1. Introduction
Chemical mechanical polishing (CMP) is an essential technology to improve the atomic-level planarization of a diverse variety of materials, such as silicon wafers, silicon carbide, sapphire, copper, gallium nitride, and glass[
1,
2,
3].In a typical CMP process, there are many influencing factors involved in polishing planarization process including pad properties, slurry characteristics and processing conditions. It is well known that chemical and mechanical mechanisms are two important mechanisms in polishing process[
4,
5,
6].Investigations of the variables in the CMP process , solid loading, particle size and distribution, modulus, modulus, hardness, asperity sizes and distribution, down-pressure, velocity are believed to be responsible for the material removal [
7,
8,
9].It is essential to develop a novel green slurry to perform CMP for sapphire wafers used for high performance devices, eliminating the environmental contamination and reducing potential harmful impact on operators. The physicochemical properties of abrasives play a critically important role in the performance of silicon wafers[
11,
12,
13].
Currently, the mechanically active silica (SiO
2), ceria (CeO
2), alumina (Al
2O
3) [
14], zirconia (ZrO
2) are widely used in preparation of abrasives[
15]. Novel environment-friendly silica slurries have been developed[
16]. The silica slurries provide high polishing rate, good planarity, and high selectivity, which have greatest influence and play an important role in the optimization of the CMP process. The regular ball like shapes SiO
2 abrasives have less scratches damage or defects during CMP[
17,
18,
19].
Substantial research efforts have been made to improve the MRR of The silicon wafers with minimally damaged surfaces during the CMP process using silica abrasives[
20,
21,
22]. Chen et al. estimated the interaction forces between the substrate surfaces and the abrasive nanoparticles, the ceria particle processed a chemical tooth and formed Si-O-Ce bonds between ceria particles and the sample surface[
23]. Chen et al. prepared parallel channels hexagonal mesoporous silica (H-mSiO
2) particles, which attached with CeO
2 nanoparticles. The H-mSiO
2-CeO
2 composite particles as abrasives revealed a reduced surface roughness, a low topographical variation, and an improved removal rate. Shi et al. reported on a novel acid based SiO
2 slurry which can simultaneously realize an ultralow Rq of only ~0.193 nm and a high MRR of ~10.9 μm h
-1 on the FS substrate. Chen et al. used the good uniformity and dispersity 30-140 nm D-mSiO
2 nanospheres as functionalized abrasives[
24].The abrasives played a key role and achieve nearly non-damage surfaces with atomic level roughness, which can avoid surface scratches commonly caused by particle agglomerations in slurries[
25].
In this work, we successfully synthesized monodispersed SiO2 nanospheres by Stöber method with the controllable 50-150 nm particle size and distribution. Furthermore, the CMP performance using silica colloid with different sizes have been studied. In addition, the possible CMP mechanism of the developed polishing slurry containing SiO2 with different sizes abrasives wafers was proposed.
2. Experimental Section
2.1. Materials
Tetraethylorthosilicate (TEOS, A.R.), ammonia aqueous solution (28 wt%), Ethanol (A.R.), Cetyltrimethylammonium chloride(CTAC, A.R.), Cetyltrimethylammonium bromide (CTAB, A.R.) , Octadecytrimethyl ammonium bromide (STAB, A.R.) , Cetyldimethyl benzyl ammonium chloride ( HDBAC, A.R.), Triethanolamine (TEA, A.R.), NH4F,NaOH, Ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used as received without further purification. Deionized water was used in all experiments.
2.2. Preparation of Silica Spheres
Silica spheres were synthesized by the Stöber method[
26]. Typically, Solution A: a combination of 100 mg NH
4F (2.7 mmol), 21.7 g H
2O (1.12 mol) and 2.41 mL of a 25% aqueous CTAC solution (1.83 mmol) was stirred at 750 rpm in a 50 mL round bottom flask equipped with a stir bar and heated to 60℃.Solution B:14.35 g TEA (97 mmol) and 2.06 mL TEOS (9.3 mmol) were statically heated in a 20 mL capped polypropylene vial at 90℃ for 30 minutes. Both components remain unmixed after heating. Solution B was then added to solution A at once while stirring vigorously, followed by removal of the oil bath, leaving the reaction solution to slowly cool down to room temperature while continuing to stir overnight. On the next day after 12 hours, 50 mL ethanol was added to the solution, which was then transferred into two 50 mL centrifuge tubes and spun down at a speed of 20000 rpm for 20 minutes at room temperature. The supernatant of the solutions was decanted, and the samples were refilled with 30 mL ethanol each, redispersed mechanically with a spatula and by sonication for 10 minutes, and centrifuged again[
27]. The sample was named CTAC-SiO
2.The CTAB-SiO
2, STAB-SiO
2, HDBAC-SiO
2 were synthesized nalogously by replacing CTAC with the equivalent molaramounts of CTAB,STAB, HDBAC ,respectively.
2.3. Polishing Tests and Evaluations
CMP experiments on silicon wafers were performed on a UNIPOL-300 CMP machine (Shenyang Kejing Instrument Co., Ltd., China) with a Rodel porous polyurethane pad. The force-volume images were recorded with the resolution typically of 10×10 pixels within a scanning area of 3×3 µm2 area. The 1 wt% solid content SiO2 abrasive particles were dispersed into deionized water and treated by sonication for 30 min before use. And the slurry pH values were adjusted to 8.5-8.6 using 0.1 M NaOH solution. The polishing parameters were: the feed rate of the polishing liquid was 180 ml/min, the pressure and table-platen speed are fixed at 6 psi and 70 rpm, and the polishing time was 2 h. After polishing, the substrates were cleaned with ultrasonic in DI water. Finally, they were dried with a stream of nitrogen prior to surface analyses.
2.4. Characterization
Fourier transform infrared spectra (FT-IR) were measured using KBr pellets on a Nicolet iS10 analyzer using a scanned area of 4000-400 cm-1 and a 4 cm-1 resolution. The samples were treated using the potassium bromide pellet technique before testing. The crystal structures of the samples were characterized by powder X-ray diffraction on a Rigaku SmartLab SE with Cu Ka radiation (λ=1.54056A).The diffraction data were collected over the angle range of 5-80°with a step size of 0.02 at 35 kV and 20 mA. Thermogravimetric Analysis was performed on an in the tempteature range of 30-900℃ under nitrogen at a heating rate of 10℃min-1 with a Netzsch STA 2500 Regulus analyzer. Nitrogen sorption desorption isotherms were obtained at 77 K using a Micromeritics ASAP 2020 sorptionmeter. The surface area based on the N2 isotherm data was analyzed by BET (Brunauer–Emment–Teller).The elemental composition of the surfaces was determined with a Kratos Analytical Axis Ultra X-ray photoelectron spectrometer equipped with a monochromatic Al Kα source. The structure and morphology of samples were investigated with Zeiss Sigma 300 scanning electron microscopy at an acceleration voltage of 15 kVa. Surface roughness and surface morphology of the polished silicon wafers were characterized by Atomic Force Microscopy with Multimode 8, Bruker, Santa Barbara, CA .
