1. Introduction
Schistosomiasis is an acute and chronic disease caused by several trematode flatworms (blood flukes) of the genus
Schistosoma. Currently, 78 countries maintain transmission of the disease, causing a global infection burden of 230 million people and up to 800 million at risk. Over 90% of them live in sub-Saharan Africa, under poverty or extreme poverty conditions [
1,
2]. Schistosomiasis is considered second, only to malaria, as the most devastating parasitic disease, and it is one of the Neglected Tropical Diseases (NTDs) [
3]. Migratory flows and international travel are triggering an increment in imported cases in non-endemic countries, 4-5% of them being severe cases [
4,
5,
6]. Moreover, population movements combined with the effects of climate change, have caused autochthonous transmission of the disease in non-tropical regions, such as France [
7] and Spain [
8].
The availability of highly sensitive diagnostic tests is crucial for the diagnosis of symptomatic cases of schistosomiasis, for mass screening of people at risk, and for the evaluation of eradication programs carried out in endemic regions [
9]. To date, there is no reference diagnosis for schistosomiasis. Direct microscopic observation of urine, feces or tissues shows a low sensitivity, particularly for intestinal schistosomiasis in adults or long-term residents outside endemic areas [
2,
10]. On the other hand, a number of serological assays detecting antibodies against
Schistosoma spp. are commercially available and they are recommended by the European Centre for Disease Prevention and Control (ECDC) for screening for schistosomiasis in migrants who have been living in Europe for less than 5 years [
11]. However, antibody detection has several limitations, including variable sensitivity, inability to distinguish past and active infections, lack of utility for post-treatment monitoring, and poor performance in the early stages of acute infections [
10,
12]. Antigenic tests, such as the urine Circulating Cathodic Antigen (CCA), also suffer from important limitations for the diagnosis of other
Schistosoma species besides
S. mansoni and for the diagnosis of patients in non-endemic regions [
10]. Finally, molecular techniques, in particular PCR-based methods, have also been used for the diagnosis of human schistosomiasis [
13], being particularly valuable for the simultaneous detection and identification of
Schistosoma species [
14]. Although very sensitive and accurate, complex PCR-based methods for schistosomiasis are expensive and require specialized personnel and equipment, and are therefore not useful for diagnosis under field conditions and are usually only available in reference laboratories [
15].
In this context, isothermal nucleic acid amplification tests, in particular the loop-mediated isothermal amplification (LAMP) technology, could be a good alternative because they have several relevant advantages over most PCR-based methods. LAMP is a powerful nucleic acid amplification technique that combines simplicity, elevated sensitivity and specificity in DNA detection [
16]. LAMP technology has all the characteristics required of a high-efficiency diagnostic assay along with simple operation for use in the clinical diagnosis of infectious diseases, including point-of-care (POC) testing under field conditions in developing countries [
17,
18].
A number of LAMP assays have already been developed and successfully used for the specific detection of the three main species causing human schistosomiasis (
Schistosoma haematobium,
S. mansoni and
S. japonicum) and have been applied to the diagnosis and evaluation of the efficacy of chemotherapy, both in animal models and human patients [
19]. Previous studies of our group have developed and successfully evaluated LAMP assays in the clinical determination of
S. mansoni in both stool [
20] and urine samples [
21], as well as
S. haematobium in urine samples [
22,
23]. In addition, we have described a genus-specific LAMP assay for simultaneous detection of the most important schistosome species affecting humans, including
S. haematobium, S. mansoni and
S. intercalatum [
24], which has also been evaluated to detect DNA from
S. haematobium-S. bovis hybrids [
25], but to date has not yet been used with clinical samples.
Thus, the main purpose of this study is to evaluate, for the first time, the performance of the LAMP technique on patients’ urine samples for the diagnosis of imported schistosomiasis in a non-endemic area in comparison to a commercial rapid diagnostic serological test based on immunochromatography (Schistosoma ICT IgG-IgM) and microscopic examination of feces and urine.
2. Results
A total of 115 patients were finally included in the study. Most of them were male (92.2%) and the mean age was 28.3 years. The mean period of residence in Europe was 30.16 months. The most frequent countries of origin were Senegal (n = 43; 37.4%), Mali (n = 39; 33.9%) and Guinea Bissau (n = 12; 10.4%). Main socio-demographic data and parasitological findings are shown in
Table 1.
