1. Introduction
T2DM is a chronic metabolic disorder characterized by high levels of glucose in the blood due to the body's inability to effectively use insulin. The incidence of T2DM has been steadily increasing, with estimates suggesting that over 422 million people worldwide currently live with this condition [
1,
2]. The increasing prevalence of T2DM is largely attributed to changes in lifestyle factors, such as physical inactivity, unhealthy diet, and obesity. Although pharmacological treatments, diets and exercise's plans, for T2DM are available [
3,
4,
5] they are often associated with high self -discipline, unwanted side effects and can be expensive. Therefore, there is a growing interest in exploring lifestyle interventions, such as Intermittent Fasting (IF), as an effective approach to managing T2DM risk parameters. IF involves periodic periods of reduced or no caloric intake alternated with periods of normal or increased caloric intake. Several studies have suggested that IF may improve various risk parameters associated with T2DM, including insulin sensitivity, glucose metabolism, and inflammation [
6,
7,
8]. Alternate-day intermittent fasting (ADF), for example, demonstrates improvements in diabetes and preservation of beta-cell function in polygenic mouse models of T2DM [
8,
9]. In addition, ADF reported as improving endothelial function in T2DM mice [
10]. Early time-restricted feeding (eTRF) is an intermittent fasting strategy restricting caloric intake to the first 6-8 hours of the day. Furthermore, previous studies in human have shown that eTRF improves glucose control in adults with prediabetes and high BMI [
11].
While the majority of randomized clinical trials studying IF interventions in humans have shown a reduction in T2DM risk parameters, such as fasting glucose and insulin levels, it is important to acknowledge that there are exceptions to this trend. Some studies may not observe significant improvements in these biomarkers following intermittent fasting interventions [
8]. The reasons for these exceptions could be multifactorial and may include variations in study design, differences in participant characteristics, or variations in the duration or intensity of the intermittent fasting protocols employed. Therefore, before advising IF intervention to a pre- diabetes or diabetes individual, it is important to consider the metabolic status of participants prior to the studies, as it can influence the outcomes and interpretation of the findings. The metabolic status of individuals with T2DM can vary widely, including factors such as the duration and severity of diabetes, baseline insulin resistance, level of glycemic control, age, weight, and BMI [
12].
This study endeavors to uncover the underlying patterns and principles that contribute to the successful improvement of T2DM risk parameters using IF. The investigation based on the outcomes of diverse randomized clinical trials that have implemented various IF interventions in human subjects. Additionally, the objective of this study is to propose a recommendation system that utilizes machine learning algorithms. The primary goal of this recommendation system is to suggest the most effective IF approach for individuals with prediabetes or diabetes, thereby reducing their type T2DM risk parameters. The recommendation system will take into consideration the impact of age, weight, and BMI on the effectiveness of intermittent fasting in improving T2DM risk parameters.
2. Materials and Methods
2.1. Intermittent Fasting Interventions
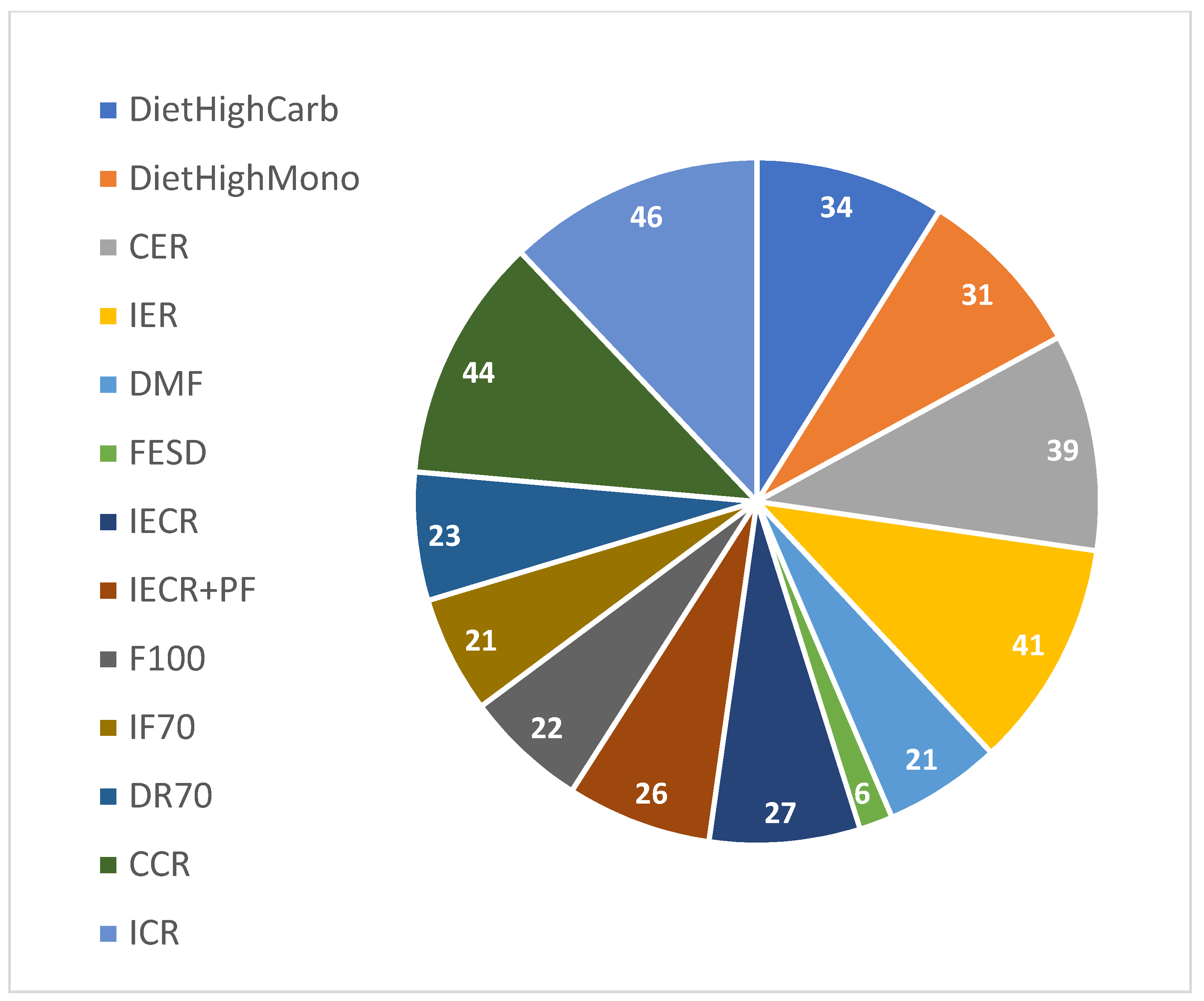