3. Results and Discussion
3.1. Structural and Textural Features
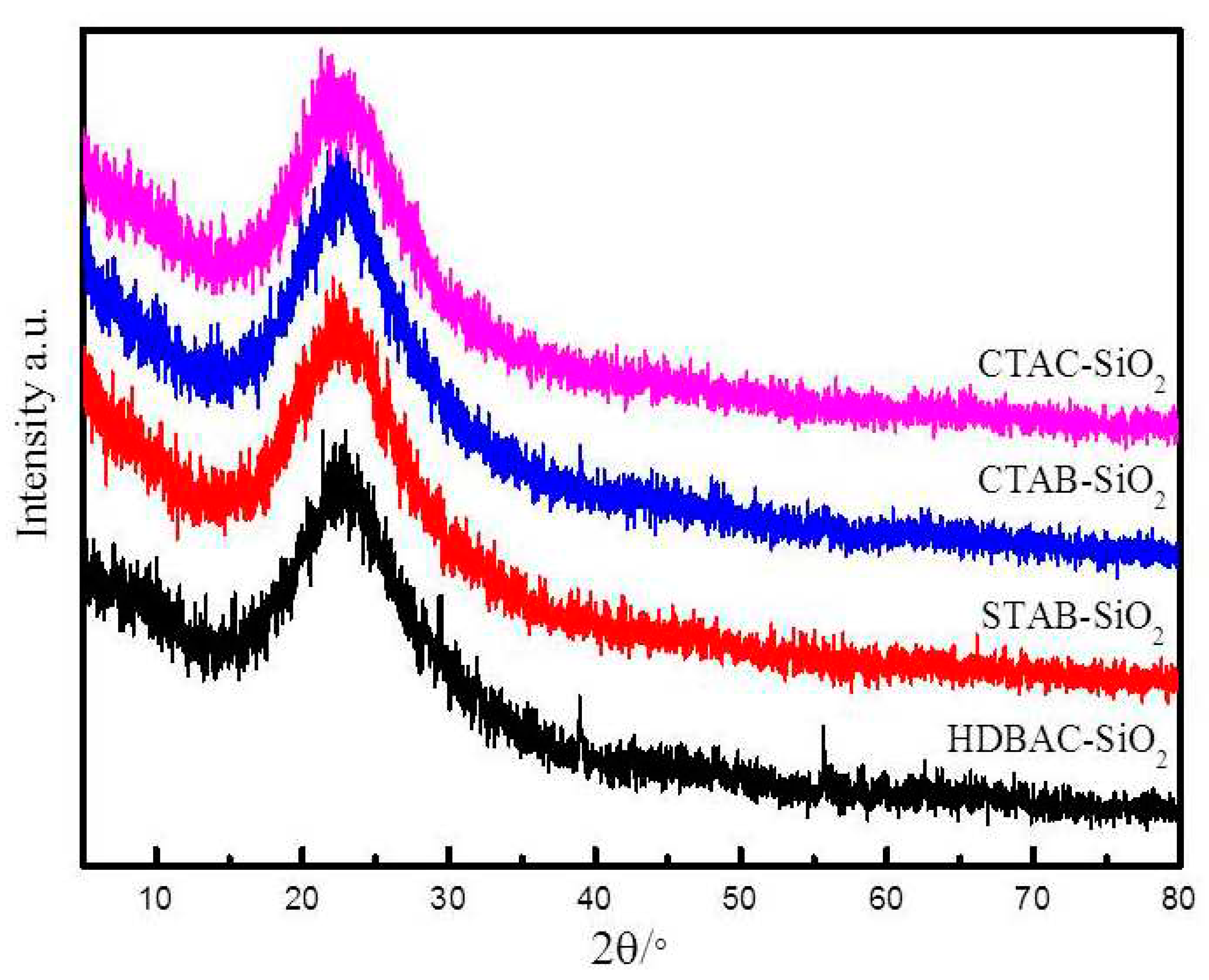
The XRD patterns of the SiO
2 sample were measured and were showed in
Figure 1. As can be seen,the strong and broad diffraction peak at 2θ of 23° in good agreement with the positions of pure amorphous silica[
28]. This result means that no discernible long-range order in the pore arrangement exists in the SiO
2. These observations illustrated that the silica microsphere were successfully synthesized using the sol-gel method.
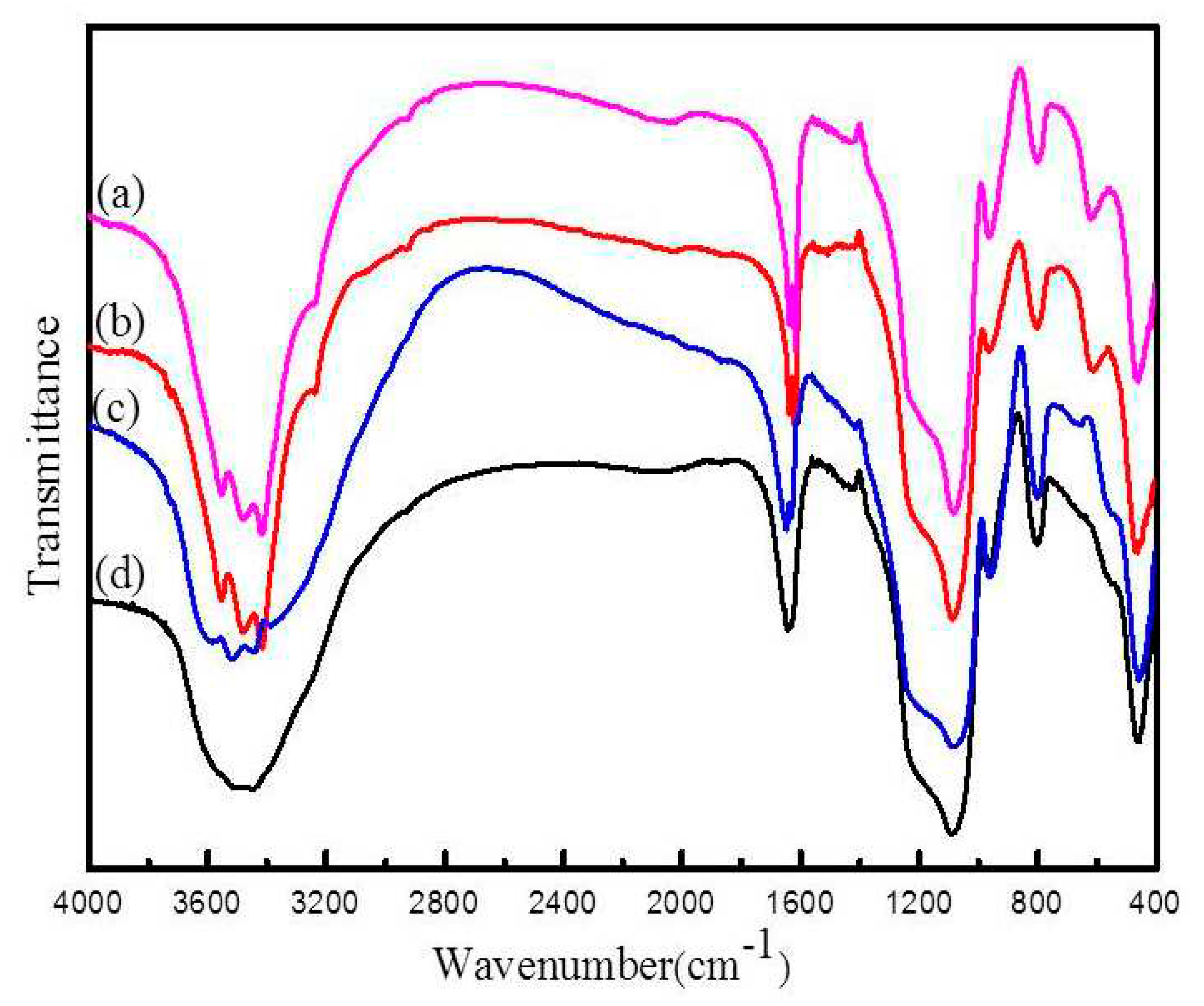
The composition of silica spheres was established by FT-IR spectrum as shown in Figure2. the strong band at 3442.2 cm
-1 can be ascribed to the absorption of -OH group of silica spheres. The absorption band appears at 2983.39 cm
-1 indicated that the stretching vibration of -CH
