1. Introduction
Sulfur is a by-product of waste from gas production and oil facilities. Predicting global sulfur production (according to the documents of the United States Geological Survey (USGS) [
1]) makes it important to seriously consider various alternative uses of sulfur as well as its complete utilization (disposal) [
2]. Based on the data of 2018, the total global production of sulfur was around 80 tons [
3]. China has been the main a contributor to sulfur as a by-product, due to an increase in the number of gas processing and refineries plants. The overall sulfur production in China was around 17 million tons [
3]. USA was the second largest producer of sulfur, with production capacity around 9.7 million tons, Russia, Saudi Arabia, and Canada produces 7.1 million tons, 6 million ton and 5.5 million tons respectively. In addition, each of Japan and Kazakhstan produces 3.5 million tons [
3].
Sulfur industry is produced primarily as a by-product, so it is a unique industry compared to other mineral industries. Demand and supply of sulfur are not constant and are not balanced. For example, in the past decade, the production of sulfur was much greater than the demand for this product. Globally, currently sulfur is almost exclusively an involuntary by-product of crude oil and sour gas processing to decrease sulfur dioxide emissions from the combustion of fossil-based energy sources [
4]. One of the most common compounds of sulfur is hydrogen sulfide (H
2S). This toxic hydrogen sulfide, among several sulfuric-based compounds, exists in sour gas. These compounds are removed by a stripping process, with subsequent transformation of hydrogen sulfide to elemental sulfur. Also, there are many organic sulfur species in crude oil in the range varies from 1% to 3%. As a result of burning these organic sulfur species, anthropogenic sulfur emissions contribute to serious pollution problems such as acid rain, air pollution, and smog. Furthermore, the planetary albedo and cloud cover increase because of sulfate aerosols [
5]. In the troposphere this taking apart in climate cooling, and thus slowing down the global warming phenomena [
6]. This has even led to propositions to use sulfuric acid/SO
2 for geoengineering to mitigate climate change.
Geopolymer or alkali activated materials are [
7,
8,
9,
10] prepared through the alkali activation of aluminum silicates precursors, where these aluminum silicates are transformed through the geopolymerisation reactions to stable and hard products of a tectosilicate nature [
11]. Geopolymers have attractive and unique characteristics such as hardening at low temperature or even at ambient conditions, excellent mechanical properties, green processing with low gas emission and low energy consumptions. They can be used in many important applications. Geopolymer-based materials are used in waste recycling and circular economy [
12], stabilization of toxic elements, water purification [
13], and passive cooling systems [
14]. However, geopolymers precursors and processing is more costly than ordinary cement. On of the main components of geopolymers is alkaline solution, which is entails certain difficulties in field applications. To overcome these challenges, researchers have worked to develop novel strategies for preparation of multi-functional and green geopolymer-based materials, which can be used for different applications at the same time such as construction and water purification purposes [
15,
16].
Geopolymers, inorganic polymers, have unique and attractive characteristics, and have been extensively investigated for the stabilization/solidification of hazardous metallic and organic pollutants. The mechanism and efficiency of stabilization and immobilization of metal ions such as Cu (II), Cd (II), Pb (II), and Cr (III) in the geopolymer matrix was studied [
17], and the results showed that these metals could be effectively stabilized and immobilized by geopolymerization. Several studies [
18,
19,
20] reported that solid waste, such as coal fly ash and slags, can be used as precursors or raw materials in geopolymer technology, concluding that these industrial wastes can be safely stabilized and capsulated through geopolymer transformation, or can be used for stabilization/solidification of other hazardous waste. Therefore, geopolymer technology is characterized by its unique advantages for the stabilization and immobilization of hazardous pollutants [
11]. The immobilization process for these ions is mainly based on the mechanisms of physical encapsulation, precipitation, sorption, and chemical bonding with lattice charges. In addition, geopolymers or alkali activated materials have also been used for the stabilization/solidification of anionic pollutants, such as As oxyanions, Se oxyanions, and Cr oxyanions [
21]. It has been confirmed that As, or Se oxyanions can be associated in geopolymer matrix through electrostatic interaction, despite that the leaching of this elements are still higher compared to cationic metals.
To date, the effect of sulfur on the geopolymer has not been explored, despite its low cost and availability. Therefore, this study aims to investigate the effects of sulfur incorporation in geopolymers. The significance of this research is improving the properties of geopolymers, reducing their production costs, and exploring new applications for both sulfur and geopolymers.