The data for this study was gathered from 7 published papers, shown in
Table 1, that perform random clinical trial to investigate the intermittent fasting effects on T2DM parameters.
The collected data included 838 individuals and 13 different types of interventions. The following interventions: CER, High Carbohydrate , High Monounsaturated, DR70 and CCR included restricted calorie diets or specific food compound' diets but did not include fasting at all , since they were used for control in the published studies of the random clinical trials. Those interventions were included in the data of this study since they provide interesting information to analyze. For example, in cases where none of the fasting methods improve the T2DM risk parameters of an individual while a calorie restriction did improve. The fasting interventions based on weekly days , which included in this study were Intermittent energy restriction - 2 days a week trail and eating free in all the other 5 days, Fasting Every Second Day; eating only four days a week, Intermittent Energy and Carbohydrate Restriction; eating restricted calories only two days a week, Intermittent Energy and Carbohydrate Restriction plus free Protein and Fat; eating restricted calories only two days a week, Fasting three non-consecutive days per week and Fasting three non-consecutive days per week and on eating days have 70% energy. The Daily Morning Fasting is a fasting intervention based on day's hours. In total 838 Individuals records were collected from 13 different intermittent fasting interventions. The distributions of the 13 different interventions are shown in
Figure 1.
2.2. Preparing and Pre-Processing the Data
2.2.1. Selecting the Features
Several features for each of the 838 individuals were collected from the different random clinical trials included in this study. The initial vector of features contained age, gender, weight, BMI, fasting glucose before and after intervention and fasting insulin before and after intervention. However, the fasting glucose and insulin after intervention have been removed to enable the machine learning classifier to learn the data and predict the results without revealing whether the intervention was successful in terms of reducing T2DM risk parameters.
2.2.2. Selecting Individuals
The motivation of this study is to select the best intervention to reduce T2DM risk parameters. Therefore, only individuals that are candidates for T2DM, pre-T2DM or having T2DM. Finally, 411 out 838 of individuals were selected with basal glucose above 5 mmol/Liter or BMI (Body Mass Index) above 25
2.2.3. Calculating HOMA-IR
T2DM is generally characterized by insulin resistance, where the body does not fully respond to insulin. HOMA-IR stands for Homeostatic Model Assessment of Insulin Resistance. HOMA-IR is calculated using fasting insulin (mU/L) multiplied by fasting glucose (mg/dL). Using HOMA-IR equation insulin resistance can be estimated from fasting glucose and insulin levels (HOMA-IR=fasting insulin*fasting glucose). High score of HOMA-IR indicates a significant Insulin resistance which usually found in people with Diabetes Type 2 For each individual out of the 411, we calculated the HOMA-IR Difference which represents the Insulin resistance reduction (the formula is shown in this slide). Finding HOMA-IR Difference was done by 2 calculations of HOMA-IR, once for the basal values of Fasting glucose and Insulin and once for the values after the Intervention or treatment .HOMA-IR difference is considered TRUE if HOMA-IR before intervention is higher than HOMA-IR after intervention. Otherwise HOMA-IR difference is considered FALSE.