3 in the CTAB. The peak at 1630.5 cm
-1 can be attributed to the bending vibration of H
2O. The peak with a wavenumber of 1404.9 and 1383.8 cm
-1 belongs to the -CH
3 and -CH
2 symmetric bending vibrations, respectively. The peak at 970.9 cm
-1 is associated with the bending vibration of Si-OH.The obvious bands located at 1054.7 and 457 cm
-1 can be assigned to the stretch vibration bands of Si-O-Si bond,respectively[
29]. It indicates that SiO
2 is hydrophilic and there are many hydroxyl groups on the surface of it.
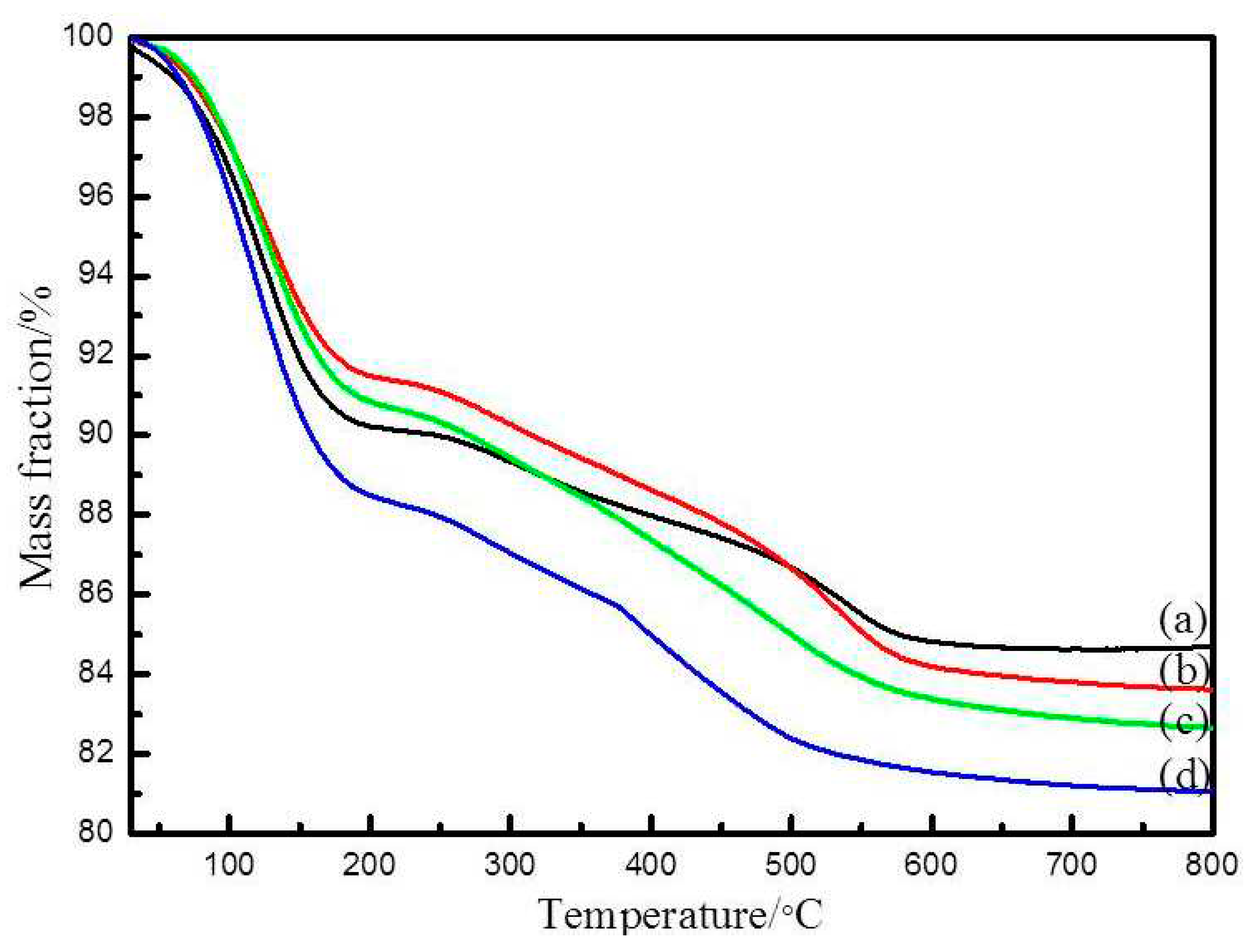
The TGA curves of SiO
2 from different preparation conditions are given in
Figure 3. The first slight weight loss of 8% below 120℃ is attributed to the dissociation of the absorbed water. The second weight loss of 15% between 120℃ to 550℃ can be attributed to the degradation of organic parts [
30].