2. Materials and Methods
2.1. Materials
Cement of geopolymer was synthesized by using kaolinitic soil and NaOH solution, and Na
2SiO
3 solution. The kaolinitic soil sample was collected from a deposit in Riyadh region (Saudi Arabia) with the assistance of Saudi Ceramic Company. The chemical composition of the kaolinitic soil is reported in
Table 1. The estimated kaolinite mass percentage in the precursor is 92% [
14]. The kaolinitic soil was heated in a furnace at 750 °C for 4 hours (Nabertherm, USA) to obtain amorphous kaolin, (i.e., metakaolin).
For geopolymerization, alkali activators (Na
2SiO
3 and NaOH) solutions, which dissolve aluminosilicates, were prepared. The solution of sodium hydroxide (NaOH), with 6 mol/L (M), was prepared by using pure pellets of sodium hydroxide (Merck, Germany) and deionized water. The Na
2SiO
3 solution consisted of 27 wt. % SiO
2, and 8 wt. % Na
2O [
22,
23].
The sulfur sample was prepared as a by-product from operations including petroleum refining, heavy oil, and natural gas processing. The purity of sample is around 99.9%. It was collected from the National Company for Sulphur Products (Riyadh, Saudi Arabia).
2.2. Preparation of geopolymers
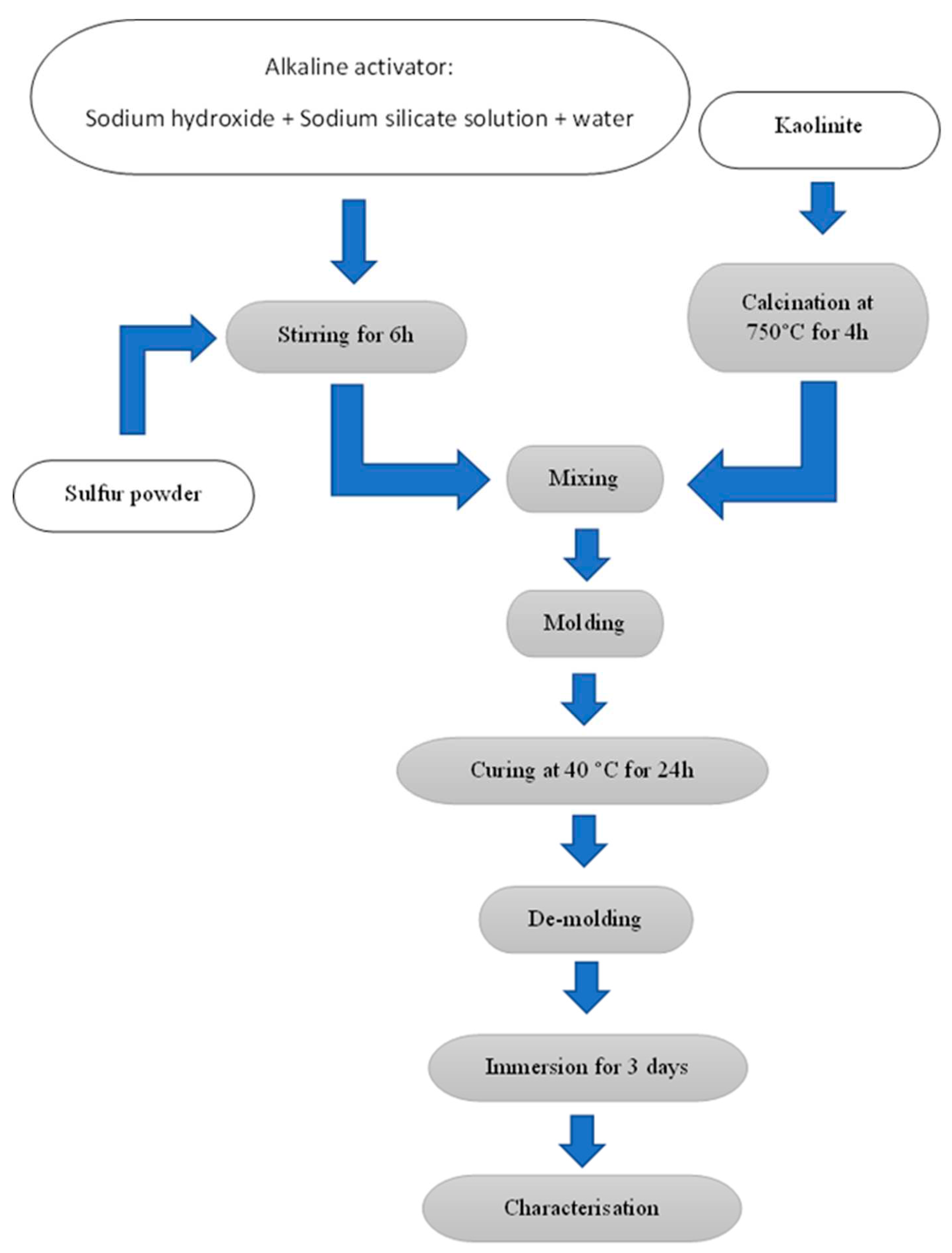
The alkali solutions of sodium hydroxide (NaOH) and sodium silicates ( Na
2SiO
3) were mixed using Si/Na/Al with molar ratio of 2/1/1 [
24] . The source of SiO
2 is the sodium silicate (Na
2SiO
3) solution, sodium oxide (Na
2O) resulting from the alkali activator solutions, and Aluminum oxide (Al
2O
3) from the calcinated kaolinite. The molar ratio of H
2O/Na
2O in the alkaline solution was 6/1. To prepare the aqueous solution of alkali, NaOH, H
2O, and Na
2SiO
3 were initially stirred for 5 minutes. Sulfur was grinded at size below 425 µm, then dissolved into the alkaline solution by magnetic stirring for 6 hours. The metakaolin were added to the sulfur- alkali solution, then mechanically mixed for 10 minutes. Lastly, the resultant mixture of geopolymer was cast into silicon molds, 20mm×20mm×40mm, sealed, and left in an oven (Raypa, Spain) at 40 °C for 1 day for curing. Afterward, the specimens were demolded and immersed for 3 days to evaluate the overall stability in presence of water. Finally, the specimens were subjected to different characterisation techniques. The description and composition of the geopolymer series are shown in
Table 1 and
Figure 1.
2.3. Characterization techniques
For studying the microstructure and the morphology of the produced geopolymers, the samples were first coated with platinum, and then scanned under scanning electron microscope (SEM), (QUANTA INSPECT F50, FEI Company, Eindhoven, The Netherlands). Thermal properties and mass loss with heating were measured by heating the samples (~100 mg) using a thermogravimetric analyzer (TGA) (Netzsch, Germany, TG 209 F1 Libra). The temperature range was 50°-800 °C with a 2 °C/min increment rate. This test was carried out in a helium environment. Qualitative mineralogical and phase analysis was carried out on the samples using a Shimadzu XRD diffractometer-6000 (Japan) with a cobalt tube and a 2-theta scanning range of 5−80° at a 2°/min scan rate. Rietveld refinement of the produced materials was carried out using the software MATCH! (Version 3.15, Crystal Impact, Bonn, Germany) with fundamental parameters approach. FTIR spectra was obtained in the range 4000–500 cm-1 using FT-IR spectrometer (Perkin–Elmer system 2000). The samples were subjected to compressive strength using a universal testing machine (HD-B615-S, China). The machine-head speed was 1 mm/min. Three specimens of each series were subjected to this mechanical testing. The specimens´ dimensions were height=40 mm, width=20 mm, and length=20 mm.
3. Results and Discussion
3.1. Effect of sulfur loading on compressive strength of geopolymers
The fraction of sulfur has a significant effect on the mechanical properties of geopolymer based on its function as a filler or reactive component. However, it is not recommended to use the sulfur as a filler because it is soluble and may resulting in mechanical failure if exposed to water [
24,
25]. Therefore, the sulfur powder was completely dissolved in the alkaline solution before the taking place of geopolymerisation,
Figure 1.
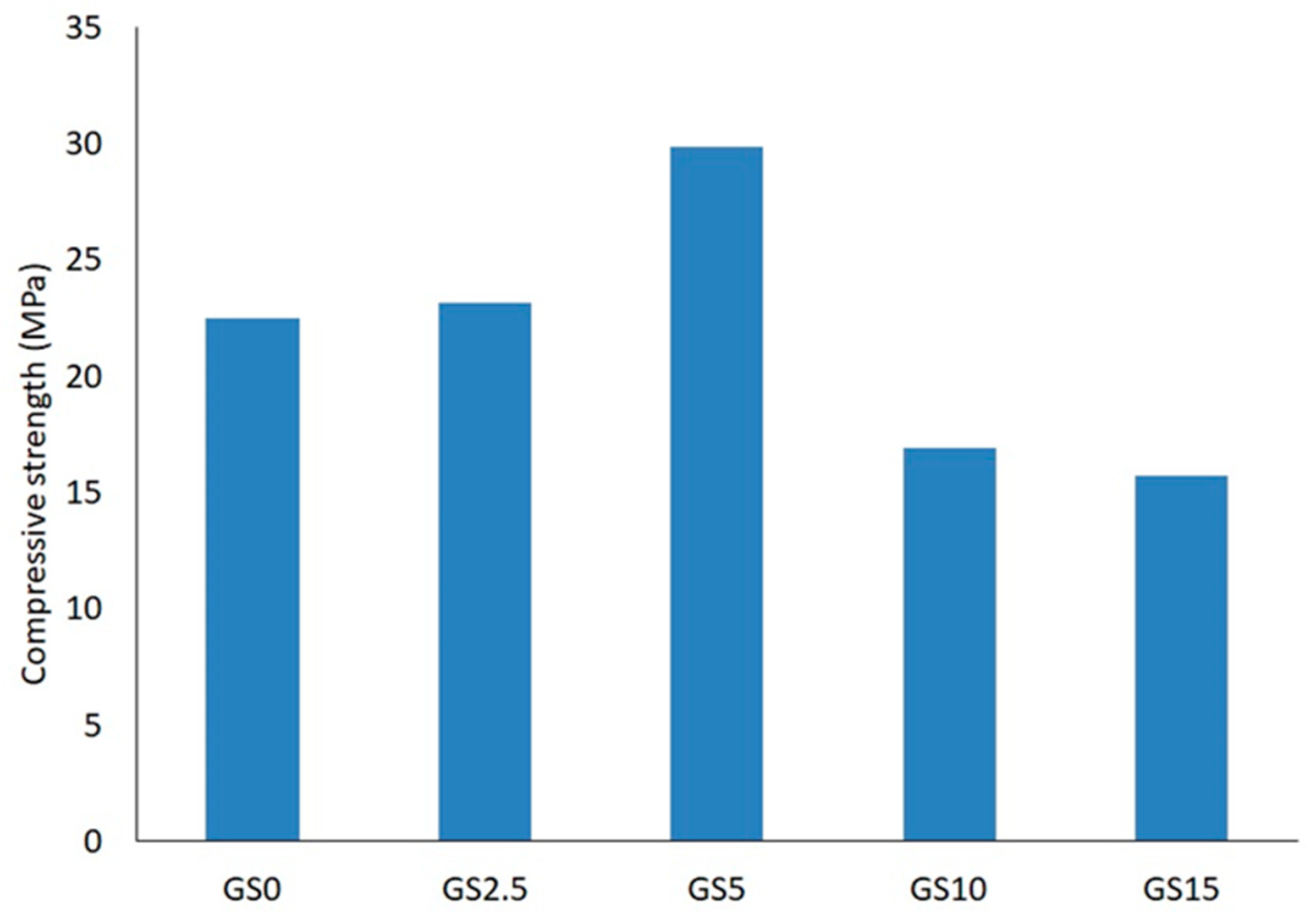
The variation in compressive strength of the specimens,
Table 1, is displayed in
Figure 2. As can be seen, increasing the loading of sulfur from 0 wt% to 5 wt% compared with metakaolin, results in an increase in the compressive strength of the geopolymer from 22.5 MPa up to 29.9 MPa respectively. However, when the content of sulfur ranged from above 5 wt% up to 15 w% in the geopolymers, a sharp decrease in compressive strength from 29.9 MPa to 15.7 is observed, which could be explained by yellow color of the water after immersion, causing defects in the geopolymer matrix through the solubility of the excess sulfur and this formation of voids and gaps. This type of defect, voids and gaps, could evolve into cracks during the compressive fracture processes of the material [
26].
