1. Introduction
Bone morphogenetic protein (BMP) is a member of the transforming growth factor β (TGF-β) superfamily. It was originally recognized as a factor in the body that can induce bone and cartilage formation, also known as "osteoblast". BMPs and its corresponding receptors were found in almost all organs during animal growth and development. Therefore, the function of BMPs goes far beyond mere bone induction. BMPs not only has obvious osteogenic effect, but also participates in the genesis and development of tumor, can inhibit cell growth, proliferation, promote cell differentiation and apoptosis, and so on [
1,
2,
3,
4,
5].
Human BMP4, as a member of the BMPs family, has the same spatial structure as BMPs. The BMP4 preproprotein is composed about 400 amino acids and consists of 3 parts: N-terminal signaling peptide, preprotein folding region and C-terminal maturation peptide. The carboxy-terminally mature BMP4 protein can be cleaved from the preproprotein to become a highly conserved BMP4 molecule consisting of 116 amino acids. Its C-terminal contains seven cysteine residues, six of which form three intramolecular disulfide bonds, called cysteine junctions, and the seventh cysteine can be glycosylated to form a covalent disulfide bond for dimerization with another monomer, thus forming a bioactive signaling molecule [
6]. BMP4 plays important roles in promoting bone tissue regeneration and repair. In addition, BMP4 is also closely related to inducing embryonic differentiation, guiding neural stem cell differentiation, regulating tumor growth and invasion, and some cardiovascular and cerebrovascular diseases [
7,
8,
9,
10] .
Recent years, the unappreciated roles of BMPs in the regulation of antiviral immunity have been demonstrated [
11,
12,
13,
14,
15,
16]. BMP6 can enhance antiviral response and suppress growth of HBV in cell culture [
17]. BMP2 may have a role in inducing virus-induced and interferon associated responses [
18]. We also found that Bmp8a plays a regulatory role in the antiviral immune response [
19]. However, whether BMP4 has the function in the regulation of immune responses is unknown. Zebrafish has been developed as a simple vertebrate system for large-scale genetic and evolution analysis [
20,
21]. In the present study, zebrafish was used as a model to answer this question. We demonstrated that Bmp4 runs a role in the host defense against virus infection.
2. Results
2.1. The identification and evolution of zebrafish bmp4
The gene of zebrafish
bmp4 is acquired from the Ensembl database (Ensembl: ENSDARG00000019995,
http://www.ensembl.org). This gene has 1 transcript encoding a protein of 400 amino acids. Orthologues of this gene were also found in mammals, birds, amphibians, teleosts and cartilaginous fish. However, no
BMP4 orthologs have been identified in jawless vertebrates. When the zebrafish Bmp4 sequence was used as a bait to search the orthologs in sea lamprey
Petromyzon marinus genome, Bmp2 was shown to be the closest to Bmp4. Thus, it is highly possible that
bmp4 genes were first present in cartilaginous fish and originated from
bmp2 during evolution (
Figure 1A). The comparison of the amino acid sequences, exon-intron organizations and three-dimensional (3D) structures among them showed that they are very similar (Fig.1B, C, D, E). Apparently, the sequences of their maturation peptides have the highest identity (Fig.1B). Also, they all contain seven cysteine residues in their C-terminal (Fig.1B). In addition, the
bmp4 loci in
D. rerio is adjacent to the genes
ddhd1a and
fermt2, which is resemblance to the
BMP4 regions of other species (Fig.1F). Both the order and direction of these genes are similar among these species, indicating the synteny encompassing
BMP4 region during vertebrate evolution.
2.2. The bmp4 expression was increased after virus or poly(I:C) challenge
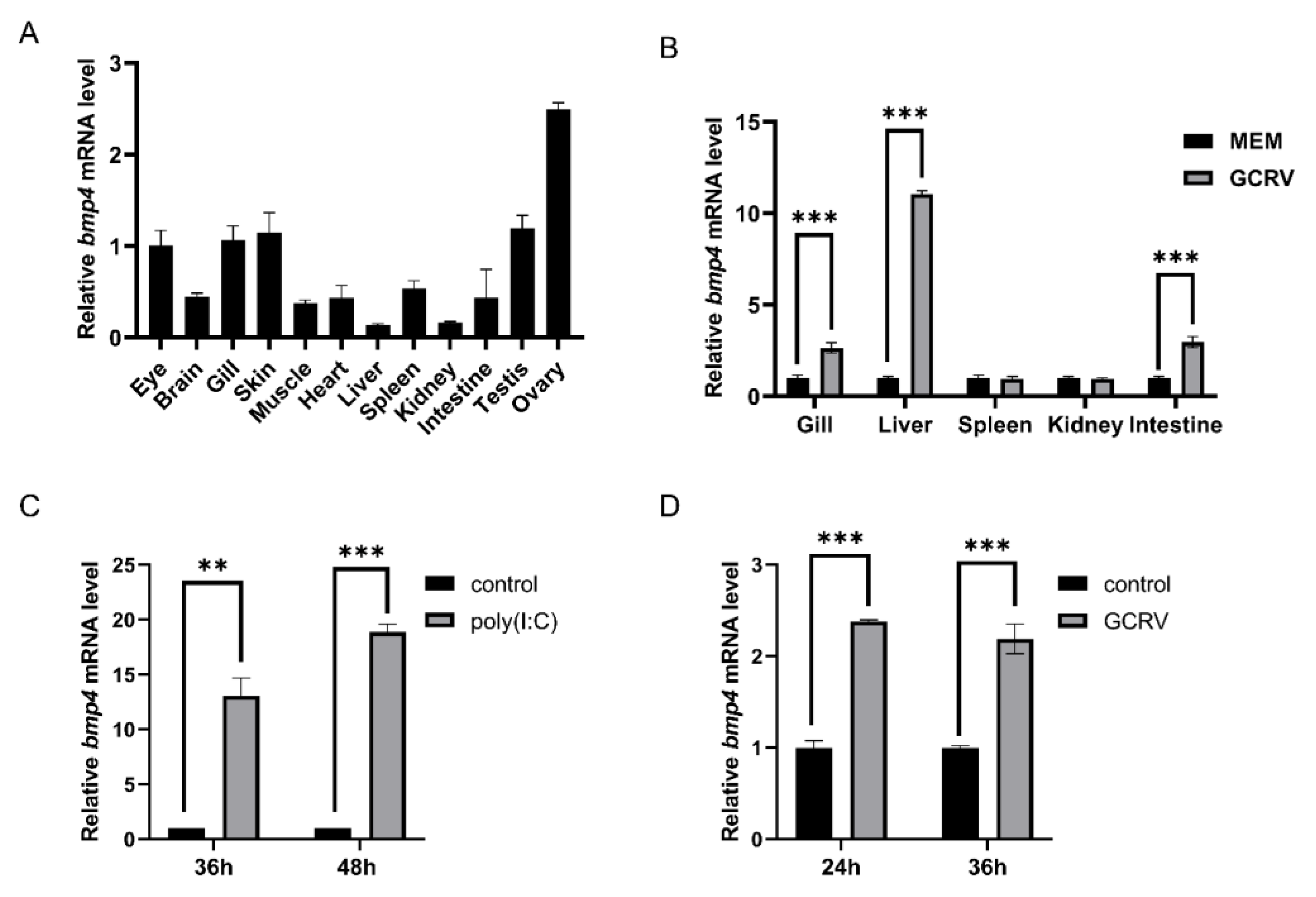
We first detected the distribution of
bmp4 mRNA in tissues of adult zebrafish by quantitative reverse transcription PCR (qRT-PCR) technology. It was found that zebrafish
bmp4 mRNA was expressed in all the detected tissues (
Figure 2A), implying multiple functions of this gene. Obviously,
bmp4 had the higher expression in the tissues of ovary, testis, skin, gill and eye (
Figure 2A). Then the expression of
bmp4 upon virus infection was measured in the immune-related tissues including gill, liver, spleen, kidney and intestine in zebrafish. After 24 h stimulation with GCRV (a dsRNA virus), the expression of
bmp4 in the tissues of gill, liver and intestine was significantly increased (
Figure 2B). The
in vitro effect of GCRV or poly(I:C) challenge on
bmp4 expression was also detected in ZFL cells. GCRV or poly(I:C) significantly elevated the expression of
bmp4 in ZFL cells (
Figure 2C, D). These suggest that the expression of
bmp4 is induced by infection with virus or its mimic poly(I:C).
