Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Collection:
2.2. Inclusion Criteria:
2.3. Study Selection:
2.4. Full-Text Review:
2.5. Data Extraction:
2.6. Data Synthesis:
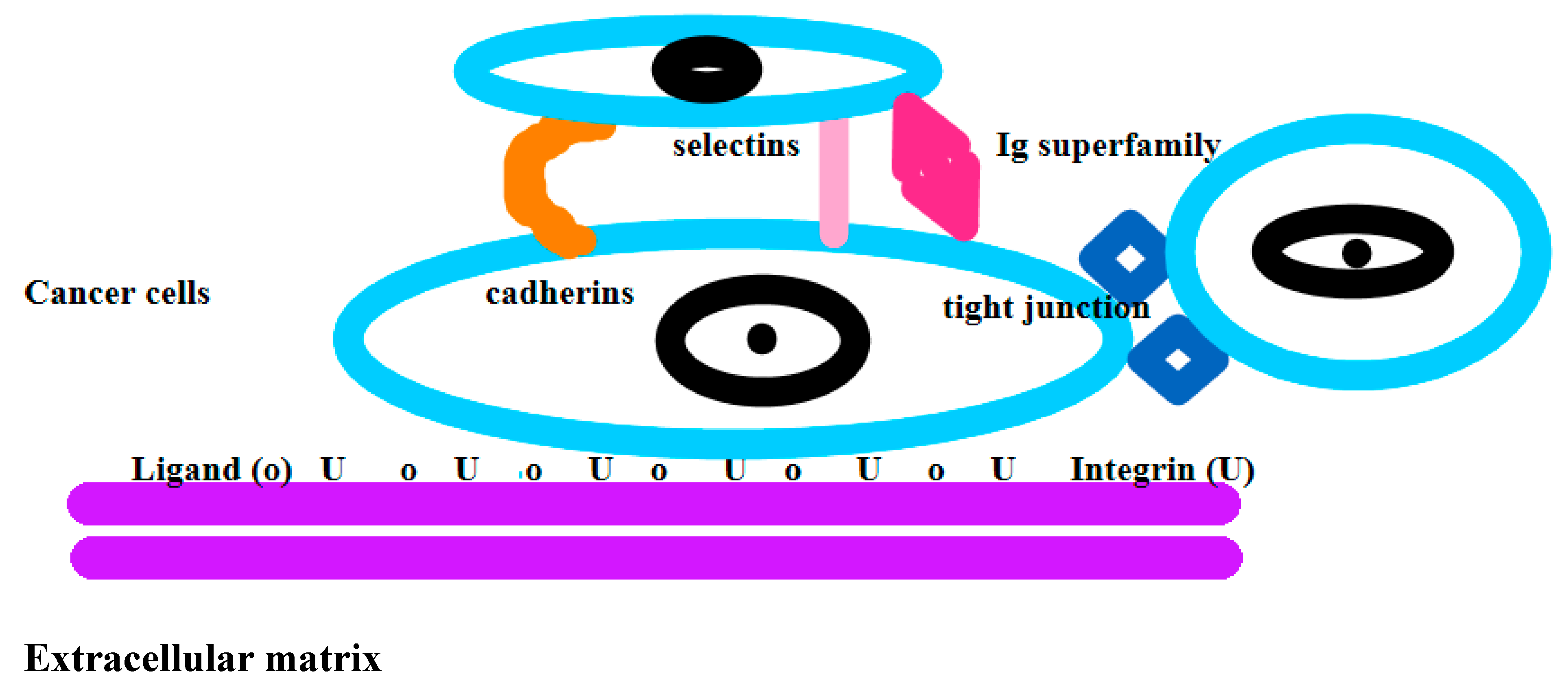
2.7. Definition of Adhesion:
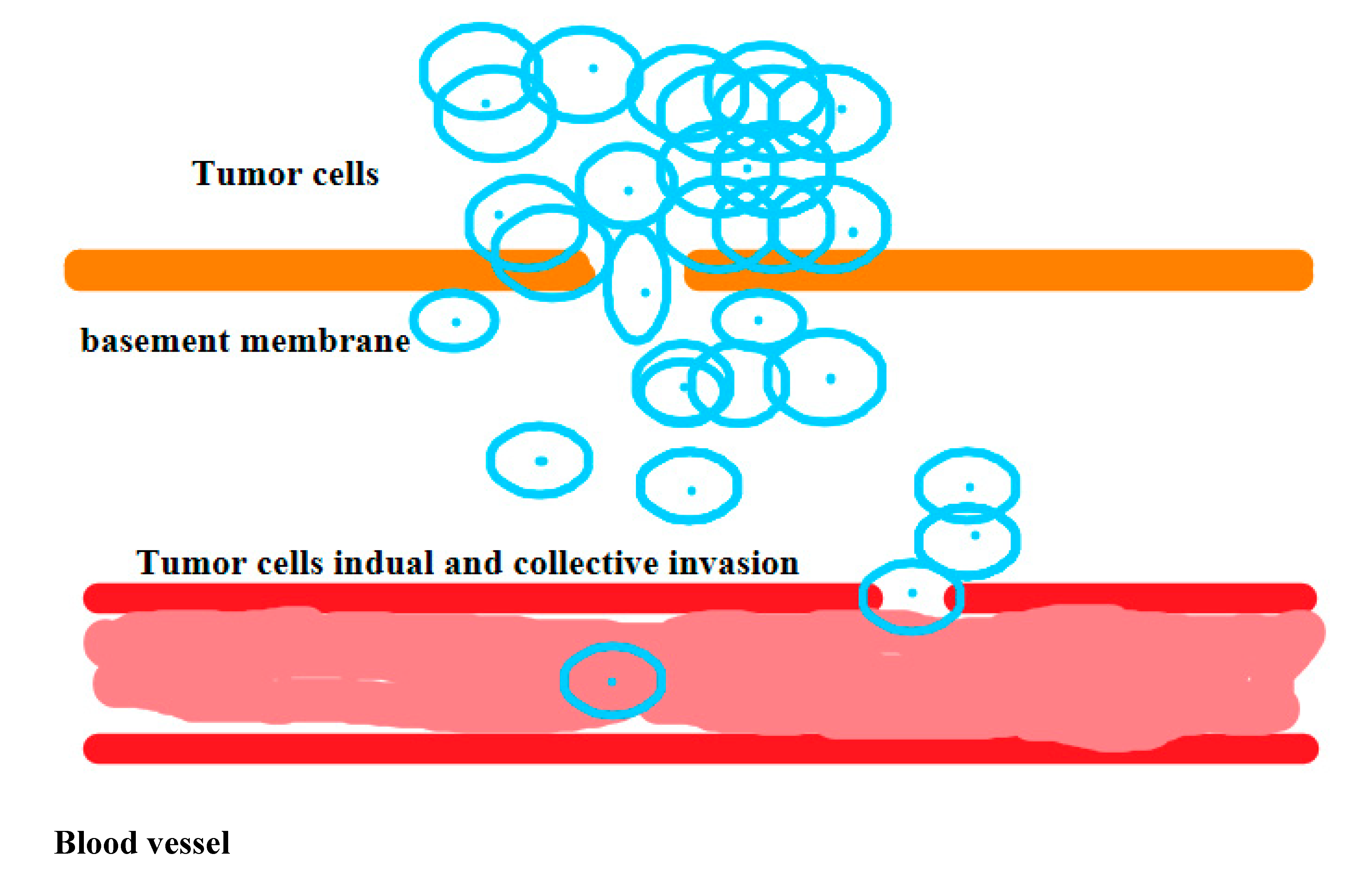
2.8. Definition of Metastasis:
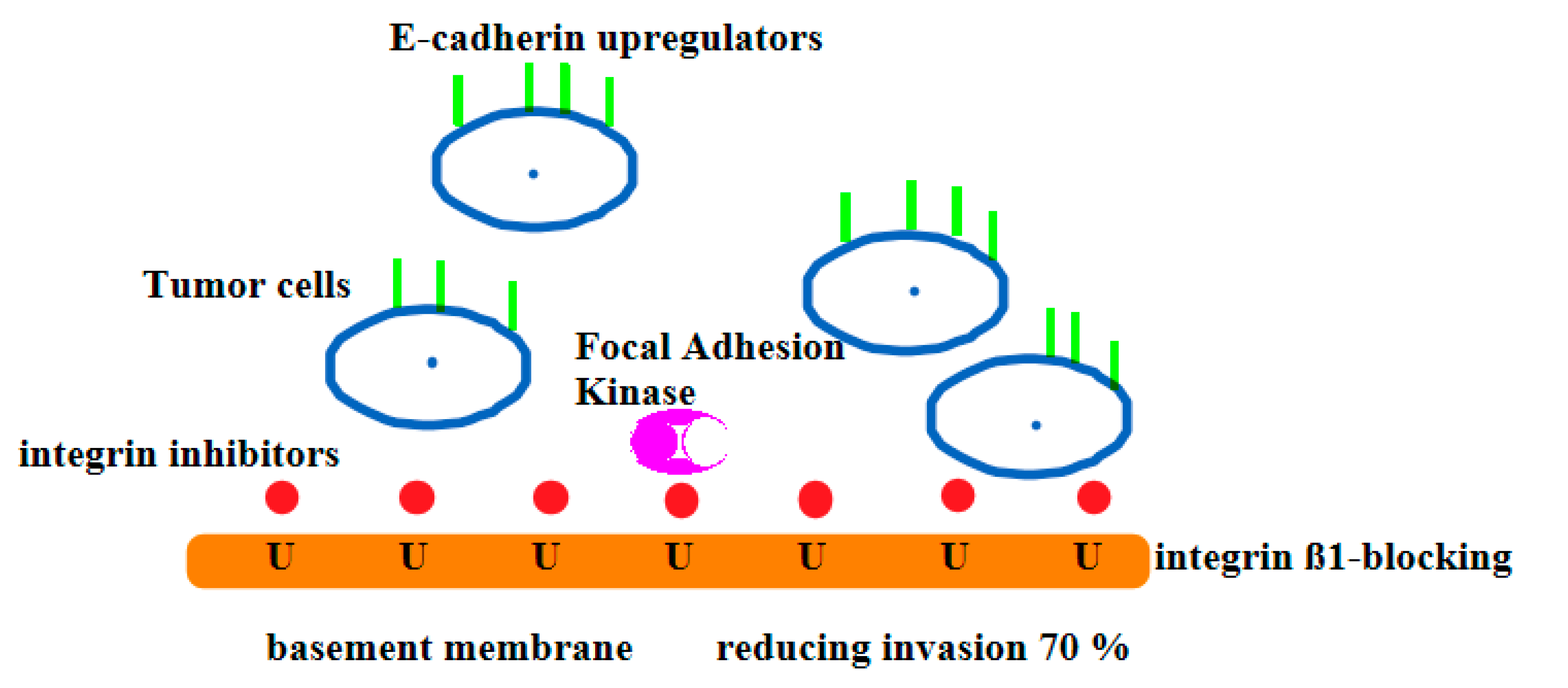
2.9. Definition Inhibitors for Cancer Cells:
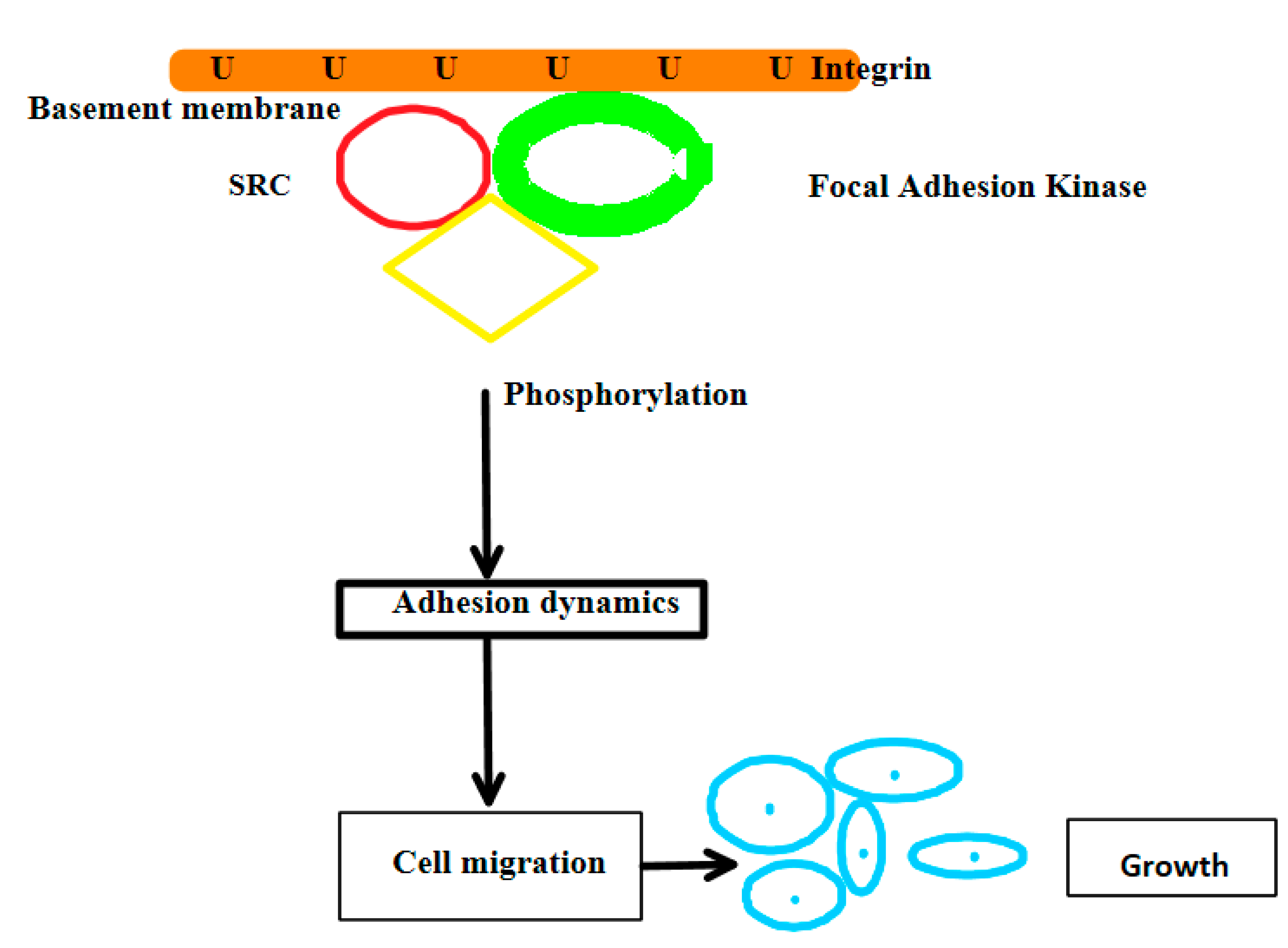
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
Consent for Publication
References
- Ma, X.; Yu, H. Global Burden of Cancer. Yale J Biol Med 2006, 79, 85–94. [Google Scholar]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R.; Kshitiz. Systems Biology of Cancer Metastasis. Cell Syst . PMID: 31465728; PMCID: PMC6716621. 2019, 28, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Petastasis: A Hallmark of Cancer Revisited. Signal Transduct Target Ther . PMID: 32296047; PMCID: PMC7067809. 2020, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging Role of Tumor Cell Plasticity in Modifying Therapeutic Response. Signal Transduct Target Ther . PMID: 33028808; PMCID: PMC7541492. 2020, 7, 228. [Google Scholar] [CrossRef]
- Shawky, J.H.; Davidson, L.A. Tissue Mechanics and Adhesion During Embryo Development. Dev Biol . Epub 2014 Dec 12. PMID: 25512299; PMCID: PMC4402132. 2015, 1, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell Adhesion in Cancer: Beyond the Migration of Single Cells. J Biol Chem . Epub 2020 Jan 14. PMID: 31937589; PMCID: PMC7039572. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Kai, F.; Drain, A.P.; Weaver, V.M. The Extracellular Matrix Modulates the Metastatic Journey. Dev Cell . PMID: 31063753; PMCID: PMC6527347. 2019, 49, 332–346. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial Steps of Metastasis: Cell Invasion and Endothelial Transmigration. Mutat Res . Epub 2011 May 12. PMID: 21605699; PMCID: PMC4028085. 