Compressive strength is a widely accepted measure to access the performance of a given mixture. This characteristic is one of the major motivations to select the best material composition [
26]. Therefore, the specimens of GS5 are selected for further studies and analysis as the optimum composition.
3.2. Analysis of microstructure and phase composition
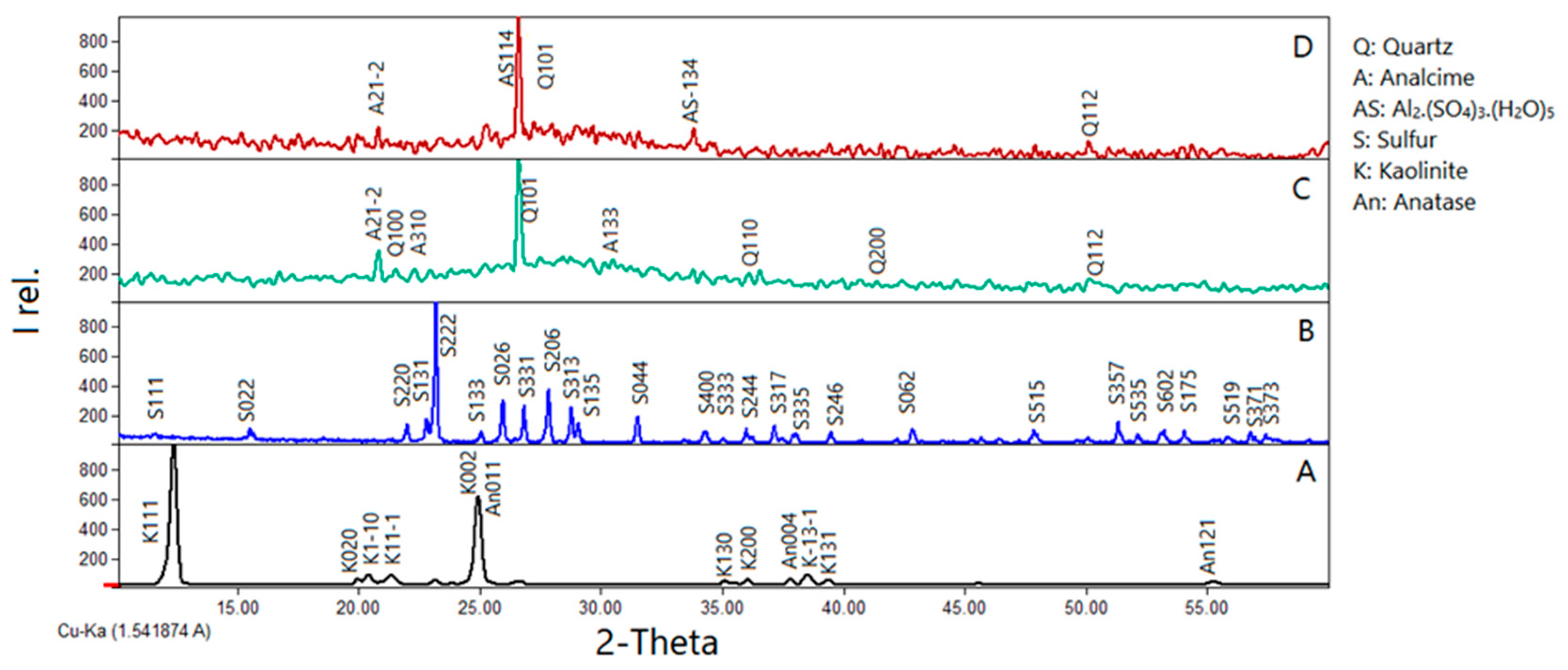
Figure 3 shows the XRD scan analyses of the precursors, kaolinite and sulfur, reference geopolymer (GS0) as well as that of sulfur-geopolymer (GS5) with optimized composition based on the compressive strength factor,
Figure 2. The characteristic XRD peaks of the kaolinite and sulfur samples are easily observable,
Figure 3A, and
Figure 3B, respectively. The XRD pattern,
Figure 3A, illustrates that kaolinite also contains some mineral (TiO
2), with Miller indices of (011) and (004), in this case 10.8% [
14], Table3. The unit cell parameters and volume are slightly differing from the typical kaolinite, where the unit cell volume is 329.4 Å
3 ,
Table 3, while the standard unit cell is 320.1 Å
3 [
27]. This change in the volume of unit cell could be a result of presence of impurities such as iron oxides as reported in
Table 1. Anatase typically exists in kaolin clay [
11]. This metastable mineral represents one of the most common of titanium dioxide (TiO
2) with a tetragonal crystal structure [
27].
The XRD pattern shows numerous peaks of sulfur at 2θ=23.13, 25.90, and 27.78. The sulfur (S8) is characterized by Orthorhombic crystal structure with a unit cell specification: a = 10.48 Å b = 12.87 Å c= 24.51 Å,
Table 3.
As a result of pre-treatment (calcination) and the subsequent chemical transformation (geopolymerization) of the precursors,
Figure 3A, the XRD peaks corresponding to kaolinite and anatase phases disappear, leading to dissolution in the alkaline environment, as illustrated in
Figure 3C, GS0. A hump observed in XRD patterns,
Figure 3C, between 20° to 40° confirms the formation of amorphous phases in the resultant geopolymer [
9,
28]. According to the Rietveld refinement analysis of the XRD data, the degree of crystallinity of GS0 is 49.9%, where two crystalline phases are detected: triclinic Analcime [
29], and trigonal (hexagonal axes) quartz with weight contents of 80.2% and 19.8 respectively,
Table 4.
Introducing sulfur to the geopolymer alkaline activator results in the diminishing of all peaks corresponding to this phase,
Figure 3D. This is an indication of the incorporation of sulfur as a reactive component, and precursor in the geopolymer matrix instead of being a water-soluble filler. The degree of crystallinity of GS5, increases slightly from 49.9% to 52.9% with adding sulfur to the alkaline activator,
Table 4. The XRD hump corresponding to amorphous phase is detected between 22°C and 35°C. The crystalline phases of GS5 are similar to metakaolin-polymers, GS0, but there is a new phase that has been associated with sulfur, which is Al
2·H
10·O
17·S
3,
Table 4. The percentage of this phase is 53.5% and it is characterized by its monoclinic crystal structure. Compared with GS0, the percentages of analcime and quartz decreased from 80.2% and 19.8% to 36.1% and 10.4% respectively. There is also a decrement in the crystalline sizes of both analcime and quartz with introducing sulfur to the geopolymer matrix. The results confirm that sulfur reacts with Aluminum from the metakaolin to form a new phase/s.