2.3. Antiviral function of Bmp4 both in vitro and in vivo
We then explored the role of zebrafish Bmp4 in the antiviral immunity. The wild-type EPC cells and EPC cells over-expressing
bmp4 were infected with GCRV virus, respectively. We found that, after 24 h of GCRV stimulation, a significant decrease in viral titers was observed in the EPC cells overexpressing
bmp4 (
Figure 3A). In addition, the CRISPR-Cas9 technology was used to generate
bmp4 deficient (
bmp4−/−) zebrafish, which 1 nucleotide was replaced by 11 nucleotides in the exon 3 of
bmp4, leading to early termination of translation to the 118th amino acid (
Figure 3B). The
bmp4 knockout zebrafish (
bmp4-/-) can survive, reproduce and breed normally under standard laboratory conditions. To study the function of Bmp4 in antiviral immunity
in vivo, the wild-type and
bmp4−/− zebrafish larvae were infected by adding GCRV in the embryo culture medium. Compared with wild-type zebrafish,
bmp4−/− zebrafish larvae exhibited significantly reduced survival rate upon GCRV infection, and most of the fish died at 36 h post infection, while only little of the wild type zebrafish was dead during the period (
Figure 3C). Moreover, the adult zebrafish were infected with the virus of GCRV (
Figure 3D). After infection with GCRV, the survival rate of
bmp4−/− zebrafish was significantly reduced (
Figure 3E). Also, the levels of virus RNA of GCRV in the liver and kidney of
bmp4−/− adult zebrafish were significantly increased than those of wild-type zebrafish (
Figure 3F, G). These data suggest that Bmp4 runs a role in the host defense against virus infection.
2.4. Bmp4 increases the expression of antiviral genes
It has revealed that
ifnφ1 and
ifnφ3 were identified to have antiviral response in zebrafish [
22], while only one type I IFN was shown to be involved in the antiviral role in carp and EPC cell [
23,
24]. The
bmp4 was over-expressed in EPC cells, and then qRT-PCR was used to detect the expressions of type I IFN gene and the antiviral protein gene
mx. It was found that EPC cells with over-expressed
bmp4 showed markedly higher expression of
ifn and
mx than that of control cells, infected with or without GCRV (
Figure 4A-4D). Moreover, when both the wild-type and
bmp4−/− zebrafish larvae or adult zebrafish were challenged with GCRV, the expression of both
ifnφ1 and
mx was all significantly down-regulated in the
bmp4−/− zebrafish larvae or the liver and spleen of
bmp4−/− adult zebrafish, compared with those of wild-type fish (
Figure 4E-4H). All these data indicate that BMP4 promotes the expression of antiviral genes.
2.5. Bmp4 activates antiviral signaling via p38 MAPK pathway
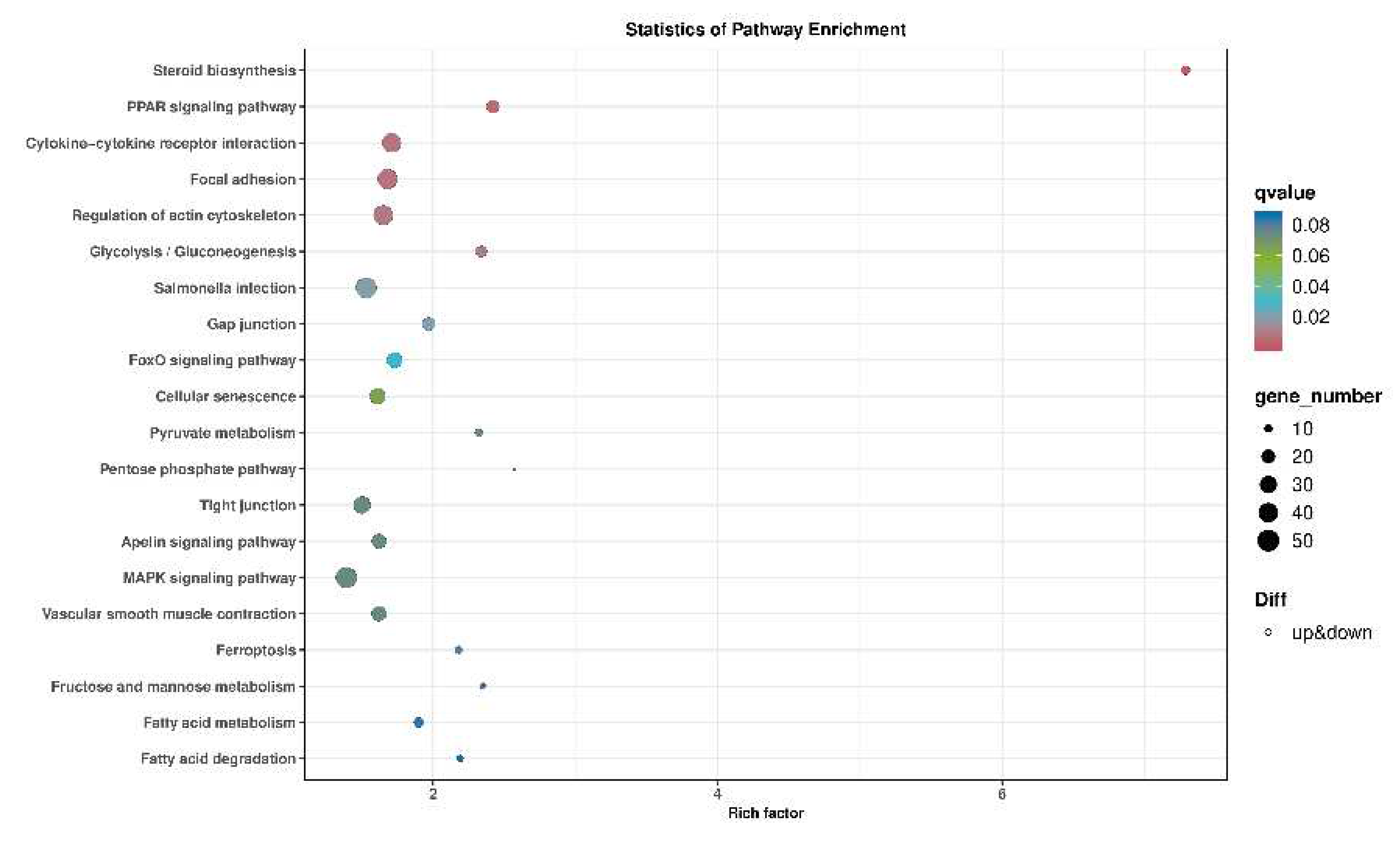
To explore the molecular mechanism of Bmp4 in antiviral immune regulation, we injected GCRV intrabitoneously into
bmp4−/− mutant and wild-type zebrafish, and then performed transcriptomic analysis of their livers. Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses revealed that the differential expressed genes (DEGs) were enriched in MAPK signaling pathway, cytokine-cytokine receptor interaction and focal adhesion that may be involved in the immune process (
Figure 5). It has been known that TBK1-IRF3/7-IFN signal plays a significant role in antiviral innate immune responses, so we then evaluated the activation of Tbk1 and Irf3 by immunoblot assays in both
bmp4-overexpressing and wild-type EPC cells, infected with or without GCRV. It was found that overexpressed
bmp4 significantly increased phosphorylation levels of Tbk1 and Irf3 (
Figure 6A,B). This was further supported by the observations
in vivo that the expression of
tbk1 and
irf3 was significantly downregulated in
bmp4−/− mutant than that in wild-type zebrafish larvae (
Figure 6C,D). This suggests that BMP4 may activate Tbk1-Irf3 antiviral signaling.
BMPs can transmit signals through smad1/5/8, smad2/3, ERK, JNK, or p38 MAPK pathways [
25,
26,
27]. Thus, the effects of p38 MAPK inhibitor SB203580, JNK inhibitor SP600125, MEK1/2 inhibitor U0126, SMAD1/5/8 inhibitor DMH1, and smad2/3 inhibitor TP0427736 HCl on the expression of
ifn was tested in
bmp4-overexpressing EPC cells. Here we found that p38 MAPK inhibitor SB203580 remarkably reduced the expression of
ifn in EPC cells (
Figure 6E). We have shown above that
bmp4 overexpression increased the expression of
ifn (
Figure 4). Therefore, we then evaluated the activation of p38 MAPK by immunoblot assays in both
bmp4-overexpressing and wild-type EPC cells, infected with or without GCRV. It was found that phosphorylation levels of p38 MAPK were significantly increased in the cells with overexpressed
bmp4 (
Figure 6F). Collectively, the data indicate that Bmp4 induces the expression of
ifn through p38 MAPK pathway.
3. Discussion
BMP4 is an extracellular polypeptide signaling molecules of BMP family. In H. sapiens, BMP4 is highly expressed in placenta, urinary bladder, prostate, ovary, colon, and stomach (human ENCODE transcriptome data in NCBI), while Bmp4 mRNA in M. musculus is mainly present in the bladder, lung, kidney and intestine including small intestine, colon and large intestine (mouse ENCODE transcriptome data in NCBI). In this paper, we showed that the zebrafish bmp4 was mainly expressed in the tissues of ovary, testis, skin, gill and eye. The expression patterns of BMP4 appear to differ across species, but it is also clear that BMP4 is expressed in almost all tissues of different species, which supports the multifunctional growth factor of BMP4.