For all 115 patients, immunological test and urine microscopy data were obtained; stool microscopy data were available for only 108 patients. A total of 21 patients (21/115; 18.3%) were diagnosed with schistosomiasis confirmed by microscopic observation, with S. haematobium being the most frequent causative agent identified (18/115; 15.7%), all of them in urine samples. Three patients were diagnosed by stool microscopy with intestinal schistosomiasis: S. mansoni was identified in two patients and S. intercalatum/S. guineensis in one patient from Equatorial Guinea.
The systematic screening protocol for searching parasites detected other helminths, including Strongyloides stercoralis (n = 12; 10.4%), hookworms (n = 3; 2.6%), Mansonella perstans (n = 1; 0.9%), and Hymenolepis nana (n = 1; 0.9%). Only one patient with confirmed schistosomiasis (S. intercalatum/S. guineenses) was coinfected with Strongyloides stercoralis. One patient was coinfected with S. stercoralis and hookworm.
The results of the
Schistosoma ICT IgG-IgM test and
Schistosoma-LAMP assays are shown in
Table 2. In patients with microscopy-confirmed schistosomiasis, the
Schistosoma ICT IgG-IgM was 100% positive and
Schistosoma-LAMP was 61.9% positive, with differences among species. In patients without microscopy-confirmed schistosomiasis, the
Schistosoma ICT IgG-IgM test was positive in 48.9% and LAMP in 63.8%.
The sensitivity, specificity, PPV and NPV values estimated by LCA are summarized in
Table 3. Overall, the highest sensitivity resulted for Schistosoma ICT IgG-IgM test, the sensitivity of
Schistosoma-LAMP resulted superior to that of microscopy, while its specificity was the lowest of the three.
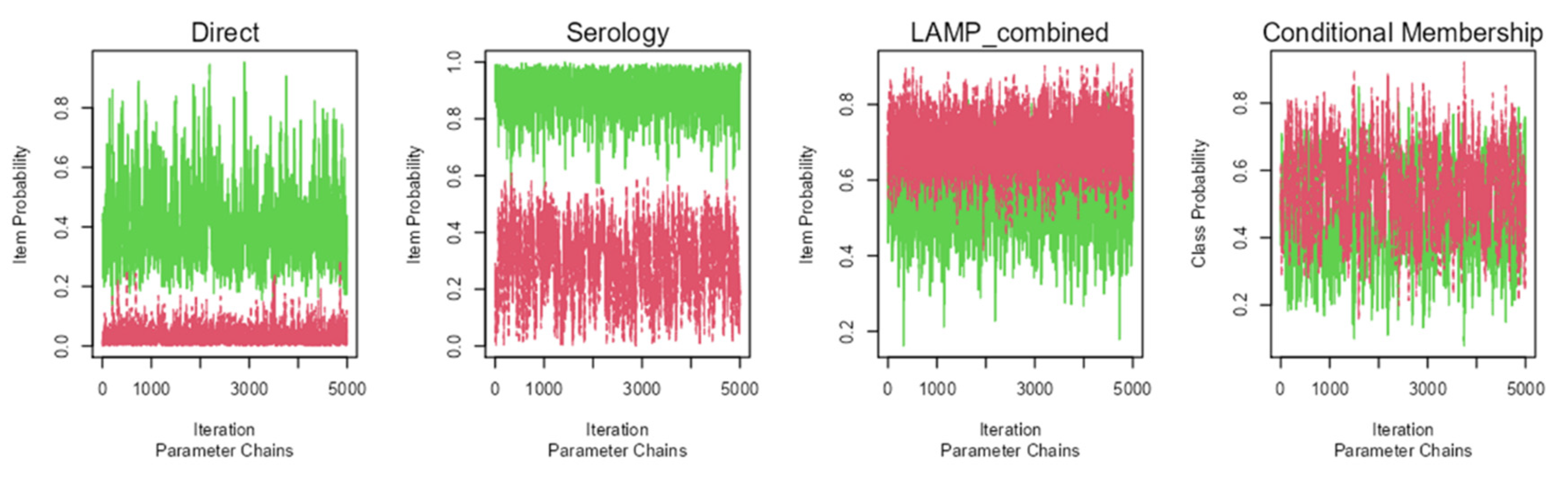
The Bayesian model showed an appropriate convergence, as none of the statistical models of Rubin and Gelman reached values over 1.01 (
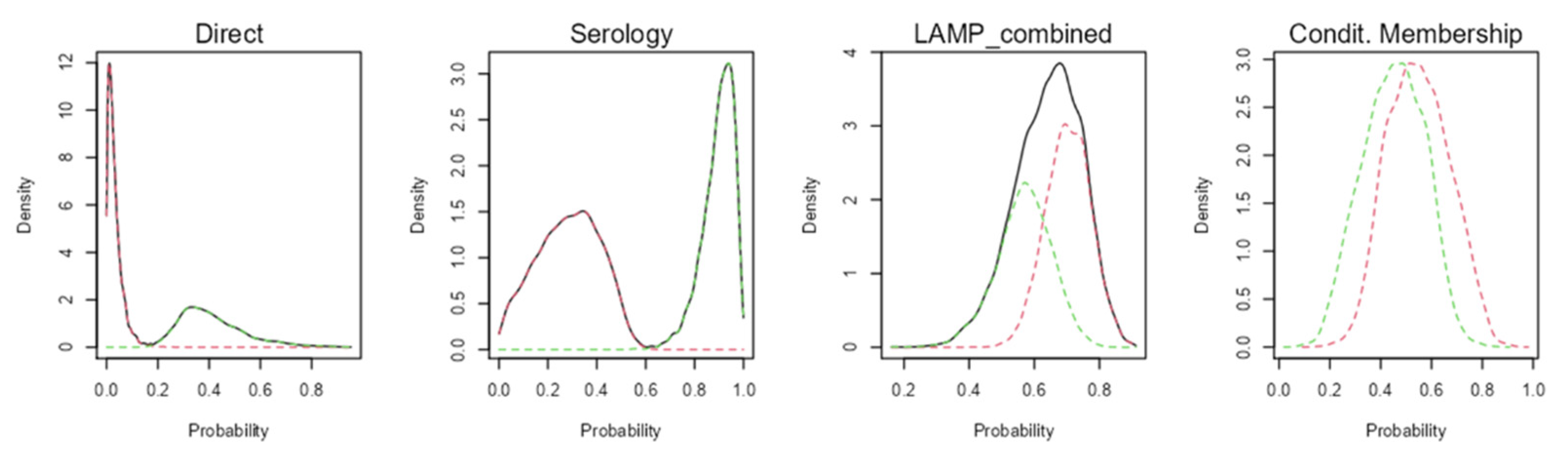
Figure 1). Probability density graphs for the classes estimated by the models are shown in