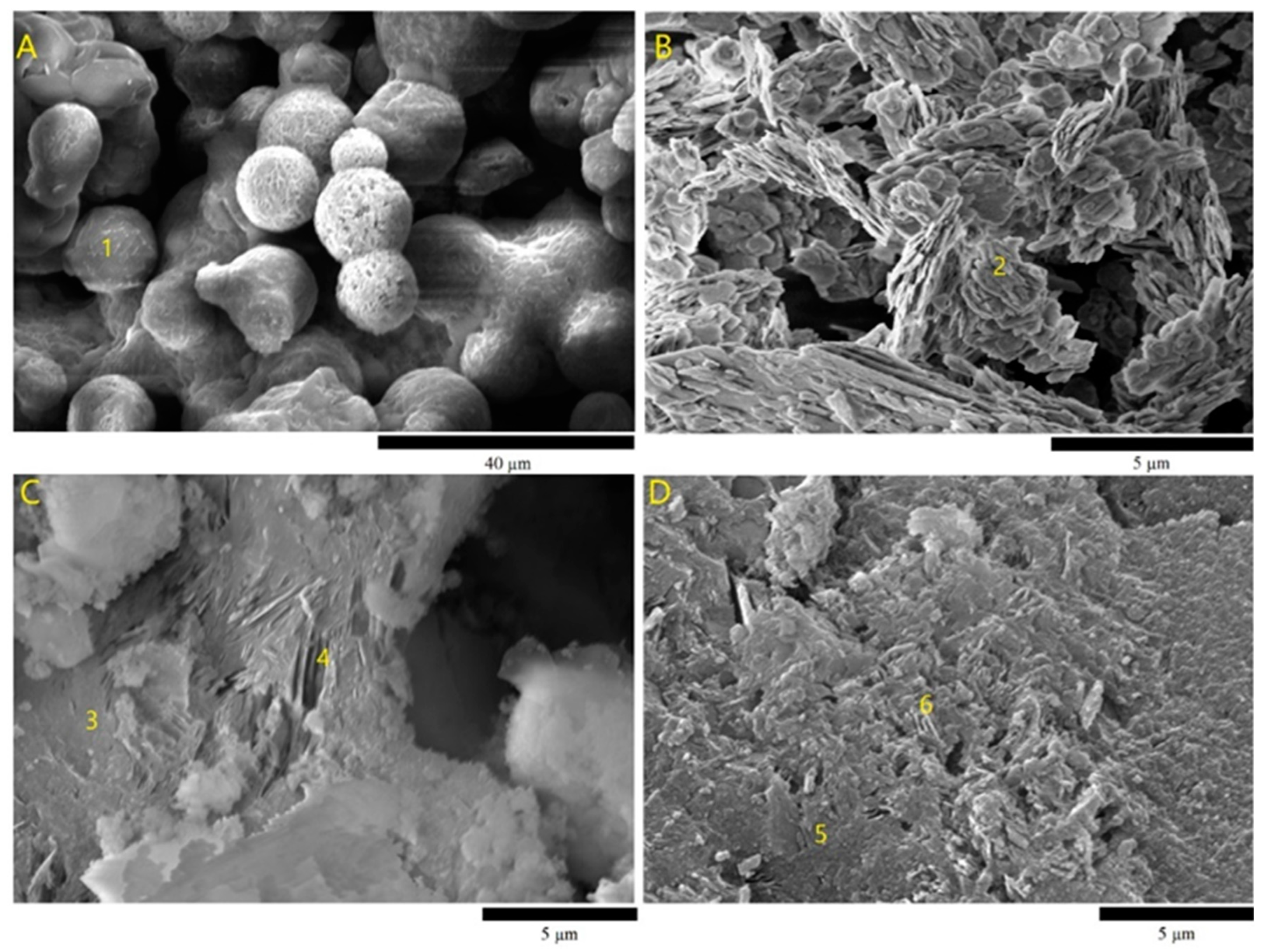
The metakaolin layers as a main precursor and the source of Al ions of the geopolymer are reported by the SEM as shown in
Figure 4B (point 2). These layers were distorted due to the calcination of kaolinite 750 °C. SEM analysis,
Figure 4C, indicates that the microstructure of GS0, is composed of binder material (geopolymer matrix) (point 3) and partially dissolved metakaolin layers (point 4). This SEM image shows also that as a result of geopolymerization and the setting reactions, the gaps between partially dissolved metakaolinite layers have been filled with formed binding material (sodium aluminosilicate matrix) [
28].
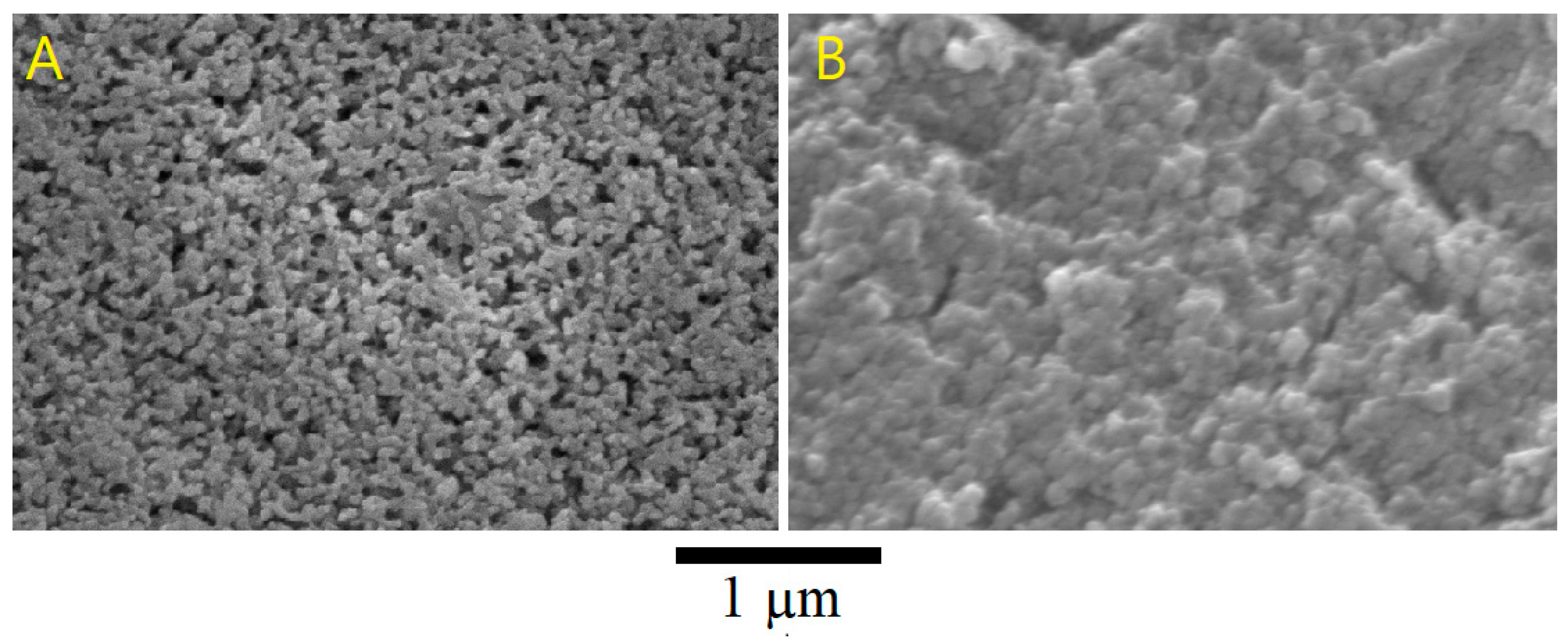
The produced geopolymer exhibit a structure of nano porous network formed by aluminosilicate particles [
30]. The size of the aluminosilicate particles (~160 nm),
Table 4 ,which are encapsulated in the geopolymeric matrix determines the nanopore pattern observed in their microstructure,
Figure 5A. The formation of a uniform pore structure and a homogeneous pore network in aluminosilicate particles can be observed in
Figure 5A. This finding is in agreement with the results reported by Alshaaer et al. [
30], who produced partially amorphous sodium aluminosilicates after activation of metakaolin with alkali activators. Sulfur micro spheres, ~10 µm, are confirmed by scanning electron microscope (SEM)
Figure 4A (point 1). The addition of sulfur showed that the microstructure of GS5 is characterized by the main phases: geopolymer matrix ,
Figure 5D (point 5), and the voids ,
Figure 5D (point 6), associated with the dissolution of metakaolin during the geopolymerization process. Increased the SEM magnification as shown in
Figure 5, observes the emergence of a more compacted, i.e. less nonporous microstructure,
Figure 5B, compared with GS0,
Figure 5A. This may have an important impact on the physical and chemical properties of the end geopolymer products.