As a model animal,zebrafish is becoming a powerful tool in the research of lots of fields [
20,
21]. In this study, we demonstrated that knockout of
bmp4 in zebrafish results in increasing of the viral load and mortality after infection with GCRV virus in both larvae and adults. Furthermore, the expression of key genes associated with antiviral innate immune were decreased in
bmp4 deficient zebrafish. Our data suggest that zebrafish
bmp4 plays roles in regulation of antiviral innate immune responses. Very recently, we also have demonstrated that Bmp8a is a positive regulator in antiviral immune responses [
19]. In addition, BMP6 was shown to block HCV replication[
17]. Thus, our finding here expands the understanding of the roles of BMP molecules in antiviral immune. It is interesting to uncover whether other members of BMP family have the function in antiviral immune in the future. In fact, the immunoregulatory roles of BMP members have also been reported [
28]. For example, the size of thymus is reduced when BMP2, 4 and 7 were mutant [
16,
17,
29,
30,
31,
32]. Moreover, BMPs, TGFβ, and activins constitute TGF-β superfamily. TGFβ can act in the regulation of inflammatory effects [
33]. Antiviral activity of activins were also been shown [
17]. Therefore, the immunoregulatory function of more members of the TGF-β superfamily would be expected.
Viral infection can be detected by pattern recognition receptors (PRRs) in host cells that trigger the activation of IRF3 and IRF7, thereby inducing the production of type I IFN and IFN-stimulated genes (ISGs) [
34,
35,
36,
37]. Here we demonstrated that Bmp4 increases the expression of antiviral genes
ifn and
mxa in vitro and
in vivo, indicating a role of BMP4 in the antiviral signals. BMPs are known to perform different functions through either the Smad-dependent pathways (SMAD1/5/8 and SMAD2/3 pathways) or the Smad-independent pathways (ERK, JNK, and p38 MAPK pathways). Our study reveals that p38 MAPK pathway may be the main pathway for BMP4 to exert antiviral immunity. We also showed that Bmp4 significantly increased phosphorylation levels of Tbk1 and Irf3. Taken the previous report that the MAPK signaling increased the phosphorylation of TBK1 and IRF3 upon viral infection into consideration [
38], we suggest that Bmp4 activates Tbk1-Irf3-Ifn antiviral signaling via p38 MAPK pathway, eventually resulting in increased synthesis of type I IFN (
Figure 6G). This is similar to the regulatory pathway of BMP8A in antiviral immunity [
19]. However, additional pathways independent of p38 MAPK-Tbk1-Irf3-IFN are not excluded. In fact, BMP6 not only enhances transcription and antiviral response to IFN, but also inhibits HCV replication independently of IFN [
17].
The expression of zebrafish
bmp4 is significantly upregulated after challenge with GCRV and poly(I:C). We have shown that STAT1 can directly bind to the IFN-γ activation sites (GAS) in the
bmp8a promoter, suggesting that
bmp8a can be activated by viruses via the Ifn-γ-Jak-Stat1 pathway [
19,
38,
39,
40]. Multiple GAS sites in
bmp4 putative promoter region were also found when searching for the transcription factor STAT1 binding sites at the web
http://jaspar.genereg.net/ (data not shown). It is highly likely that the transcripts of
bmp4 can be activated by Stat1. Thus, it is possible that
bmp4 can also be activated by viruses via the Ifn-γ-Jak-Stat1 pathway.
4. Materials and Methods
4.1. Cell culture
We bought the epithelioma papulosum cyprini cells (EPC) and zebrafish liver cells (ZFL) from the China Zebrafish Resource Center (CZRC). EPC cells were cultured in MEM media supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin. ZFL cells were maintained in DMEM/F-12 media supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. They were grown at 28 °C in an incubator supplied with 5% of CO2.
4.2. Viruses
Grass carp reovirus (GCRV) were kindly provided by professor Yibing Zhang in Institute of Hydrobiology, Chinese Academy of Sciences. The GCRV is a dsRNA virus. The 50% tissue culture-infective dose (TCID
50) assay was performed to determine the viral titer in EPC cells [
41].
4.3. Sequence comparison and phylogenetic analysis of BMP4 proteins
Multiple BMP4 protein sequences were aligned by the Clustal W method in the DNASTAR software [
42]. The exon-intron information of
BMP4 genes was obtained from the Ensembl database (
http://www.ensembl.org). Using the Sequence Viewer (
http://www.ncbi.nlm.nih.gov/projects/sviewer/) and Ensembl Genome Browser to retrieve the synteny data among BMP4 orthologs in different species. Three-dimensional structure of BMP4 proteins was predicted by SWISS-MODEL program of Expert Protein Analysis System on line (
http://www.expasy.org/) [
43]. MEGA (version 11) software was used to construct the phylogenetic tree with the neighbor-joining method. Sea lamprey Bmp2 was selected as an outgroup for rooting. Bootstrap 1000 times to estimate the confidence of each node.
4.4. Generation of bmp4 mutant zebrafish
The zebrafish
Danio rerio bmp4 knockout mutant lines (
bmp4-/-) were established from the zebrafish AB line using CRISPR/Cas9 technology [
44]. The
bmp4 target in this study was 5′-agtcgagccaacaccgtgag-3′ in the third exon. Mixtures of Cas9 protein and gRNA were microinjected into 1-cell stage zebrafish embryos. Mutant sites were verified by comparison to the WT zebrafish sequences. Heterozygous F1 mutant of the same mutation were crossed to obtain homozygous germline mutants. The primers used in this study were listed in
Table 1.
4.5. Plasmid construction
For the eukaryotic expression, the coding sequence (CDS) of zebrafish
bmp4 was amplified by PCR and cloned into pcDNA3.1(+) vector. The primer sequences were listed in
Table 1.
4.6. Transcriptome Sequencing and Identification of DEGs
Adult zebrafish were randomly divided into wild type group and bmp4
-/- group. Each fish was intraperitoneally injected (i.p.) with 40 μl of GCRV (2.5 x 10
7TCID
50/ml). After 36 h, liver tissue from three fish in each group was collected together as a separate sample for total RNA extraction. Transcriptome sequencing was performed by BioMarker (Beijing, China). RPKM (reads per kb per million reads) method was used to calculate and normalize gene abundance. DEGs (differentially expressed genes) between groups were determined by the edgeR package (
http://www.r-project.org/). Fold change≥2 and false discovery rate (FDR) < 0.05 was considered statistically significant. Then the enrichment analysis of KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway was performed.
4.7. Viral infection in vitro
EPC and ZFL cells were infected with GCRV (106 TCID50 per ml) in the medium without FBS (Gibco), and after 1 h, they continued to be cultured in 2% culture medium. Collect the virus until the cytopathic effect reaches around 70% for subsequent experiments.
4.8. Viral infection in vivo
Zebrafish larvae were cultured in embryo culture medium (CaCl2 0.4 g, KCl 0.3 g, MgSO4 0.79 g, NaCl 10 g in 10 L deionized water). GCRV was added to the embryo culture medium (final concentration 6 x 106TCID50/ml) to infect WT and bmp4-/- zebrafish larvae (4 dpf). In addition, WT and bmp4-/- adult zebrafish were infected with 40 μL of GCRV (2.5 x 107TCID50/ml) per fish by intraperitoneal injection. Total RNA was extracted from adult zebrafish and the expression of viral RNA in liver and spleen was detected by qRT-PCR. The survival of zebrafish infected with GCRV was monitored at different time points as indicated.
4.9. Crystal violet staining
EPC cells were transfected with zebrafish bmp4 and empty vector plasmid respectively. After transfection for 24 h, the cells were challenged with GCRV (106 TCID50/ml). After 48 h of challenge, cells were fixed with 4% formaldehyde for 2 h, followed by staining with 0.5% crystal violet for 2 h. After flushing with water, the experimental results were observed and photographed.
4.10. Inhibitor treatment and analysis
The inhibitors used in this study were: SMAD1/5/8 inhibitor DMH1(Selleck, #S7146), smad2/3 inhibitor TP0427736 HCl (Selleck, #S8700), p38 MAPK inhibitor SB203580 (th, #S1863), JNK inhibitor SP600125 (Beyotime, #S1876), and ERK1/2 inhibitor U0126 (Cell Signaling Technology). They were dissolved in dimethyl ulfoxide (DMSO). EPC cells were treated with inhibitors (SP600125, U0126 and DMH1) in a concentration of 10 μM for 24 h, SB203580 and TP0427736 HCl were used in 5 μM for 24 h. Total RNA was extracted from the cells for qRT-PCR analysis.
4.11. Quantitative Real-time PCR
Total RNA from zebrafish or EPC cells was extracted with the Total RNA Kit I (Omega). RNA was treated with DNase. Then PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, #RP047A) was used to synthesize cDNAs. At the same time, samples without reverse transcriptase were added as controls. To detect the gene expression, the cDNA was amplified with ChamQ SYBR Color qPCR Master Mix (Vazyme, #Q431-02) by ABI 7500 Fast Real-Time PCR System (Applied Biosystems). The expression of zebrafish actb1 or EPC actin was used as the internal reference. The relative expression was calculated using the 2
−ΔΔCt method. All qRT-PCR experiments were triplicate and repeated three times. Primers were listed in
Table 1.