2.3. Constructing the Datasets
2.3.1. Dataset to Predict Whether a Specific Intervention Can Improve HOMA-IR
The dataset contained 411 individuals' records. The properties in each record were: age, weight, BMI, fasting glucose before intervention, fasting insulin before intervention and the intervention(one out of the 13 interventions). The class column was True or False while True means the HOMA-IR before intervention was higher than the HOMA-IR before intervention. A dataset of 350 derived from the original 411 records dataset. In the smaller dataset 61 records of control interventions (CER, DR70 and CCR) were taken out. In the control intervention (CER, DR70 and CCR) no fasting intervention was applied on the participants.
2.3.2. Continuous Target Column: Improve Fasting Glucose
As described in 4.1 the class for each individual and specific intervention in our dataset was true if the intervention improved HOMA-IR otherwise it was false. However, in case we want to compare between different interventions a true or false target column is not useful. Therefore, Comparison between different intervention should be done using target column which represents the difference between HOMA-IR values before and after the intervention. Larger difference indicates a more effective intervention. In that case the target column in our dataset is continuous instead of binary (true or false). We choose the random forest classifier to predict the difference, since random forest is capable to deal with continuous values in the target column.
2.3.3. Excluding the Interventions Feature
Wondering what is the percentage of the population that our dataset can recommend any effective intervention to reduce HOMA-IR we built a new dataset. The dataset was based on the original dataset with target column : true or false, but the column intervention was excluded.
2.3.4. Increasing the Threshold for Improvement in HOMA-IR or Fasting Glucose
To ensure HOMA-IR improvement post-intervention, we redefined it as a decrease >15% of normal HOMA-IR (48038). Normal HOMA-IR is calculated based on fasting glucose (5.56 mmol/liter) and insulin (80 pmol/liter normal HOMA-IR = 5.56 *6 * 80 * 18 = 48038). Cases with HOMA-IR difference ≥7206 are considered successful (True) in our dataset; others are False.
2.4. Machine Learning Classifiers
This study focuses on classification, determining if specific interventions improve HOAM-IR for pre-diabetes and diabetes individuals. Four classifiers were chosen: decision tree (J48), logistic model tree, random forest, and logistic regression. Decision trees are simple and interpretable, while J48 avoids overfitting. Logistic model trees handle complex relationships, and random forests excel with high-dimensional data. Logistic regression is straightforward. These classifiers were selected due to their advantages and compatibility with the dataset. Decision trees handle missing values, random forests are accurate in high dimensions, J48 avoids overfitting, logistic model trees manage complex relationships, and logistic regression is easy to implement.
2.5. Testing Approach
The test approach which is used is 10-fold which is building the model using 9 over 10 of the data and testing with 1 over 10. This is done 10 times.3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3. Results
3.1. Predicting Whether a Certain Intervention Can Improve HOMA-IR
The initial step in selecting the optimal intervention for a patient with reported prediabetes is to address the question: does a specific intervention improve HOMA-IR? Four machine learning classifiers were chosen to answer this question: J48 decision tree, Logistic Model Tree (LMT), Random Forest, and Logistic. Detailed information about each classifier can be found in the Method section. The dataset described in the Methods section was trained and tested, utilizing the 10-fold test to assess the predictive ability of each classifier for HOMA-IR improvement. Improvement in HOMA-IR was defined as a decrease in HOMA-IR after the intervention compared to before the intervention. The results for each classifier can be found in
Table 2 under the "Discrete difference" row and "HOMA-IR (with control)" column. The results show modest significance, with AUC values ranging from 0.6 to 0.7 (J48: 0.68, LMT: 0.6, Random Forest: 0.68, and Logistic: 0.7), and accuracy ranging from 70% to 72% (J48: 70%, LMT: 72%, Random Forest: 70%, and Logistic: 71%). To reduce data noise, 61 records of control interventions (CER, DR70, and CCR) were removed from the dataset. These control interventions did not involve fasting. After removing these records (see Methods section for details), slight improvements in prediction were observed for all four classifiers. The results can be found in
Table 2 under the "Discrete difference" row and "HOMA-IR (no control)" column. The significance remained moderate, with AUC values ranging from 0.65 to 0.71 and accuracy ranging from 68% to 74%. Interestingly, when the difference between post-intervention and baseline HOMA-IR increased, the results became more significant in terms of AUC and accuracy. In this case, improvement in HOMA-IR was defined as a decrease in HOMA-IR after the intervention by more than 15% compared to the normal HOMA-IR (calculated using fasting glucose of 5.56 mmol/liter and fasting insulin of 80 pmol/liter; calculation explained in the Methods section). Using a cutoff of greater than 15% decrease, the results are presented in
Supplementary Tables S3A, S4A, and S5A for different fasting glucose and HOMA-IR differences (before and after interventions) with cutoffs of 15%, 10%, and 20% respectively. The supplementary tables demonstrate that the 15% cutoff performs the best. The results for each classifier, using the cutoff of 15%, can be found in
Table 1 under the "Discrete difference above 15%" row and "HOMA-IR (no control)" column. AUC values ranged from 0.73 to 0.89, and accuracy ranged from 74% to 82%. The Logistic Model Tree classifier exhibited the most favorable results, with an AUC of 0.89 and an accuracy of 82%. These findings suggest that the proposed method can serve as a robust foundation for a recommendation system.