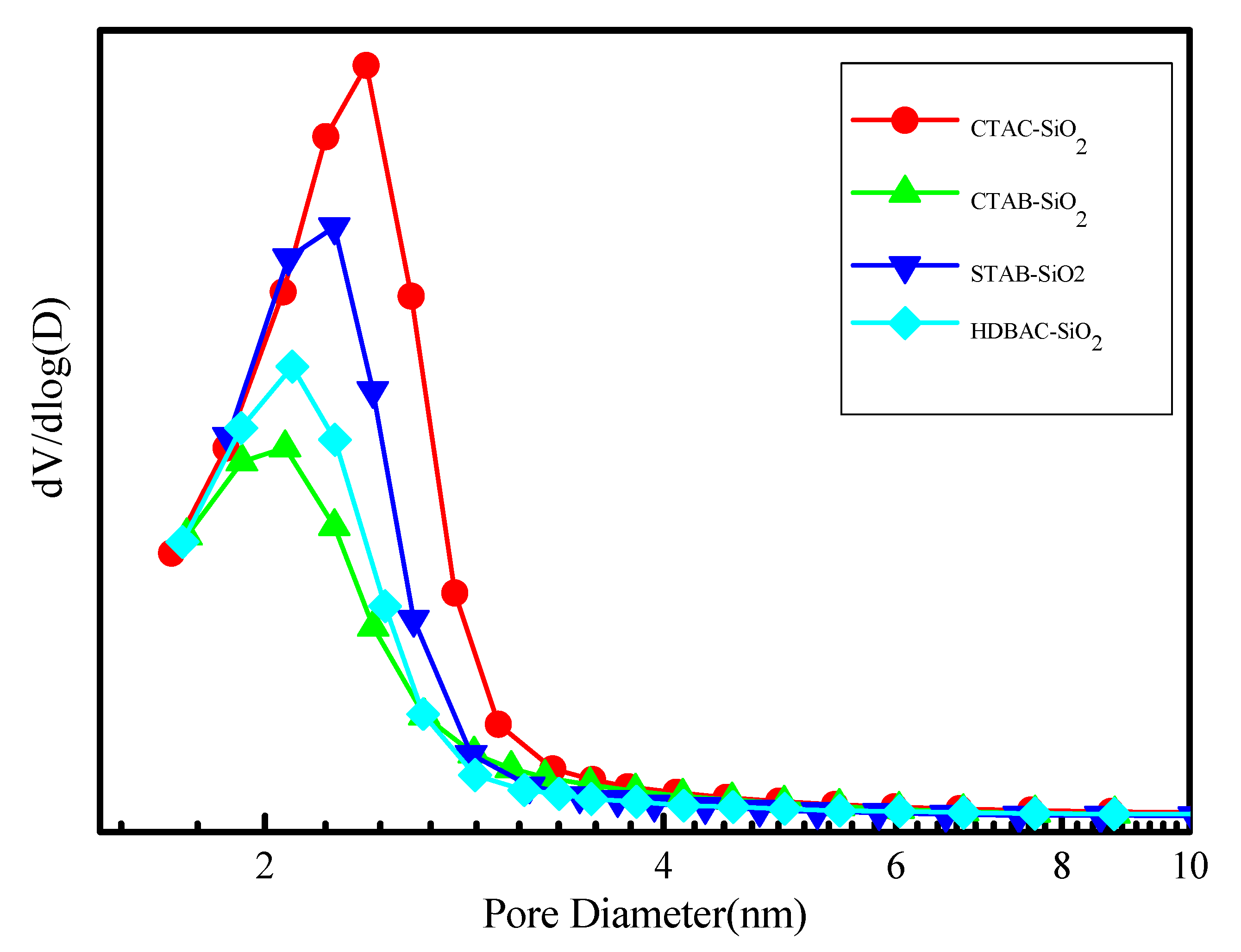
The Brunauer-Emmett-Teller (BET) specific surface area during the synthesis of silica spheres were monitored by the nitrogen adsorption desorption isotherms Figure 5. The adsorption isotherms of silica spheres show a typical type IV adsorption isotherms, which are normally attributed to the characteristics of ordered mesoporous channels. The sorption showing a pore-condensation step around the relative pressure range p/p0 = 0.3-0.4. Specifically, the BET surface areas of The CTAC-SiO2,CTAB-SiO2,STAB-SiO2,HDBAC-SiO2 were comparably decreased from 1155.9, 1059.0 and 1119.2 to 796.9 m2 g-1.The pore size of these samples were reduced from 2.4,2.4, and 2.38 to2.35 nm. The pore size did not change significantly.
Table 1.
The structure parameters of all related samples.
Table 1.
The structure parameters of all related samples.
|
Samples. |
SBET(m2 g-1) |
Pore size(nm)
|
| CTAC-SiO2
|
1155.9 |
2.40 |
| CTAB-SiO2
|
1119.2 |
2.40 |
| STAB-SiO2
|
1059.0 |
2.38 |
| HDBAC-SiO2
|
796.9 |
2.35 |
Figure 6.
The corresponding pore size distributions.
Figure 6.
The corresponding pore size distributions.
