Submitted:
07 August 2023
Posted:
09 August 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction
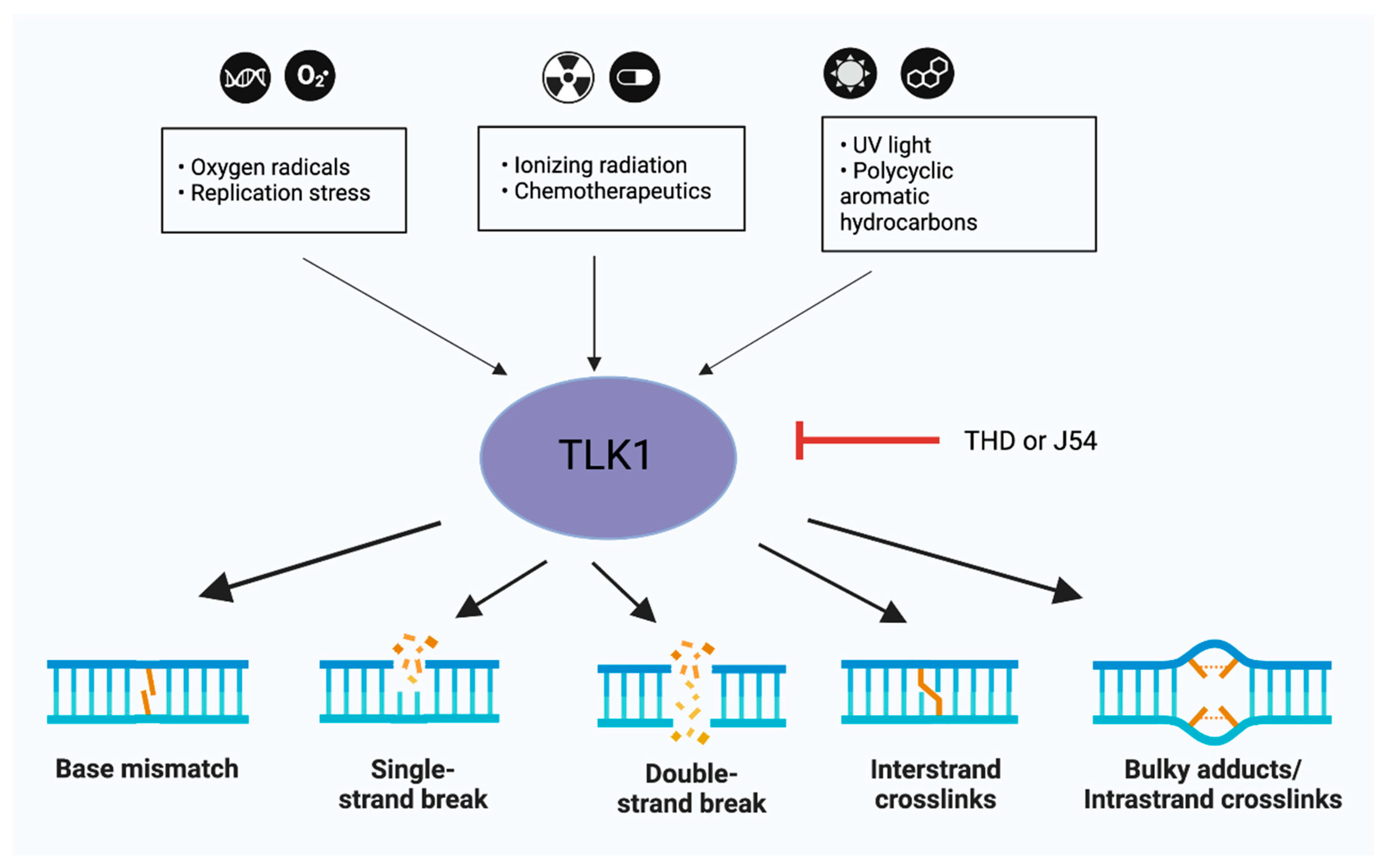
Role of TLK1 in DSB repair
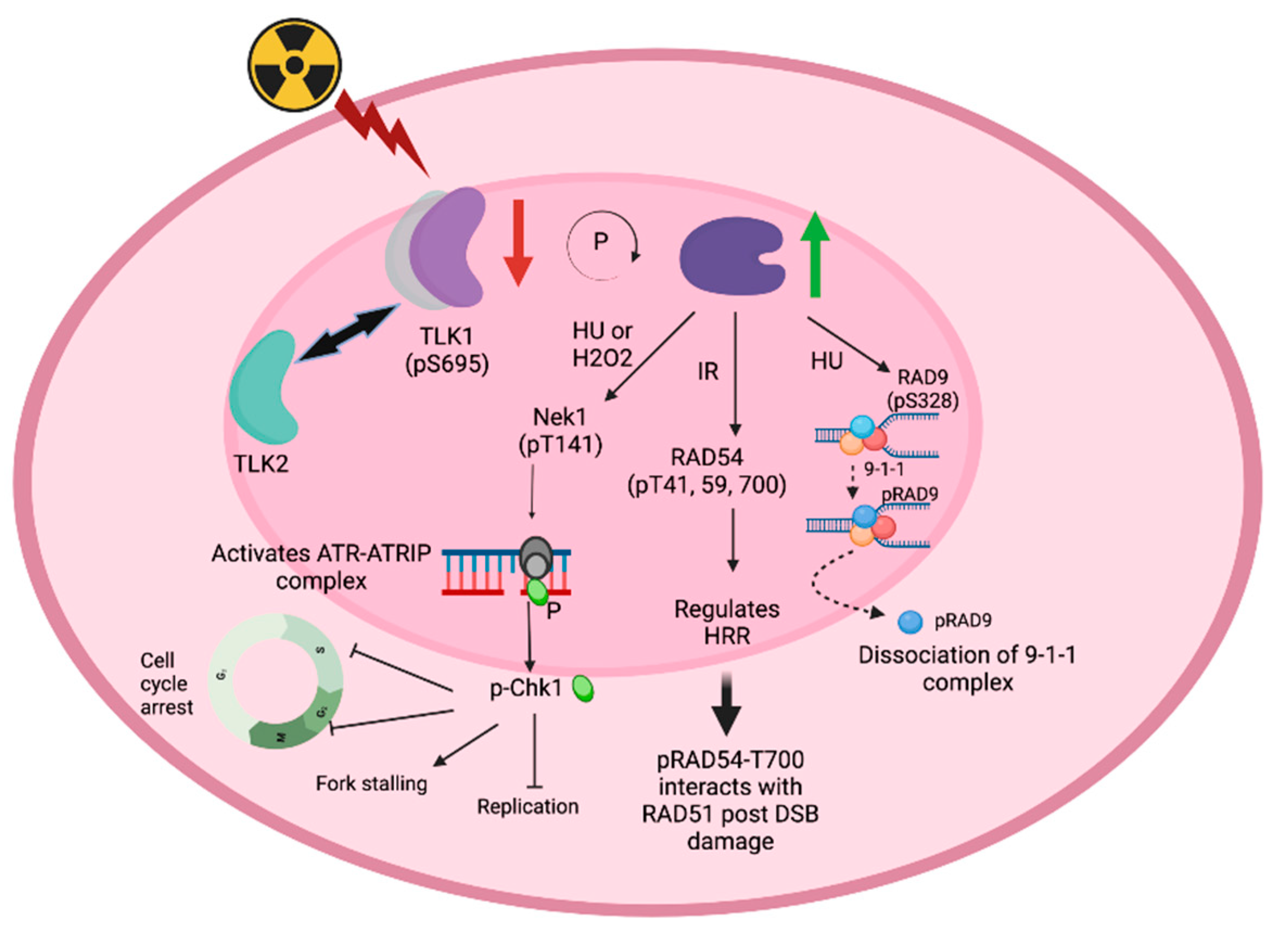
Role of TLK1 in regulating HRR factors
Role of TLK1 in eukaryotic recombination
Role of TLK1 in cancer
Conclusion: Targeting TLK1 for cancer treatment
Funding
Conflicts of Interest
References
- Silljé, H.H.W., Takahashi, K., Tanaka, K., Van Houwe, G. and Nigg, E.A. Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. The EMBO Journal, 1999, 18, 5691–5702. [CrossRef] [PubMed]
- Li, Y. , DeFatta, R., Anthony, C., Sunavala, G. and De Benedetti, A. A translationally regulated Tousled kinase phosphorylates histone H3 and confers radioresistance when overexpressed. Oncogene 2001, 20, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Groth, A. , Lukas, J., Nigg, E.A., Silljé, H.H.W., Wernstedt, C., Bartek, J. and Hansen, K. Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. The EMBO Journal 2003, 22, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Segura-Bayona, S. and Stracker, T.H. The Tousled-like kinases regulate genome and epigenome stability: implications in development and disease. Cellular and Molecular Life Sciences 2019, 76, 3827–3841. [Google Scholar] [CrossRef]
- Li, Z. , Gourguechon, S.p. and Wang, C.C. Tousled-like kinase in a microbial eukaryote regulates spindle assembly and S-phase progression by interacting with Aurora kinase and chromatin assembly factors. Journal of Cell Science 2007, 120, 3883–3894. [Google Scholar] [CrossRef]
- Awate, S. and De Benedetti, A. TLK1B mediated phosphorylation of Rad9 regulates its nuclear/cytoplasmic localization and cell cycle checkpoint. BMC Molecular Biology 2016, 17, 3. [Google Scholar] [CrossRef]
- Sunavala-Dossabhoy, G. and De Benedetti, A. Tousled homolog, TLK1, binds and phosphorylates Rad9; TLK1 acts as a molecular chaperone in DNA repair. DNA Repair 2009, 8, 87–102. [Google Scholar] [CrossRef]
- Klimovskaia, I.M. , Young, C., Strømme, C.B., Menard, P., Jasencakova, Z., Mejlvang, J., Ask, K., Ploug, M., Nielsen, M.L., Jensen, O.N. et al. Tousled-like kinases phosphorylate Asf1 to promote histone supply during DNA replication. Nature Communications 2014, 5, 3394. [Google Scholar] [CrossRef]
- Sen, S.P. and De Benedetti, A. TLK1B promotes repair of UV-damaged DNA through chromatin remodeling by Asf1. BMC Molecular Biology 2006, 7, 37. [Google Scholar] [CrossRef]
- Singh, V. , Connelly, Z.M., Shen, X. and De Benedetti, A. Identification of the proteome complement of humanTLK1 reveals it binds and phosphorylates NEK1 regulating its activity. Cell Cycle 2017, 16, 915–926. [Google Scholar] [CrossRef]
- Ghosh, I., Kwon, Y., Shabestari, A.B., Chikhale, R., Chen, J., Wiese, C., Sung, P. and DeBenedetti, A. TLK1-mediated RAD54 phosphorylation spatio-temporally regulates Homologous Recombination Repair. bioRxiv 2022, 2022.2009.2019.508551.