Figure 2 in order to identify how classes are discriminated
In brackets: bootstrap 95% confidence interval (CI95).
3. Discussion
In Western travel clinics and hospital settings, where schistosomiasis is found as a generally mild imported disease, early diagnosis is an essential priority. This study presents the evaluation of a simple colorimetric LAMP technique in urine samples for the diagnosis of imported schistosomiasis, comparing it with direct microscopic observation and a highly sensitive serological technique. Based on the results, the LAMP technique presents a higher sensitivity than microscopy for the diagnosis of urinary schistosomiasis, which could improve the diagnosis both in specialized centers and in centers with little experience or scarce resources and infrastructure.
According to data from reference centers in Europe, the prevalence of schistosomiasis by microscopy in series of patients from schistosomiasis-endemic regions ranges from 9% to 17%. However, many of these patients had positive serology with negative microscopy, indicating that schistosomiasis could be misdiagnosed [
26,
27,
28]. Bearing in mind that the reference centers have personnel with expertise in the microscopic diagnosis of schistosomiasis, the number of potential cases diagnosed in primary care, with technical staff less experienced in the management of imported diseases, is likely to be significantly lower; this could result in an increase in the number of persons with severe complications of the disease [
6].
Currently, serological techniques are recommended for screening of schistosomiasis because of their higher sensitivity [
11], being especially useful in individuals with light infections with a negative parasitological test [
29]. However, serological tests do not discriminate between active or past infection [
2,
10,
15,
29], so this strategy typically results in the treatment of people who are not really sick. Thus, serological data should be considered with caution, since an over-diagnosis may occur.
