1. Introduction
Hydraulic systems are a series of fluid-powered components that generate, control, and transmit mechanical energy. They find wide-ranging applications in industries such as aerospace, automobiles, power plants, and industrial manufacturing. One of the primary and inherent components of every hydraulic system is hydraulic fluid or hydraulic oil. Hydraulic fluid not only transmits power from one component to another but also provides lubrication to reduce friction and offers protection against cavitation and corrosion. The properties of hydraulic oil have a significant impact on the overall performance of the hydraulic circuit. Parameters like viscosity, temperature range, and pressure rating are important considerations that depend on the specific application of machine. Another utmost important parameter of every hydraulic oil, which should be monitored carefully, is cleanliness during its operation. Wear or deterioration of the sealing surfaces of the circuit will allow internal and external leakage of fluid causing contamination which will lead to severe implications for the service life and operational safety of the machine. To measure overall contamination of in-service hydraulic fluids several laboratory testing methods exist. [
1]
The first testing method formerly developed in 1964 to define contamination classes in aircraft hydraulic components is called NAS 1638. It defines oil cleanliness by using coding system with differential counts which defines the maximum numbers permitted of 100mL of oil sample. This method is now more often replaced with ISO 4406 standard, which uses cumulative counts and is generally considered to be more representative.
It must be state that both mentioned methods are not able to detect particles smaller than 5 µm therefore cannot detect varnish or oxidation products which can be as harmful for hydraulic and turbine oils as larger metal particles and other contaminants. [
2,
3]
Laboratory testing method used to determine concentration of insoluble contaminants in in-service oils using MPC is described by standard ASTM D7843 – 21 [
4]. However, according to ASTM this standard is only applicable for in-service turbine oils. For insoluble contaminants in hydraulic fluids there is a standard ASTM D4898 – 16 that uses gravimetric analysis where contaminated oil is filtered through a membrane filter disc to measure the overall weight of contaminants [
5].
This study applies testing method described in ASTM D7843 – 21 which according to ASTM can be applied for measurement of lubricant generated insoluble color bodies in in-service turbine oils for testing varnish concentration in in-service hydraulic oil samples taken from rubber vulcanizing press machine. These samples were not chosen by coincidence, but as a representative example of a machine with hydraulic components that are exposed to a wide operating temperature range.
Rubber vulcanization is a process of cross-linking rubber molecules chemically with organic/inorganic substance through the action of heat and pressure to increase mechanical strength of the final rubber article. In manufacturing process of various rubber products, especially tires of road vehicles, the heat and pressure are generated by special vulcanizing presses in combination with vulcanizing agent. Initially as the vulcanization agent elemental sulfur was used with curing time of over 5h for one vulcanizing process. Modern vulcanizing machines can still use sulfur as vulcanizing agent, but the curing time has been significantly shortened with the use of accelerators to as short as 1-3 min [
6].