- Kiianitsa, K. , Solinger Jachen, A. and Heyer, W.-D. Terminal association of Rad54 protein with the Rad51–dsDNA filament. Proceedings of the National Academy of Sciences 2006, 103, 9767–9772. [Google Scholar] [CrossRef] [PubMed]
- Elisabeth, K. , Reinhard, K., Hak, C. and Debabrata, S. Sustained Metaphase Arrest in Response to Ionizing Radiation in a Non-small Cell Lung Cancer Cell Line. Radiation Research 2008, 169, 46–58. [Google Scholar]
- Kelly, R. and Davey, S.K. Tousled-Like Kinase-Dependent Phosphorylation of Rad9 Plays a Role in Cell Cycle Progression and G2/M Checkpoint Exit. PLOS ONE 2013, 8, e85859. [Google Scholar] [CrossRef] [PubMed]
- Lisby, M. , Barlow, J.H., Burgess, R.C. and Rothstein, R. Choreography of the DNA Damage Response: Spatiotemporal Relationships among Checkpoint and Repair Proteins. Cell 2004, 118, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Delacroix, S. , Wagner, J.M., Kobayashi, M., Yamamoto, K.-i. and Karnitz, L.M. The Rad9–Hus1–Rad1 (9–1–1) clamp activates checkpoint signaling via TopBP1. Genes & Development 2007, 21, 1472–1477. [Google Scholar]
- Zhu, A. , Zhang, C.X. and Lieberman, H.B. Rad9 Has a Functional Role in Human Prostate Carcinogenesis. Cancer Research 2008, 68, 1267–1274. [Google Scholar] [CrossRef]
- Post, S.M. , Tomkinson, A.E. and Lee, E.Y.H.P. The human checkpoint Rad protein Rad17 is chromatin-associated throughout the cell cycle, localizes to DNA replication sites, and interacts with DNA polymerase ϵ. Nucleic Acids Research 2003, 31, 5568–5575. [Google Scholar] [CrossRef]
- Lin, C.-Y. , Chang, H.-H., Wu, K.-J., Tseng, S.-F., Lin, C.-C., Lin, C.-P. and Teng, S.-C. Extrachromosomal Telomeric Circles Contribute to Rad52-, Rad50-, and Polymerase δ-Mediated Telomere-Telomere Recombination in Saccharomyces cerevisiae. Eukaryotic Cell 2005, 4, 327–336. [Google Scholar] [CrossRef]
- Ghosh, I., Kwon, Y., Shabestari, A.B., Chikhale, R., Chen, J., Wiese, C., Sung, P. and De Benedetti, A. TLK1-mediated RAD54 phosphorylation spatio-temporally regulates Homologous Recombination Repair. Nucleic Acids Research 2023, gkad589.
- Goyal, N. , Rossi, M.J., Mazina, O.M., Chi, Y., Moritz, R.L., Clurman, B.E. and Mazin, A.V. RAD54 N-terminal domain is a DNA sensor that couples ATP hydrolysis with branch migration of Holliday junctions. Nature Communications 2018, 9, 34. [Google Scholar] [CrossRef]
- Selemenakis, P. , Sharma, N., Uhrig, M.E., Katz, J., Kwon, Y., Sung, P. and Wiese, C. RAD51AP1 and RAD54L Can Underpin Two Distinct RAD51-Dependent Routes of DNA Damage Repair via Homologous Recombination. Frontiers in Cell and Developmental Biology 2022, 10. [Google Scholar] [CrossRef]
- Sunavala-Dossabhoy, G. , Li, Y., Williams, B. and De Benedetti, A. A dominant negative mutant of TLK1 causes chromosome missegregation and aneuploidy in normal breast epithelial cells. BMC Cell Biology 2003, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B. , Segura-Bayona, S., Villamor-Payà, M., Saredi, G., Todd, M.A.M., Attolini, C.S.-O., Chang, T.-Y., Stracker, T.H. and Groth, A. Tousled-like kinases stabilize replication forks and show synthetic lethality with checkpoint and PARP inhibitors. Science Advances 2023, 4, eaat4985. [Google Scholar] [CrossRef] [PubMed]
- Uhrig, M., Sharma, N., Maxwell, P., Selemenakis, P. and Wiese, C. (2023) RAD54L regulates replication fork progression and nascent strand degradation in BRCA1/2-deficient cells.
- Timiri Shanmugam, P.S. Timiri Shanmugam, P.S., Nair, R.P., De Benedetti, A., Caldito, G., Abreo, F. and Sunavala-Dossabhoy, G. Tousled kinase activator, gallic acid, promotes homologous recombinational repair and suppresses radiation cytotoxicity in salivary gland cells.
