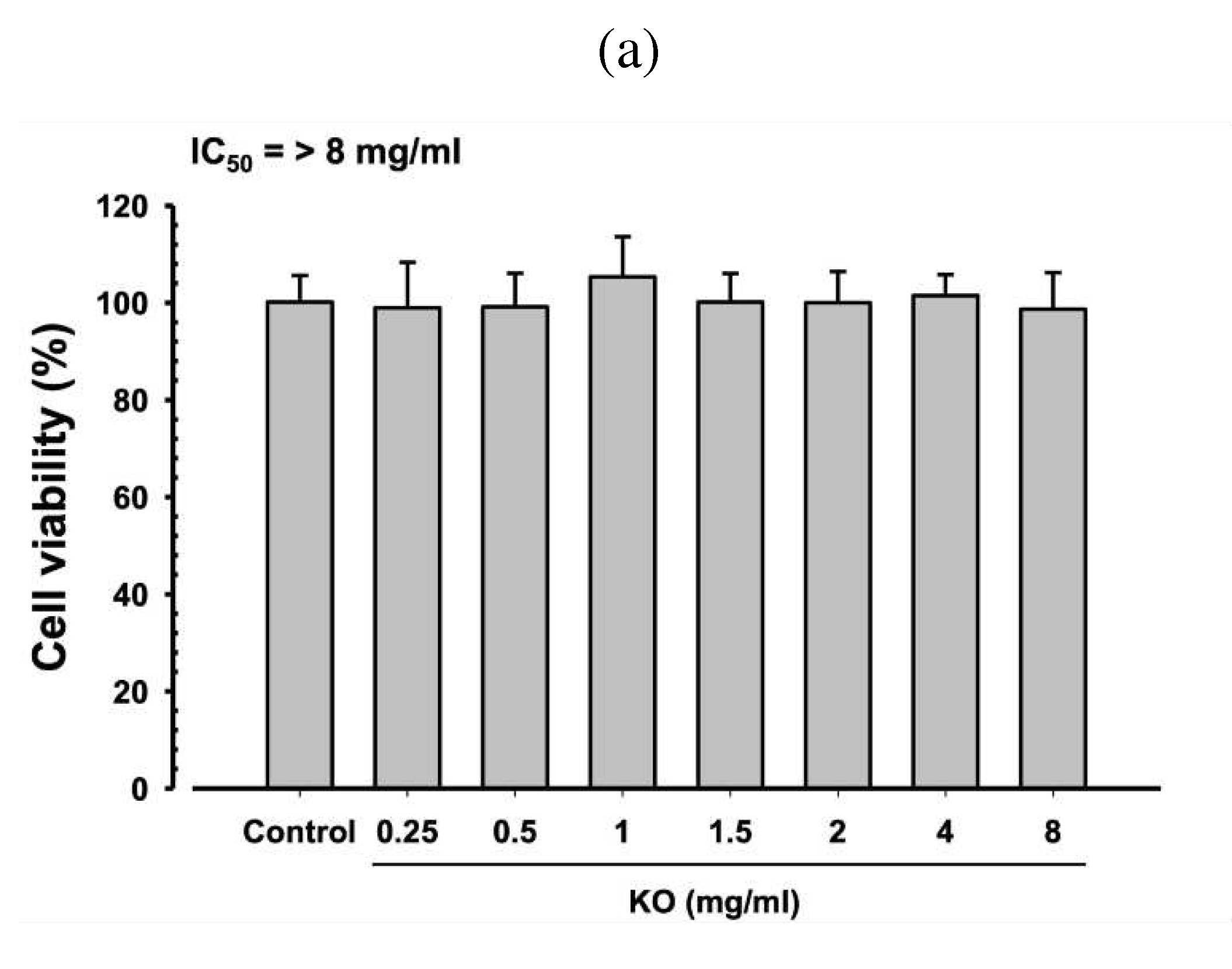
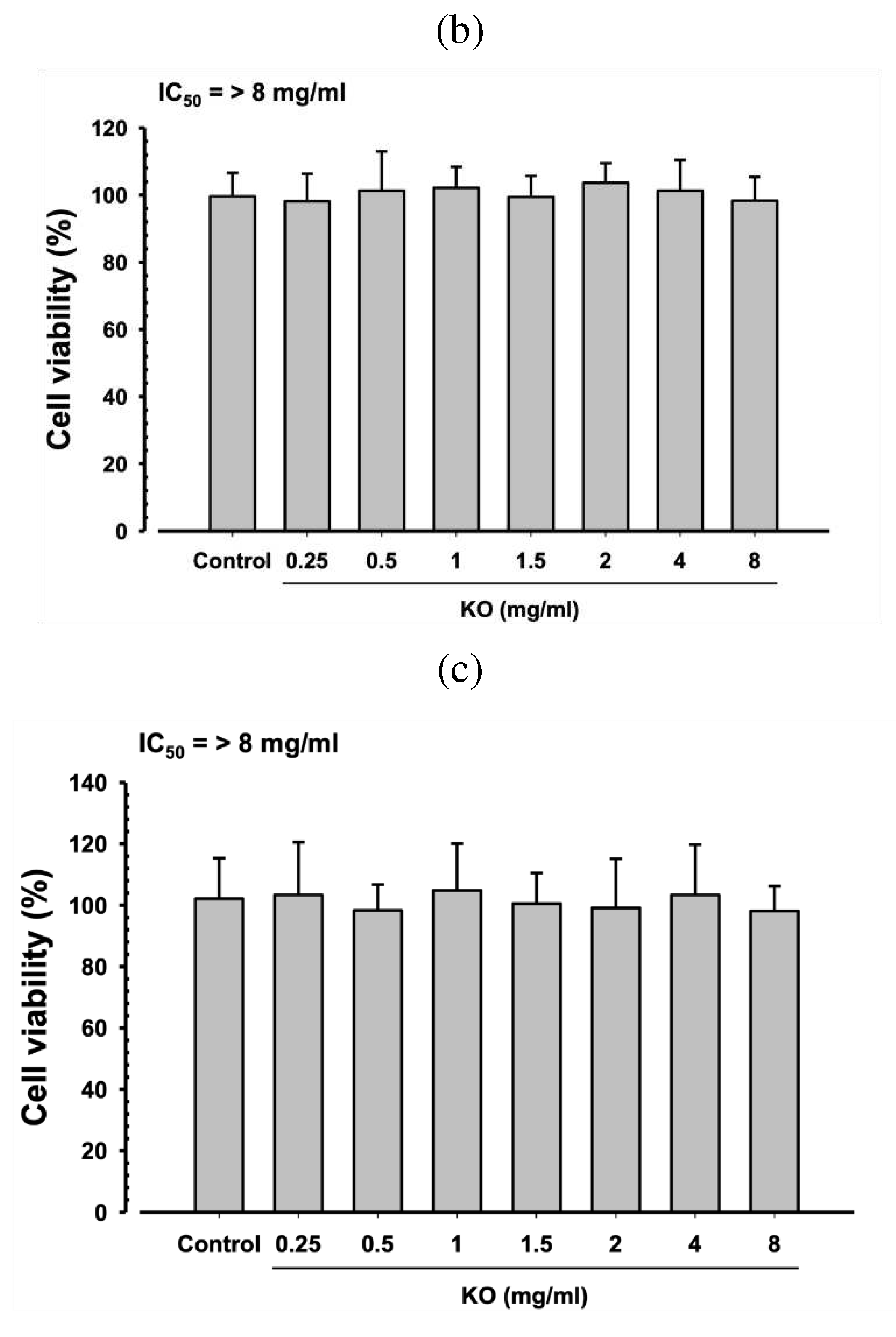
2.1.1. Cytotoxicity of KO in HDF, HaCaT, and B16/F10 Cells
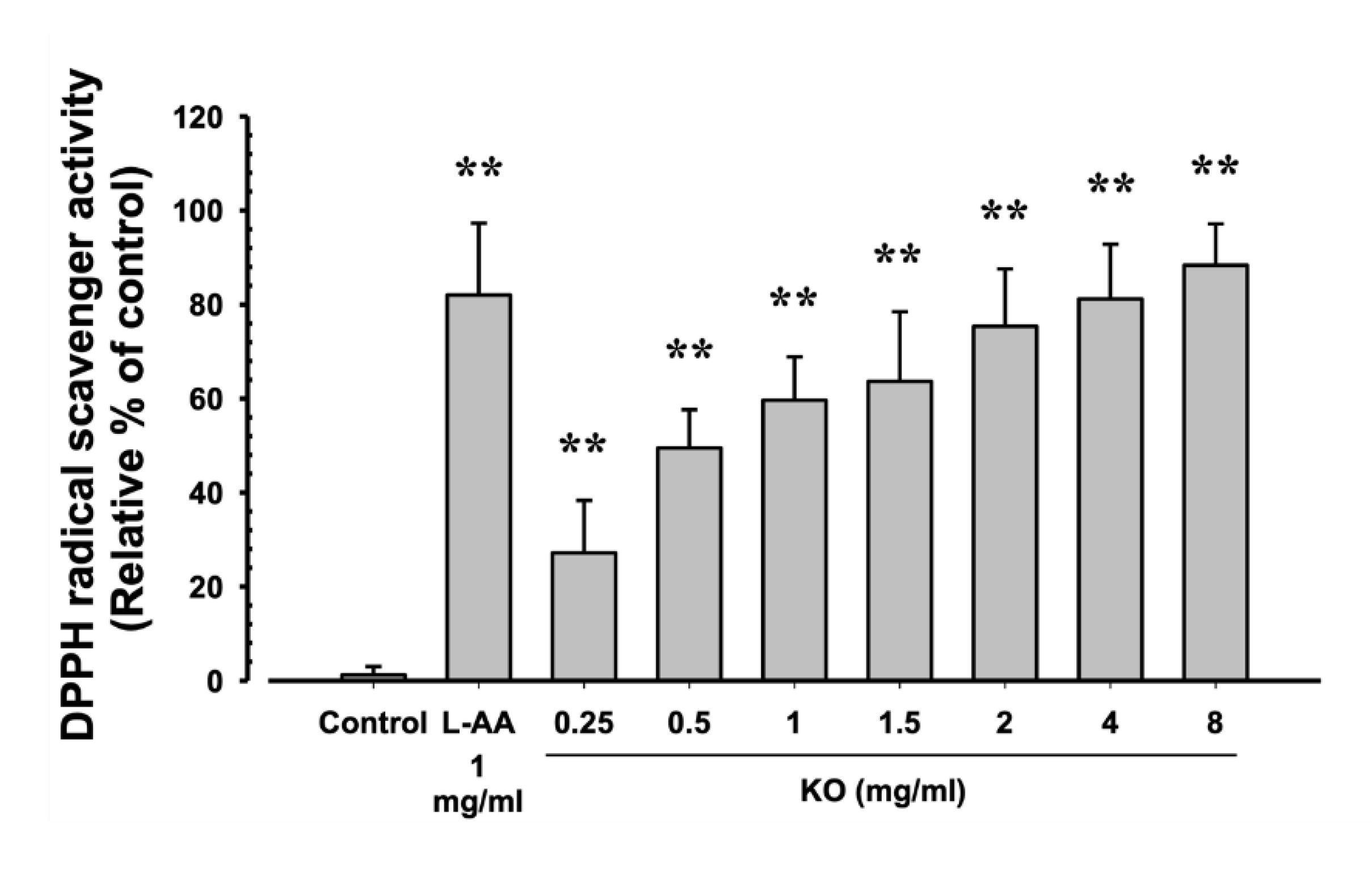
2.1.2. Free Radical Scavenging Activity of KO
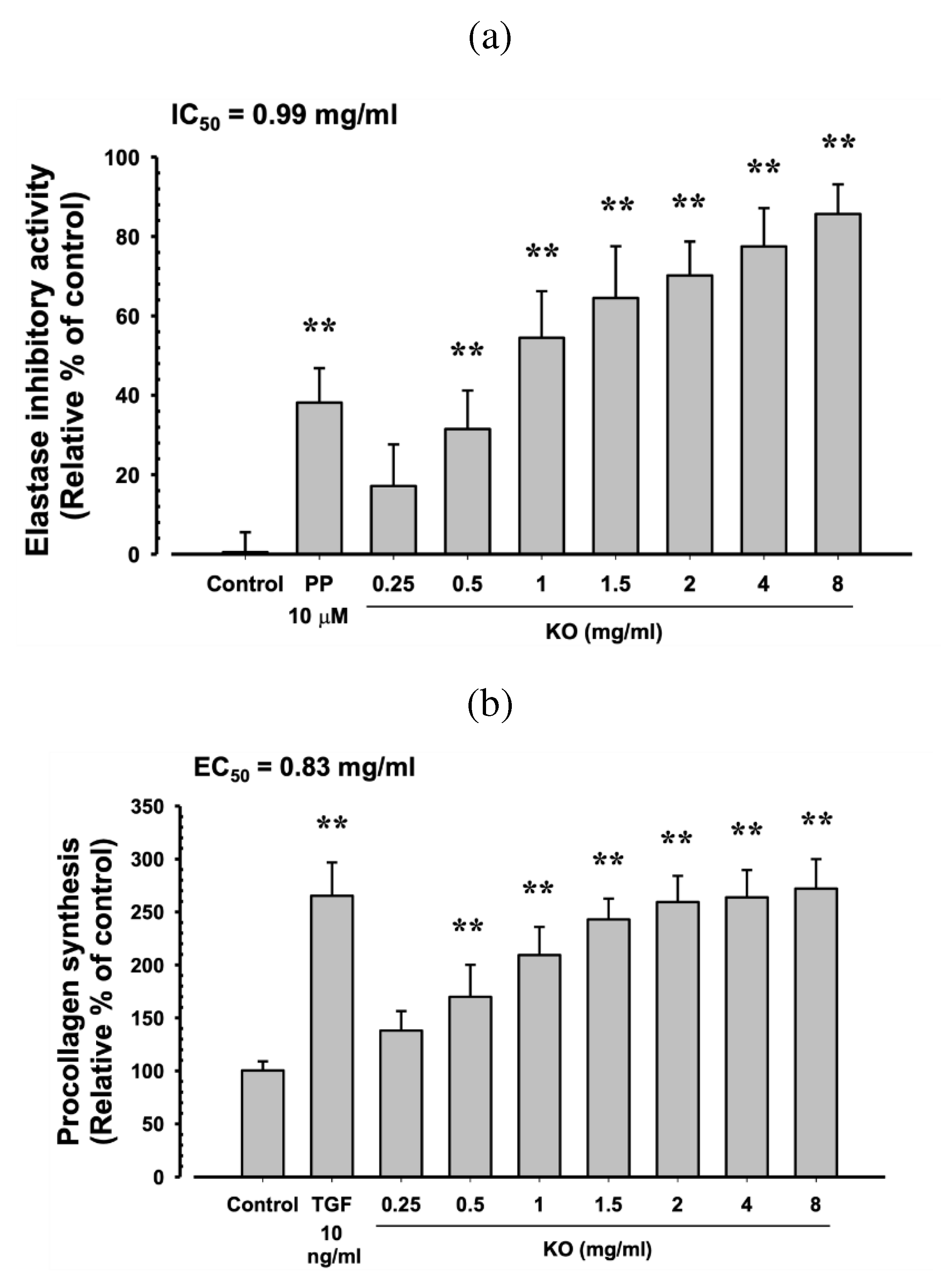
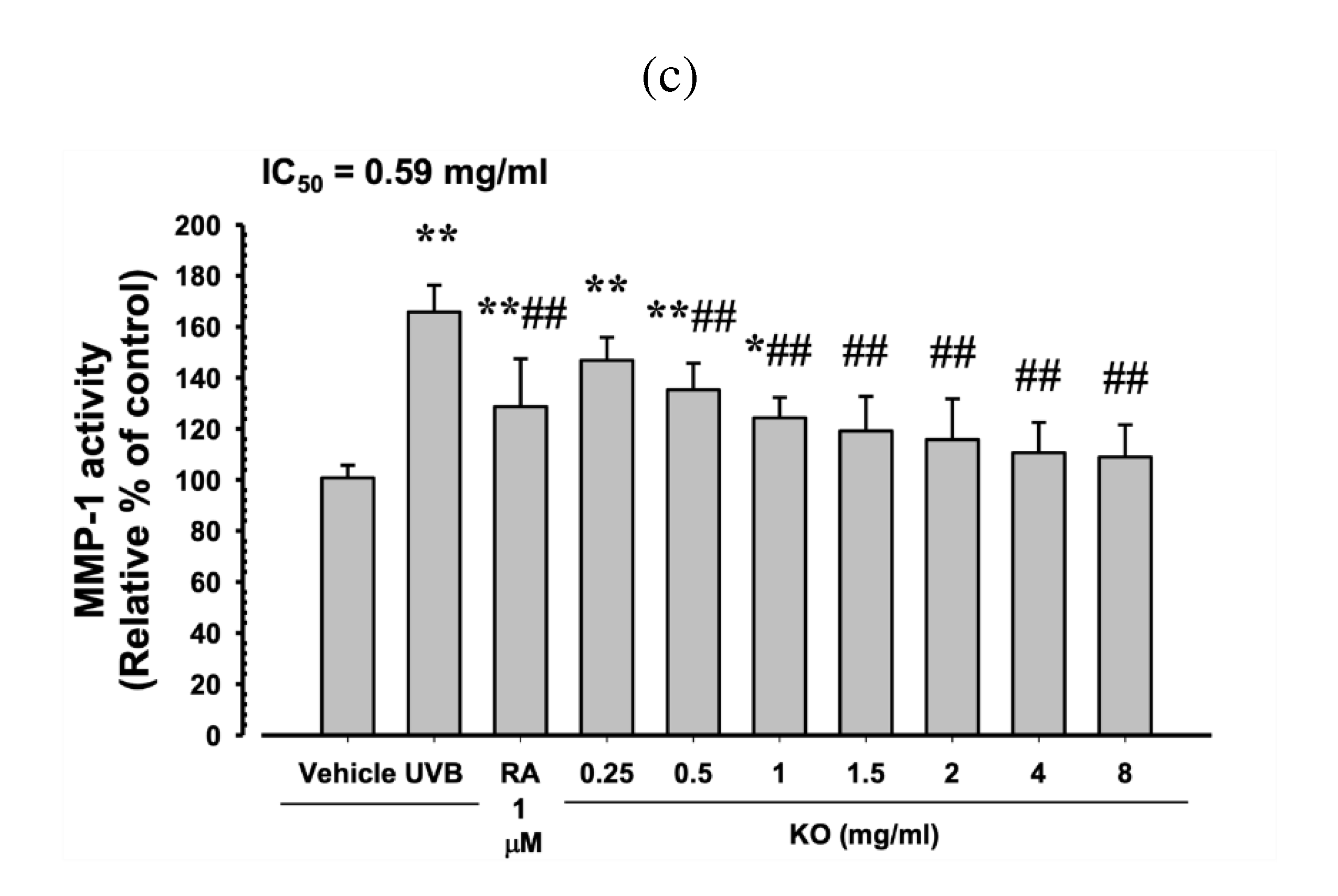
2.1.3. Anti-Wrinkle Benefits of KO
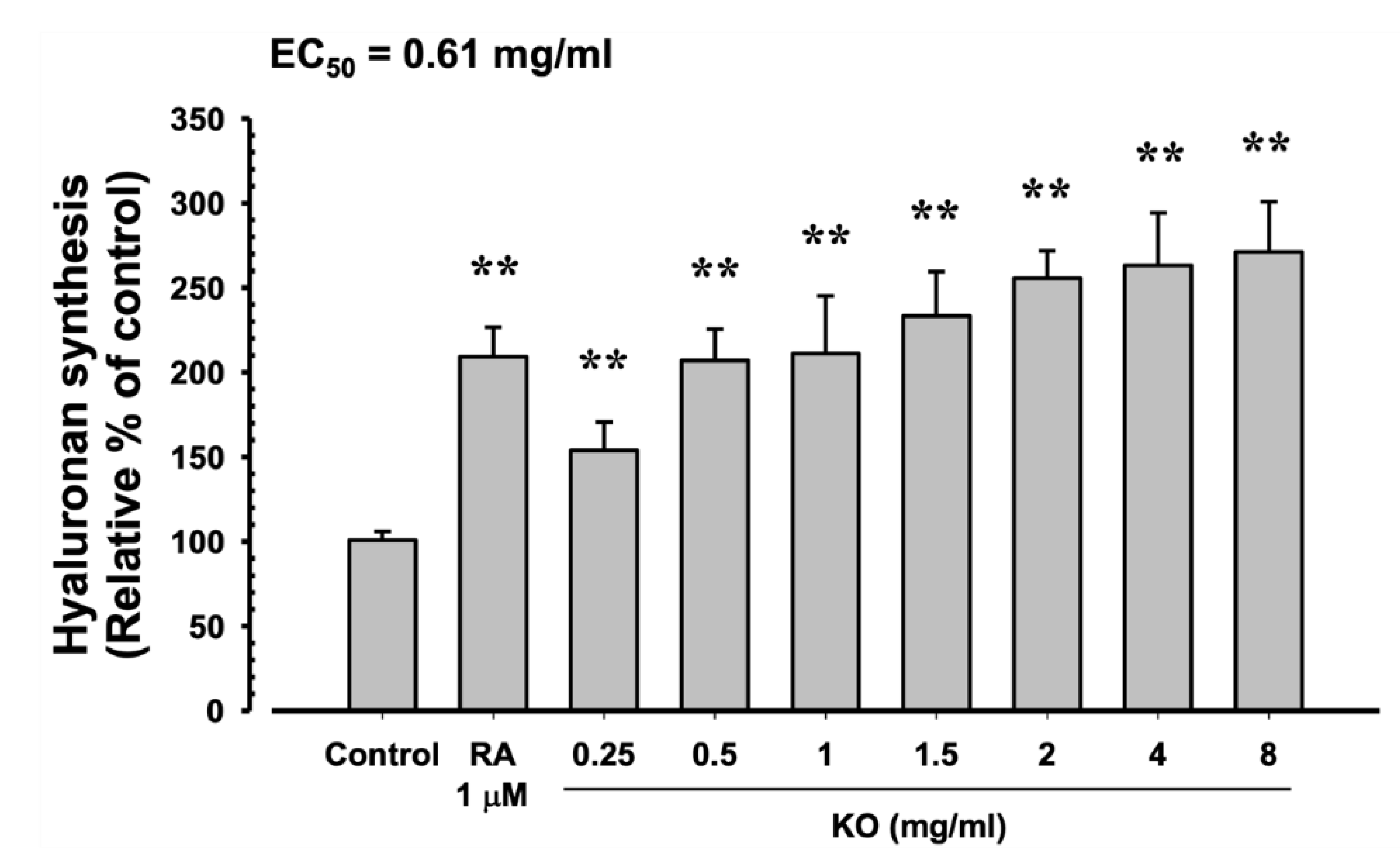
2.1.4. Moisturizing Benefits of KO
2.2. In vivo Evidence Anti-Photoaging
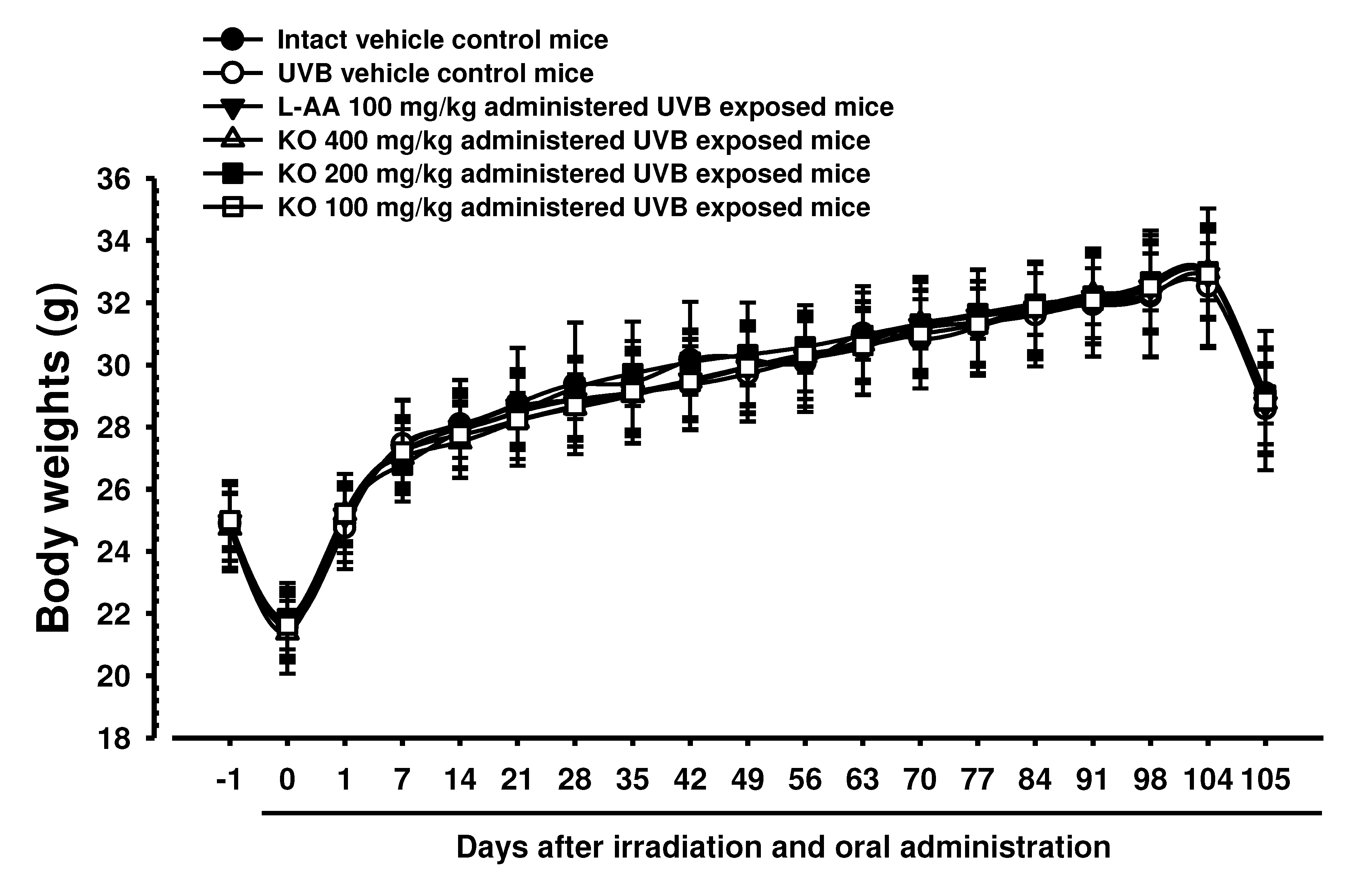
2.2.1. Effects of KO on Body Weight Changes
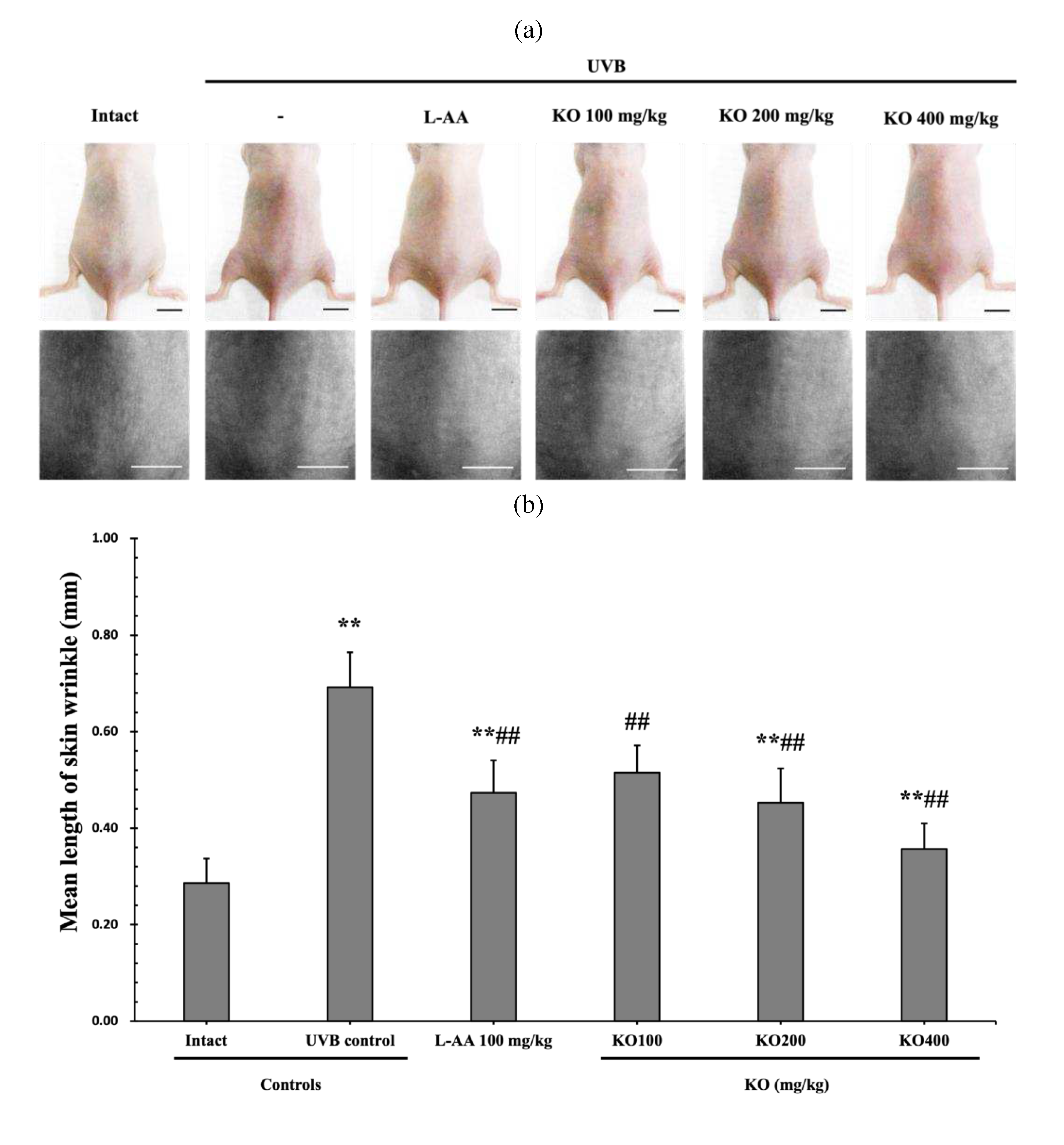
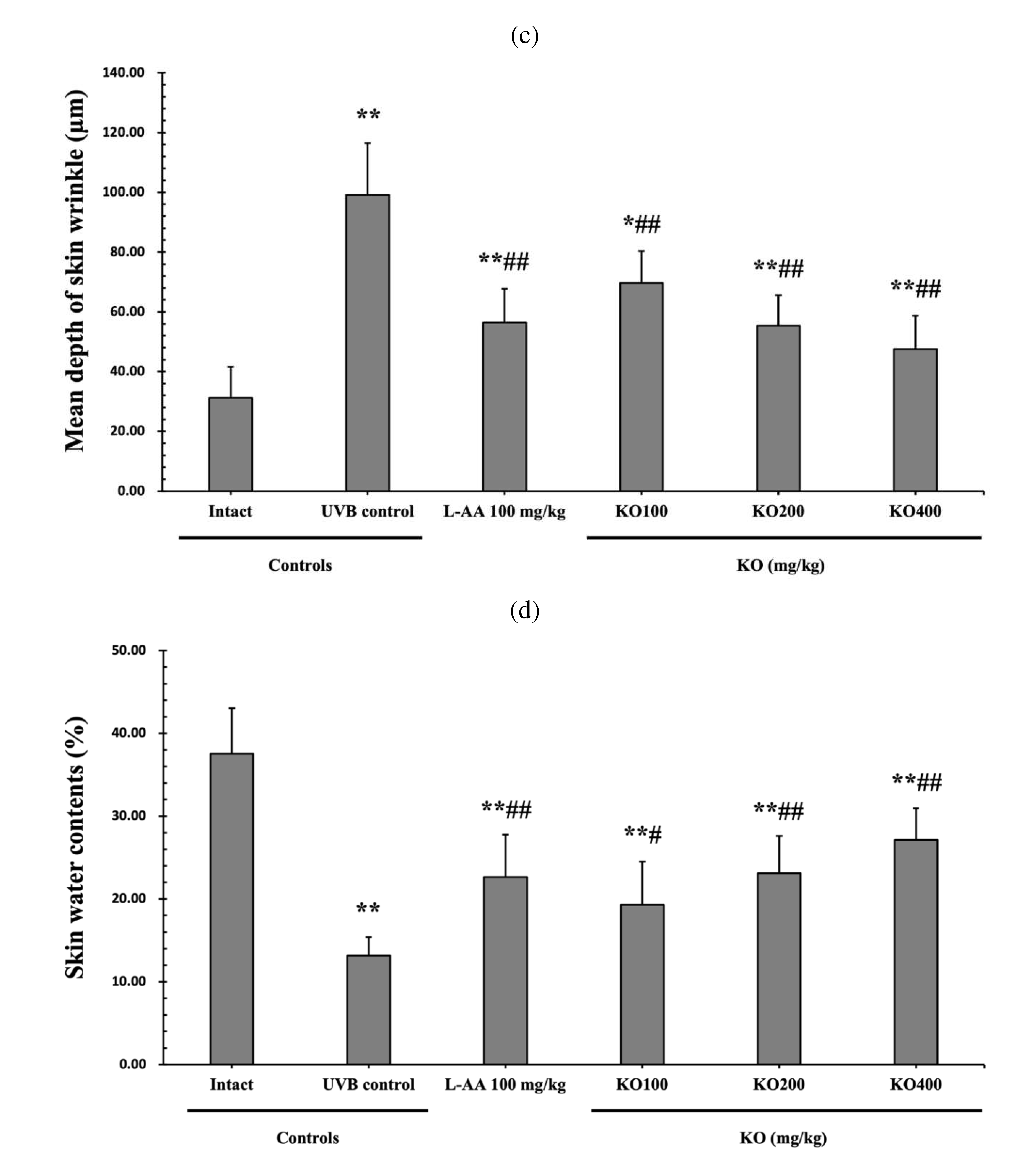
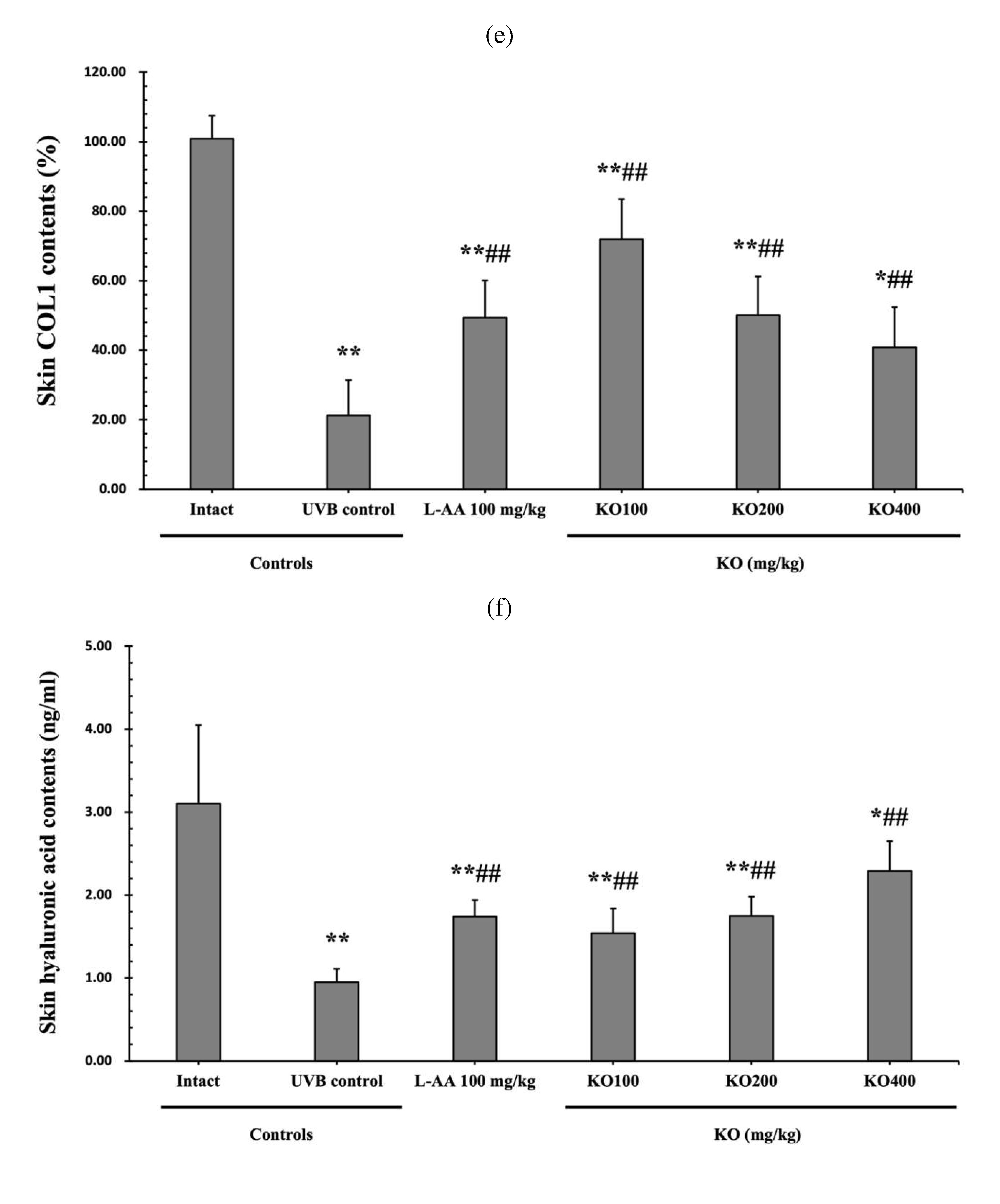
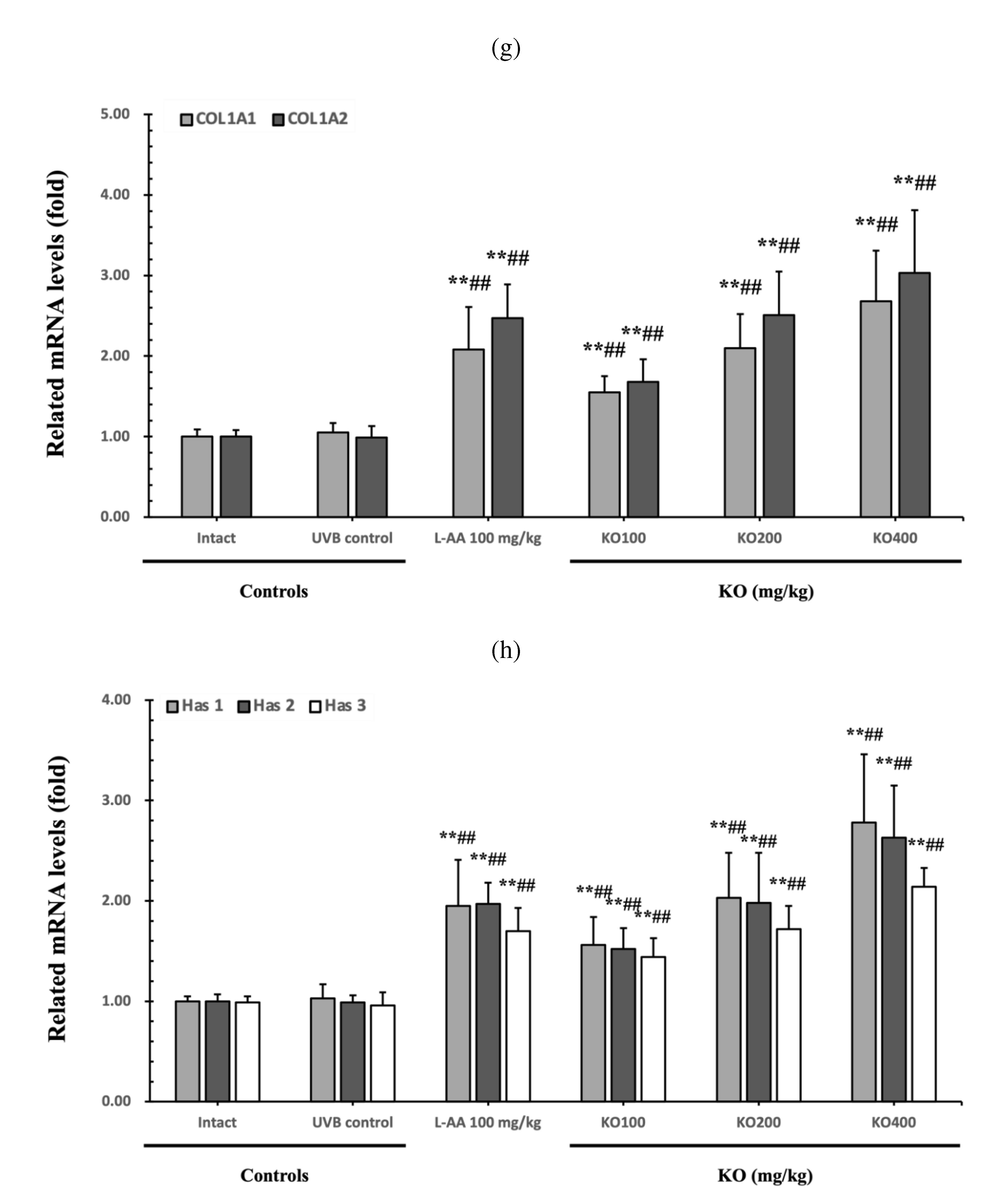
2.2.2. Effects of KO on UVB-Induced Skin Wrinkle Formation and Skin Moisturization
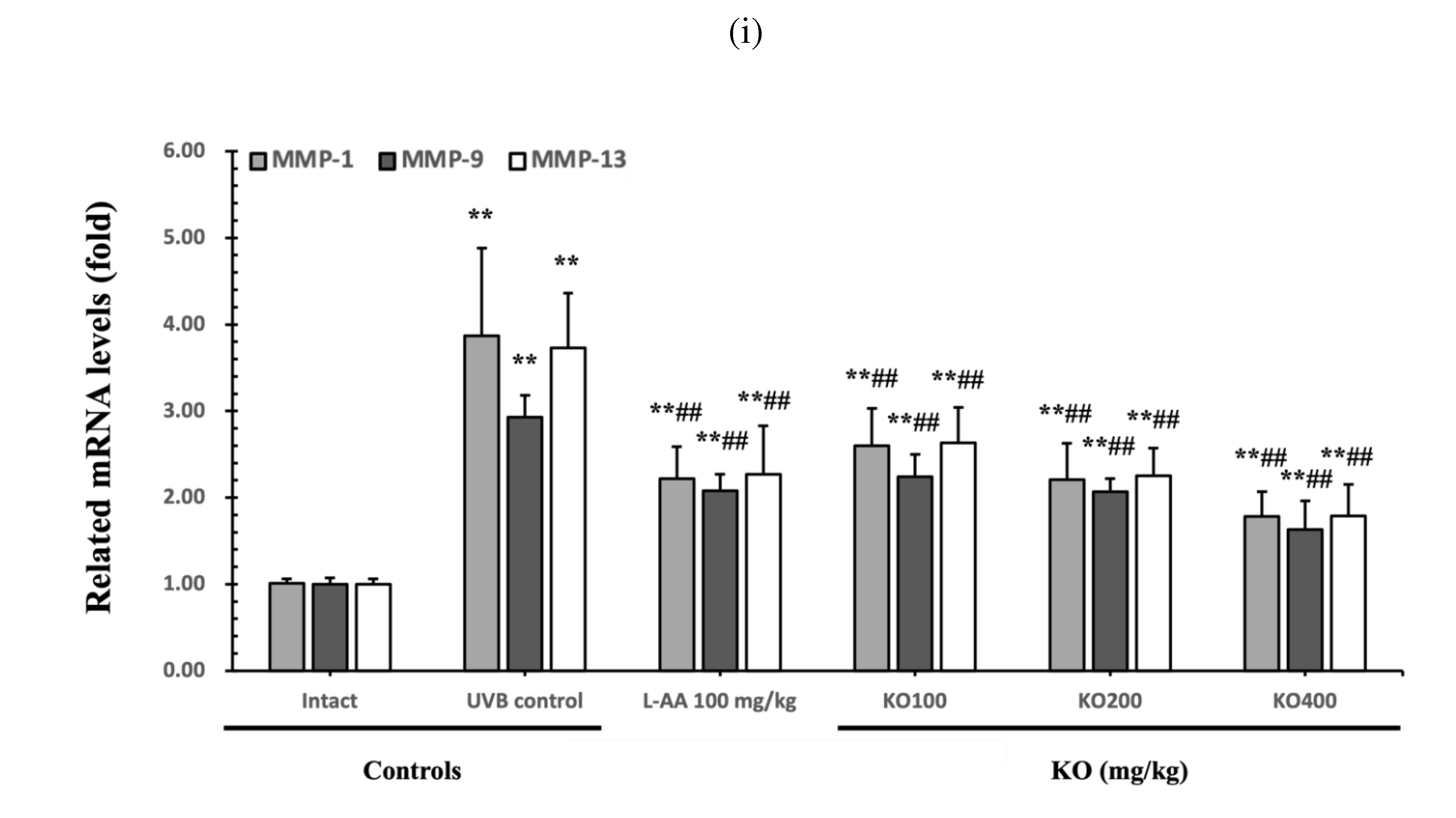
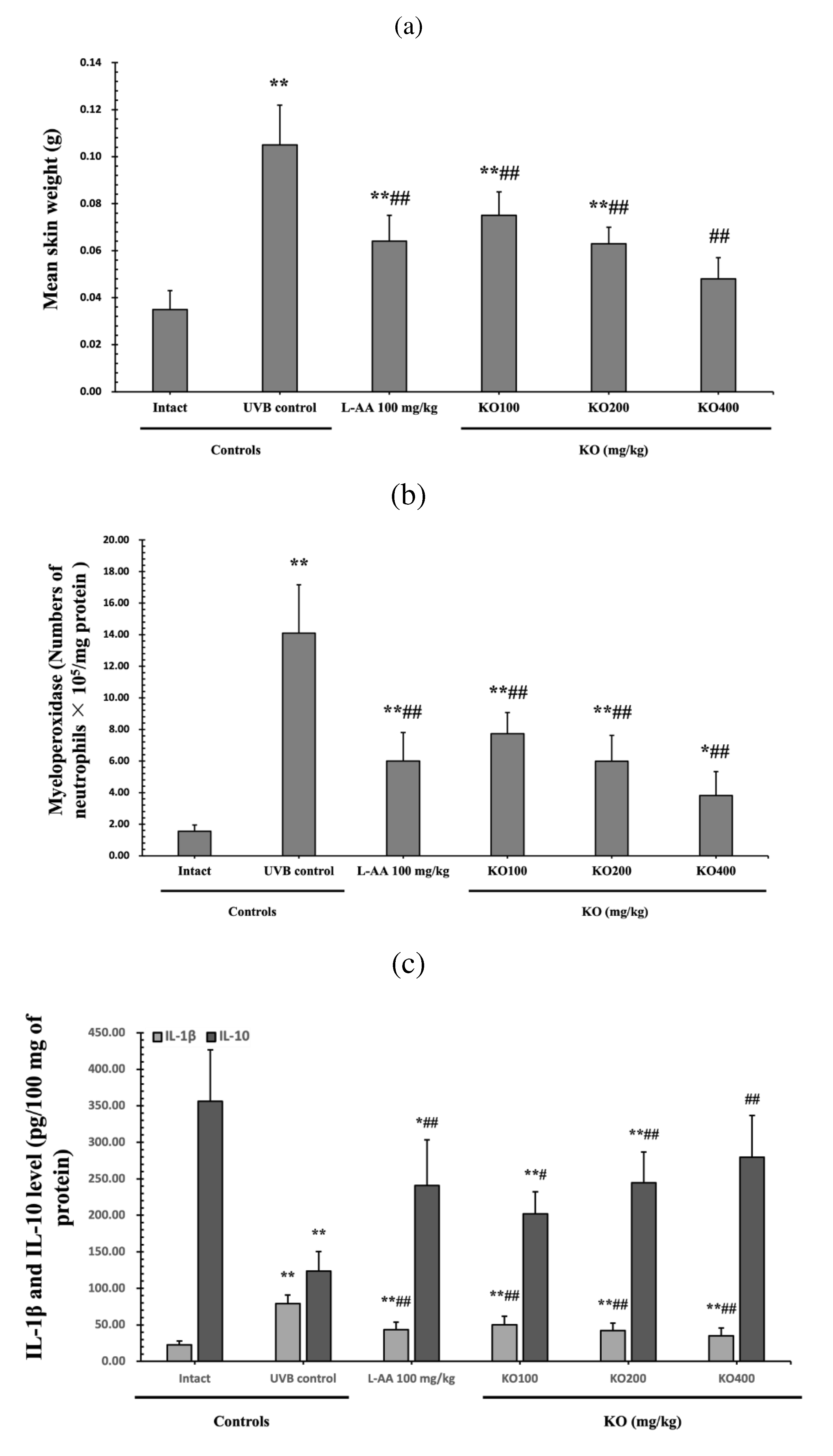
2.2.3. Effects of KO on UVB-Induced Skin Inflammation
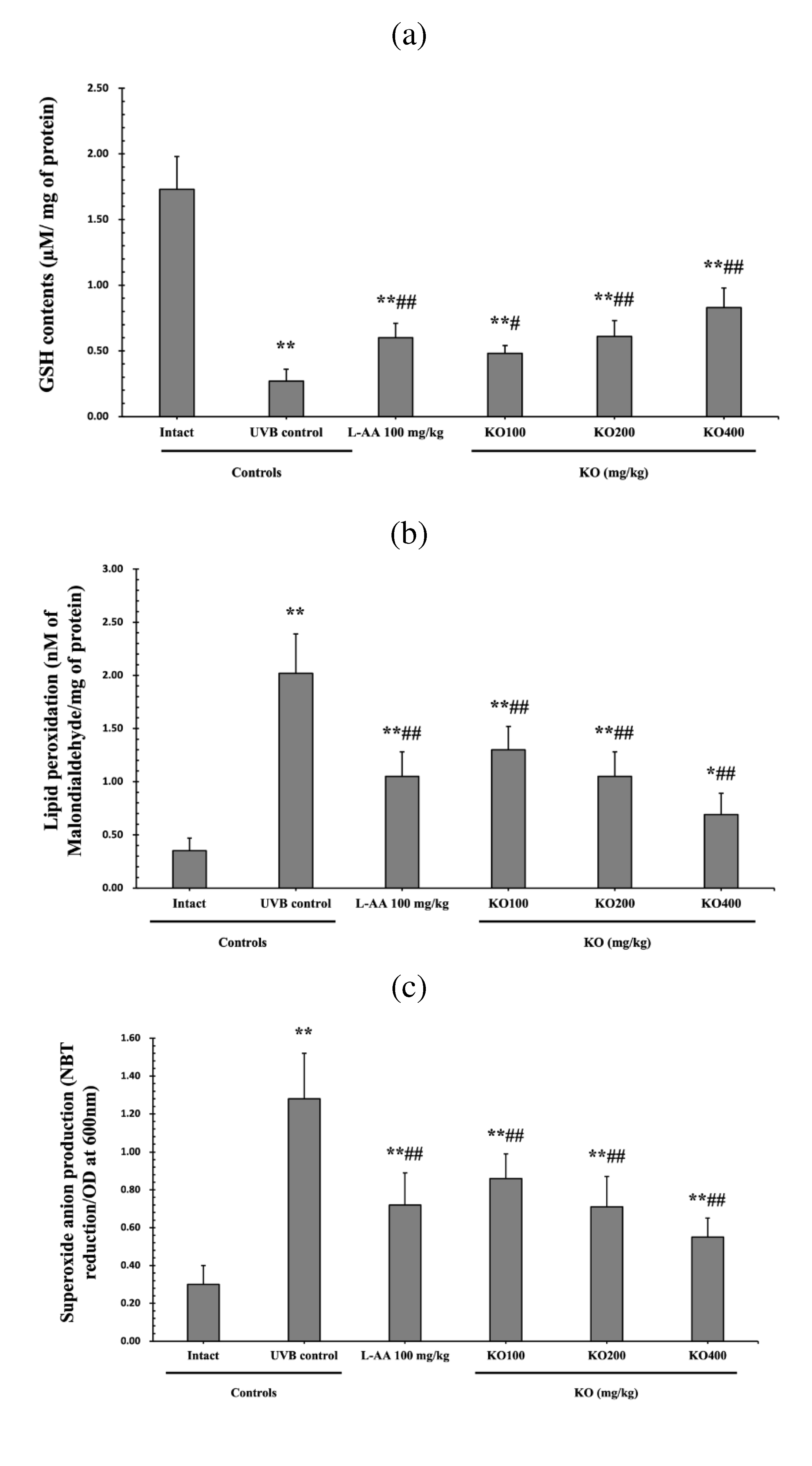
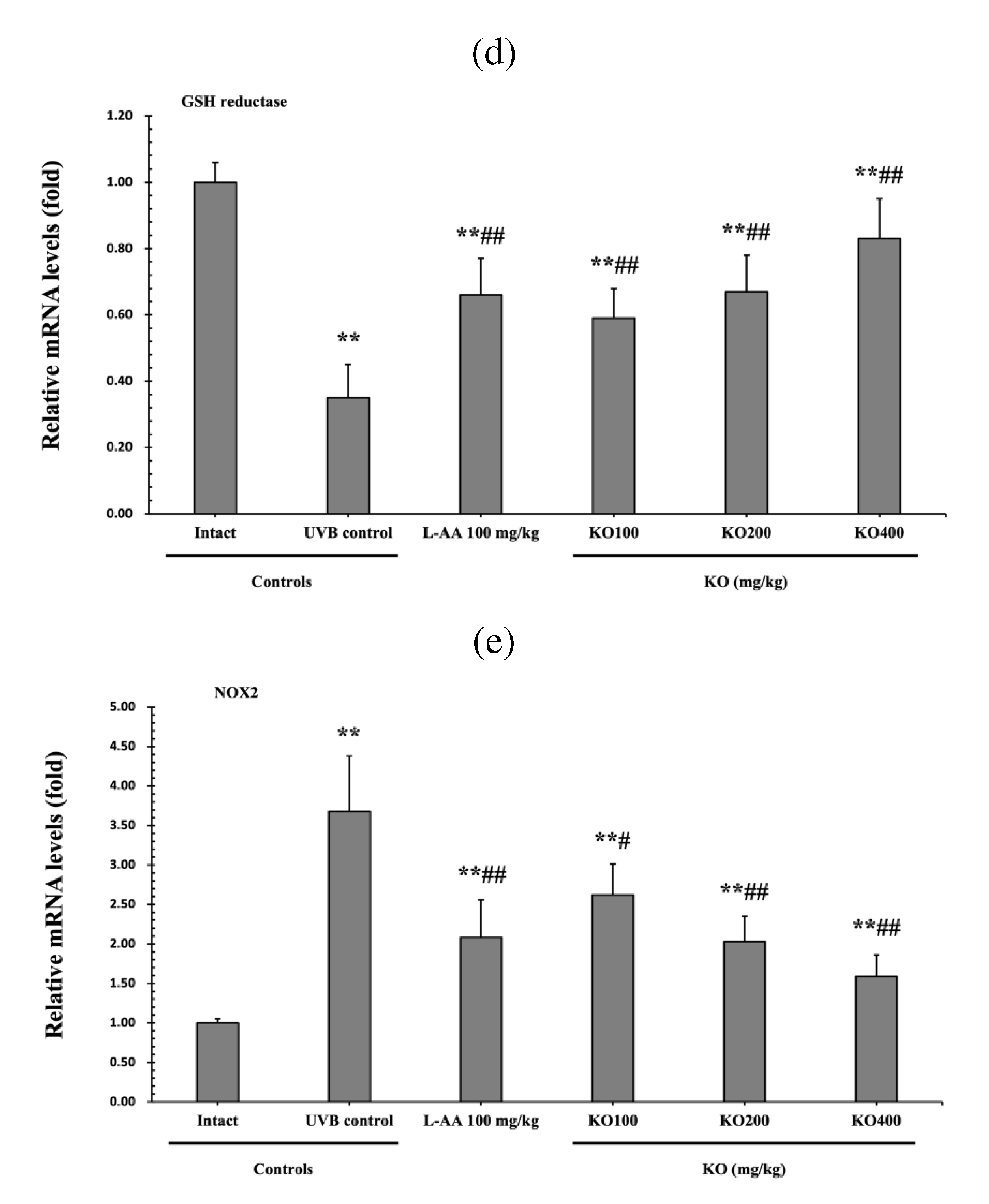
2.2.4. Effects of KO on UVB-Induced Oxidative Stress
2.2.5. Effects of KO on UVB-Induced Histopathological Changes in Skin Tissue
3. Discussion
4. Materials and Methods
4.1. In Vitro
4.1.1. Preparation of KO
4.1.2. Cell Cultures