4.12. Western blot and antibodies
Western blot was performed according to the description previously [
19]. In briefly, whole cell lysates were separated by 12.5% SDS-PAGE gels, electrotransferred to PVDF membrane, blocked with 5% fat-free milk solution for 2 h at room temperature, incubated with the primary antibody at 4℃ overnight. Non-specific binding was washed by 1× PBST, and incubated with the second antibody at room temperature for 1 h, and detected by enhanced chemiluminescence (ECL). Antibodies used in this paper were as follows: TBK1 (#3504T) and p-TBK1(Ser172) (#5483T) from Cell Signaling Technology, IRF3 (#bs-2993R), p-IRF3 (Ser386) (#bsm-52170R), p38MAPK (#bs-0637R), p-p38MAPK (Thr180 + Tyr182) (#bs-2210R) and Actin (#bs-0061R) from Bioss and goat anti-rabbit IgG HRP secondary antibody (1:3000, #CW0103S) from CWBIO.
4.13. Statistical analysis
Graphpad Prism 9.0.0 software was used to perform all statistical analyses. Data are expressed as mean±SD. Statistical significance between two groups was analyzed by two-tailed student’s t-test. For the comparison of multiple groups, one-way ANOVA was used for statistical analysis, and then Games-Howell post hoc test was performed. Survival was analyzed using Kaplan-Meier method and evaluated using log-rank (Mantel-Cox) test. *p < 0.05, **p < 0.01, *** p< 0.001; ns, not significant, p > 0.05.
5. Conclusions
Zebrafish Bmp4 was demonstrated to have roles in antiviral innate immune responses in this paper. The transcripts of bmp4 are activated upon viral infection. The bmp4-/- mutant zebrafish significantly increases the viral load and mortality after infection with GCRV virus in both larvae and adults. Furthermore, Bmp4 promotes the phosphorylation level of Tbk1 through p38 MAPK pathway and increases the production of type I IFN. Our study expands the understanding of the function and regulation of BMPs in antiviral immune and may provide new targets to confront viral infection.
Author Contributions
Investigation, methodology, visualization, validation, writing—original draft: L.C.; visualization, validation, writing—review and editing: S.Z., Y.W and X.W.; conceptualization, formal analysis, methodology, visualization, validation, writing—review and editing, project administration, funding acquisition: Z.L. and G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants of National Key Research and Development Project of the Ministry of Science and Technology (SQ2022YFE012560), Science & Technology Innovation Project of Laoshan Laboratory (LSKJ202203204) and Shandong Provincial Natural Science Foundation (ZR2022MC032).
Institutional Review Board Statement
The experimental animals zebrafish follow the ethical guidelines formulated by the Animal Protection and Use Committee of Ocean University of China (permit number, SD2007695).
Data Availability Statement
All relevant data are available from the authors upon request and the corresponding author will be responsible for replying to the request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bragdon, B.; Moseychuk, O.; Saldanha, S.; King, D.; Julian, J.; Nohe, A. Bone morphogenetic proteins: a critical review. Cell Signal 2011, 23, 609–620. [Google Scholar] [CrossRef]
- Crisan, M.; Kartalaei, P.S.; Vink, C.S.; Yamada-Inagawa, T.; Bollerot, K.; van Ijcken, W.; van der Linden, R.; de Sousa Lopes, S.M.C.; Monteiro, R.; Mummery, C.; et al. BMP signalling differentially regulates distinct haematopoietic stem cell types. Nat Commun 2015, 6, 8040. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 2016, 4, 16009. [Google Scholar]
- Xue, Y.; Zheng, X.; Huang, L.; Xu, P.; Ma, Y.; Min, Z.; Tao, Q.; Tao, Y.; Meng, A. Organizer-derived Bmp2 is required for the formation of a correct Bmp activity gradient during embryonic development. Nat Commun 2014, 5, 3766. [Google Scholar] [CrossRef]
- Yu, Y.; Mutlu, A.S.; Liu, H.; Wang, M.C. High-throughput screens using photo-highlighting discover BMP signaling in mitochondrial lipid oxidation. Nat Commun 2017, 8, 865. [Google Scholar] [CrossRef] [PubMed]
- Shore, E.M.; Xu, M.; Shah, P.B.; Janoff, H.B.; Hahn, G.V.; Deardorff, M.A.; Sovinsky, L.; Spinner, N.B.; Zasloff, M.A.; Wozney, J.M.; et al. The human bone morphogenetic protein 4 (BMP-4) gene: molecular structure and transcriptional regulation. Calcif Tissue Int 1998, 63, 221–229. [Google Scholar] [CrossRef]
- Bakrania, P.; Efthymiou, M.; Klein, J.C.; Salt, A.; Bunyan, D.J.; Wyatt, A.; Ponting, C.P.; Martin, A.; Williams, S.; Lindley, V.; et al. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet 2008, 82, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Mao, Y.; Gao, P. The Role of Bone Morphogenetic Protein 4 in Lung Diseases. Curr Mol Med 2023, 23, 324–331. [Google Scholar] [CrossRef]
- Godoy-Parejo, C.; Deng, C.; Xu, J.; Zhang, Z.; Ren, Z.; Ai, N.; Liu, W.; Ge, W.; Deng, C.; Xu, X.; et al. Protein Kinase C Modulation Determines the Mesoderm/Extraembryonic Fate Under BMP4 Induction From Human Pluripotent Stem Cells. Stem Cells 2023, 41, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, D.; Kunishige, R.; Taguchi, Y.; Shinozaki-Narikawa, N.; Osaka, K.; Yokomizo, K.; Ishida, M.; Takei, S.; Yamasaki, S.; Hagiya, K.; et al. BMP4-SMAD1/5/9-RUNX2 pathway activation inhibits neurogenesis and oligodendrogenesis in Alzheimer's patients' iPSCs in senescence-related conditions. Stem Cell Reports 2023, 18, 1246. [Google Scholar] [CrossRef]
- Huber, S.; Schramm, C. Role of activin A in the induction of Foxp3+ and Foxp3- CD4+ regulatory T cells. Crit Rev Immunol 2011, 31, 53–60. [Google Scholar] [PubMed]
- Martínez, V.G.; Hernández-López, C.; Valencia, J.; Hidalgo, L.; Entrena, A.; Zapata, A.G.; Vicente, A.; Sacedón, R.; Varas, A. The canonical BMP signaling pathway is involved in human monocyte-derived dendritic cell maturation. Immunol Cell Biol 2011, 89, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.G.; Sacedón, R.; Hidalgo, L.; Valencia, J.; Fernández-Sevilla, L.M.; Hernández-López, C.; Vicente, A.; Varas, A. The BMP Pathway Participates in Human Naive CD4+ T Cell Activation and Homeostasis. PLoS One 2015, 10, e0131453. [Google Scholar] [CrossRef]
- Phillips, D.J.; de Kretser, D.M.; Hedger, M.P. Activin and related proteins in inflammation: not just interested bystanders. Cytokine Growth Factor Rev 2009, 20, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Seeger, P.; Musso, T.; Sozzani, S. The TGF-β superfamily in dendritic cell biology. Cytokine Growth Factor Rev 2015, 26, 647–657. [Google Scholar] [CrossRef]
- Takabayashi, H.; Shinohara, M.; Mao, M.; Phaosawasdi, P.; El-Zaatari, M.; Zhang, M.; Ji, T.; Eaton, K.A.; Dang, D.; Kao, J.; et al. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology 2014, 147. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, L.A.; Al-Hourani, K.; Ramamurthy, N.; Frankish, J.; Baddock, H.T.; Sandor, C.; Ryan, J.D.; Fusco, D.N.; Arezes, J.; Giannoulatou, E.; et al. Antiviral activity of bone morphogenetic proteins and activins. Nat Microbiol 2019, 4, 339–351. [Google Scholar] [CrossRef]
- Olsavszky, V.; Ulbrich, F.; Singh, S.; Diett, M.; Sticht, C.; Schmid, C.D.; Zierow, J.; Wohlfeil, S.A.; Schledzewski, K.; Dooley, S.; et al. GATA4 and LMO3 balance angiocrine signaling and autocrine inflammatory activation by BMP2 in liver sinusoidal endothelial cells. Gene 2017, 627, 491–499. [Google Scholar] [CrossRef]
- Zhong, S.; Li, H.; Wang, Y.-S.; Wang, Y.; Ji, G.; Li, H.-Y.; Zhang, S.; Liu, Z. Bmp8a is an essential positive regulator of antiviral immunity in zebrafish. Commun Biol 2021, 4, 318. [Google Scholar] [CrossRef]