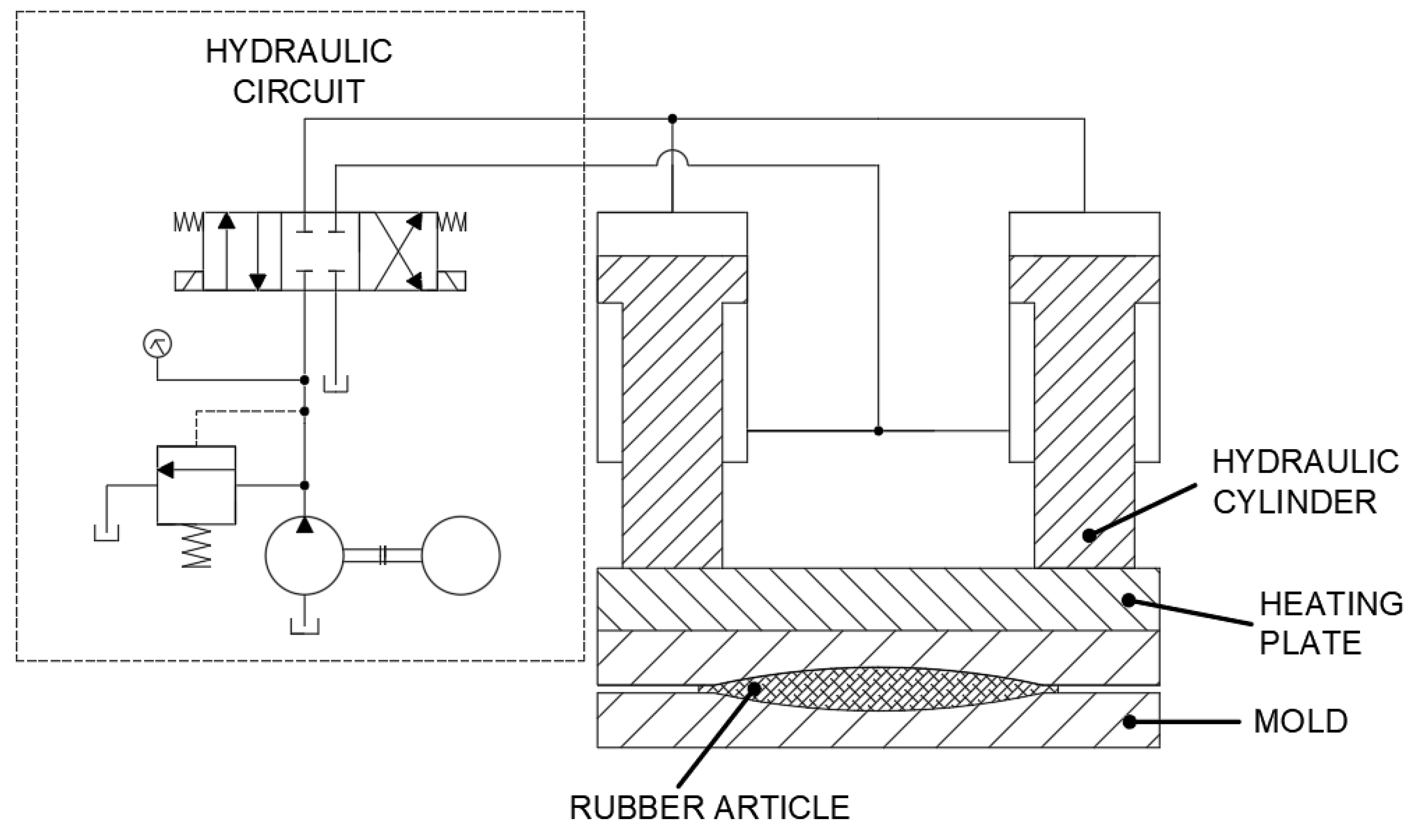
Vulcanizing machines are generally operated using two-way hydraulic cylinders parallelly driven by a hydraulic pump that creates pressure and a heating plate to crate sufficient heat (see
Figure 1.)
During the rubber vulcanization process, a natural rubber part is placed inside a mold attached to a heating plate. The heating plate is heated to operating temperature between 140–160 °C while simultaneously pushing on the rubber product inside the mold. This is also a point of heat transfer which not only happens between the heating plate and the mold but also between the heating plate and the hydraulic piston rod.
The main source of oil oxidation can be identified here as well – when the piston rods of hydraulic cylinders are heated it subsequently affects the hydraulic fluid within them.
Base oil slow oxidation is a chemical process of oil degradation in which the long chains of hydrocarbon molecules that make up the oil lose hydrogen molecules and gain oxygen molecules through a series of organic reactions. Antioxidants are almost always used in formulating synthetic hydraulic fluids however their efficiency decreases with time and due to oil contamination by products that act as free radicals in oil oxidation processes. For oxidation stability testing, number of methods has been developed such ASTM D2272 that uses rotating pressure vessel or ASTM D4310 that measures oil oxidation stability and sludge formation by following the acid number of oil [
1,
8].