4.1.3. Cell Viability Assay
4.1.4. DPPH Radical Scavenging Activity Test
4.1.5. Elastase Inhibition Assay
4.1.6. Procollagen Content Test.
4.1.7. MMP-1 Inhibition Test
4.1.8. Hyaluronan Production Assay
4.2. In Vivo
4.2.1. Preparation of Experimental Group
4.2.2. Skin Photoaging
4.2.3. Experimental Substances and Administration
4.2.4. Antioxidant and Anti-Inflammatory
4.2.5. Moisturizing
4.2.6. Anti-Wrinkle
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolarsick, P.A.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. JDNA 2011, 3, 203–213. [Google Scholar] [CrossRef]
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery (Oxford) 2022, 40, 8–12. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005, 19, 176–194. [Google Scholar] [CrossRef]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Kawada, S.; Ohtani, M.; Ishii, N. Increased oxygen tension attenuates acute ultraviolet-B-induced skin angiogenesis and wrinkle formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R694–R701. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinol. 2012, 4, 109–117. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Boelsma, E.; Hendriks, H.F.; Roza, L. Nutritional skin care: health effects of micronutrients and fatty acids. Am. J. Clin. Nutr. 2001, 73, 853–864. [Google Scholar] [CrossRef]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 1–11. [Google Scholar] [CrossRef]