A retrospective study of Beltrame et al (2017) [
10] conducted on African migrants for the screening of human schistosomiasis using different tests, LCA assessment indicated an ICT sensitivity of 96% for the diagnosis of imported schistosomiasis, when compared to circulating cathodic antigen (CCA) urine cassette (29%), microscopy (48%), ELISA (76%), and Western-blot (94%) [
10]. According to these results, authors suggests that dipstick CCA test in a schistosomiasis non-endemic setting is inadequate for screening purpose and the rapid diagnostic test ICT is a suitable screening tool for schistosomiasis, although a positive result should ideally be confirmed by a second test such as ELISA and microscopy. Although CCA urine cassette test has been recommended as an appropriate tool for monitoring schistosomiasis control programs in endemic areas [
15], its low sensitivity obtained in African migrants is probably related to the fact that the parasitic load in the migrant population is much lower than in the population of schistosomiasis-endemic regions of origin. In this sense, it is important to note that the sensitivity values estimated by LCA in our study for microscopy (46.27%) and Schistosoma ICT IgG-IgM test (92.46%) are in agreement with those reported by Beltrame et al (2017) [
10].
The limitations of serological methods make it a priority to develop molecular diagnostic techniques for schistosomiasis that have high sensitivity, detect active infections and, ideally, do not require expert personnel and large technical resources to carry out. An easy, rapid and simple colorimetric LAMP assay could meet these requirements [
19]. In addition, the use of urine samples instead of other more difficult to access (such as feces, blood or biopsies) greatly simplifies the process, reducing the logistics required in schistosomiasis-endemic regions with limited resources and also at a reference laboratory. Furthermore, in clinical practice, the use of urine specimens would greatly simplify sampling, as a very significant number of sub-Saharan migrants are reluctant to repeat blood sampling.
There are already numerous well-established applications of LAMP technology in the diagnostic of bacterial, viral, fungal, and parasitic diseases in humans, animals, and plants, being particularly useful as a point-of-care (POC) molecular tool for parasitic diseases in resource-limited regions [
30]. Moreover, the World Health Organization (WHO) has also recommended the development of LAMP technology for several NTDs, including schistosomiasis [
31]. Different studies have been conducted to evaluate the clinical application of LAMP in the diagnosis of human schistosomiasis [
19]. In a previous study of our group, a colorimetric LAMP assay was evaluated under field conditions in Cubal (Angola) to detect
S. haematobium using both purified DNA and crude urine samples in comparison with microscopy [
23]. The overall prevalence by LAMP was significantly higher than microscopy when testing both purified DNA (73.8%
vs. 50.6%) and crude urine (63.4%
vs. 50.6%) samples, respectively. Bayoumi et al [
33] also evaluated a LAMP test to detect
S. haematobium DNA in urine samples collected from patients suspected of urogenital schistosomiasis attending outpatient clinic in Egypt. LAMP resulted in a 100% sensitivity and 63.16% specificity when compared with conventional urine filtration followed by microscopy for eggs detection.
To the best of our knowledge, the present study is the first to use the LAMP technique on urine samples in real clinical practice for the diagnosis of imported schistosomiasis. Here, the overall sensitivity for Schistosoma-LAMP estimated by the Bayesian LCA approach was 68.8%, decreasing slightly to 61.9% when using microscopy as the reference method for diagnosis. However, if only S. haematobium infections are considered, the sensitivity of Schistosoma-LAMP is as high as 72.2%. These results seem logical, as for the rest of the schistosome species (although detection of eggs in urine is possible) urine is not the ideal sample for detection and LAMP would have a lower sensitivity and therefore less diagnostic utility. Given the biological characteristics of the Schistosoma species that cause intestinal schistosomiasis, it also seems logical that the LAMP technique would show a higher sensitivity when performed on stool or blood samples.