As oil oxidation is the primary source of insoluble contaminants, also known as varnish, it is essential to comprehend the process involved. Basically, all lubricant based oils can be classified as organic compounds because they primarily consist of long chains of hydrocarbon molecules. These chains of molecules consist of hydrogen and carbon atoms in nearly infinite number of variations. Each atom is linked to another with a bond. The bonded structure of atoms is referred to as a compound. Usually, the oxidation happens at the end of the chain, but it can occur in the middle as well creating two carboxylic acids as a product [
8]. The oxidation process can be described chemically using R which represents undefined chain of hydrocarbon molecules (see
Table 1).
The most significant effect of oxidation in lubricants happens when a single chain is broken down into two chains. By breaking down the chain, oil molecules start to fail at carrying the load between two moving parts therefore it significantly debilitates the lubricating process itself. Another problem occurs when new compounds with higher molecular weight are formed due to polymerization processes. These compounds are responsible for formation of insoluble contaminants (varnish and sludge) [
8].
Insoluble contaminants and varnish build-up create a challenging problem for oil manufactures as well as maintenance workers and oil laboratory technicians. In case of hydraulic circuits, they can form layers of polymers in small cavities, valves and filters which are very difficult to dissolve and can have a devastating impact on lubricating properties of hydraulic fluids as well as on operation reliability of the hydraulic circuit itself.
The first step in degradation process of majority of lubricants can be recognized by formation of amber-brown color bodies that indicate the higher potential for varnish build-up caused by thermo-oxidative degradation or hydrolysis or combination of both. Modern hydraulic systems often use fire resistant fluids that are based on phosphate esters, in this case the insoluble contaminants tend to create brown or black (soot) colored insoluble bodies [
5]. These can be recognized using MPC – Membrane Patch Colorimetry. The process of MPC Measurement Methodology is described in the next chapter.
Before proceeding with further investigation into insoluble contaminants, it is essential to acknowledge that hydraulic circuits and the fluids circulating within them are subject to several issues beyond oil oxidation alone. An optimal strategy for monitoring the condition of hydraulic circuits involves employing multiparametric diagnostics. This approach integrates modern diagnostic methods that offer valuable insights into the machinery's overall health and operating condition [
12].
2. Materials and Methods
Validation of proposed insoluble contaminants concentration testing method using colorimetry was performed by laboratory workers from Department of Machine and Industrial Design, Faculty of Mechanical Engineering, VSB – Technical University of Ostrava.
First, unnamed local company which deals with manufacturing of rubber products was addressed. This manufacturer has had lasting problems with their hydraulic vulcanizing presses. They faced problems described in previous chapter as oil in hydraulic circuits of these presses degraded quickly due to oxidation initiating varnish formation. This resulted in shortening oil replacement intervals and caused repeated cleaning process of hydraulic components affected by insoluble contaminants.
Our laboratory workers took 8 samples of hydraulic oil from 3 hydraulic circuits of vulcanizing presses using sampling practice ASTM D4057-22 – Standard Practice for Manual Sampling of Petroleum and Petroleum Products. Each of 3 groups of samples represents different level of insoluble contaminants contamination. Special sampling containers were used to preclude exposure to UV light from indoor and outdoor sources as it can increase deposit formation in oil sample. Each sample contained approximately 150mL of in-service hydraulic oil. According to this standard each sample should contain at least 60mL of the material to be tested [
10].
After sampling procedure, all samples were brought to the Laboratory of Technical Diagnostics at VSB – Technical University of Ostrava and placed into a laboratory oven.
According to ASTM D7843-21 samples shall be heated to 65°C for 24 hours, after the heating procedure, samples shall be placed away from UV light for an incubation period of 68 h to 78 h [
4,
10]. Here we focus the main part our research when we try to determine whether this period can be shortened or prolonged to receive more valid results of this test. By standard it was stated that samples analyzed prior to this aging period may produce fewer color bodies resulting in lower value of trend analysis [
4], therefore gaining inaccurate results from this test.