- Fasano, E.; Serini, S.; Mondella, N.; Trombino, S.; Celleno, L.; Lanza, P.; Cittadini, A.; Calviello, G. Antioxidant and anti-inflammatory effects of selected natural compounds contained in a dietary supplement on two human immortalized keratinocyte lines. Biomed Res. Int. 2014. [Google Scholar] [CrossRef]
- Watanabe, N.; Suzuki, T.; Yamazaki, Y.; Sugiyama, K.; Koike, S.; Nishimukai, M. Supplemental feeding of phospholipid-enriched alkyl phospholipid from krill relieves spontaneous atopic dermatitis and strengthens skin intercellular lipid barriers in NC/Nga mice. Biosc. Biotechnol. Biochem. 2019, 83, 717–727. [Google Scholar] [CrossRef]
- Farage, M.; Miller, K.; Elsner, P.; Maibach, H. Intrinsic and extrinsic factors in skin ageing: a review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, J.K.; Lee, J.K.; Choi, B.R.; Ku, S.K.; Jegal, K.H. The Protective Effects of Unripe Apple (Malus pumila) Extract on Ultraviolet B-Induced Skin Photoaging Mouse Model. Appl. Sci. 2023, 13, 4788. [Google Scholar] [CrossRef]
- Berge, K.; Musa-Veloso, K.; Harwood, M.; Hoem, N.; Burri, L. Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutr. Res. 2014, 34, 126–133. [Google Scholar] [CrossRef]
- Mödinger, Y.; Schön, C.; Wilhelm, M.; Hals, P.-A. Plasma kinetics of choline and choline metabolites after a single dose of SuperbaBoostTM krill oil or choline bitartrate in healthy volunteers. Nutrients 2019, 11, 2548. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, J.W.; Chun, Y.S.; Kim, J.; Lim, T.G.; Shim, S.M. Krill oil inhibited adipogenic differentiation by inducing the nuclear Nrf2 expression and the AMPK activity. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Kim, Y.U.; Kim, J.-K.; Chun, Y.-S.; Kwon, Y.-S.; Ku, S.-K.; Song, C.-H. Preventive and Therapeutic Effects of Krill Oil on Obesity and Obesity-Induced Metabolic Syndromes in High-Fat Diet-Fed Mice. Mar. Drugs 2022, 20, 483. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, G.S.; Quayle, C.; Garcia, C.; Fotoran, W.L.; Dos Santos, J.F.; van der Horst, G.T.; Hoeijmakers, J.H.; Menck, C.F. Photorepair of either CPD or 6-4PP DNA lesions in basal keratinocytes attenuates ultraviolet-induced skin effects in nucleotide excision repair deficient mice. Front. Immunol. 2022, 13, 800606. [Google Scholar] [CrossRef]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Wu, P.