Table 2 shows that using the Logistic Model Tree classifier, HOMA-IR improvement can be predicted with an accuracy of 82% and an AUC of 0.89. The other three classifiers also provided similar predictive results.
3.2. Can We Predict Improvement in HOMA-IR without Knowing the Intervention?
Establishing a solid foundation for a recommendation system requires assessing the predictability of HOMA-IR improvement and the accuracy of the predictions. To determine this, we can leverage our dataset without the intervention feature. Additionally, it would be intriguing to investigate the decision rules for HOMA-IR improvement by training and testing our data without the intervention column. The results of this test, using the four classifiers, are presented in
Table 2 under the "Discrete difference above 15%. No Interventions" row and "HOMA-IR (no control)" column. The AUC values range from 0.71 to 0.88, with accuracy ranging from 72% to 83%. While these results are slightly less significant than when utilizing the intervention column (AUC between 0.73 and 0.89, accuracy between 74% and 82%), it is reasonable since omitting the intervention column leads to some information loss. Nonetheless, these results are meaningful and support the recommendation system. For instance, the logistic model tree classifier demonstrates an accuracy of 83% and an AUC of 0.88, while the Random Forest yields an accuracy of 79% and an AUC of 0.86. Interestingly,
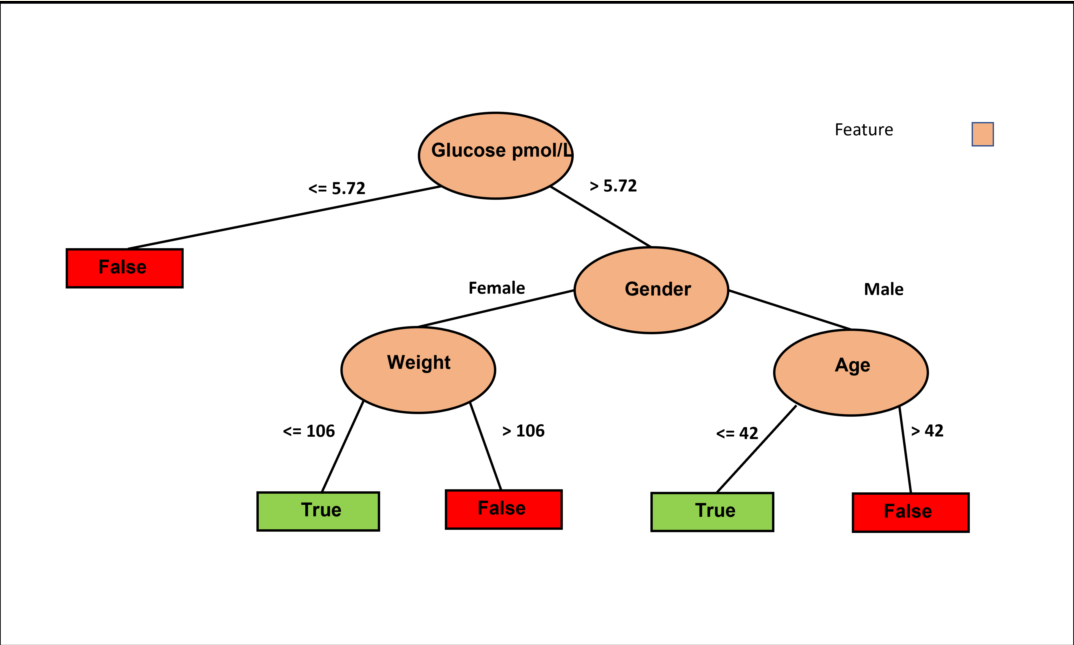
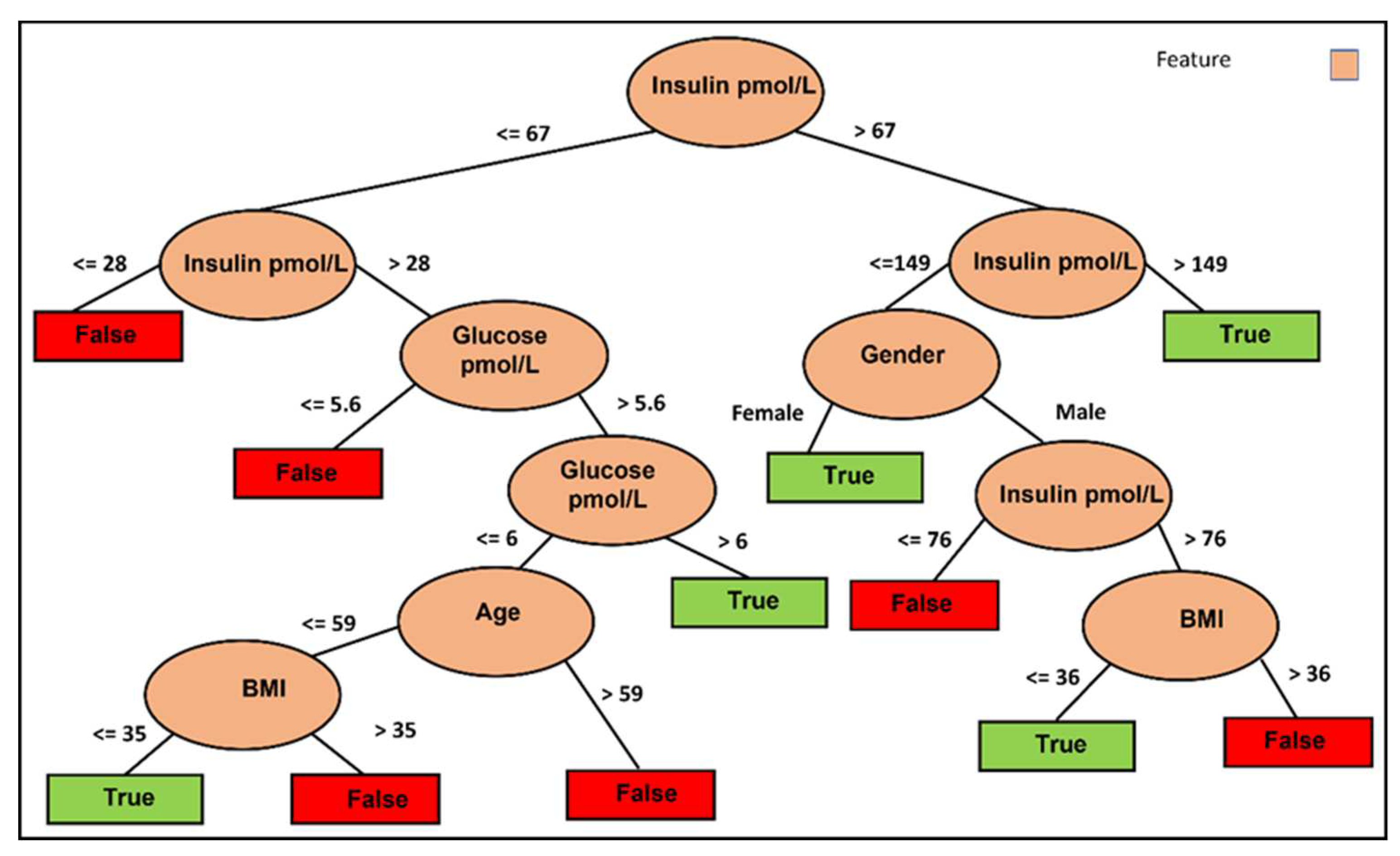
Figure 2 highlights the significance of fasting glucose and fasting insulin as influential factors in predicting HOMA-IR improvement using the J48 classifier. Furthermore, the figure reveals the substantial role of BMI in determining the appropriateness of IF for reducing HOMA-IR in individuals below 60 years of age. In contrast, for individuals aged 60 and above, gender, specifically women, appears to have a higher likelihood of benefiting from an IF approach to reduce HOMA-IR.