2.1. Measurement Methodology
We created a measurement methodology based on ASTM D7843-21 which was used in this test. The methodology is described in
Table 2,
Table 3,
Table 4 and
Table 5. First after the sampling procedure we proceeded to the heating and incubation procedures.
After the heating and incubation procedure, the sample shall be prepared for the filtration process.
After the heating, incubation, and preparation procedures all samples shall be filtrated through 47 mm, 0.45 μm membrane filter using filtration vacuum apparatus.
The final part of this laboratory test determinates color of the membrane patch using 3NH NR 200 precision colorimeter. Any type of standardized precision colorimeter can be used for this task as long as it provides results in CIELAB color space.
CIELAB color space or CIE L*a*b* is a 3D color space that allows accurate measurement and comparison of all perceivable colors using three color values. It was developed by International Commission on Illumination (CIE) in 1976 with intend of improving Munsell color system and creating a new standard for color communication [
11]. CIELAB color space is produced by plotting in rectangular coordinates three quantities – L*, a*, b*, calculated using (1,2,3).
Values
Xn, Yn and Zn define the color of the normally white object-color stimulus, in case of MPC colorimetry, it is a color of a new, clean, and dry membrane patch using relationship (4). Very significant role plays the ΔE* or ΔE*
ab MPC value that shows poor oil condition therefore shows the increasing concentration of both varnish and soot [
4]. The total difference ΔE*
ab between two colors is calculated using relationship (5). Usually, this value is used to describe aging process of oil by comparing a color of a new unused oil and oil degraded by varnish. In our case we only compare colors of more samples of used oil to check for accuracy based on sample incubation period.
The total difference Δ(a*+b*) is used to distinguish amber brown patches (colored by varnish) from black patches with high ΔE value due to soot or high-temperature breakdown products [
4], the MPC value Δ(a*+b*) is calculated using equation (6).