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Bissell, M.J.; Hines, W.C. Why Don't We Get More Cancer? A Proposed Role of the Microenvironment in Restraining Cancer Progression. Nat Med . PMID: 21383745; PMCID: PMC3569482. 2011, 17, 320–339. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors Involved in Cancer Metastasis: A Better Understanding to "Seed and Soil" Hypothesis. Mol Cancer . PMID: 29197379; PMCID: PMC5712107. 2017, 16, 176. [Google Scholar] [CrossRef]
- Venning, F.A.; Wullkopf, L.; Erler, J.T. Targeting ECM Disrupts Cancer Progression. Front Oncol . PMID: 26539408; PMCID: PMC4611145. 2015, 5, 224. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.S.; Banerji, U. Combine and Conquer: Challenges for Targeted Therapy Combinations in Early Phase Trials. Nat Rev Clin Oncol . Epub 2016 Jul 5. PMID: 27377132; PMCID: PMC6135233. 2017, 2017 14, 57–66. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting Metastatic Cancer. Nat Med . Epub 2021 Jan 13. PMID: 33442008; PMCID: PMC7895475. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med . PMID: 34408877; PMCID: PMC8366192. 2021, 12, 9:20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.F.; Marshall, J.F. Integrin-Mediated TGFβ Activation Modulates the Tumour Microenvironment. Cancers (Basel) . PMID: 31438626; PMCID: PMC6769837. 2019, 11, 1221. [Google Scholar] [CrossRef]
- Wan, X.; Kim, S.Y.; Guenther, L.M.; Mendoza, A.; Brigg, J.; Yeung, C.; Currier, D.; Zhang, H.; Mackall, C.; Li, W.J.; Tuan, R.S.; Deyru, A.T.; Khanna, C.; Helman, L. Beta4 Integrin Promotes Osteosarcoma Metastasis and Interacts with Ezrin. Oncogene . Epub 2009 Jul 13. PMID: 19597468; PMCID: PMC2753583. 2009, 28, 3401–3411. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol Ther . Epub 2020 Nov 28. PMID: 33259885; PMCID: PMC8084948. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Beri, P.; Popravko, A.; Yeoman, B.; Kumar, A.; Chen, K.; Hodzic, E.; Chiang, A.; Banisadr, A.; Placone, J.K.; Carter, H.; Fraley, S.I.; Katira, P.; Engler, A.J. Cell Adhesiveness Serves as a Biophysical Marker for Metastatic Potential. Cancer Res . Epub 2019 Dec 19. PMID: 31857292; PMCID: PMC7024658. 2020, 80, 901–911. [Google Scholar] [CrossRef]