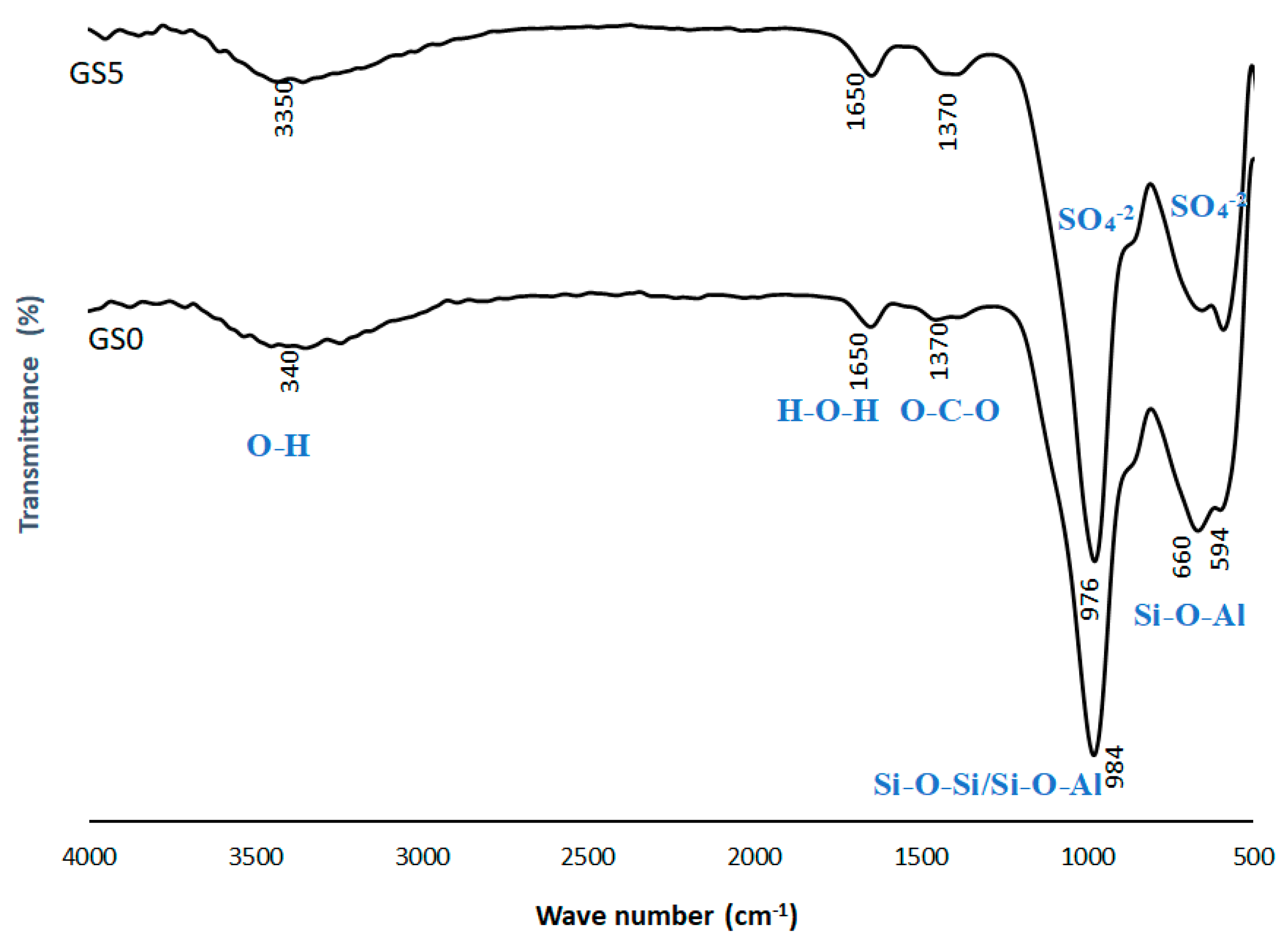
3.3. FTIR Spectrum of geopolymers (GS0 and GS5)
FTIR spectra of GS0, and GS5 are exhibited in
Figure 6. In the spectrum of GS0, the peak at 594 cm
−1, 660 cm
−1 and 984 cm
−1 are attributed to Si-O-Al, Si-O-Al and the Si–O-Si bonds, respectively [
31]. The appearance of these three peaks is also observed in the result of the GS5, which are indicative of the limited change in the bonding by adding sulfur to the alkaline activator (GS5). These absorption bands at about 594 cm
−1, and 660 cm
−1 on both GS0 and GS5 spectra are respectively attributed to asymmetric and symmetric vibrations of Si-O-Al and Si-O-Si bonds that provide the cohesion between AlO
4 and SiO4 tetrahedrons in geopolymeric structure [
32].
In the spectrum of geopolymer (GS0) in
Figure 6, the band at 1370 cm
−1 can be assigned to O-C-O, which is also observed for the GS5. This band is related to the presence of sodium carbonate via the reaction of alkali metal hydroxide with atmospheric CO
2 [
33,
34]. These peaks are clearly presented in the spectrum of the GS5, which suggests the existence of geopolymer. The absorption broadband at 1650 cm
−1 and 3340 cm
−1 are the stretching and bending vibration frequencies of OH groups associated to water [
32]. The strong and broad absorption band, GS5, centered at 613 cm
−1 probably also resulted from the combined absorptions of SO4
-2, and the Si-O-Al stretching vibrations. The strong band centered at 998 cm
-1 could be assigned to combination of sulfate absorptions and Si-O-Al band [
35].
The FTIR spectrum confirmed the geopolymer structure of both GS0, and GS5 [
32]. There is a clear similarity between the FTIR spectrum of geopolymers, GS0 and GS5. This similarity is probably due to the small proportion of sulfur, about 1.8% of the total mix, which has been added to precursors of GS5. Another possibility is the apparent overlap in sulfate spectrums with silica and aluminum bonds [
35].