- Ronald, S. , Awate, S., Rath, A., Carroll, J., Galiano, F., Dwyer, D., Kleiner-Hancock, H., Mathis, J.M., Vigod, S. and De Benedetti, A. Phenothiazine Inhibitors of TLKs Affect Double-Strand Break Repair and DNA Damage Response Recovery and Potentiate Tumor Killing with Radiomimetic Therapy. Genes Cancer 2013, 4, 39–53. [Google Scholar] [PubMed]
- Yang, S. , Liu, L., Cao, C., Song, N., Wang, Y., Ma, S., Zhang, Q., Yu, N., Ding, X., Yang, F. et al. USP52 acts as a deubiquitinase and promotes histone chaperone ASF1A stabilization. Nature Communications 2018, 9, 1285. [Google Scholar] [CrossRef]
- Sukackaite, R. , Cornacchia, D., Jensen, Malene R., Mas, P.J., Blackledge, M., Enervald, E., Duan, G., Auchynnikava, T., Köhn, M., Hart, D.J. et al. Mouse Rif1 is a regulatory subunit of protein phosphatase 1 (PP1). Scientific Reports 2017, 7, 2119. [Google Scholar] [CrossRef]
- Escribano-Díaz, C. , Orthwein, A., Fradet-Turcotte, A., Xing, M., Young, Jordan T.F., Tkáč, J., Cook, Michael A., Rosebrock, Adam P., Munro, M., Canny, Marella D. et al. A Cell Cycle-Dependent Regulatory Circuit Composed of 53BP1-RIF1 and BRCA1-CtIP Controls DNA Repair Pathway Choice. Molecular Cell 2013, 49, 872–883. [Google Scholar]
- Batenburg, N.L. , Walker, J.R., Noordermeer, S.M., Moatti, N., Durocher, D. and Zhu, X.-D. ATM and CDK2 control chromatin remodeler CSB to inhibit RIF1 in DSB repair pathway choice. Nature Communications 2017, 8, 1921. [Google Scholar] [CrossRef]
- Tang, M., Chen, Z., Wang, C., Feng, X., Lee, N., Huang, M., Zhang, H., Li, S., Xiong, Y. and Chen, J. Histone chaperone ASF1 acts with RIF1 to promote DNA end-joining in BRCA1-deficient cells. Journal of Biological Chemistry 2022, 101979.
- Xu, L. and Blackburn, E.H. Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. Journal of Cell Biology 2004, 167, 819–830. [Google Scholar] [CrossRef]
- Segura-Bayona, S. , Villamor-Payà, M., Attolini, C.S.-O., Koenig, L.M., Sanchiz-Calvo, M., Boulton, S.J. and Stracker, T.H. Tousled-Like Kinases Suppress Innate Immune Signaling Triggered by Alternative Lengthening of Telomeres. Cell Reports 2020, 32, 107983. [Google Scholar] [CrossRef]
- Carrera, P. , Moshkin, Y.M., Grönke, S., Silljé, H.H.W., Nigg, E.A., Jäckle, H. and Karch, F. Tousled-like kinase functions with the chromatin assembly pathway regulating nuclear divisions. Genes & Development 2003, 17, 2578–2590. [Google Scholar]
- Han, Z. , Riefler, G.M., Saam, J.R., Mango, S.E. and Schumacher, J.M. The C. elegans Tousled-like Kinase Contributes to Chromosome Segregation as a Substrate and Regulator of the Aurora B Kinase. Current Biology 2005, 15, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, L.M. , Gál, Z., Nieto, B., Quevedo, O., Boukoura, S., Lund, C.C. and Larsen, D.H. Recent advances in the nucleolar responses to DNA double-strand breaks. Nucleic Acids Research 2020, 48, 9449–9461. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. , Jaiswal, P.K., Ghosh, I., Koul, H.K., Yu, X. and De Benedetti, A. The TLK1-Nek1 axis promotes prostate cancer progression. Cancer Letters 2019, 453, 131–141. [Google Scholar] [CrossRef]