-Y.; Hou, C.-W.; Chien, T.-Y.; Chang, Q.-X.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protective effects of sesamin against UVB-induced skin inflammation and photodamage in vitro and in vivo. Biomolecules 2019, 9, 479. [Google Scholar] [CrossRef]
- Choi, H.-J.; Alam, M.B.; Baek, M.-E.; Kwon, Y.-G.; Lim, J.-Y.; Lee, S.-H. Protection against UVB-induced photoaging by Nypa fruticans via inhibition of MAPK/AP-1/MMP-1 signaling. Oxid. Med. Cell. Longev. 2020. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investiga. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Sullivan, G.W.; Sarembock, I.J.; Linden, J. The role of inflammation in vascular diseases. J. Leukoc. Biol. 2000, 67, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Íşeri, S.O.; Sener, G.; Yuksel, M.; Contuk, G.; Cetinel, S.; Gedik, N.; Yeğen, B.C. Ghrelin against alendronate-induced gastric damage in rats. J. Endocrinol. 2005, 187, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.; Rao, V.; Carvalho, C.; Bezerra-Filho, J.; Fonseca, S.; Vale, M.; Montenegro, D.; Cunha, F.; Ribeiro, R.; Brito, G. Lippia sidoides and Myracrodruon urundeuva gel prevents alveolar bone resorption in experimental periodontitis in rats. J. Ethnopharmacol. 