- Hölttä-Vuori, M.; Salo, V.T.V.; Nyberg, L.; Brackmann, C.; Enejder, A.; Panula, P.; Ikonen, E. Zebrafish: gaining popularity in lipid research. Biochem J 2010, 429, 235–242. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Z. Receptor, signal transduction and evolution of sweet, umami and bitter taste. Marine Life Science & Technology 2020, 2, 6–15. [Google Scholar] [CrossRef]
- Aggad, D.; Mazel, M.; Boudinot, P.; Mogensen, K.E.; Hamming, O.J.; Hartmann, R.; Kotenko, S.; Herbomel, P.; Lutfalla, G.; Levraud, J.-P. The two groups of zebrafish virus-induced interferons signal via distinct receptors with specific and shared chains. J Immunol 2009, 183, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Robertsen, B. The interferon system of teleost fish. Fish Shellfish Immunol 2006, 20, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Jiang, J.; Chen, Y.-D.; Zhu, R.; Shi, Y.; Zhang, Q.-Y.; Gui, J.-F. The innate immune response to grass carp hemorrhagic virus (GCHV) in cultured Carassius auratus blastulae (CAB) cells. Dev Comp Immunol 2007, 31, 232–243. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Oh, S.P.; Yeo, C.-Y.; Lee, Y.; Schrewe, H.; Whitman, M.; Li, E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev 2002, 16, 2749–2754. [Google Scholar] [CrossRef]
- Drummond, A.E. TGFbeta signalling in the development of ovarian function. Cell Tissue Res 2005, 322, 107–115. [Google Scholar] [CrossRef]
- Tu, E.; Chia, P.Z.C.; Chen, W. TGFβ in T cell biology and tumor immunity: Angel or devil? Cytokine Growth Factor Rev 2014, 25, 423–435. [Google Scholar] [CrossRef]
- Bleul, C.C.; Boehm, T. BMP signaling is required for normal thymus development. J Immunol 2005, 175, 5213–5221. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Pardoux, C.; Hall, M.C.; Lee, P.S.; Warburton, D.; Qing, J.; Smith, S.M.; Derynck, R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J 2007, 26, 3957–3967. [Google Scholar] [CrossRef]
- Gordon, J.; Patel, S.R.; Mishina, Y.; Manley, N.R. Evidence for an early role for BMP4 signaling in thymus and parathyroid morphogenesis. Dev Biol 2010, 339, 141–154. [Google Scholar] [CrossRef]
- Aleman-Muench, G.R.; Soldevila, G. When versatility matters: activins/inhibins as key regulators of immunity. Immunol Cell Biol 2012, 90, 137–148. [Google Scholar] [CrossRef]
- Worthington, J.J.; Fenton, T.M.; Czajkowska, B.I.; Klementowicz, J.E.; Travis, M.A. Regulation of TGFβ in the immune system: an emerging role for integrins and dendritic cells. Immunobiology 2012, 217, 1259–1265. [Google Scholar] [CrossRef]
- Honda, K.; Yanai, H.; Negishi, H.; Asagiri, M.; Sato, M.; Mizutani, T.; Shimada, N.; Ohba, Y.; Takaoka, A.; Yoshida, N.; et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005, 434, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yanai, H.; Savitsky, D.; Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 2008, 26, 535–584. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hofer, M.J.; Jung, S.R.; Lim, S.-L.; Campbell, I.L. IRF7-dependent type I interferon production induces lethal immune-mediated disease in STAT1 knockout mice infected with lymphocytic choriomeningitis virus. J Virol 2014, 88, 7578–7588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Ding, X.; Wan, X.; Liu, L.; Yuan, X.; Zhang, W.; Hui, X.; Meng, G.; Xiao, H.; Li, B.; et al. MLL5 suppresses antiviral innate immune response by facilitating STUB1-mediated RIG-I degradation. Nat Commun 2018, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Du, L.; Lv, D.; Li, H.; Shang, J.; Lu, J.; Zhou, L.; Bai, L.; Tang, H. Exosomal Interferon-Induced Transmembrane Protein 2 Transmitted to Dendritic Cells Inhibits Interferon Alpha Pathway Activation and Blocks Anti-Hepatitis B Virus Efficacy of Exogenous Interferon Alpha. Hepatology 2019, 69, 2396–2413. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu Rev Immunol 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Ruan, B.Y.; Chen, S.N.; Hou, J.; Huang, B.; Laghari, Z.A.; Li, L.; Nie, P. Two type II IFN members, IFN-γ and IFN-γ related (rel), regulate differentially IRF1 and IRF11 in zebrafish. Fish Shellfish Immunol 2017, 65, 103–110. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A SIMPLE METHOD OF ESTIMATING FIFTY PER CENT ENDPOINTS12. American Journal of Epidemiology 1938, 3. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR's Lasergene sequence analysis software. Methods Mol Biol 2000, 132, 71–91. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Zhang, Y.; Lin, S.; Shi, D.-L.; Shao, M. Highly efficient genome editing using oocyte-specific zcas9 transgenic zebrafish. J Genet Genomics 2018, 45, 509–512. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Bioinformatic analysis of D. rerio bmp4. A. Phylogenetic tree of BMP4 proteins was constructed by MEGA (version 11) using the neighbor-joining method and sea lamprey Bmp2 was rooted as an outgroup. The reliability of each node was estimated by bootstrapping with 1000 replications. The numbers shown at each node indicate the bootstrap values (%). The bars represent the distance. B. Multiple alignment of zebrafish Bmp4 and other known BMP4 amino acid sequences using the Clustal W program within the MegAlign of the DNASTAR software package. Shaded residues are the amino acids that match the consensus. (C) Sequence similarity and sequence divergence of the BMP4 proteins in different species. Numbers in the table are calculated with the method of Clustal W in the software package DNASTAR. (D) Schematic representation of the gene organization comparisons of BMP4 genes in different species. Exons are indicated with boxes and introns are represented as horizontal lines. (E) The three dimensional structures of D. rerio Bmp4, H. sapiens BMP4 and M. musculus Bmp4. This diagram was generated by SWISS-MODEL online software. (F) Synteny map of the genomic segment where BMP4 genes in different species. Genes are represented by boxes. Transcription direction is indicated by arrow. GenBank accession numbers: Homo sapiens BMP4 (NP_001193.2); Mus musculus Bmp4 (NP_031580.2); Gallus gallus Bmp4 (NP_990568.4); Xenopus tropicalis Bmp4 (NP_001017034.2); Cyprinus carpio Bmp4 (XP_042630442.1); Callorhinchus milii Bmp4 (XP_007886339); Petromyzon marinus Bmp2 (XP_032811632).
Figure 1.
Bioinformatic analysis of D. rerio bmp4. A. Phylogenetic tree of BMP4 proteins was constructed by MEGA (version 11) using the neighbor-joining method and sea lamprey Bmp2 was rooted as an outgroup. The reliability of each node was estimated by bootstrapping with 1000 replications. The numbers shown at each node indicate the bootstrap values (%). The bars represent the distance. B. Multiple alignment of zebrafish Bmp4 and other known BMP4 amino acid sequences using the Clustal W program within the MegAlign of the DNASTAR software package. Shaded residues are the amino acids that match the consensus. (C) Sequence similarity and sequence divergence of the BMP4 proteins in different species. Numbers in the table are calculated with the method of Clustal W in the software package DNASTAR. (D) Schematic representation of the gene organization comparisons of BMP4 genes in different species. Exons are indicated with boxes and introns are represented as horizontal lines. (E) The three dimensional structures of D. rerio Bmp4, H. sapiens BMP4 and M. musculus Bmp4. This diagram was generated by SWISS-MODEL online software. (F) Synteny map of the genomic segment where BMP4 genes in different species. Genes are represented by boxes. Transcription direction is indicated by arrow. GenBank accession numbers: Homo sapiens BMP4 (NP_001193.2); Mus musculus Bmp4 (NP_031580.2); Gallus gallus Bmp4 (NP_990568.4); Xenopus tropicalis Bmp4 (NP_001017034.2); Cyprinus carpio Bmp4 (XP_042630442.1); Callorhinchus milii Bmp4 (XP_007886339); Petromyzon marinus Bmp2 (XP_032811632).