In a previous work of our group with patients attending at the Hospital Universitario Insular de Las Palmas de Gran Canaria (Spain) as part of public health diagnostic activities, colorimetric
S. haematobium-specific LAMP was retrospectively evaluated in 18 urine samples from sub-Saharan migrants with parasitological proven
S. haematobium infection. The diagnostic parameters for LAMP resulted in a sensitivity of 100% and a specificity of 86.67% [
22]. Considering that the referred LAMP method is the same as the one used in this study (specifically, the
Sh-LAMP) the different sensitivity value in comparison to that obtained here (100%
vs. 72%, for species-specific
S. haematobium) could be due to the fact that the patients included in the present study have an average stay in Europe of more than 2,5 years, whereas the patients included in the study conducted in the Canary Islands were newly arrived patients, so they are expected to have a much higher parasite load. The difference in the specificity values obtained between the two studies (86.67%, in Canary Islands
vs. 44.60%, in this study), could be due to the fact that specificity is estimated by the Bayesian LCA approach considering together possible detection of
S. haematobium, S. mansoni and
S. intercalatum/
S.guineensis, which would considerably decrease the specificity.
A number of molecular techniques for the detection and quantification of schistosome-specific DNA in clinical samples have been described [
15,
29,
33] and have proven to be particularly useful for the diagnosis of acute schistosomiasis, especially in travellers, where there is a need for ultra-sensitive blood-based diagnostic tests that can detect
Schistosoma infection at an early stage [
34]. In a recent study with 376 travelers and African migrants from a non-endemic setting in Germany, different diagnostic tests, including microscopy, serology, POC-CAA urine tests and a serum in-house PCR were performed and the results were analyzed by LCA [
33]. For serum in-house PCR (including serology in the calculation) a LCA analysis assessed a sensitivity and specificity of 94.9% and 98.4%, respectively, thus demonstrating that PCR in serum is a highly reliable diagnostic method for schistosomiasis in travelers and migrants. However, to date there are no studies assessing the efficacy of urine PCR for the diagnosis of schistosomiasis in the migrant population. Notwithstanding this, PCR-based methods are technically demanding, require well-equipped laboratories, well-trained technicians and are quite expensive, so PCR tests are limited to reference laboratories. Ideally, to overcome these difficulties, PCR-based methods could be replaced by simpler isothermal methods such as LAMP assay. Unfortunately, until now there are still no studies evaluating the diagnostic ability of LAMP
versus microscopy and/or serology in a non-schistosomiasis endemic clinical setting to compare with our results with migrant population.
We are aware of some limitations of our work. On one hand, the prevalence estimates in a Tropical Medicine Unit scenario such as the one in which we conducted this study, may not be representative of what occurs in the general population. On the other hand, the use of a single sample and the small amount used for molecular analysis by LAMP (2 µL of DNA extracted from only 2 mL of total urine volume per patient) might have underestimated the diagnosis. This could be especially relevant considering the low parasite load and therefore the minimal presence of free circulating parasite DNA in the urine of our patients. Additionally, frozen storage conditions of urine samples without a preserving solution or repetitive freeze-thaw cycles for different trials, could be also the reason for a decrease in sensitivity of DNA detection by LAMP. We described similar complications in obtaining positive results in
S. mansoni DNA detection in urine samples not only by LAMP [
21] but also by PCR [
35]. As we also previously pointed out, because the urine is collected by the patients themselves, molecular analysis can be very susceptible to preanalytical contamination issues. In this respect, it would be interesting to use reagents that stabilize cell-free DNA in urine specimens, which would allow efficient collection, transport and storage of samples for subsequent molecular analysis [
21]. It is also important to note that processes such as hybridization among
Schistosoma species, mainly
S. haematobium-
S. bovis, could cause the sharing of different ITS (Internal Transcribed Spacer) types [
36], which would likely affect the sensitivity of LAMP in detecting genomic DNA from the different hybrid specimens. It would be very interesting to test if LAMP assay could amplify the increasingly surprising hybridizations between schistosomes species that infect humans [
37,
38], considering that this is an emerging problem both in endemic [
39] and non-endemic areas of schistosomiasis [
7]. It would also be very interesting to evaluate the correlation of the sensitivity of LAMP in urine compared to its application in blood and stool samples, which are much more suitable (particularly stool samples) for the detection of genomic DNA of other schistosome species than
S. haematobium. Finally, further studies are needed to determine the potential usefulness of LAMP in the follow-up of patients after treatment. If a positive LAMP result is proven negative after treatment, this could be a very important step towards obtaining an easy and inexpensive molecular test that would assure clinicians of treatment success. It would also allow clinical trials to establish the best treatment regimen for patients with urinary schistosomiasis.