2007, 113, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Campanini, M.Z.; Pinho-Ribeiro, F.A.; Ivan, A.L.; Ferreira, V.S.; Vilela, F.M.; Vicentini, F.T.; Martinez, R.M.; Zarpelon, A.C.; Fonseca, M.J.; Faria, T.J. Efficacy of topical formulations containing Pimenta pseudocaryophyllus extract against UVB-induced oxidative stress and inflammation in hairless mice. J. Photochem. Photobiol. B. 2013, 127, 153–160. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Meguro, S.; Arai, Y.; Masukawa, Y.; Uie, K.; Tokimitsu, I. Relationship between covalently bound ceramides and transepidermal water loss (TEWL). Arch. Dermatol. Res. 2000, 292, 463–468. [Google Scholar] [CrossRef]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, S.J.; Lee, J.E.; Lee, Y.J.; Song, C.H.; Choi, S.H.; Ku, S.K.; Kang, S.J. Anti-skin-aging benefits of exopolymers from Aureobasidium pullulans SM2001. J. Cosmet. Sci. 2014, 65, 285–298. [Google Scholar]
- Kang, S.J.; Choi, B.R.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Park, S.J.; Song, C.H.; Park, J.H.; Lee, Y.J.; Kwang, S. Inhibitory effects of pomegranate concentrated solution on the activities of hyaluronidase, tyrosinase, and metalloproteinase. J. Cosmet. Sci. 2015, 66, 145–159. [Google Scholar] [CrossRef]