- Duś-Szachniewicz, K.; Drobczyński, S.; Woźniak, M.; Zduniak, K.; Ostasiewicz, K.; Ziółkowski, P.; Korzeniewska, A.K.; Agrawal, A.K.; Kołodziej, P.; Walaszek, K.; Bystydzieński, Z.; Rymkiewicz, G. Differentiation of Single Lymphoma Primary Cells and Normal B-cells Based on Their Adhesion to Mesenchymal Stromal Cells in Optical Tweezers. Sci Rep . PMID: 31285461; PMCID: PMC6614388. 2019, 9, 9885. [Google Scholar] [CrossRef]
- Wojtowicz, W.M.; Vielmetter, J.; Fernandes, R.A.; Siepe, D.H.; Eastman, L.; Chisholm, G.B.; Cox, S.; Klock, H.; Anderson, P.W.; Rue, S.M.; Miller, J.J.; Glaser, S.M.; Bragstad, M.L.; Vance, J.; Lam, A.W.; Lesley, S.A.; Zinn, K.; Garcia, K.C. A Human IgSF Cell-Surface Interactome Reveals a Complex Network of Protein-Protein Interactions. Cell . PMID: 32822567; PMCID: PMC7440162. 2020, 182, 1027–1043. [Google Scholar] [CrossRef]
- Mierke, C.T.; Frey, B.; Fellner, M.; Herrmann, M.; Fabry, B. Integrin α5β1 Facilitates Cancer Cell Invasion Through Enhanced Contractile Forces. J Cell Sci . Epub 2011 Jan 11. PMID: 21224397; PMCID: PMC3021998. 2011, 124(Pt 3) Pt 3, 369–383. [Google Scholar] [CrossRef]
- Wendt, M.K.; Taylor, M.A.; Schiemann, B.J.; Schiemann, W.P. Downregulation of Epithelial Cadherin is Required to Initiate Metastatic Outgrowth of Breast Cancer. Mol Biol Cell . Epub 2011 May 25. PMID: 21613543; PMCID: PMC3135469. 2011, 22, 2423–2435. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Paul, C.D.; Stahl, R.; Vanmeerbeeck; G. ; Reumers, V.; Liu, C.; Konstantopoulos, K.; Lagae, L. Time-lapse Lens-free Imaging of Cell Migration in Diverse Physical Microenvironments. Lab Chip . PMID: 27436197; PMCID: PMC4987231. 2016, 16, 3304–3316. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Hoskin, V.; Turashvili, G.; Varma, S.; Mewburn, J.; Mullins, G. , Greer, P.A.; Kiefer, F.; Day, A.G.; Madarnas, Y.; SenGupta, S.; Elliott, B.E. Intravital Imaging Reveals Systemic Ezrin Inhibition Impedes Cancer Cell Migration and Lymph Node Metastasis in Breast Cancer. Breast Cancer Res . PMID: 30678714; PMCID: PMC6345049. 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat Rev Cancer 2014, 14, 430. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int J Mol Sci 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Morand du Puch, C.B.; Vanderstraete, M.; Giraud, S.; Lautrette, C.; Christou, N.; Mathonnet, M. Benefits of Functional Assays in Personalized Cancer Medicine: More Than Just a Proof-of-Concept. Theranostics 2021, 11, 9538–9556. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.S.; Jarajapu, Y.; Rao, R.; Mathew, S. Integrin β1 Promotes Pancreatic Tumor Growth by Upregulating Kindlin-2 and TGF-β Receptor-2. Int J Mol Sci 2021, 22, 10599. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat Rev Mol Cell Biol 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Khera, N.; Rajput, S. Therapeutic Potential of Small Molecule Inhibitors. J Cell Biochem 2017, 118, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Mousson, A.; Legrand, M.; Steffan, T.; Vauchelles, R.; Carl, P.; Gies, J.P.; Lehmann, M.; Zuber, G.; De Mey, J.; Dujardin, D.; Sick, E.; Rondé, P. Inhibiting FAK–Paxillin Interaction Reduces Migration and Invadopodia-Mediated Matrix Degradation in Metastatic Melanoma Cells. Cancers (Basel) 2021, 13, 1871. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, U.; Kowanetz, M.; Niimi, H.; Heldin, C.H.; Moustakas, A. TGF-Beta and the Smad Signaling Pathway Support Transcriptomic Reprogramming During Epithelial-Mesenchymal Cell Transition. Mol Biol Cell 2005, 16, 1987–2002, . Epub 2005 Feb 2. PMID: 15689496; PMCID: PMC1073677. [Google Scholar] [CrossRef] [PubMed]