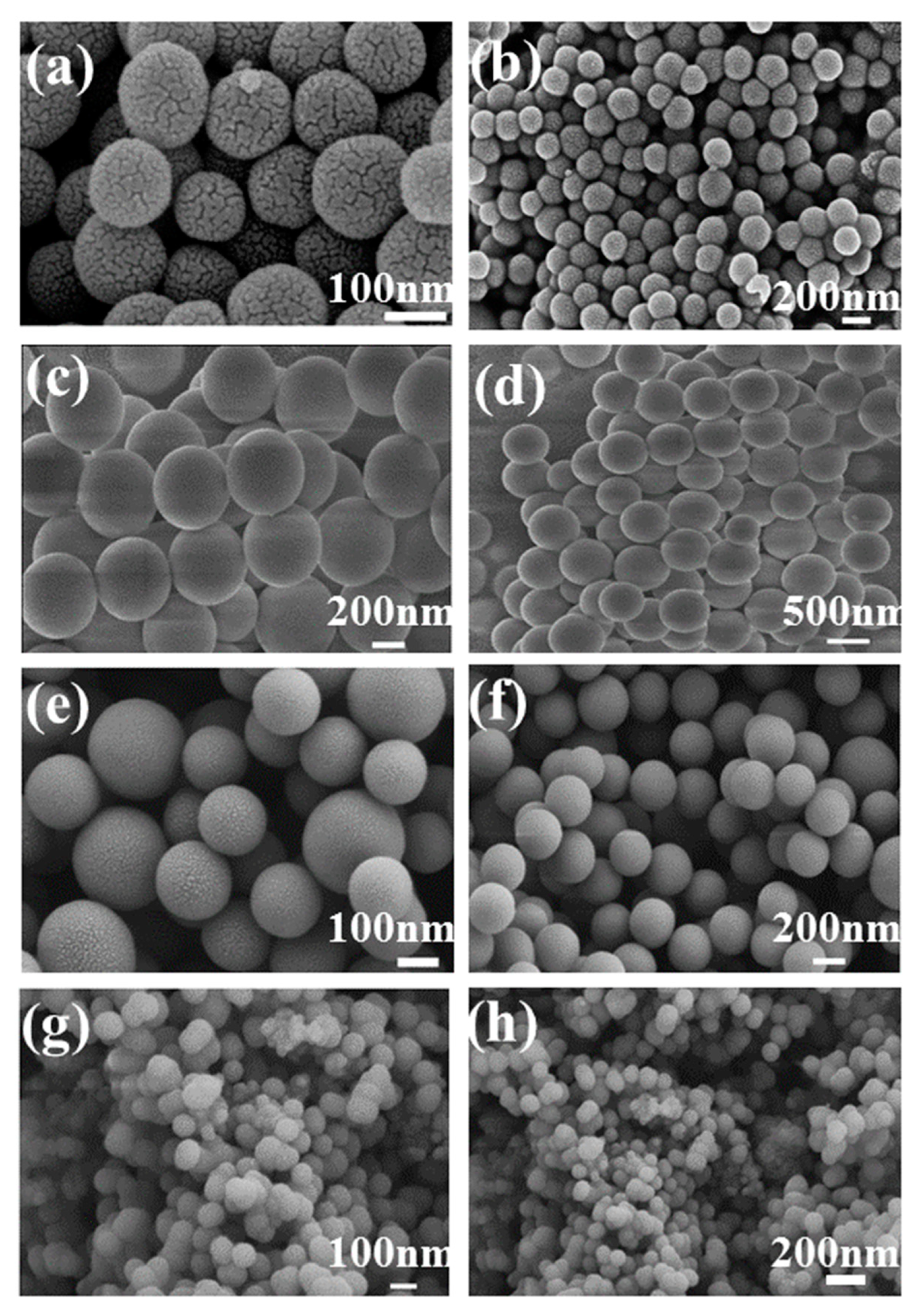
The particle size and morphology of SiO
2 microspheres were confirmed by SEM, as shown in
Figure 7. All samples have a regular spherical shape[
31]. The surface of CTAB-SiO
2 and CTAC-SiO
2 are smoother than that of other surfactants-SiO
2. The average particle sizes of SiO
2 microspheres samples are ca. 30 nm-150 nm, respectively.
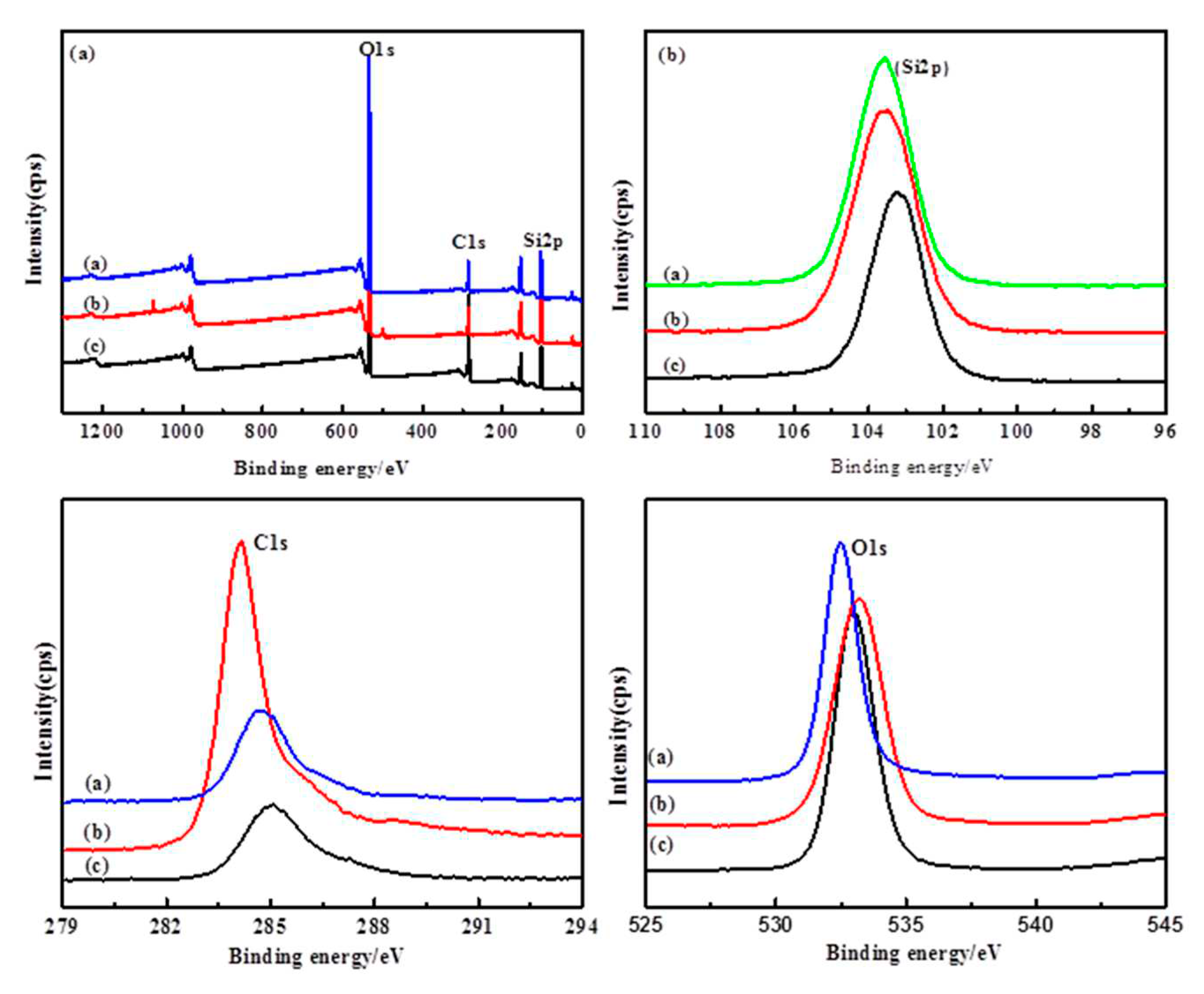
The X-ray photoelectron spectroscopy (XPS) is a highly sensitive technique to explore the chemical changes in the element surroundings. The XPS spectra of the elements of Si2p,O1s and C1s are shown in
Figure 8. The spectrum of SiO
2 microspheres shows strong peaks at 531.9 eV corresponding to the binding energy of O1s[
32,
33]. The spectrum at the BE of 533.19 eV and 532.94 eV, corresponding to Si-O. The electronic binding energy of C1s peak at 284.6 eV was corresponded to C–C bonding[
34]. The peaks at 283.4 and 286.3 eV corresponded to C-Si and H-C bonding. The peaks of 103.75 and 103.9 eV were assigned to Si2p. The small shoulder peak of 102.9 eV was reported to H
6C
2Si
2O
3.Slurry actively reacts with the oxide surface leading dissolution of Si–O bonds to H–C–O–Si bonds[
35]. This hydro-carbonated surface of oxide film was easier to be removed by mechanical parts of CMP process.