- Jin-Chul, H.; Sang-Mi, A.; Chi-Young, Y.; Heung-Mook, S.; Taeg-Kyu, K. Anti-oxidant and anti-tumor activities of crude extracts by Gastrodia elata Blume. Korean J. Food Preserv. 2006, 13, 83–87. [Google Scholar]
- Losso, J.N.; Munene, C.N.; Bansode, R.R.; Bawadi, H.A. Inhibition of matrix metalloproteinase-1 activity by the soybean Bowman–Birk inhibitor. Biotechnol. lett. 2004, 26, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.-S.; Kang, N.J.; Bode, A.M.; Dong, Z. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Oh, W.-S.; Song, P.H.; Yun, S.; Kwon, Y.-S.; Lee, Y.J.; Ku, S.-K.; Song, C.-H.; Oh, T.-H. Anti-photoaging effects of low molecular-weight fucoidan on ultraviolet B-irradiated mice. Mar. Drugs 2018, 16, 286. [Google Scholar] [CrossRef] [PubMed]
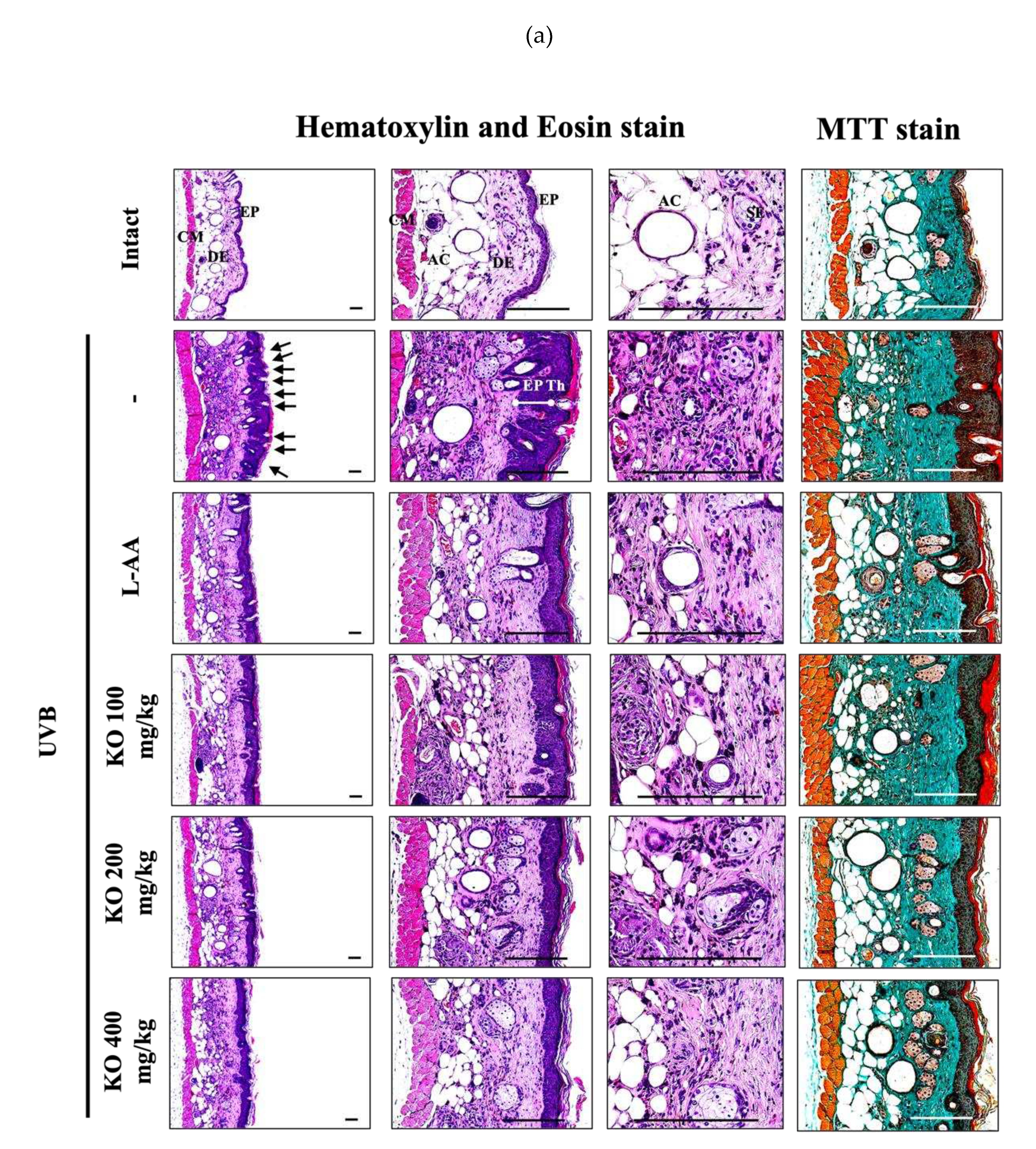
| Items (Unit) Groups |
Number of Microfolds (Folds/mm of Epidermis) |
Mean Epithelial Thickness (μm/Epidermis) | Mean Inflammatory Cells (Cells/mm2 of Dermis) |
Collagen Fiber Occupied Regions (%/mm2 of Dermis) |
|---|---|---|---|---|
| Controls | ||||
| Intact | 3.30±1.06 | 24.45±4.21 | 43.40±11.12 | 31.76±6.58 |
| UVB | 17.60±2.37** | 120.25±13.37** | 525.20±118.05** | 75.73±7.50** |
| Reference | ||||