- Aksorn, N.; Losuwannarak, N.; Tungsukruthai, S.; Roytrakul, S.; Chanvorachote, P. Analysis of the Protein-Protein Interaction Network Identifying c-Met as a Target of Gigantol in the Suppression of Lung Cancer Metastasis. Cancer Genomics Proteomics 2021, 18, 261–272. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Zulehner, G.; Petz, M.; Schneller, D.; Kornauth, C. : Hau, M.; Machat, G.; Grubinger, M.; Huber, H.; Mikulits, W. Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Future Oncol 2009, 5, 1169–1179. [Google Scholar] [CrossRef]
- Chen, C.T.; Liao, L.Z.; Lu, C.H.; Huang, Y.H.; Lin, Y.K.; Lin, J.H.; Chow, L.P. Quantitative Phosphoproteomic Analysis Identifies the Potential Therapeutic Target EphA2 for Overcoming Sorafenib Resistance in Hepatocellular Carcinoma Cells. Exp Mol Med 2020, 52, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Feldman, AM. Bench-to-Bedside; Clinical and Translational Research; Personalized Medicine; Precision Medicine-What's in a Name? Clin Transl Sci 2015, 8, 171–173. [Google Scholar] [CrossRef]
- Barbosa MAG, Xavier CPR, Pereira RF, Petrikaitė V, Vasconcelos MH. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers (Basel) 2021, 14, 190. [Google Scholar] [CrossRef]
- Frigault MM, Barrett JC. Is target validation all we need? Curr Opin Pharmacol 2014, 17, 81–6. [Google Scholar] [CrossRef] [PubMed]
- Ong FS, Das K, Wang J, Vakil H, Kuo JZ, Blackwell WL, Lim SW, Goodarzi MO, Bernstein KE, Rotter JI, Grody WW. Personalized medicine and pharmacogenetic biomarkers: progress in molecular oncology testing. Expert Rev Mol Diagn 2012, 12, 593–602. [Google Scholar] [CrossRef]
- Goetz LH, Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Chen J, Mullins CD, Novak P, Thomas SB. Personalized Strategies to Activate and Empower Patients in Health Care and Reduce Health Disparities. Health Educ Behav 2016, 43, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat Rev Cancer 2019, 18, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Wörthmüller, J.; Rüegg, C. The Crosstalk Between FAK and Wnt Signaling Pathways in Cancer and its Therapeutic Implication. Int J Mol Sci 2020, 21, 9107. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.; Somarelli, J.A.; Hanna, G.; Palmer, G.M.; Garcia-Blanco, M.A. Cellular Migration and Invasion Uncoupled: Increased Migration is not an Inexorable Consequence of Epithelial-to-Mesenchymal Transition. Mol Cell Biol 2014, 34, 3486–3499. [Google Scholar] [CrossRef]
- Banyard, J.; Bielenberg, D.R. The Role of EMT and MET in Cancer Dissemination. Connect Tissue Res 2015, 56, 403–413. [Google Scholar] [CrossRef]
- Turner, K.M.; Yeo, S.K.; Holm, T.M.; Shaughnessy, E.; Guan, J.L. Heterogeneity Within Molecular Subtypes of Breast Cancer. Am J Physiol Cell Physiol 2021, 321, C343–C354. [Google Scholar] [CrossRef]
- Smart, J.A.; Oleksak, J.E.; Hartsough, E.J. Cell Adhesion Molecules in Plasticity and Metastasis. Mol Cancer Res 2021, 19, 25–37. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat Rev Drug Discov 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Sabnis, A.J.; Bivona, T.G. Principles of Resistance to Targeted Cancer Therapy: Lessons From Basic and Translational Cancer Biology. Trends Mol Med 2019, 25, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hua, F.; Hu, Z.W. The Regulation of β-catenin Activity and Function in Cancer: Therapeutic Opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct Target Ther 2021, 6, 425. [Google Scholar] [CrossRef]
- Yip, H.Y.K.; Papa, A. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina (Kaunas) 2019, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Manduca, N.; Maccafeo, E.; De Maria, R.; Sistigu, A.; Musella, M. 3D Cancer Models: One Step Closer to in Vitro Human Studies. Front Immunol 2023, 14, 1175503. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kong, D.; Liu, J.; Zhan, L.; Luo, L.; Zheng, W.; Zheng, Q.; Chen, C.; Sun, S. Breast Cancer Heterogeneity and its Implication in Personalized Precision Therapy. Exp Hematol Oncol 2023, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.M.; Mobley, W.C.; Nolan, G.P.; Rosen, S.T.; Tan, P.; Yen, Y.; Zarrinpar, A. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dignam, J.J. Biomarker-Driven Oncology Clinical Trials: Key Design Elements, Types, Features, and Practical Considerations. JCO Precis Oncol 2019, 3, PO–19. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina (Kaunas) 2019, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front Bioeng Biotechnol 2016, 4, 12, . PMID: 26904541; PMCID: PMC4751256. [Google Scholar] [CrossRef]
- Jacquemin V, Antoine M, Dom G, Detours V, Maenhaut C, Dumont JE. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers (Basel) 2022, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Strickaert A, Saiselet M, Dom G, De Deken X, Dumont JE, Feron O, Sonveaux, P. , Maenhaut, C. Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene 2017, 36, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: one size does not fit all. J Biopharm Stat 2009, 19, 530–542. [Google Scholar] [CrossRef]
- Janiszewska, M. , Primi MC, Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Sabnis AJ, Bivona TG. Principles of Resistance to Targeted Cancer Therapy: Lessons from Basic and Translational Cancer Biology. Trends Mol Med 2019, 25, 185–197. [Google Scholar] [CrossRef]
- Xiao, Y. , Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 2021, 221, 107753. [Google Scholar] [CrossRef]
- Fontana F, Marzagalli M, Sommariva M, Gagliano N, Limonta P. In Vitro 3D Cultures to Model the Tumor Microenvironment. Cancers (Basel) 2021, 13, 2970. [Google Scholar] [CrossRef]
- Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 2020, 5, 28. [Google Scholar] [CrossRef]