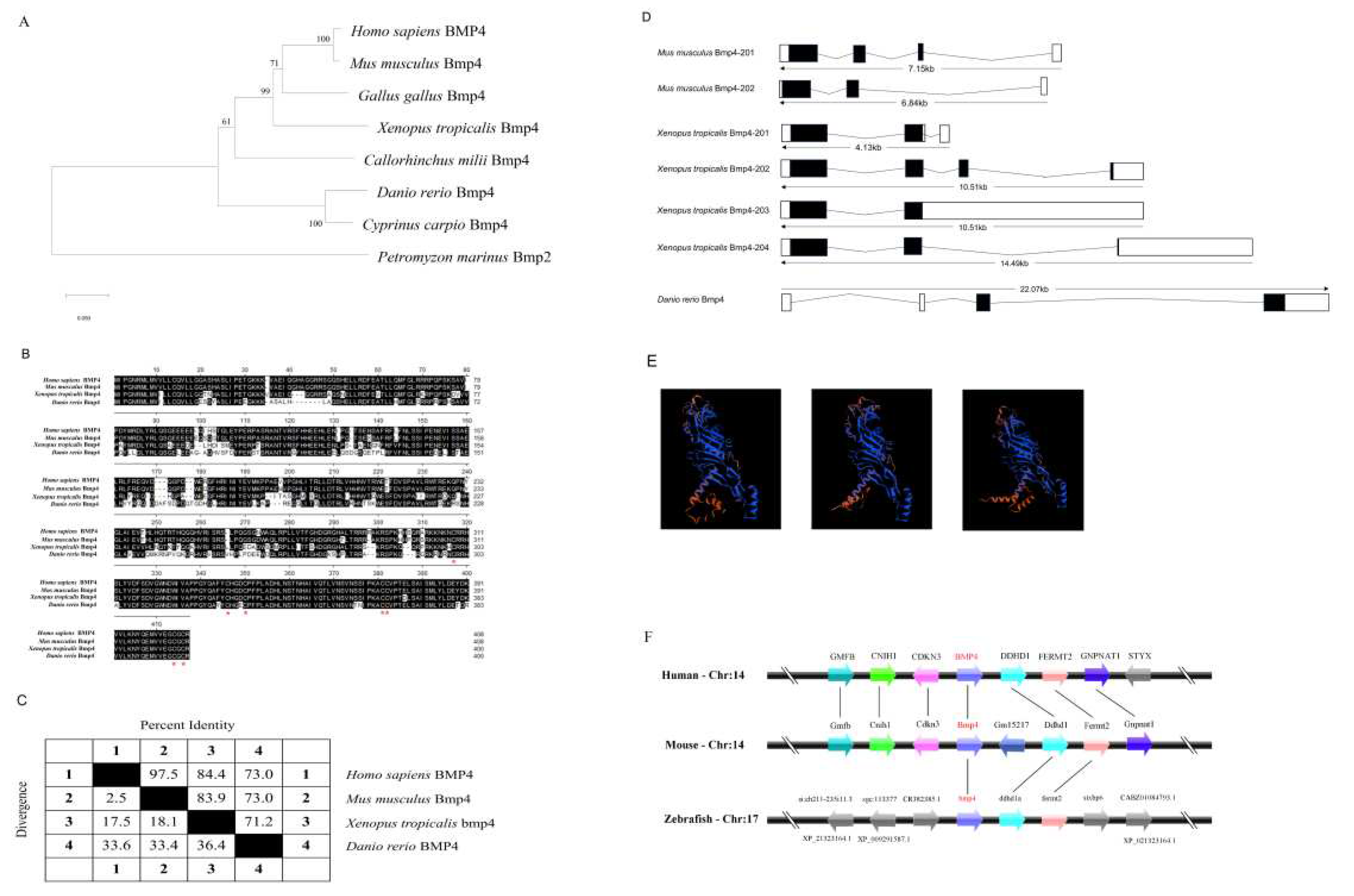
Figure 2.
The bmp4 expression was increased after virus or poly(I:C) challenge. A. Distribution of bmp4 mRNA in different tissues of adult zebrafish. B. The expression of bmp4 in the gill, liver, spleen, kidney, and intestine from zebrafish challenged with GCRV. Zebrafish injected i.p. with MEM were used as the control. C, D. Expression of bmp4 mRNA in the ZFL cells challenged with poly(I:C) (C) or GCRV virus (D). ZFL cells challenged with PBS (C) or MEM (D) were used as the control. The expression of actb1 served as an internal control for the qRT-PCR. Data were from three independent experiments and were analyzed by Student’s t-test (two-tailed). All data were presented as mean ± SD (**p < 0.01, ***p < 0.001).
Figure 2.
The bmp4 expression was increased after virus or poly(I:C) challenge. A. Distribution of bmp4 mRNA in different tissues of adult zebrafish. B. The expression of bmp4 in the gill, liver, spleen, kidney, and intestine from zebrafish challenged with GCRV. Zebrafish injected i.p. with MEM were used as the control. C, D. Expression of bmp4 mRNA in the ZFL cells challenged with poly(I:C) (C) or GCRV virus (D). ZFL cells challenged with PBS (C) or MEM (D) were used as the control. The expression of actb1 served as an internal control for the qRT-PCR. Data were from three independent experiments and were analyzed by Student’s t-test (two-tailed). All data were presented as mean ± SD (**p < 0.01, ***p < 0.001).
Figure 3.
Antiviral function of Bmp4. A. Overexpressing bmp4 significantly decreased the viral titers in EPC cells. EPC cells were transfected with bmp4 or empty vector, and 24 h later the cells were infected with GCRV (106TCID50/ml). The cells and culture supernatants were collected at 24 h post-infection and the viral titers were determined by TCID50 assays. B. The generation bmp4 mutation zebrafish using CRISPR/Cas9 technology. a. The knockout gene target is located in exon 3. The black box represents exons. The fold line represents introns. The white square represents un-translated regions. b and c. Compared with the wild type, 1 nucleotide was replaced by 11 nucleotides in the exon 3 of bmp4 in the mutant, leading to early termination of translation to the 118th amino acid. C. Kaplan–Meier analysis of the overall survival of WT (n = 30) or bmp4-/- zebrafish larvae (n = 30) which were infected by adding GCRV into the embryo culture medium (final 6 x 106TCID50/ml) and monitored every 6 h after infection. D,E. The symptoms of adult zebrafish which were infected with the virus of GCRV. And Kaplan–Meier analysis of the overall survival of WT (n = 30) or bmp4-/- zebrafish (n = 30) which were injected i.p. with 40 µl of GCRV (2.5 x 107TCID50/ml) and monitored everyday after infection. F, G. The expression of GCRV RNA in the liver and spleen from wild-type (WT) or bmp4-/- zebrafish injected i.p. with 40 µl of GCRV (2.5 x 107TCID50/ml). The expression of zebrafish actb1 was used as an internal control for the qRT-PCR. Data were from three independent experiments. Data were analyzed by Student’s t-test (two-tailed) and were presented as mean ± SD (*p < 0.05, **p < 0.01, ***p < 0.001).
Figure 3.
Antiviral function of Bmp4. A. Overexpressing bmp4 significantly decreased the viral titers in EPC cells. EPC cells were transfected with bmp4 or empty vector, and 24 h later the cells were infected with GCRV (106TCID50/ml). The cells and culture supernatants were collected at 24 h post-infection and the viral titers were determined by TCID50 assays. B. The generation bmp4 mutation zebrafish using CRISPR/Cas9 technology. a. The knockout gene target is located in exon 3. The black box represents exons. The fold line represents introns. The white square represents un-translated regions. b and c. Compared with the wild type, 1 nucleotide was replaced by 11 nucleotides in the exon 3 of bmp4 in the mutant, leading to early termination of translation to the 118th amino acid. C. Kaplan–Meier analysis of the overall survival of WT (n = 30) or bmp4-/- zebrafish larvae (n = 30) which were infected by adding GCRV into the embryo culture medium (final 6 x 106TCID50/ml) and monitored every 6 h after infection. D,E. The symptoms of adult zebrafish which were infected with the virus of GCRV. And Kaplan–Meier analysis of the overall survival of WT (n = 30) or bmp4-/- zebrafish (n = 30) which were injected i.p. with 40 µl of GCRV (2.5 x 107TCID50/ml) and monitored everyday after infection. F, G. The expression of GCRV RNA in the liver and spleen from wild-type (WT) or bmp4-/- zebrafish injected i.p. with 40 µl of GCRV (2.5 x 107TCID50/ml). The expression of zebrafish actb1 was used as an internal control for the qRT-PCR. Data were from three independent experiments. Data were analyzed by Student’s t-test (two-tailed) and were presented as mean ± SD (*p < 0.05, **p < 0.01, ***p < 0.001).