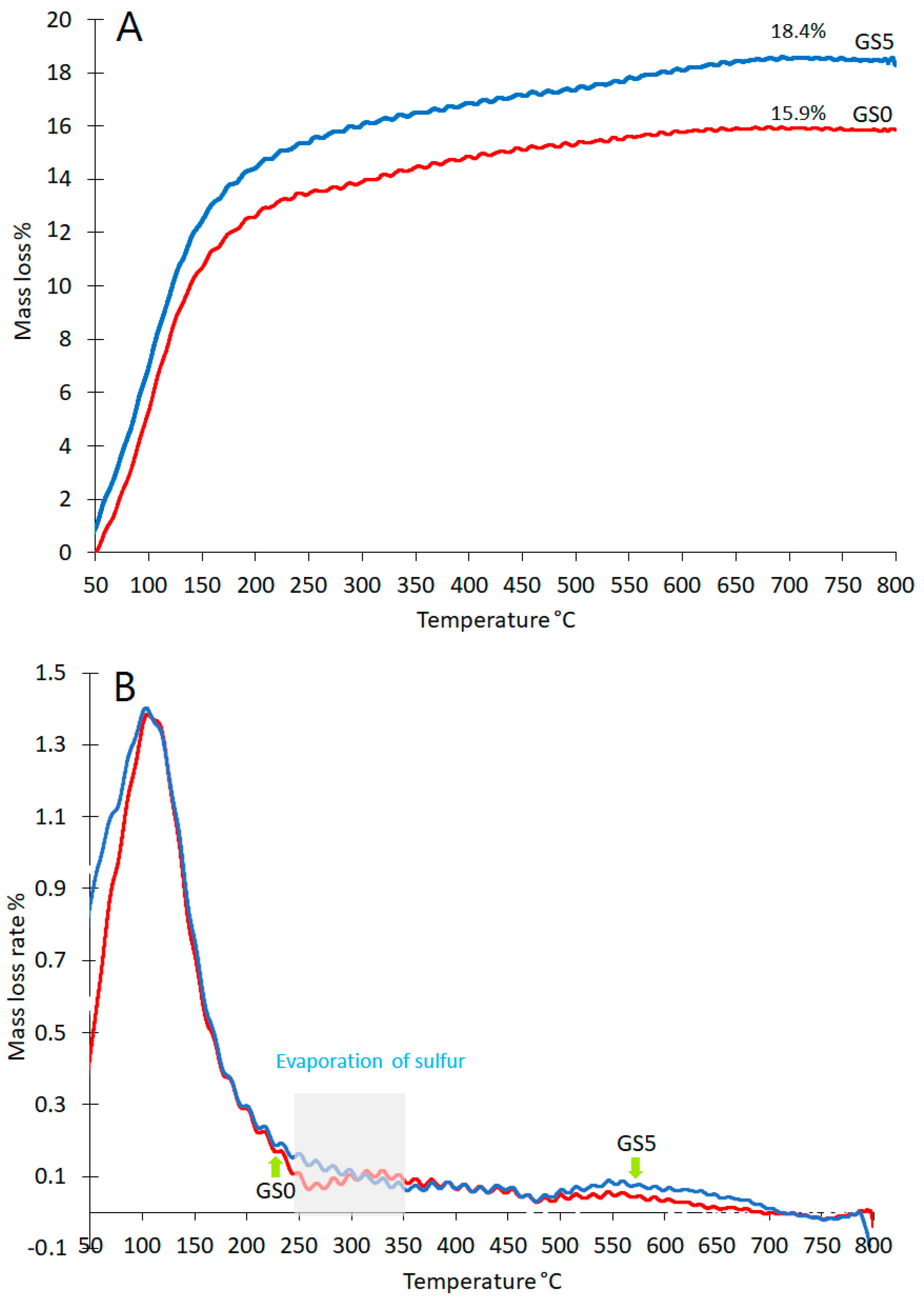
3.4. Thermogravimetric Analysis (TGA)
Thermal decomposition process of GS0 and GS5 under air were investigated by TG tests and the corresponding results are given in
Figure 7. The total weight loss as a result of heating the GS0 up to 800 °C is around 15.9%,
Figure 7A. This cumulative mass loss increases by adding sulfur (GS5) to 18.4%. This overall increment in mass loss due to the presence of more structural water attributed to the new phases, i.e. Al
2·H
10·O
17·S
3,
Table 4. Evaporation of sulfur at relatively low temperature, less than 300 °C [
36], may be one of the causes of this increase in weight loss, but it is certainly not the main reason total sulfur constituents, 1.8%wt, is less than the difference in weight loss, 2.5%.
The derivative of the TGA curve reveals that thermal events occur in two main temperatures ranges, 50–250 °C and 500–700 °C, respectively, as shown in
Figure 7B. In the first range (25–200 ◦C), the decrease in weight is associated with the release of fine-pore moisture. In the second range (500–700 ◦C), the decrease in weight can be attributed to the release of zeolitic water from the nano-porous network [
30].
Previous studies [
36] have concluded that the sulfur begins to evaporate at 250 °C and disappears completely at about 350 °C. This rapid weight loss area of sulfur is attributable to the collapse of S-S bonds. But it is observed in
Figure 7B that there is no increase in the percentage of weight loss for GS5 compared to GS0, so sulfur as an element can be considered to have no presence in the structure of GS0. Thus, sulfur has been associated with essential elements in geopolymers such as aluminum as confirmed by the formation of Al
2·H
10·O
17·S
3,
Table 4.
The presence of elemental sulfur in the microstructure of geopolymers leads to its dissolution in water, which turns yellow as shown by GS10 and GS15,
Table 1. Thus, elemental sulfur leaves voids resulting in a decrease in its mechanical performance,
Figure 1. Increase in weight loss in the second range, 500–700 °C, is likely due to the release of Al
2·H
10·O
17·S
3 structural water [
37].
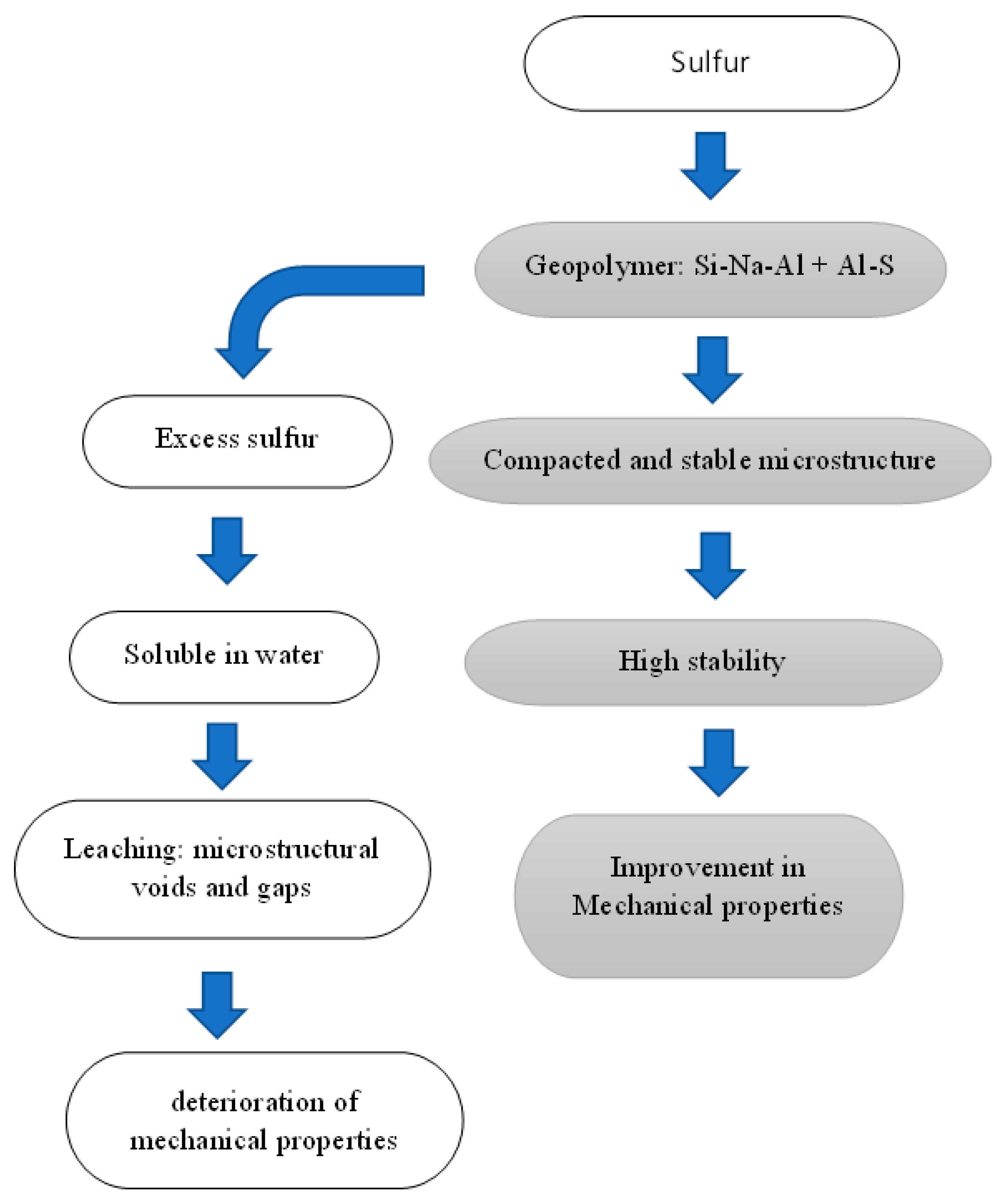
3.5. Influence of S/Al molar ratio on geopolymer properties
The above-mentioned results show that sulfur could have both positive and negative effects on the mechanical properties and stability of the geopolymers. Sulfur is first dissolved in the alkaline solution and then the metakaolin is added. It is observed that preparation of S-geopolymer with S/Al molar ratio around 0.17,
Table 5, results in the formation of stable S-Al-Si-Na matrix. Sulfur reacts with Al to form new phases, such as Al
2·H
10·O
17·S
3. The resultant microcrystalline is composed from compacted nano-crystalline/amorphous phases. Sulfur decreases the crystalline sizes of both quartz and analcime. As a result of these microstructural changes an improvement of the mechanical properties is observed, where the compressive strength increases by ~32%,
Figure 2.