| L-AA 100 mg/kg | 8.90±1.79**## | 65.94±10.15**## | 188.60±74.90**## | 51.47±8.96**## |
| Test materials | ||||
| KO 100 mg/kg | 12.10±1.60**## | 77.69±10.98**## | 242.80±67.05**## | 56.78±5.30**## |
| KO 200 mg/kg | 8.10±1.45**## | 65.03±11.28**## | 186.40±82.58**## | 51.43±7.23**## |
| KO 400 mg/kg | 6.10±1.29**## | 55.00±10.87**## | 134.20±38.05**## | 42.95±8.00*## |
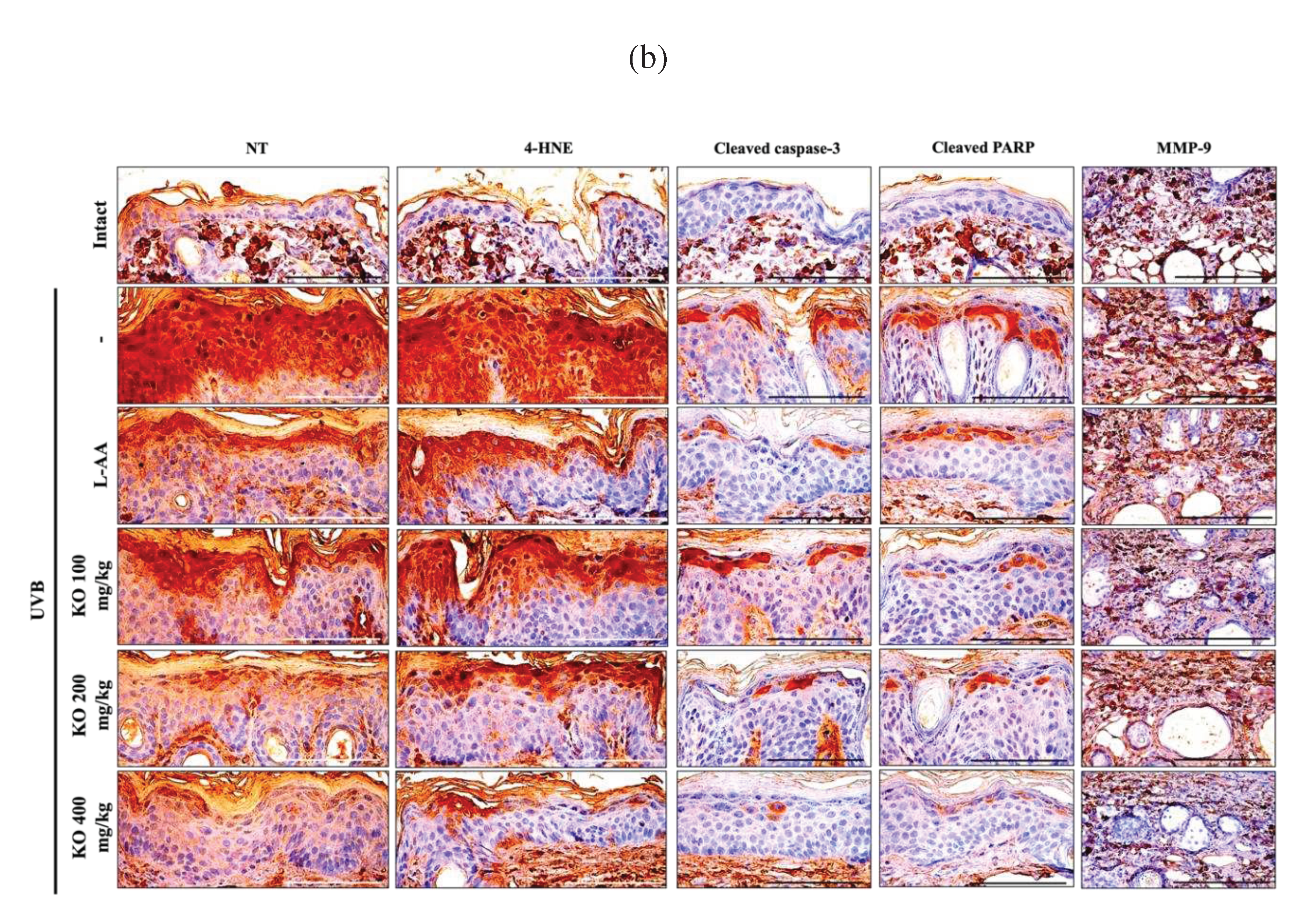
| Groups Items |
Controls | Reference | Test materials | |||
|---|---|---|---|---|---|---|
| Intact | UVB | L-AA 100 mg/kg | KO 100mg/kg | KO 200 mg/kg | KO 400 mg/kg | |
| Epidermis (cells/100 epithelial cells) |
||||||
| Nitrotyrosine | 18.20±4.47 | 80.20±7.80** | 41.40±10.98**## | 53.60±10.45**## | 40.80±11.52**## | 30.20±10.69## |
| 4-HNE | 14.00±2.49 | 86.20±4.94** | 52.00±6.86**## | 63.00±10.17*## | 51.20±10.92**## | 28.00±7.60**## |
| Cleaved caspase-3 | 4.60±1.90 | 36.60±4.22** | 18.40±5.40**## | 24.80±4.12**## | 18.00±5.25**## | 10.00±4.32## |
| Cleaved PARP | 4.60±1.90 | 41.00±5.68** | 22.00±3.77**## | 27.00±3.68**## | 21.00±3.92**## | 12.20±2.20**## |
| Dermis (%/mm2) | ||||||
| MMP-9 | 20.60±5.17 | 70.14±7.05** | 43.50±9.50**## | 53.95±8.33**## | 41.98±10.77**## | 32.75±9.27*## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