Besides the MPC test, it has become a standard when measuring varnish concentration in oils to record MPC patch weight or to provide a gravimetric analysis according to ASTM D4898 – 16. However, in this test we only focus on MPC analysis, and its accuracy based on incubation period of hydraulic oil samples.
3. Results
Using measurement methodology described in
Table 2,
Table 3,
Table 4 and
Table 5, we analyze 3 groups each with 8 hydraulic oil samples obtained from 3 hydraulic circuits of rubber vulcanizing presses. After the sampling procedure, all samples were provided with a description and a number, for e.g.,
Group 1, Sample 4, and stored in a dark, dust free cabin away from UV light sources in appropriate containers. Sample 8 was analyzed prior to the heating procedure after the standard 72 h incubation period. Other 7 samples were heated in a laboratory oven for approximately 24h at 65 °C. After the heating procedure described in
Table 2. samples were placed back into the cabin, then sample from each group was randomly removed from the cabin and proceeded to a sample preparation process after certain incubation period was achieved (12 h, 24 h, 48 h, 72 h, 96 h, 120 h). Then each sample was prepared as described in
Table 3. and filtrated as described in
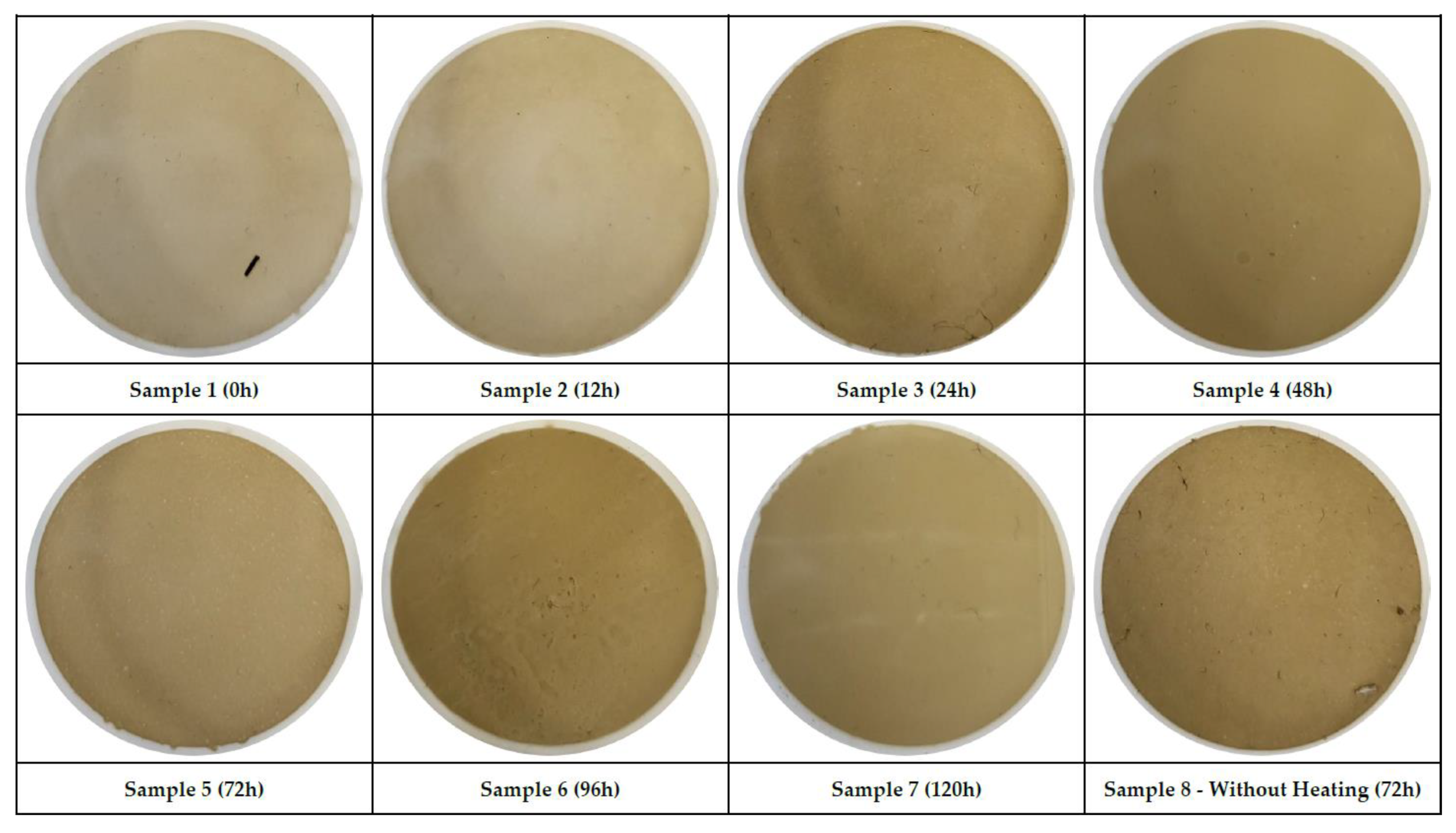
Table 4. As a result of this filtration process, we received 24 varnish-colored patches (see
Figure 2,
Figure 3 and
Figure 4) that were used to measure MPC values using 3NH NR 200 precision colorimeter. Before measuring L*, a*, b* we calibrated the colorimeter using new, dry patch therefore we received L*, a*, b* blank values for a calculation of all delta values. In tables each group of samples is described by one Table with the first row showing values of blank filter as reference value for further measurements. Other rows show measured L*, a*, b* of each patch in first two columns, delta values ΔL*, Δa*, Δb*in another three columns and the last two columns show MPC values ΔE* and Δ(a*+b*). Each table is accompanied by a Figure showing the pictures of all patches after the filtration process.