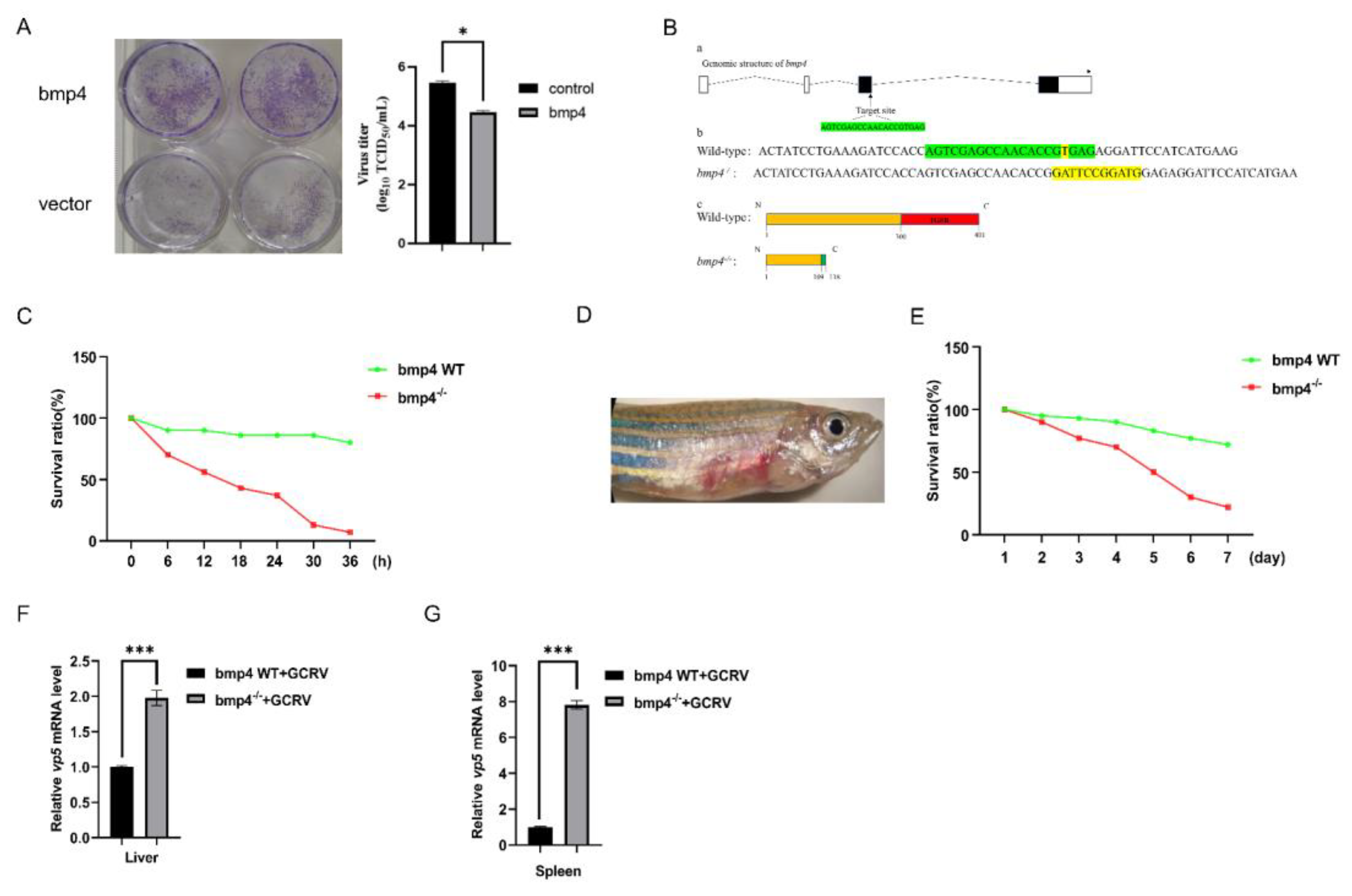
Figure 4.
Bmp4 promotes the expression of antiviral genes. A, B. Expression of EPC ifn and EPC mx mRNA after transfected with bmp4 (2 μg) or empty vector (2 μg) in EPC cells. The cells were collected at 24 h or 36 h post transfection. C,D. Expression of EPC ifn and EPC mx mRNA after transfected with bmp4 (2 μg) or empty vector (2 μg) in EPC cells for 24 h, followed by infection with GCRV for another 24 h or 48 h. E, F. Expression of ifnφ1 and mxa mRNA from WT or bmp4-/- zebrafish larvae challenged with GCRV for 24 h or 36 h. G, H. Expression of ifnφ1 and mxa mRNA in the liver and spleen from WT or bmp4-/- adult zebrafish challenged by injecting i.p. with 40 µl of GCRV (2.5 x 107TCID50/ml) for 36 h. The expression of zebrafish actb1 or EPC actin was used as an internal control for the qRT-PCR. Data were from three independent experiments and were analyzed by Student’s t-test (two-tailed) for comparison of two groups. All data were presented as mean ± SD (*p < 0.05, **p < 0.01, ***p < 0.001).
Figure 4.
Bmp4 promotes the expression of antiviral genes. A, B. Expression of EPC ifn and EPC mx mRNA after transfected with bmp4 (2 μg) or empty vector (2 μg) in EPC cells. The cells were collected at 24 h or 36 h post transfection. C,D. Expression of EPC ifn and EPC mx mRNA after transfected with bmp4 (2 μg) or empty vector (2 μg) in EPC cells for 24 h, followed by infection with GCRV for another 24 h or 48 h. E, F. Expression of ifnφ1 and mxa mRNA from WT or bmp4-/- zebrafish larvae challenged with GCRV for 24 h or 36 h. G, H. Expression of ifnφ1 and mxa mRNA in the liver and spleen from WT or bmp4-/- adult zebrafish challenged by injecting i.p. with 40 µl of GCRV (2.5 x 107TCID50/ml) for 36 h. The expression of zebrafish actb1 or EPC actin was used as an internal control for the qRT-PCR. Data were from three independent experiments and were analyzed by Student’s t-test (two-tailed) for comparison of two groups. All data were presented as mean ± SD (*p < 0.05, **p < 0.01, ***p < 0.001).
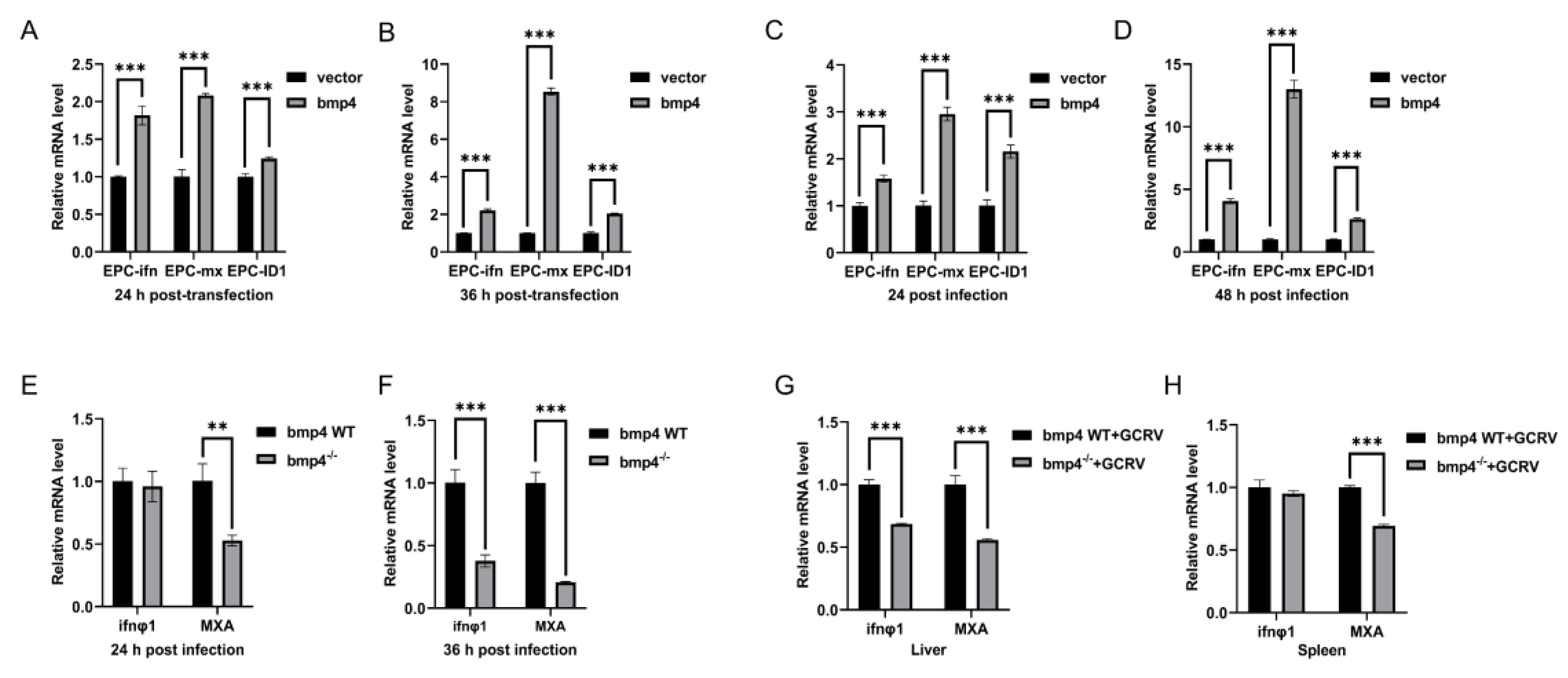
Figure 5.
KEGG analysis of the pathways by enrichment of DEGs between bmp4-/- and WT liver tissue of zebrafish challenged by GCRV. Rich factor is the ratio of differentially expressed gene numbers annotated in this pathway terms to all gene numbers annotated in this pathway terms. The q-value means corrected p-value, q < 0.05 as significantly enriched.
Figure 5.
KEGG analysis of the pathways by enrichment of DEGs between bmp4-/- and WT liver tissue of zebrafish challenged by GCRV. Rich factor is the ratio of differentially expressed gene numbers annotated in this pathway terms to all gene numbers annotated in this pathway terms. The q-value means corrected p-value, q < 0.05 as significantly enriched.
Figure 6.