- Alday-Parejo, B. , Stupp R., Rüegg C. Are Integrins Still Practicable Targets for Anti-Cancer Therapy? Cancers (Basel) 2019, 11, 978. [Google Scholar] [CrossRef]
- Allison KH, Sledge GW. Heterogeneity and cancer. Oncology (Williston Park) 2014, 28, 772–8. [Google Scholar] [PubMed]
- Hu, C. Hu C., Dignam JJ. Biomarker-Driven Oncology Clinical Trials: Key Design Elements, Types, Features, and Practical Considerations. JCO Precis Oncol 2019, 3, PO.19.00086 PMID: 32923854; PMCID: PMC7446374. [CrossRef]
- Kwon YW, Jo HS, Bae S, Seo Y, Song P, Song M. , Yoon JH. Application of Proteomics in Cancer: Recent Trends and Approaches for Biomarkers Discovery. Front Med (Lausanne) 2021, 8, 747333, . PMID: 34631760; PMCID: PMC8492935. [Google Scholar] [CrossRef]
- Berger MF, Mardis ER. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol 2018, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Yip HYK, Papa A. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef]
- Hulsen T, Jamuar SS, Moody AR, Karnes JH, Varga O, Hedensted S, Spreafico R, Hafler DA, McKinney EF. From Big Data to Precision Medicine. Front Med (Lausanne) 2019, 6, 34, . PMID: 30881956; PMCID: PMC6405506. [Google Scholar] [CrossRef]
- Johnson KB, Wei WQ, Weeraratne D, Frisse ME, Misulis K, Rhee K, Zhao J, Snowdon JL. Precision Medicine, AI, and the Future of Personalized Health Care. Clin Transl Sci 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Liu W, Kovacevic Z, Peng Z, Jin R, Wang P, Yue F, Zheng M, Huang ML, Jansson PJ, Richardson V, Kalinowski DS, Lane DJ, Merlot AM, Sahni S. , Richardson DR. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: new therapeutic targets. Oncotarget 2015, 6, 35522–41. [Google Scholar] [CrossRef]
- Bohr, A. , Memarzadeh K. The rise of artificial intelligence in healthcare applications. Artificial Intelligence in Healthcare, . Epub 2020 Jun 26. PMCID: PMC7325854. [CrossRef]
- Verma, M. Personalized medicine and cancer. J Pers Med 2012, 2, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