3.3. Predicting Whether a Certain Intervention Can Improve only Fasting Glucose
In real life, patients typically undergo only fasting glucose blood tests as part of their routine health check-ups, while fasting insulin tests are not commonly included in the standard blood tests conducted by Health Maintenance Organizations (HMOs). Therefore, a recommendation system based solely on fasting glucose levels would have lower performance compared to a system based on HOMA-IR. However, it is still intriguing to evaluate the performance of our data when using only fasting glucose. The results of the glucose test can be found in
Table 2 under the "Discrete difference above 15%. No Interventions" row and "Fasting Glucose (control)" column. Interestingly, the accuracy of the prediction ranges from 93% to 95%, compared to 74% to 77% with the same test for HOMA-IR. Additionally, the AUC values show improvement, ranging from 0.64 to 0.91, compared to 0.75 to 0.83 for the HOMA-IR test. The results of the glucose test without control or interventions, as shown in
Table 2, are similar to the HOMA-IR tests but with better accuracy and AUC values. The higher accuracy when using only fasting glucose can be attributed to the smaller number of successful cases in improving fasting glucose (24 out of 474), which is lower compared to the number of cases with improved HOMA-IR (150 out of 471). However, the improvement in AUC is not affected by these numbers, indicating that the data contains relevant information.
The random forest classifier performs best for the dataset using only fasting glucose, where the class is considered True if the difference between basal and post-intervention is higher than 15% of normal fasting glucose. In
Table 2, under the "Discrete difference above 15%" row and "Fasting Glucose (no control)" column, the random forest classifier achieves an AUC of 0.93 and an accuracy of 96%. The J48 classifier, although slightly less accurate than the random forest, has the advantage of providing a visible decision-making process.
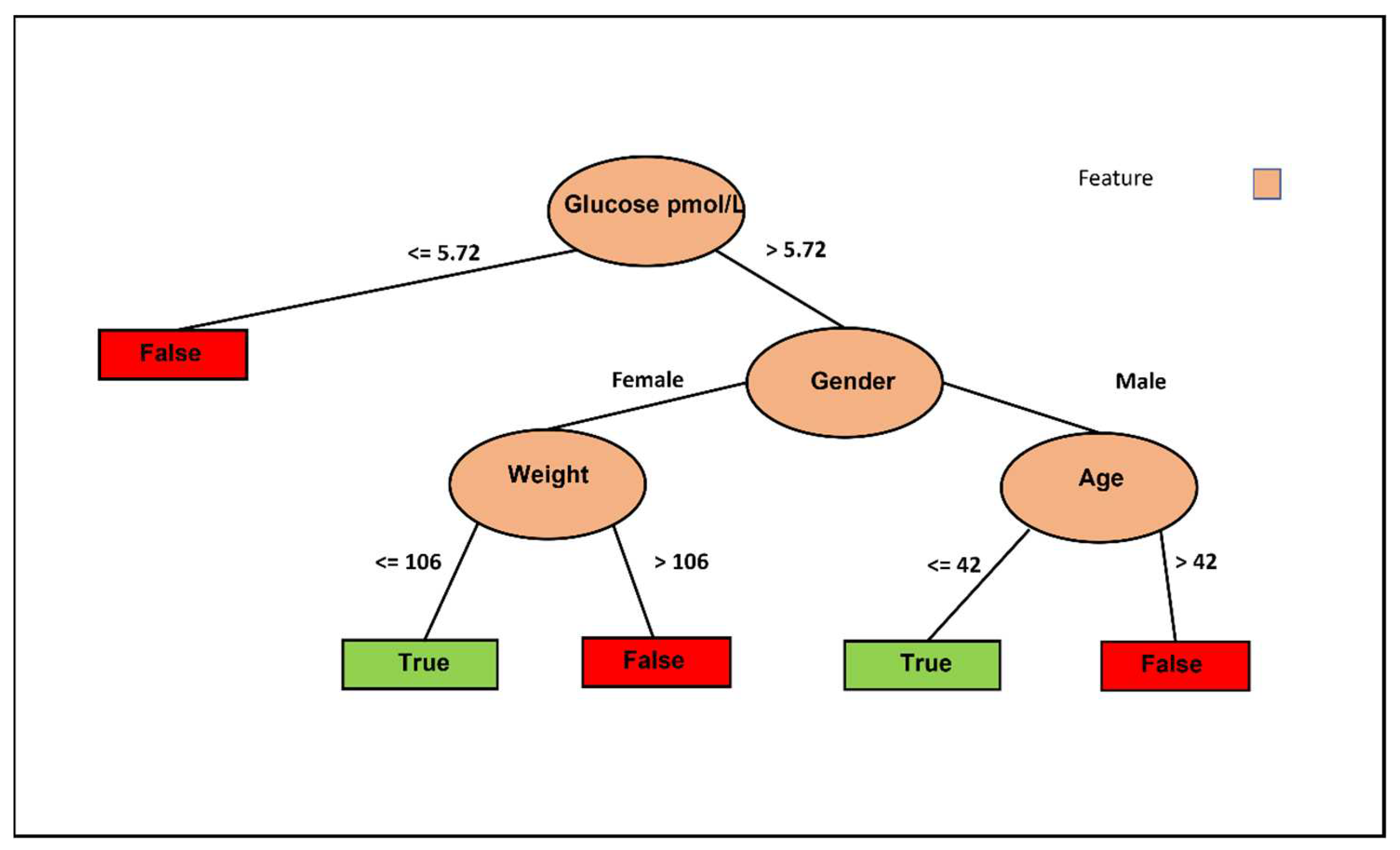
Figure 3 illustrates the decision tree based on the J48 classifier using only fasting glucose without the intervention columns but with control. Six decision pathways are depicted, with four resulting in a False decision and two leading to a True decision. Lower basal fasting glucose tends to lead to a False decision, while being a female with lower weight favors a True decision. Conversely, being a female with higher weight leans towards a False decision. Additionally, older males tend to result in a False decision, whereas younger males tend to result in a True decision. These results are logical and allow us to conclude that weight is the crucial feature for females, while age is the determining factor for males.