3.2. Polishing Performance of SiO2 Microsphere
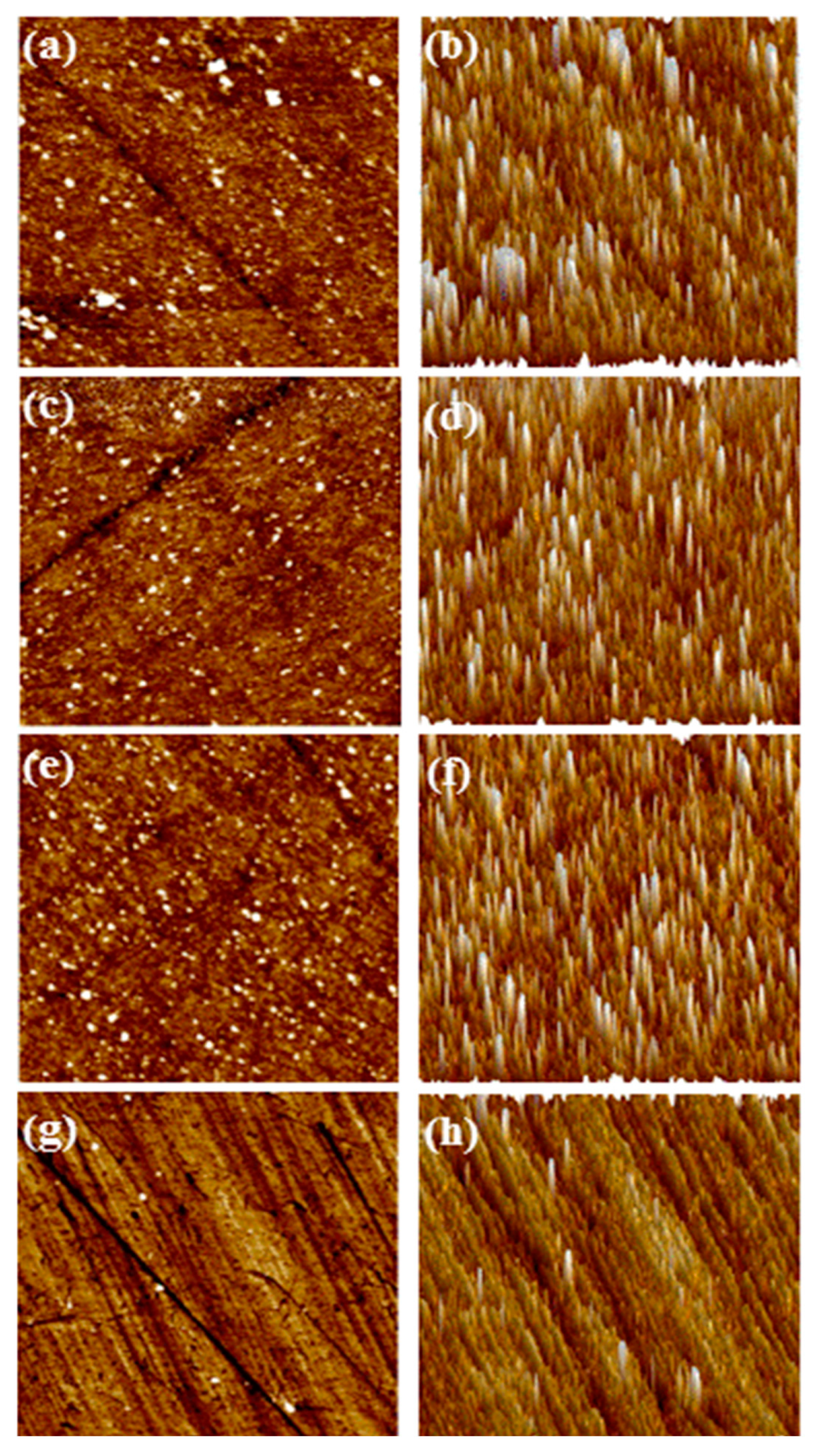
The surface quality of polished silicon wafers after polishing with CTAB-SiO
2, CTAC-SiO
2, STAB-SiO
2 and HDBAC-SiO
2 particles can be investigated through their surface topographies, which were achieved by AFM optical microscope. As shown in Figure9, the AFM micrographs reveal that flat and smooth surfaces without distinct scratches residual particles are achieved by using the as-obtained composite particles as abrasives[
36,
37]. The scratches as well as other microdefects could hardly be observed[
38,
39]. The silica abrasive particle size plays an essential role during CMP process. It is clearly seen that CTAB-SiO
2 ,CTAC-SiO
2 abrasives have much more regular round shapes and remain independent for each particle[
40,
41]. There are some mechanical scratches and cracks are distributed deeply on HDBAC-SiO
2 abrasives. The HDBAC-SiO
2 particle size were about 140 nm, which was larger than the other there SiO
2 abrasives[
42,
43]. The surfactants group may be adsorbed on the surface of SiO
2 abrasive under the action of electrostatic attraction. The Si–OH in the slurry interacts with the silicon wafer surface in a similar fashion to the Si–OH on the SiO
2 grain surface, both in the form of bridge bonds to reduce the breakage bond energy of the Si–Si bonds inside the silicon[
44,
45].It is noteworthy that the optimal polishing performance can be achieved by controlling the balance between the chemical effect and the mechanical effect[
46].
4. Conclusions
In summary, the controllable 50-150 nm sizes SiO2 microsphere were successfully synthesized by the Stöber method with a series of cationic surfactants. The spherical SiO2 exhibited an improved surface quality in the chemical-mechanical polishing process. The polishing results indicate that the SiO2 abrasives can achieve a substantial improvement of surface planarization. The novel SiO2 abrasives were successful applications in CMP.
Author Contributions
J.G. carried the main responsibility for the writing of the manuscript. X.J. supervised the preparation work and contributed to writing the manuscript. L.Z., Y.J.,Q.W. Y.W. assisted J. G. carried out the synthesized and characterized experiment. J. G. was responsible for analyzing the obtained data. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the top notch talent grants programme from Anhui Provincial Education Department , China (Grant No. gxbjZD2020092) ; The excellent innovative scientific research team of silicon-based materials(2022AH010101); The Major Research Project of Natural Science from the Anhui Provincial Education Department , China (Grant No. KJ2019ZD62, No. KJ2021ZD0140); The key research and development project of Anhui Province (Grant No. 202004a05020017); The high-level scientific research and cultivation projects of Bengbu University (Grant No. 2021pyxm09, 2021pyxm08).