4. Materials and Methods
4.1. Study design and study population
A prospective observational study comparing diagnostic tests for the detection and screening of schistosomiasis was carried out in sub-Saharan migrant patients attended at the Tropical Medicine Unit (TMU) of the Hospital Universitario Poniente (El Ejido, Almería, Spain) from January 2020 to June 2021. The Poniente area is an administrative area located in Southeast Spain holding a population close to 300,000 inhabitants with a migrant share of 21%, many of them coming from sub-Saharan countries to work in horticultural greenhouses. Patients included were sub-Saharan migrants older than 14 years of age who agreed to participate in the study and signed the informed consent. Patients with HIV infection were excluded.
4.2. Definitions and collected data
A screening protocol for infectious diseases was systematically applied to all migrant patients referred to the TMU. For sub-Saharan migrant patients, the screening protocol comprised medical history, epidemiological data, complete physical examination, and several additional tests: blood count, liver and renal function tests, syphilis, HIV, HBV and HCV serologies, tuberculin skin test and search for parasites in stool (three concentrated stool samples) and urine (one concentrated urine sample [10 cc]), Strongyloides (ELISA DRG® Strongyloides IgG) and Schistosoma serological tests, and Knott and/or saponin tests for microfilariae. Chest and abdominal X-rays were routinely performed too. If any other specific disease was suspected (e.g., onchocerciasis, malaria, etc.), further proper diagnostic procedures were performed. Diagnosis of strongyloidiasis was considered either when larvae were isolated from stool samples or when serology was positive.
Diagnosis of schistosomiasis was confirmed when Schistosoma spp. eggs were microscopically detected in urine and/or feces. Patients with confirmed schistosomiasis and those with a serology- and/or Schistosoma-LAMP-positive result were treated with praziquantel at the usual doses (40 mg/kg, 1 day), completing the study with abdominal and bladder ultrasound in cases of confirmed schistosomiasis or when they presented genitourinary symptoms.
4.3. Microscopy
Urine microscopy. The urine samples were collected between 9 a.m. and 12 a.m. and processed on the day of the sample collection. After centrifugation and concentration, each urine sample was placed on a labeled slide and examined under a microscope (100x) for Schistosoma eggs. Aliquots of urine samples were reserved and stored at -80ºC until shipment to the laboratory at IBSAL-CIETUS (Salamanca, Spain) for further DNA extraction for molecular analyses.
Stool microscopy. Three stool samples per patient were collected in formol on alternate days. Each sample was submitted to formol-ether concentration and examined (100x) using Ritchie’s method.
4.4. Schistosoma ICT IgG-IgM testing
Serum samples were tested for antibodies against Schistosoma spp. using the commercial rapid immunochromatographic test Schistosoma ICT IgG-IgM (LDBIO Diagnostics, Lyon, France) according to the manufacturer's instructions. Briefly, 30 μL of each serum sample were added to a cassette, followed by 3 drops of the supplied eluent in the kit. The result was read after 20-30 min as positive or negative depending on whether or not a colored band appeared.
4.5. LAMP testing
Aliquots of 2 mL of frozen patients’ urine samples were used for DNA extraction for molecular analysis by using the NZY Tissue gDNA Isolation kit (NZYtech, Lda., Lisbon, Portugal) following the manufacturer’s instructions. Subsequently, 2 μL of purified DNA thus obtained were added as template for LAMP amplifications. LAMP technique was performed using the combination of three different LAMP assays (hereafter,
Schistosoma-LAMP assays) previously described elsewhere by our research group (IBSAL-CIETUS) for the specific detection of
Schistosoma mansoni (
Sm-LAMP) [
21], the specific detection of
S. haematobium (
Sh-LAMP) [
22] and an assay for the genus
Schistosoma (
Schisto-LAMP) [
24].