Increasing the S/Al molar ratio from 0.17 up to 0.5 leading to precipitation of excess sulfur, or unreacted sulfur, in the matrix. The excess sulfur dissolves when the material is exposed to water, and a leaching process takes place. As a result of this sulfur leaching, defects and voids are leftover the microstructure and thus deteriorate it mechanical properties,
Figure 2.
4. Conclusions
In this preliminary study, sulfur, a material available as waste by-product was added to the precursors of geopolymers. The sulfur was dissolved in different proportions to the alkaline activator used to produce geopolymers. Clearly, there is an improvement in the mechanical properties of geopolymers by adding sulfur up to 5% wt compared with metakaolin. The improvement in the mechanical properties increases until the percentage reaches 5%wt of metakaolin (source of aluminum). Different tests have confirmed that sulfur interacts with aluminum ions from the metakaolin and incorporates in the microstructure of geopolymers. The crystalline phases of the resulting geopolymers change clearly after the addition of sulfur. The analysis of the SEM showed that the microstructure of geopolymers became denser and more compacted after the addition of sulfur. FTIR spectra analysis did not show significant differences after the addition of sulfur and was explained by interference bands of sulfate, and silica and aluminum bands. The TG analysis confirmed that there was no unreacted sulfur in the microstructure of geopolymers, although it was added to the precursors and dissolved in the alkaline solution. From this study we conclude that sulfur can be used initially by improving the properties of geopolymers and the emergence of new phases and microstructural characteristics. Adding sulfur to alkaline solution to higher levels leads to precipitation of elemental sulfur in the geopolymer microstructure and thus soluble in water resulting in a deterioration of the geopolymer mechanical performance.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used Conceptualization, M.A., A.A. and I.A..; methodology, M.A. ; software, M.A.; validation, M.A., A.A. and I.A.; formal analysis, M.A.; investigation, M.A. , A.A., and I.A.; resources, M.A., and A.A.; data curation, M.A. , and A.A.; writing—original draft preparation, M.A., I.A. and A.A. ; writing—review and editing, M.A. ; visualization, M.A. ; supervision, M.A. ; project administration, M.A.; funding acquisition, M.A. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Prince Sattam bin Abdulaziz University, grant number 2022/01/17935.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [M. Alshaaer], upon reasonable request.
Acknowledgments
This study is supported via funding from Prince Sattam bin Abdulaziz Uni-versity project number (2022/01/17935) Financial support for the project, grant 2022/01/17935S Specialized Research Grant program, provided by the Deanship of Scientific Research at the Prince Sattam bin Abdulaziz University is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ober, J. Materials Flow of Sulfur; U.S. Geological Surevey: Reston, VA, USA, 2002. [Google Scholar]
- Fediuk, R.; Amran, Y.H.M.; Mosaberpanah, M.A.; Danish, A.; El-Zeadani, M.; Klyuev, S.V.; Vatin, N. A Critical Review on the Properties and Applications of Sulfur-Based Concrete. Materials 2020, 13, 4712. [Google Scholar] [CrossRef]
- Fediuk, R.S.; Yevdokimova, Y.G.; Smoliakov, A.K.; Stoyushko, N.Y.; Lesovik, V.S. Use of geonics scientific positions for designing of building composites for protective (fortification) structures. IOP Conf. Series: Mater. Sci. Eng. 2017, 221, 012011. [Google Scholar] [CrossRef]
- Wagenfeld, J.-G.; Al-Ali, K.; Almheiri, S.; Slavens, A.F.; Calvet, N. Sustainable applications utilizing sulfur, a by-product from oil and gas industry: A state-of-the-art review. Waste Manag. 2019, 95, 78–89. [Google Scholar] [CrossRef]
- Stern, D.I. Reversal of the trend in global anthropogenic sulfur emissions. Glob. Environ. Chang. 2006, 16, 207–220. [Google Scholar] [CrossRef]
- Eliseev, A.V. Climate change mitigation via sulfate injection to the stratosphere: impact on the global carbon cycle and terrestrial biosphere. Atmospheric Ocean. Opt. 2012, 25, 405–413. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2006, 42, 3073–3082. [Google Scholar] [CrossRef]
- Alshaaer, M. Two-phase geopolymerization of kaolinite-based geopolymers. Appl. Clay Sci. 2013, 86, 162–168. [Google Scholar] [CrossRef]
- Alshaaer, M.; Cuypers, H. ; Wastiels. In Stabilisation of kaolinitic soil for construction purposes by using mineral polymerisation technique, in Proceedings of the 6th International Conference Technology for Developing Countries, Amman; 2002. [Google Scholar]
- Alshaaer, M.; El-Eswed, B.; Yousef, R.; Khalili, F.; Rahier, H. Development of functional geopolymers for water purification, and construction purposes. J. Saudi Chem. Soc. 2016, 20, S85–S92. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Alshaaer, M.; Khalili, F.; Khoury, H. The influence of using Jordanian natural zeolite on the adsorption, physical, and mechanical properties of geopolymers products. J. Hazard. Mater. 2009, 165, 379–387. [Google Scholar] [CrossRef]
- M. Alshaaer, m.; S. Abu Mallouh, S.; J. Al-Kafawein, J.; Y. Al-Faiyz, Y.; T. Fahmy, T.; A. Kallel, A.; Rocha, F. Fabrication, microstructural and mechanical characterization of Luffa Cylindrical Fibre - Reinforced geopolymer composite. Applied Clay Science 2017, 143, 125–133. [CrossRef]
- Alshaaer, M.; Alkafawein, J.; Al-Fayez, Y.; Fahmy, T.; Hamaideh, A. Synthesis of geopolymer cement using natural resources for green construction materials, in Recent Advances in Earth Sciences. Environment and Development. Proceedings of the 8th International Conference on Engineering Mechanics, Structures, Engineering Geology (EMESEG'15), Konya, 2015.
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The circular economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- El-Eswed, B.; Yousef, R.; Alshaaer, M.; Hamadneh, I.; Al-Gharabli, S.; Khalili, F. Stabilization/solidification of heavy metals in kaolin/zeolite based geopolymers. Int. J. Miner. Process. 2015, 137, 34–42. [Google Scholar] [CrossRef]
- Kinnunen, P.; Ismailov, A.; Solismaa, S.; Sreenivasan, H.; Räisänen, M.-L.; Levänen, E.; Illikainen, M. Recycling mine tailings in chemically bonded ceramics – A review. J. Clean. Prod. 2018, 174, 634–649. [Google Scholar] [CrossRef]
- Tian, Q.; Bai, Y.; Pan, Y.; Chen, C.; Yao, S.; Sasaki, K.; Zhang, H. Application of Geopolymer in Stabilization/Solidification of Hazardous Pollutants: A Review. Molecules 2022, 27, 4570. [Google Scholar] [CrossRef]
- Shi, C.; Fernández-Jiménez, A.M. Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements. J. Hazard. Mater. 2006, 137, 1656–1663. [Google Scholar] [CrossRef]
- Luukkonen, T.; Runtti, H.; Niskanen, M.; Tolonen, E.-T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; Lassi, U. Simultaneous removal of Ni(II), As(III), and Sb(III) from spiked mine effluent with metakaolin and blast-furnace-slag geopolymers. J. Environ. Manag. 2016, 166, 579–588. [Google Scholar] [CrossRef]
- Alshaaer, M. Synthesis and characterization of self-healing geopolymer composite. Constr. Build. Mater. 2020, 245, 118432. [Google Scholar] [CrossRef]
- Alshaaer, M. Synthesis, Characterization, and Recyclability of a Functional Jute-Based Geopolymer Composite. Front. Built Environ. 2021, 7. [Google Scholar] [CrossRef]
- Maldonado-Zagal, S.B.; Boden, P.J. Hydrolysis of Elemental Sulphur in Water and its Effect on the Corrosion of Mild Steel. Br. Corros. J. 1982, 17, 116–120. [Google Scholar] [CrossRef]
- Ferreira, A.; Lobo, L. The low-pressure phase diagram of sulfur. J. Chem. Thermodyn. 2011, 43, 95–104. [Google Scholar] [CrossRef]
- Ashby, M.F.; Sammis, C.G. The Damage Mechanics of Brittle Solids in Compression. Pure and Applied Geophysics 1990, 133, 489–521. [Google Scholar]
- Nesse, W.D. Introduction to mineralogy. New York: Oxford University Press, 2000, 254–255.