Judging by the MPC values ΔE*ab and Δ(a*+b*) the first group of samples show high varnish concentration. Generally, it can be said that samples with ΔE* value higher than 35 show critical concentration of insoluble contaminants. The hydraulic oil shall be changed, and the hydraulic circuit shall be flushed immediately which was also suggested to the operator of the machine.
The second group of samples show significantly lower varnish concentration than the first group. MPC value is not greater than 21 in case of both ΔE*ab and Δ(a*+b*) which shows elevated results. The machine should be monitored carefully and the MPC test shall be performed again to validate measured results. If concentration crosses MPC value of 35, the oil shall be replaced immediately.
Table 7.
MPC results of patch Group 3.
Table 7.
MPC results of patch Group 3.
Sample
(According to the Incubation Period)
|
L* |
a* |
b* |
Δa* |
Δb* |
ΔL* |
ΔE* |
Δ(a*+b*) |
| Reference (Blank) Value |
95,58 |
0,41 |
1,13 |
|
| Sample 1 (0 h) |
80,89 |
4,35 |
16,54 |
3,94 |
15,41 |
-14,69 |
21,65 |
19,35 |
| Sample 2 (12 h) |
81,91 |
3,88 |
15,71 |
3,47 |
14,58 |
-13,67 |
20,28 |
18,05 |
| Sample 3 (24 h) |
72,11 |
7,1 |
24,9 |
6,69 |
23,77 |
-23,47 |
34,07 |
30,46 |
| Sample 4 (48 h) |
70,23 |
6,49 |
25,06 |
6,08 |
23,93 |
-25,35 |
35,38 |
30,01 |
| Sample 5 (72 h) |
75,17 |
6,53 |
22,19 |
6,12 |
21,06 |
-20,41 |
29,95 |
27,18 |
| Sample 6 (96 h) |
66,31 |
6,83 |
25,11 |
6,42 |
23,98 |
-29,27 |
38,38 |
30,4 |
| Sample 7 (120 h) |
72,75 |
5,8 |
22,91 |
5,39 |
21,78 |
-22,83 |
32 |
27,17 |
| Sample 8 (72 h - Without Heating) |
71,83 |
6,24 |
22,38 |
5,83 |
21,25 |
-23,75 |
32,4 |
27,08 |
Last group of samples show similar results to the second group. MPC values can be considered elevated. The recommendation given to the operator was the same as well in this case.
To quantify accuracy of our test methodology we compare results between groups of samples using average deviation as a comparative tool. The average deviation can be calculated as seen below in relationship (7) for ΔE*
ab values and relationship (8) for Δ(a*+b*) values.
4. Discussion
Membrane Patch Colorimetry as a laboratory method for varnish potential testing has been used for many years. It provides important information about oil condition while being quick and user-friendly. Methodology described in ASTM D7843 - 21 standard describes usage of MPC for turbine oils testing therefore it creates a basepoint for many oil-laboratories. This research describes our test methodology which was based on ASTM D 7843 – 21. Tests performed according to this test methodology proofed increased concentration of varnish in samples of hydraulic oil obtained from rubber vulcanizing presses. It can be stated that samples served as a sufficient source of information about insoluble contaminants formation in hydraulic circuits as presumed in Introduction.
The focus of this study was based around incubation time of oil samples after the sampling and heating procedure. We tested 3 groups of 8 samples from 3 different rubber vulcanizing presses all with increased concentration of varnish. In tests performed we compare primary ΔE*ab and Δ(a*+b*) values which are based on CIELAB color space, and which provide the main information about varnish potential of each oil sample.
Each sample was tested based on methodology described in
Table 2,
Table 3,
Table 4 and
Table 5 and analyzed using precision colorimeter. Results of this analysis were presented using
Tables and
Figures with filter patch pictures. The first group of samples shows the highest varnish and soot concentration in oil represented with increased ΔE*
ab and Δ(a*+b*) values. Results presented in
Table 5. point out at relatively low variance of L*, a* and b* values. The same can be stated about delta values as well. Average deviation of ΔE*
ab and Δ(a*+b*) is also relatively low and comparable to the third group of samples. The second group of samples represents not only the lowest concentration of varnish and soot, but also the best results in terms of average deviation of ΔE*
ab and Δ(a*+b*) values. It leads to the question whether the low varnish concentration in oil is also related to sample resistance to incubation time variations and we expect to study this in future research. Analysis results of the last group of samples show similar progress to the first group in terms of ΔE*
ab and Δ(a*+b*) values and their average deviations.