All the Schistosoma-LAMP assays were performed similarly. In brief, LAMP reaction mixtures (15 μL) contained 1.6 M FIP/BIP primers, 0.2 μM F3/B3 primers, 0.4 μM LF/LB primers (if applicable), 1.4 mM of each dNTP, (Bioron, GmBH, Römerberg, Germany), 6 mM MgSO4, and 1× Isothermal Amplification Buffer (20 mM Tris-HCl (pH 8.8), 50 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Tween20) for Bst 2.0 Warm Start (WS) (0.32 U/μL) (New England Biolabs Ltd., Ipswich, MA, USA) and 2 µL of template DNA. Reactions were performed in 0.5-mL micro-centrifuge tubes by incubation in a heating block at 65°C for 60 min followed at 80°C for 5-10 min to stop the reaction. The LAMP amplification results were visually inspected by adding 1 μL of 1:10 diluted 10,000X concentration fluorescent dye SYBR Green I (Invitrogen, Waltham, MA, USA) in each reaction tube post-amplification. Green fluorescence was observed in LAMP-positive reactions and original orange in LAMP-negative reactions. Positive (DNA from S. mansoni, S. haematobium or both) and negative controls (purified water instead DNA template) were included in all trials. The tubes were briefly centrifuged and carefully opened before adding the dye to avoid possible cross-contamination with amplified products.
4.6. Statistical analysis
For descriptive statistical analysis, quantitative variables were expressed as means ± standard deviations (SD) and qualitative variables as frequencies and percentages. To evaluate Schistosoma-LAMP assays diagnostic capacity compared to direct microscopic observation and immunological test, a Latent Class Analysis (LCA) was performed. LCA combine the results of multiple diagnostic tests through a probabilistic model to obtain estimates of disease prevalence and diagnostic test accuracy in situations where there is no single, accurate reference standard [
40]. LCA enables the creation of a hypothetical standard (as it is not known whether patients are infected or not) and determine the test diagnostic capacity based on that standard.
Initially, the apparent prevalence (function of the positives in the evaluated test) and the real prevalence (function of the positives by direct observation, considered “real positives”), sensitivity and specificity (95% confidence interval [CI95%]), positive predictive value (PPV) and negative predictive value (NPV) were obtained. Diagnostic accuracy of each test was also estimated, defined as the true positives fraction in relation to the reference diagnosis.
Then, a LCA was performed, assuming that the number of latent classes were 2 (presence/absence of infection). A Bayesian approach was taken, first defining the frequency of the different test combination and, secondly determining the initial conditions of the model. In this case, to properly handle the different scenarios, initial conditions included assumptions of low (10%), medium (50%) and high (90%) prevalence, sensitivity and specificity. Lastly, a priori prevalence, sensitivity and specificity informative distributions of each test were obtained, for which estimations of α and β parameters were obtained from the literature on the different tests and the confidence in the truthfulness of the indicated values. Thus, a Bayesian LCA analysis with a priori informative distributions was performed. To obtain the parameters and Beta distribution, the following values were selected:
(i) Prevalence: considering 20% as the most plausible value, with a CI95%, real prevalence was considered to be over 8%;
(ii) Sensitivity: for direct observation, considering the most plausible value 50% [
2] and a CI95%, real sensitivity was considered to be over 40%. For immunological test, the most plausible value considered was 95% [
10], CI95%, real sensitivity was estimated at 90%. For Schistosoma-LAMP, the most plausible value considered was 90% [
19], CI95%, real sensitivity was estimated at 70%;
(iii) Specificity: for direct observation, considering the most plausible value 98% and a CI95%, real sensitivity was considered to be over 40%. For Schistosoma-LAMP and immunological tests, the most plausible value considered was 90% (CI95%), real sensitivity was estimated at 70% [
19,
10].
Statistical analysis was performed in R (version 4.2.2) using packages R2jags, BayesLCA and randomLCA to analyze the data; α and β parameters of the Beta distribution were obtained with the package epiR.
Author Contributions
Conceptualization, J.S.-C., P.F.-S.; methodology, J.S.-C., P.F.-S.; validation, J.S.-C., P.F.-S.; formal analysis, J.S.-C., P.F.-S.; investigation and data curation, J.S.-C., M.P.L.G., B.C.-V., M.J.S., B.F.-S., J.V.-V., J.G.-B.D., M.I.C.-B., N.C.-F., A.M., M.D.B., P.F.-S.; writing—original draft preparation, J.S.-C., P.F.-S.; writing—review and editing, J.S.-C., P.F.-S., M.P.L.G., B.C.-V., M.J.S., B.F.-S., J.V.-V., J.G.-B.D., M.I.C.-B., N.C.-F., A.M., M.D.B.; supervision, J.S.-C., P.F.-S.; project administration, J.S.-C.; funding acquisition, J.S.-C., P.F.-S., M.D.B.. All authors have read and agreed to the published version of the manuscript.