- Alshaaer, M.; Zaharaki, D.; Komnitsas, K. Microstructural characteristics and adsorption potential of a zeolitic tuff–metakaolin geopolymer. Desalination Water Treat. 2014, 56, 338–345. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E.N. Impact of Alkali Silica Reaction on Fly Ash-Based Geopolymer Concrete. J. Mater. Civ. Eng. 2013, 25, 131–139. [Google Scholar] [CrossRef]
- Alshaaer, M.; Al-Fayez, Y.J.; Fahmy, T.; Hamaideh, A. Synthesis of geopolymer cement using natural resources for green construction materials, in Recent Advances in Earth Sciences, Environment and Development, Konya, 2015.
- Huang, Y.; Gong, L.; Pan, Y.; Li, C.; Zhou, T.; Cheng, X. Facile construction of the aerogel/geopolymer composite with ultra-low thermal conductivity and high mechanical performance. RSC Adv. 2018, 8, 2350–2356. [Google Scholar] [CrossRef]
- Nmiri, A.; yazoghli, O.; Duc, M.; Hamdi, N.; Srasra, E. Temperature effect on mechanical and physical proprieties of Na or K alkaline silicate activated metakaolin-based geopolymers. Italian Journal of Engineering Geology a Engineering Geology and Environment. 2016, 1, 5–15. [Google Scholar]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Kumar, S.; Kristály, F.; Mucsi, G. Geopolymerisation behaviour of size fractioned fly ash. Adv. Powder Technol. 2015, 26, 24–30. [Google Scholar] [CrossRef]
- Serna, C.J; White, J.L.; Hem, S.L. Anion-Aluminum Hydroxide Interactions. Soil Science Society of America Journal. 1977, 42, 1009–1013. [Google Scholar] [CrossRef]
- Zhang, S.S. Understanding of Sulfurized Polyacrylonitrile for Superior Performance Lithium/Sulfur Battery. Energies 2014, 7, 4588–4600. [Google Scholar] [CrossRef]
- Van essen, M.; Gores, J.; Bleijendaal, L.; Zondag, H.; Schuitema, R.; Helden, W. Characterization of salt hydrates for compact seasonal thermochemical storage., in Effstock 2009, Stockholm, 2009.
Figure 1.
Experimental procedure.
Figure 1.
Experimental procedure.
Figure 2.
Compressive strength as a function of sulfur weight percentage (Series GS0, GS2.5, GS5, GS10, and GS15,
Table 1)
.
Figure 2.
Compressive strength as a function of sulfur weight percentage (Series GS0, GS2.5, GS5, GS10, and GS15,
Table 1)
.
Figure 3.
Qualitative XRD patterns of: (A) powdered kaolinite (K), (B) sulfur (S), (C) reference geopolymer (GS0), and (D) sulfur-geopolymer (GS5).
Figure 3.
Qualitative XRD patterns of: (A) powdered kaolinite (K), (B) sulfur (S), (C) reference geopolymer (GS0), and (D) sulfur-geopolymer (GS5).
Figure 4.
SEM images of (A) sulfur, (B) metakaolin, (C) GS0 geopolymer, and (D) GS5 geopolymer; (1) sulfur, (2) metakaolin layers, (3, 5) geopolymer matrix, and (4,6) voids resulting from the dissolution of metakaolin.
Figure 4.
SEM images of (A) sulfur, (B) metakaolin, (C) GS0 geopolymer, and (D) GS5 geopolymer; (1) sulfur, (2) metakaolin layers, (3, 5) geopolymer matrix, and (4,6) voids resulting from the dissolution of metakaolin.
Figure 5.
SEM images with high magnification of: (A) metakaolin-based geopolymer (GS0), and (B) sulfur-based geopolymer, GS5.
Figure 5.
SEM images with high magnification of: (A) metakaolin-based geopolymer (GS0), and (B) sulfur-based geopolymer, GS5.
Figure 6.
FTIR spectra of geopolymers (GS0 and GS5).
Figure 6.
FTIR spectra of geopolymers (GS0 and GS5).
Figure 7.
TGA of geopolymers (GS0 and GS1); (A) cumulative TGA, and (B) Differential TGA.
Figure 7.
TGA of geopolymers (GS0 and GS1); (A) cumulative TGA, and (B) Differential TGA.
Figure 8.
Influence of sulfur on the geopolymer properties.
Figure 8.
Influence of sulfur on the geopolymer properties.
Table 1.
Chemical analysis of kaolinite.
Table 1.
Chemical analysis of kaolinite.
| Compound |
Composition% |
| MnO |
0.34
|
| Cr2O3
|
0.45
|
| CaO |
1.11
|
| K2O |
0.12
|
| P2O5
|
0.93
|
| Fe2O3
|
9.37
|
| Al2O3
|
22.56
|
| SiO2
|
38.41
|
| TiO2
|
14.22
|
Table 2.
Composition of the synthesized geopolymers.
Table 2.
Composition of the synthesized geopolymers.
| ID |
Composition of geopolymer mixture [wt.%] |
| Metakaolin |
Na2SiO3 solution |
NaOH |
H2O |
S |
| GS0 |
100 |
100 |
25 |
48 |
0 |
| GS2.5 |
100 |
100 |
25 |
48 |
2.5 |
| GS5 |
100 |
100 |
25 |
48 |
5 |
| GS10 |
100 |
100 |
25 |
48 |
10 |
| GS15 |
100 |
100 |
25 |
48 |
15 |
Table 3.
Results of XRD analysis of the precursors (Rietveld refinement, MATCH! software).
Table 3.
Results of XRD analysis of the precursors (Rietveld refinement, MATCH! software).
| Precursor |
Phase |
Phase% |
Crystal structure |
a (Å) |
b (Å) |
c (Å) |
V (Å3) |
| Kaolinite |
Kaolinite Al2Si2O5(OH)4
|
89.2 |
Triclinic * |
5.15 |
8.94 |
7.40 |
329.4 |
| |
Anatase TiO2
|
10.8 |
tetragonal |
3.78 |
- |
9.51 |
135.9 |
| Sulfur |
S8 |
100 |
orthorhombic |
10.46 |
12.87 |
24.49 |
3296.8 |
Table 4.
Results of XRD analysis showing crystallinity, phase composition, and crystal structure (Rietveld refinement, MATCH! software).
Table 4.
Results of XRD analysis showing crystallinity, phase composition, and crystal structure (Rietveld refinement, MATCH! software).
| |
Degree of Crystallinity |
Crystalline Phase composition |
Phase% |
Crystal system |
Unit cell size (nm3) |
Crystalline size (nm) |
| GS0 |
49.9% |
Al1.81·H4·Na1.71·O14 (Analcime) |
80.2 |
triclinic |
2.22835 |
152.7 |
| SiO2 (Quartz) |
19.8 |
Hexagonal |
0.113526 |
179.5 |
| GS5 |
52.9% |
Al1.81·H4·Na1.71·O14 (Analcime) |
36.1 |
triclinic |
2.22835 |
93.5 |
| SiO2 (Quartz) |
10.4 |
Hexagonal |
0.113526 |
68.7 |
| Al2·H10·O17·S3
|
53.5 |
monoclinic |
1.226255 |
75.9 |
Table 5.
S/Al molar ratio of the geopolymers.
Table 5.
S/Al molar ratio of the geopolymers.
| ID |
S/Al molar ratio |
| GS0 |
0.00 |
| GS2.5 |
0.09 |
| GS5 |
0.17 |
| GS10 |
0.35 |
| GS15 |
0.52 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).