Acknowledgments
We extend our appreciation to Anhui Province Engineering Laboratory of Silicon-based Materials; Functional Powder Material Lab of Bengbu City; Engineering Technology Research Center of Silicon-based Materials, Anhui Provincial for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, Z.; Ryde, N.P.; Babu, S.V.; Matijević, E. Particle Adhesion Studies Relevant to Chemical Mechanical Polishing. Langmuir 2005, 21, 9866–9872. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Fink, M.J.; Shchukin, D.; Mitchell, B.S. Functionalized silicon nanoparticles from reactive cavitation erosion of silicon wafers. Chem. Commun. 2014, 51, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Chen, G.; Xu, L.; Kang, C.; Luo, G.; Luo, H.; Zhou, Y.; Dargusch, M.S.; Pan, G. Achieving ultralow surface roughness and high material removal rate in fused silica via a novel acid SiO2 slurry and its chemical-mechanical polishing mechanism. Appl. Surf. Sci. 2019, 500, 144041. [Google Scholar] [CrossRef]
- Stavreva, Z.; Zeidler, D.; Plötner, M.; Drescher, K. Characteristics in chemical-mechanical polishing of copper: comparison of polishing pads. Appl. Surf. Sci. 1997, 108, 39–44. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Wang, Y.; Xu, J.; Ootani, Y.; Higuchi, Y.; Ozawa, N.; Kubo, M. Atom-by-Atom and Sheet-by-Sheet Chemical Mechanical Polishing of Diamond Assisted by OH Radicals: A Tight-Binding Quantum Chemical Molecular Dynamics Simulation Study. ACS Appl. Mater. Interfaces 2021, 13, 41231–41237. [Google Scholar] [CrossRef]
- Luo, Q.; Mackay, R.A.; Babu, S.V. Copper Dissolution in Aqueous Ammonia-Containing Media during Chemical Mechanical Polishing. Chem. Mater. 1997, 9, 2101–2106. [Google Scholar] [CrossRef]
- Penta, N.K.; Veera, P.R.D.; Babu, S.V. Role of Poly(diallyldimethylammonium chloride) in Selective Polishing of Polysilicon over Silicon Dioxide and Silicon Nitride Films. Langmuir 2011, 27, 3502–3510. [Google Scholar] [CrossRef]
- Huang, C.J.; Mu, W.X.; Zhou, H.; Zhu, Y.W.; Xu, X.M.; Jia, Z.T.; Zheng, L.; Tao,X. T. Effect of OH- on chemical mechanical polishing of β-Ga2O3 (100) substrate using an alkaline slurry. RSC Adv. 2018, 8, 6544–6550. [Google Scholar] [CrossRef]
- Tseng, K.C.; Yen, Y.T.; Thomas, S.R.; Tsai, H.W.; Hsu, C.H.; Tsai, W.C.; Shen, C.H.; Shieh, J.M.; Wang, Z.M.; Chueh,Y. L. A facile chemical mechanical polishing lift off transfer process toward large scale Cu(In,Ga)Se2 thin-film solar cells on arbitrary substrates. Nanoscale 2016, 8, 5181–5188. [Google Scholar] [CrossRef]
- He, J.; Li, X.L.; Su, D.; Ji, H.M.; Zhang, X.; Zhang, W.S. Super hydrophobic hexamethyl disilazane modified ZrO2-SiO2 aerogels with excellent thermal stability. J. Mater. Chem. A 2016, 4, 5632–5638. [Google Scholar] [CrossRef]
- Luo, J.; Dornfeld, D. Material removal mechanism in chemical mechanical polishing: theory and modeling. IEEE Trans. Semicond. Manuf. 2001, 14, 112–133. [Google Scholar] [CrossRef]
- Luo, Q.; Mackay, R.A.; Babu, S.V. Copper dissolution in aqueous ammonia containing media during chemical mechanical polishing. Chem. Mater. 1997, 9, 2101–2106. [Google Scholar] [CrossRef]
- Miller, M.S.; Ferrato, M.-A.; Niec, A.; Biesinger, M.C.; Carmichael, T.B. Ultrasmooth Gold Surfaces Prepared by Chemical Mechanical Polishing for Applications in Nanoscience. Langmuir 2014, 30, 14171–14178. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ma, T.; Zhang, W.; van Duin, A.C.T.; Lu, X. Surface Orientation and Temperature Effects on the Interaction of Silicon with Water: Molecular Dynamics Simulations Using ReaxFF Reactive Force Field. J. Phys. Chem. A 2017, 121, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, H.A.; Degen, G.D.; Berkson, Z.J.; Kristiansen, K.; Schrader, A.M.; Oey, T.; Sant, G.; Chmelka, B.F.; Israelachvili, J.N. Electrochemically Enhanced Dissolution of Silica and Alumina in Alkaline Environments. Langmuir 2019, 35, 15651–15660. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, R.Y.; Kawai, K.; Arima, K.; Yamamura, K. Surface modification and microstructuring of 4H-SiC(0001) by anodic oxidation with sodium chloride aqueous solution. ACS Appl. Mater. Inter. 2019, 11, 2535–2542. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Qi, F.; Zhao, D.; Liu, W. A material removal model for silicon oxide layers in chemical mechanical planarization considering the promoted chemical reaction by the down pressure. Tribol. Int. 2016, 93, 11–16. [Google Scholar] [CrossRef]
- Xu, L.; Lei, H.; Wang, T.; Dong, Y.; Dai, S. Preparation of flower-shaped silica abrasives by double system template method and its effect on polishing performance of sapphire wafers. Ceram. Int. 2019, 45, 8471–8476. [Google Scholar] [CrossRef]
- Lei, H.; Luo, J. CMP of hard disk substrate using a colloidal SiO2 slurry: preliminary experimental investigation. Wear 2004, 257, 461–470. [Google Scholar] [CrossRef]
- Kim, R.; Sung, Y.I.; Lee, J.S.; Lim, H.B. Chemiluminescence system for direct determination and mapping of ultra-trace metal impurities on a silicon wafer. Anal. 2010, 135, 2901–2906. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, G.; Gong, H.; Shi, X.; Zou, C. Characterization of sapphire chemical mechanical polishing performances using silica with different sizes and their removal mechanisms. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 513, 153–159. [Google Scholar] [CrossRef]