5. Conclusions
In this research paper we validated the effect of oil sample incubation time on accuracy of colorimetric measurement of filter patches for insoluble contaminant concentration. Results of the colorimetric analysis showed relatively low average deviation between MPC values of samples with long and short incubation time in all 3 groups of samples. The group with the lowest varnish and soot concentration also shows the best results in terms of average deviation of MPC values ΔE*ab and Δ(a*+b*). Our test methodology shows that it is suitable for varnish potential testing of hydraulic oils. In case of incubation time which was another main parameter validated in this study we can state that in certain conditions the incubation period can be shortened although this eventuality should always be mentioned in documentation as a deviation of Standard’s methodology.
Author Contributions
“Conceptualization, J.B. and S.P.; methodology, D.S.; software, S.P.; validation, J. D.S.; formal analysis, J.B.; investigation, J.B.; resources, S.P.; data curation, S.P.; writing—original draft preparation, J.B.; writing—review and editing, S.P.; visualization, S.P.; supervision, J.B.; project administration, D.S.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Youth and Sports of The Czech Republic, Grant No. SP2023/003.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
This work has been supported by the Ministry of Education, Youth and Sports of the Czech Republic from the Specific Research Project SP2023/003.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leslie, R. Rudnick Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technology; CRC Press: Boa Raton, FL, USA, 2020; ISBN 978-1-138-06821-6. [Google Scholar]
- Sasaki, A. Contaminants in Used Oils and Their Problems. In Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology; SAGE Publications, 2006; 220, pp. 471–478. [Google Scholar] [CrossRef]
- Comparison NAS/ISO - cleanliness codes [online]. Swift Filters, Inc. Available from: https://www.swiftjbinternational.com/comparison-NAS-ISO-cleanliness-codes.php.
-
ASTM D7843-21; Standard Test Method for Measurement of Lubricant Generated Insoluble Color Bodies in In-Service Turbine Oils using Membrane Patch Colorimetry. American Society for Testing and Materials, 2021; 05.
-
ASTM D4898-16; Standard Test Method for Insoluble Contamination of Hydraulic Fluids by Gravimetric Analysis. American Society for Testing and Materials, 2016; 12.
- A.Y. Coran. The Science and Technology of Rubber (Fourth Edition), 2013.
- Sasaki, A.; Aoyama, H.; Honda, T.; Iwai, Y.; Yong, C.K. (2013). A Study of the Colors of Contamination in Used Oils. In Tribology Transactions (Vol. 57, Issue 1, pp. 1–10). Informa UK Limited. [CrossRef]
- Lubricant Oxidation Analysis and Control. Machinery Lubrication [online]. Available from: https://www.machinerylubrication.com/Read/14/lubricant-oxidation.
- Nasser, R.M. (2015). The behavior of Some Acrylate Copolymers as Lubricating Oil Additives. OmniScriptum GmbH & Co.KG, Germany. [CrossRef]
-
ASTM D4057-22; Standard Practice for Manual Sampling of Petroleum and Petroleum Products. American Society for Testing and Materials, 2022; 06.
- What Is CIELAB Color Space? HunterLab [online]. Available from: https://www.hunterlab.com/blog/whatis-cielab-color-space/.
- Opocenska, H.; Nahodil, P.; Hammer, M. USE OF MULTIPARAMETRIC DIAGNOSTICS IN PREDICTIVE MAINTENANCE. In MM Science Journal (Vol. 2017, Issue 05, pp. 2090–2093). MM Publishing, s.r.o. 2017. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).