Bmp4 increases Tbk1-Irf3 antiviral signaling via p38 MAPK pathway. A, B. Immunoblot analysis of phosphorylated (p-) Tbk1 (A) and Irf3 (B) was performed 24 h after transfection of 2 μg bmp4 or empty vector in EPC cells, followed by infection with or without GCRV for 24 h. C, D. Expression of tbk1 and irf3 mRNA from WT or bmp4-/- zebrafish larvae challenged with GCRV. E. Expression of EPC ifn mRNA 24 h after transfection of bmp4 (2 μg) in EPC cells, followed by treatment with SB203580, SP600125, U0126, DMH1 and TP0427736 HCl for 24 h. F. Representative western blot analysis in p-p38 MAPK expression in bmp4-overexpressed EPC cells. G. Schematic illustration of the regulation of Bmp4 in the antiviral immune. The Bmp4 promotes phosphorylation of Tbk1 and Irf3 to induce the expression of ifn through p38 MAPK pathway. The expression of zebrafish actb1 or EPC actin was used as an internal control for the qRT-PCR. Data were from three independent experiments and were analyzed by Student’s t-test (two-tailed) for comparison of two groups or one-way ANOVA followed by Games-Howell post hoc tests for comparison of multiple groups. All data were presented as mean ± SD (**p < 0.01, ***p < 0.001).
Figure 6.
Bmp4 increases Tbk1-Irf3 antiviral signaling via p38 MAPK pathway. A, B. Immunoblot analysis of phosphorylated (p-) Tbk1 (A) and Irf3 (B) was performed 24 h after transfection of 2 μg bmp4 or empty vector in EPC cells, followed by infection with or without GCRV for 24 h. C, D. Expression of tbk1 and irf3 mRNA from WT or bmp4-/- zebrafish larvae challenged with GCRV. E. Expression of EPC ifn mRNA 24 h after transfection of bmp4 (2 μg) in EPC cells, followed by treatment with SB203580, SP600125, U0126, DMH1 and TP0427736 HCl for 24 h. F. Representative western blot analysis in p-p38 MAPK expression in bmp4-overexpressed EPC cells. G. Schematic illustration of the regulation of Bmp4 in the antiviral immune. The Bmp4 promotes phosphorylation of Tbk1 and Irf3 to induce the expression of ifn through p38 MAPK pathway. The expression of zebrafish actb1 or EPC actin was used as an internal control for the qRT-PCR. Data were from three independent experiments and were analyzed by Student’s t-test (two-tailed) for comparison of two groups or one-way ANOVA followed by Games-Howell post hoc tests for comparison of multiple groups. All data were presented as mean ± SD (**p < 0.01, ***p < 0.001).
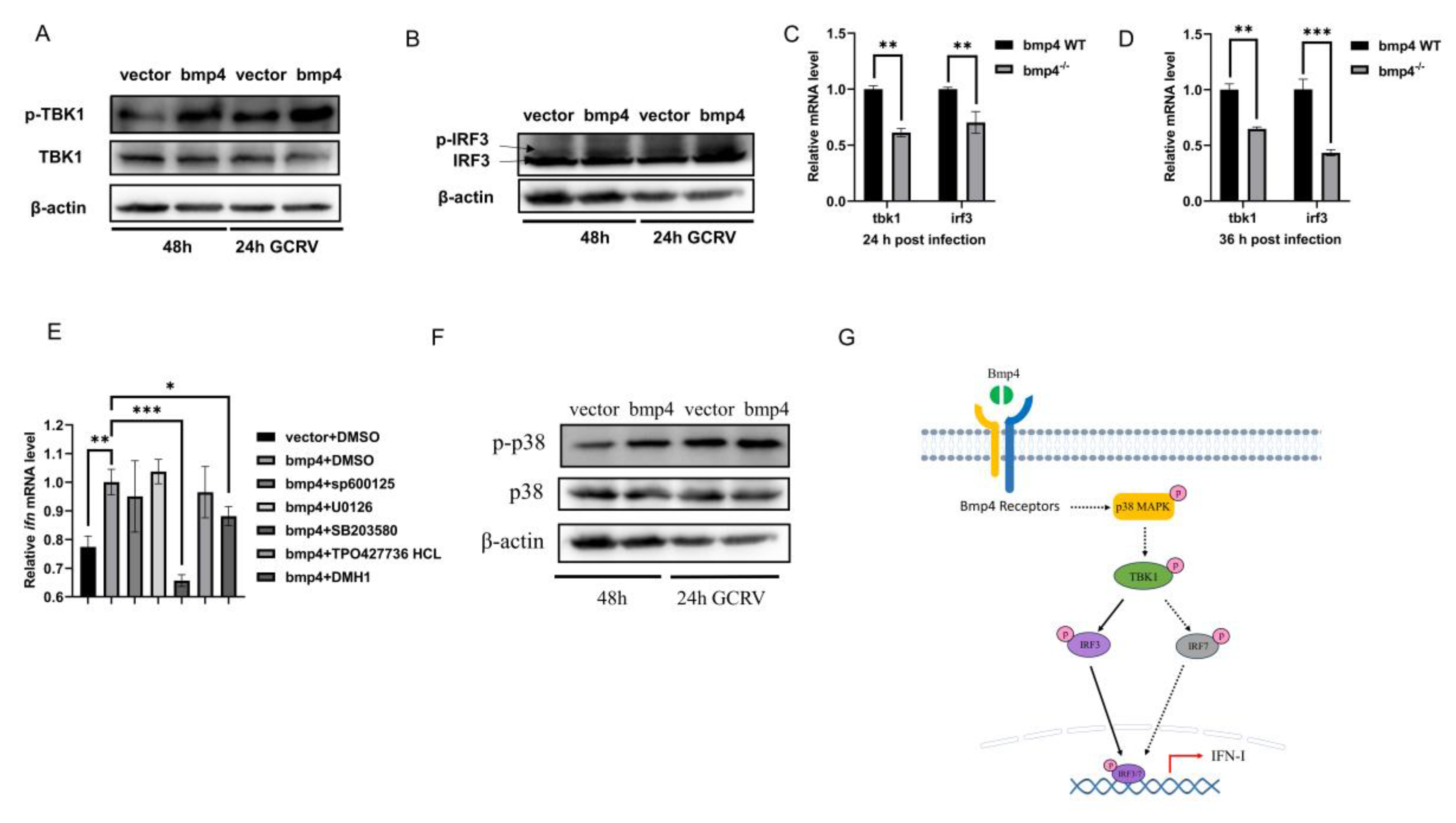
Table 1.
Sequences and applications of the primers used in this study.
Table 1.
Sequences and applications of the primers used in this study.
| Primer name |
Primer sequence (5'--3') |
Application |
| EPC-bmp4-F |
GTAGGCTGGAACGACTGGATTG |
|
| EPC-bmp4-R |
GCGTGATTGGTGGAGTTGAGA |
|
| zebrafish-bmp4-F |
CGCAGCCCTAAACAAAGAG |
|
| zebrafish-bmp4-R |
TGATTGGTGGAGTTGAGATGAT |
|
| GCRV-vp5-F |
CTCCCCGTGAGCGTATTT |
|
| GCRV-vp5-R |
GTTAGCAGCGGTAGTGACTTG |
|
| EPC-ifn-F |
ATAGACAACGCTAAGGTGGAGG |
|
| EPC-ifn-R |
TTCCGACGACTGCCTGTTC |
|
| EPC-mx-F |
GGGAGAAGGGATCAGTCATG |
|
| EPC-mx-R |
GGTTTAGTCAGAATACCGAGGG |
|
| EPC-ID1-F |
GATGTTGTCCGCTGCCTCT |
qRT-PCR |
| EPC-ID1-R |
CATGGTCATTTGCTCGTCC |
|
| EPC-tbk1-F |
TCAGAAGTTTGAGAACGGGAAGA |
|
| EPC-tbk1-R |
CGTAGACCACGATGCGGTGTAAG |
|
| EPC-irf3-F |
AACAAGAATGACACTGCGGA |
|
| EPC-irf3-R |
AACTCGGGAGGGACTTTCAT |
|
| ZFL-ifnφ1-F |
GTGGAGGACCAGGTGAAGTT |
|
| ZFL-ifnφ1-R |
GATTGACCCTTGCGTTGC |
|
| ZFL-MXA-F |
ATGGCTGGAGCAGGTGTT |
|
| ZFL-MXA-R |
TCTGTGGTGGCGATGTCA |
|
| EPC-actin-F |
TGTTCCAGCCATCCTTCTTG |
|
| EPC-actin-R |
TGATTTTCATTGTGCTGGGG |
|
| ZFL-actin-F |
GGTATTGTGATGGACTCTGGTGAT |
|
| ZFL-actin-R |
TCGGCTGTGGTGGTGAAG |
|
| zebrafish-bmp4-F |
ATGATTCCTGGTAATCGAATGCTG |
cDNA cloning |
| zebrafish-bmp4-R |
TTAGCGGCAGCCACACCC |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).