- Lv, M.X.; Yu, S.T.; Liu, S.W.; Li, L.; Yu, H.H.; Wu, Q.; Pang, J.H.; Liu, Y.X.; Xie, C.X.; Liu,Y. One-pot synthesis of stable Pd@mSiO2 core-shell nanospheres with controlled pore structure and their application to the hydrogenation reaction. Dalton Trans. 2019, 48, 7015–7024. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, S.; Cai, W.; Mu, Z.; Chen, Y. Tunable synthesis, characterization, and CMP performance of dendritic mesoporous silica nanospheres as functionalized abrasives. Colloids Surfaces A: Physicochem. Eng. Asp. 2022, 638, 128322. [Google Scholar] [CrossRef]
- Chen, A.; Wang, W.; Ma, X.; Chen, Y. Ceria coated hexagonal mesoporous silica core–shell composite particle abrasives for improved chemical–mechanical planarization performance. J. Porous Mater. 2018, 26, 1005–1015. [Google Scholar] [CrossRef]
- Ng, H.T.; Han, J.; Yamada, T.; Nguyen, P.; Chen, Y.P.; Meyyappan, M. Single Crystal Nanowire Vertical Surround-Gate Field-Effect Transistor. Nano Lett. 2004, 4, 1247–1252. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2016, 29, 371–388. [Google Scholar] [CrossRef]
- Yoo, W.C.; Stein, A. Solvent Effects on Morphologies of Mesoporous Silica Spheres Prepared by Pseudomorphic Transformations. Chem. Mater. 2011, 23, 1761–1767. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Z.; Liao, L.; Liu, J.; Su, H.; Wang, S.; Guo, D. Green chemical mechanical polishing of sapphire wafers using a novel slurry. Nanoscale 2020, 12, 22518–22526. [Google Scholar] [CrossRef]
- Pan, G.; Gu, Z.; Zhou, Y.; Li, T.; Gong, H.; Liu, Y. Preparation of silane modified SiO2 abrasive particles and their Chemical Mechanical Polishing (CMP) performances. Wear 2011, 273, 100–104. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, W.; Yun, J.; Lee, K.; Lee, J.; Yu, H.; Kim, J.J.; Jang, J. Fabrication of Uniform Wrinkled Silica Nanoparticles and Their Application to Abrasives in Chemical Mechanical Planarization. ACS Appl. Mater. Interfaces 2018, 10, 11843–11851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhang, Z.; Qin, F.; Liu, W.; Song, Z. Preparation of monodisperse polystyrene/silica core–shell nano-composite abrasive with controllable size and its chemical mechanical polishing performance on copper. Appl. Surf. Sci. 2011, 258, 1217–1224. [Google Scholar] [CrossRef]
- Kim, N.-H.; Ko, P.-J.; Choi, G.-W.; Seo, Y.-J.; Lee, W.-S. Chemical mechanical polishing (CMP) mechanisms of thermal SiO2 film after high-temperature pad conditioning. Thin Solid Films 2006, 504, 166–169. [Google Scholar] [CrossRef]
- Myong, K.K.; Byun, J.; Choo, M.J.; Kim, H.; Kim, J.Y.; Lim, T.; Kim, J.J.; Myong,K. Direct and quantitative study of ceria SiO2 interaction depending on Ce3+ concentration for chemical mechanical planarization (CMP) cleaning. Mat. Sci. Semicon. Proc. 2021, 122, 105500–105509. [Google Scholar] [CrossRef]
- Chen, G.M.; Ni, Z.F.; Bai, Y.W.; Li, Q.Z.; Zhao, Y.W. The role of interactions between abrasive particles and the substrate surface in chemical-mechanical planarization of Si-face 6H-SiC. RSC Adv. 2017, 7, 16938–16952. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, A.L.; Ma, X.Y.; Wang, T.Y.; Chen, A. Copper incorporated dendritic mesoporous silica nanospheres and enhanced chemical mechanical polishing (CMP) performance via Cu2+/H2O2 heterogeneous Fenton-like system. Appl. Surf. Sci. 2022, 601, 154262–154273. [Google Scholar] [CrossRef]
- Matovu, J.B.; Ong, P.; Leunissen, L.H.A.; Krishnan, S.; Babu, S.V. Use of Multifunctional Carboxylic Acids and Hydrogen Peroxide To Improve Surface Quality and Minimize Phosphine Evolution During Chemical Mechanical Polishing of Indium Phosphide Surfaces. Ind. Eng. Chem. Res. 2013, 52, 10664–10672. [Google Scholar] [CrossRef]
- Shi, X.; Xu, L.; Zhou, Y.; Zou, C.; Wang, R.; Pan, G. An in situ study of chemical-mechanical polishing behaviours on sapphire (0001) via simulating the chemical product-removal process by AFM-tapping mode in both liquid and air environments. Nanoscale 2018, 10, 19692–19700. [Google Scholar] [CrossRef]
- Myers, J.N.; Zhang, X.; Bielefeld, J.; Lin, Q.; Chen, Z. Nondestructive in Situ Characterization of Molecular Structures at the Surface and Buried Interface of Silicon-Supported Low-k Dielectric Films. J. Phys. Chem. B 2015, 119, 1736–1746. [Google Scholar] [CrossRef]
- Wu, L.; Cui, L.; He, W.; Guo, J.; Yu, B.; Qian, L. Toward Controllable Wet Etching of Monocrystalline Silicon: Roles of Mechanically Driven Defects. ACS Appl. Mater. Interfaces 2022, 14, 29366–29376. [Google Scholar] [CrossRef]
- Qin, K.; Moudgil, B.; Park, C.-W. A chemical mechanical polishing model incorporating both the chemical and mechanical effects. Thin Solid Films 2003. [Google Scholar] [CrossRef]
- Gao, P.; Liu, T.; Zhang, Z.; Meng, F.; Ye, R.-P.; Liu, J. Non-spherical abrasives with ordered mesoporous structures for chemical mechanical polishing. Sci. China Mater. 2021, 64, 2747–2763. [Google Scholar] [CrossRef]
- Wang, H.; Hu, L.; Cao, G.; Xia, R.; Cao, J.; Zhang, J.; Pan, G. Experimental and Computational Studies on Octyl Hydroxamic Acid as an Environmentally Friendly Inhibitor of Cobalt Chemical Mechanical Polishing. ACS Appl. Mater. Interfaces 2022, 14, 28321–28336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, Z.; Guo, J. The effect of the interface reaction mode on chemical mechanical polishing. CIRP J. Manuf. Sci. Technol. 2020, 31, 539–547. [Google Scholar] [CrossRef]
- Bu, Z.; Niu, F.; Chen, J.; Jiang, Z.; Wang, W.; Wang, X.; Wang, H.; Zhang, Z.; Zhu, Y.; Sun, T. Single crystal silicon wafer polishing by pretreating pad adsorbing SiO2 grains and abrasive-free slurries. Mater. Sci. Semicond. Process. 2021, 141, 106418. [Google Scholar] [CrossRef]
- Wang, M.; Duan, F. Atomic-Level Material Removal Mechanisms of Si(110) Chemical Mechanical Polishing: Insights from ReaxFF Reactive Molecular Dynamics Simulations. Langmuir 2021, 37, 2161–2169. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).