3.4. Comparison between Different Intervention in Ability to Improve T2DM Risk Parameters Using Continuous Difference
The previous sections extensively discussed the ability and success in predicting whether an intervention would improve T2DM risk parameters for a patient with reported pre-diabetes. The next crucial question to address in building a recommendation system is determining the most effective intermittent fasting method for an individual. This question can be answered using continuous difference analysis. In this approach, the target column represents the difference between the basal fasting glucose level and the fasting glucose level. In previous analyses, the target column was labeled as TRUE when the basal fasting glucose level was higher than the fasting glucose level; otherwise, it was labeled as FALSE. However, with the continuous column as the target, we can select the method with the highest difference as the best match. By using a continuous column as the target, the solution shifts from binary classification (True or False) to predicting the value of the difference. The success of this prediction can be measured using the correlation coefficient between the real and predicted values. For this prediction, the random forest algorithm was employed. The results can be found in
Table 2 under the "Continuous difference" row and "Fasting Glucose (control)" column, with the random forest algorithm achieving a correlation coefficient of 0.51. The significance of these results will be discussed in the next section, where the correlation coefficients of these algorithms will be compared with a random dataset.
The training set for our recommendation system utilized the original Fasting Glucose dataset with interventions and without control, with the class column replaced by the difference between the basal and post-intervention fasting glucose levels. The test set consisted of 13 records with the same features: age, gender, weight, BMI, and basal fasting glucose level. However, the intervention feature varied across the 13 records since our study included 13 different interventions (without control). As an example, let's consider a pre-diabetic woman who is 54 years old, weighs 73 kg, has a BMI of 26.7, and a basal fasting glucose level of 6.1 mmol/L (110 mg/dL). Inputting this data into our recommendation system yields the output shown in
Table 2 under the "Continuous difference Random Forest" column. The random forest algorithm recommends the 'IECR+PF' intervention as having the largest difference, making it the best intervention for this case. Two different cases for men are shown in
Tables S1 and S2, where S1 recommends the 'diet high mono' intervention according to the random forest algorithm, while S2 suggests the 'CCR' intervention.
3.5. Random Test
An interesting and essential question in prediction queries is whether the same results can be obtained randomly. To address this, we utilized the same dataset but with a randomized target column while maintaining the proportion between TRUE and FALSE labels identical to the original target column in each test. The results of all random tests can be found in
Tables S3-S6 in the supplementary materials. Notably, all random tests consistently exhibited significantly lower success rates compared to the real tests. For instance,
Table S3A presents the results of the dataset with a target column indicating a 15% difference, while
Table S3B displays the random results for the same dataset. In the real test, the AUC for fasting glucose without control and with intervention ranged from 0.82 to 0.93, whereas in the random test, the AUC ranged from 0.47 to 0.57. Accuracy in the random test is not informative since the TRUE and FALSE labels are not equally distributed in the target set. Therefore, if the TRUE labels account for 20% of the data, we could achieve 80% accuracy by predicting FALSE for all cases.
Table S6 demonstrates the random test for continuous difference, where the target column was replaced by random continuous values within the range of the highest and lowest differences found in the original column of the dataset (-2 to 2 mg/dL). As seen in
Table S6, the correlation coefficients for all random tests are significantly lower than the results of the original test. These outcomes emphasize the fact that the successful predictions described in the previous sections cannot be achieved randomly.