- Singh, V. , Jaiswal, P.K., Ghosh, I., Koul, H.K., Yu, X. and De Benedetti, A. Targeting the TLK1/NEK1 DDR axis with Thioridazine suppresses outgrowth of androgen independent prostate tumors. International Journal of Cancer 2019, 145, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K. , Abdul Murad, N.A., Harun, R., Jamal, R., Ibrahim, K., Abdul Murad, N.A., Harun, R., Jamal, R., Ibrahim, K., Abdul Murad, N.A. et al. Knockdown of Tousled-like kinase 1 inhibits survival of glioblastoma multiforme cells. Int J Mol Med 2020, 46, 685–699. [Google Scholar] [CrossRef]
- Jiang, J. , Jia, P., Zhao, Z. and Shen, B. Key regulators in prostate cancer identified by co-expression module analysis. BMC Genomics 2014, 15, 1015. [Google Scholar] [CrossRef]
- Kim, J.-A. , Anurag, M., Veeraraghavan, J., Schiff, R., Li, K. and Wang, X.-S. Amplification of TLK2 Induces Genomic Instability via Impairing the G2–M Checkpoint. Molecular Cancer Research 2016, 14, 920–927. [Google Scholar] [CrossRef]
- Lin, M. , Yao, Z., Zhao, N. and Zhang, C. TLK2 enhances aggressive phenotypes of glioblastoma cells through the activation of SRC signaling pathway. Cancer Biol Ther 2019, 20, 101–108. [Google Scholar] [CrossRef]
- Van Roy, N. , Vandesompele, J., Berx, G., Staes, K., Van Gele, M., De Smet, E., De Paepe, A., Laureys, G., van der Drift, P., Versteeg, R. et al. Localization of the 17q breakpoint of a constitutional 1;17 translocation in a patient with neuroblastoma within a 25-kb segment located between the ACCN1 and TLK2 genes and near the distal breakpoints of two microdeletions in neurofibromatosis type 1 patients. Genes Chromosomes Cancer 2002, 35, 113–120. [Google Scholar]
- Ghosh, I., Khalil, M.I., Mirza, R., King, J., Olatunde, D. and De Benedetti, A. NEK1-Mediated Phosphorylation of YAP1 Is Key to Prostate Cancer Progression. Biomedicines 2023. [CrossRef]
- Singh, V. , Bhoir, S., Chikhale, R.V., Hussain, J., Dwyer, D., Bryce, R.A., Kirubakaran, S. and De Benedetti, A. Generation of Phenothiazine with Potent Anti-TLK1 Activity for Prostate Cancer Therapy. iScience 2020, 23, 101474. [Google Scholar] [CrossRef]
- Mortuza, G.B. , Hermida, D., Pedersen, A.-K., Segura-Bayona, S., López-Méndez, B., Redondo, P., Rüther, P., Pozdnyakova, I., Garrote, A.M., Muñoz, I.G. et al. Molecular basis of Tousled-Like Kinase 2 activation. Nature Communications 2018, 9, 2535. [Google Scholar] [CrossRef] [PubMed]
- Bhoir, S. and De Benedetti, A. Targeting Prostate Cancer, the ‘Tousled Way&rsquo. International Journal of Molecular Sciences 2023. [CrossRef]
- Takayama, Y. , Kokuryo, T., Yokoyama, Y., Ito, S., Nagino, M., Hamaguchi, M. and Senga, T. Silencing of Tousled-like kinase 1 sensitizes cholangiocarcinoma cells to cisplatin-induced apoptosis. Cancer Letters 2010, 296, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I. and De Benedetti, A. Tousled-like kinase 1: a novel factor with multifaceted role in mCRPC progression and development of therapy resistance. Cancer Drug Resistance 2022, 5, 93–101. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