4. Discussion
IF primary mechanisms to improve T2DM risk parameters involve metabolic changes that enhance overall metabolism and trigger tissue-specific metabolic adaptations. These adaptations include modifications in the gut microbiota, remodeling of adipose tissue, restoration of circadian rhythm balance, and increased autophagy in peripheral tissues. (L. Checn et al. European Review for Medical and Pharmacological Sciences. 2023). (Lisandra Joaquim et al. Journal of Physiology and Biochemistry 2022). Age plays a crucial role as older individuals may have different physiological responses to intermittent fasting compared to younger individuals. Weight and BMI can influence the outcomes, as individuals with higher body weight and BMI may experience greater improvements in insulin sensitivity and glycemic control through IF. The pre-existing metabolic conditions, mentioned above, along with age weight and BMI, can have a significant impact on how individuals respond to IF interventions. Hence, when evaluating the effects of IF, it is crucial to account for the heterogeneity in the metabolic status of participants, as this can help explain the conflicting findings observed across different studies. In addition, considering the baseline metabolic status of participants allows for a more comprehensive understanding of the potential benefits and limitations of IF as an intervention for T2DM management. The results of this study allow us to conclude that weight is the crucial feature for females, while age is the determining factor for males to reduce glucose levels in blood. Furthermore, the results reveal the substantial role of BMI in determining the appropriateness of IF for reducing HOMA-IR in individuals below 60 years of age. In contrast, for individuals aged 60 and above, gender, specifically women, appears to have a higher likelihood of benefiting from an IF approach to reduce HOMA-IR. By leveraging advanced machine learning techniques, such a recommendation system holds the potential to provide highly personalized and customized recommendations, thereby optimizing the advantages of intermittent fasting for various subgroups within the population. Moreover, the development of such a recommendation system will contribute to our understanding of the underlying mechanisms behind T2DM and explore potential clinical applications of intermittent fasting in a more precise and individualized manner.5. Conclusions
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1-S2: examples of recommendation system's results. Table S3: Different cutoff values for difference 15%. Table S4: Different cutoff values for difference 10%. Table S5: Different cutoff values for difference 20%. Table S6: Random test for continuous difference.
Acknowledgments
I want to thank Michelle Harvie, Nils Halberg, Flemming Dela, Peter M. Clifton, Eric Ravussin, Leonie Kaye Heilbronn, Enhad Chowdhury and Tilman Kuhn who send me the Individual’s detailed data of their studies.
Conflicts of Interest
The author declares no conflict of interest.
References
- Organization, W.H. World Health Organization. 2023.
- Federation, I.-I.D. , Diabetes Atlas 10th. 2022.
- Vieira-Lara, M.A.; et al. Age and Diet Modulate the Insulin-Sensitizing Effects of Exercise: A Tracer-Based Oral Glucose Tolerance Test. Diabetes 2023, 72, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Weir, M.R. Multimodal efforts to slow the progression of chronic kidney disease in patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2023, 37, 108515. [Google Scholar] [CrossRef] [PubMed]
- Pfützner, A.; et al. De-Escalation Treatment (DET) – A personalized pharmacological intervention to stop disease progression in patients with type 2. Diabetes 2020, 69. [Google Scholar] [CrossRef]
- Harris, C.; Czaja, K. Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D? Nutrients 2023, 15, 1762. [Google Scholar] [CrossRef] [PubMed]
- Ojo, T.K.; et al. Role of Intermittent Fasting in the Managementof Prediabetes and Type 2 Diabetes Mellitus. Cureus 2022, 4, e28800. [Google Scholar] [CrossRef]
- Borgundvaag, E. Metabolic Impact of Intermittent Fastingin Patients with Type2 Diabetes Mellitus A Systematic Review and Meta analysis of Interventional Studies. J. Clin. Metab. 2021, 106, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Alternate-Day Intermittent Fasting Improves Diabetes and Protects Beta-Cell Function in Polygenic Mouse Models of T2DM. Diabetes. 2022, 71. [Google Scholar] [CrossRef]
- Cui, J.; et al. Alternate Day Fasting Improves Endothelial Function in Type 2 Diabetic Mice: Role of Adipose-Derived Hormones. Front. Cardiovasc. Med. 2022, 9, 925080. [Google Scholar] [CrossRef]
- Barua, S.; et al. Early Time-Restricted Feeding Reduces Time in Elevated Glucose Range in Adults With Prediabetes. Diabetes 2023, 72. [Google Scholar] [CrossRef]
- Joaquim, L.; et al. , Benefits, mechanisms, and risks of intermittent fasting in metabolic syndrome and type 2 diabetes. J. Physiol. Biochem. 2022, 78, 295–305. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).