Submitted:
28 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
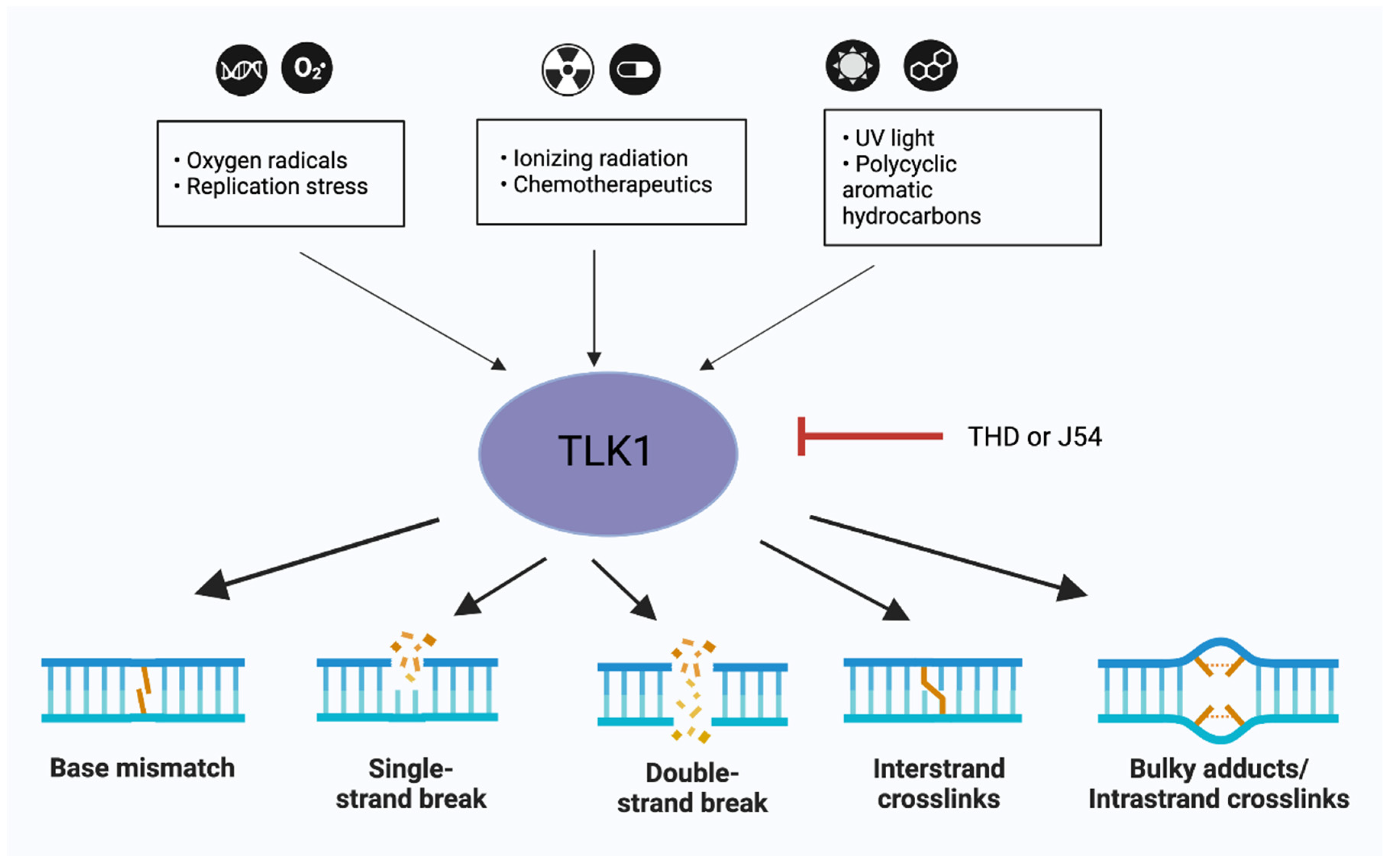
1. Introduction
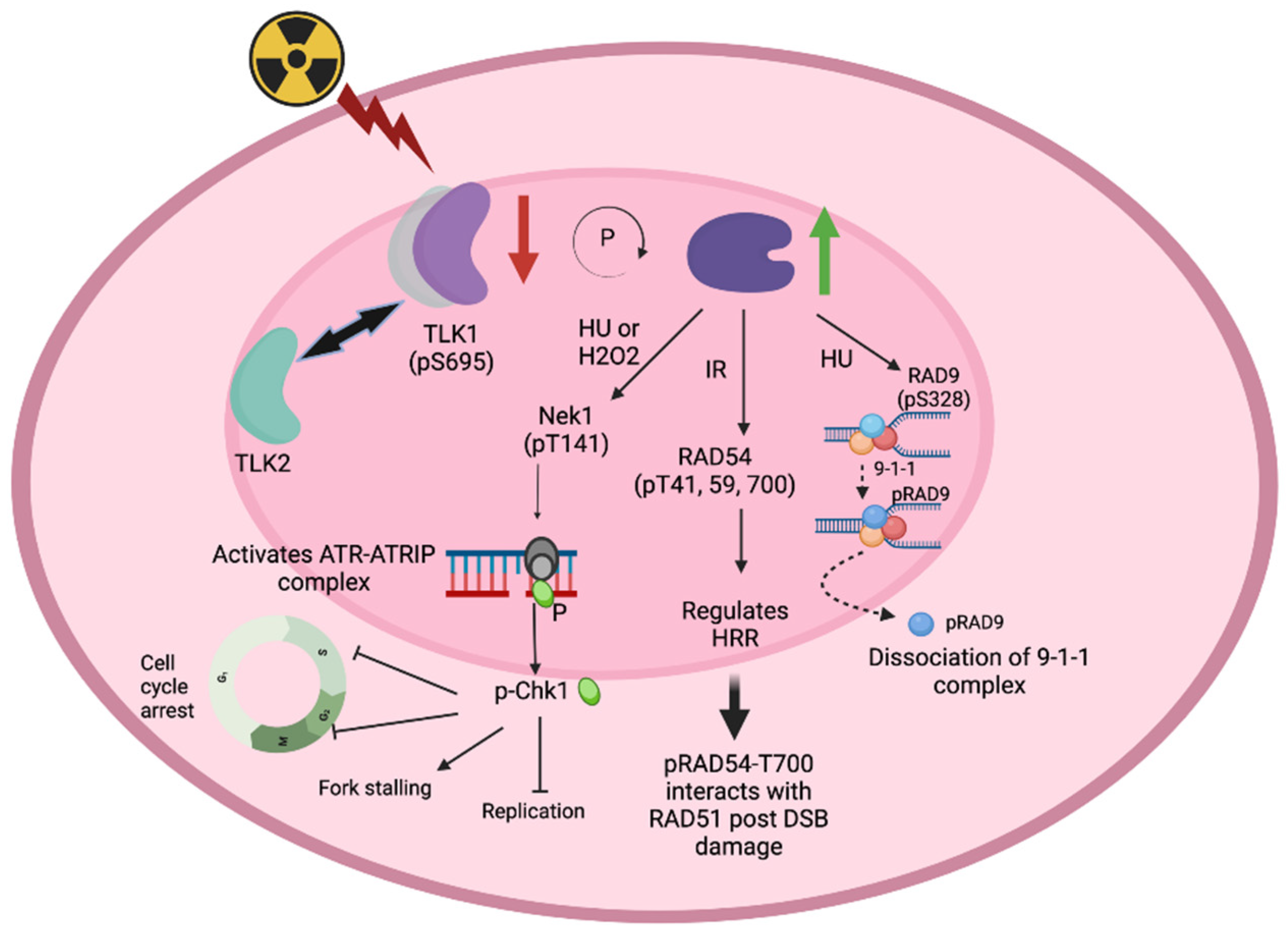
2. Role of TLK1 in DSB repair
3. Role of TLK1 in regulating HRR factors
4. Role of TLK1 in eukaryotic recombination repair
5. TLKs and DNA damage and checkpoint functions
6. Role of TLK1 in cancer
7. Conclusion: Targeting TLK1 for cancer treatment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Silljé, H.H.W., Takahashi, K., Tanaka, K., Van Houwe, G. and Nigg, E.A. [1999] Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. The EMBO Journal, 18, 5691-5702. [CrossRef]
- Li, Y., DeFatta, R., Anthony, C., Sunavala, G. and De Benedetti, A. [2001] A translationally regulated Tousled kinase phosphorylates histone H3 and confers radioresistance when overexpressed. Oncogene, 20, 726-738. [CrossRef]
- Groth, A., Lukas, J., Nigg, E.A., Silljé, H.H.W., Wernstedt, C., Bartek, J. and Hansen, K. [2003] Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. The EMBO Journal, 22, 1676-1687. [CrossRef]
- Ghosh, I., Kwon, Y., Shabestari, A.B., Chikhale, R., Chen, J., Wiese, C., Sung, P. and De Benedetti, A. [2023] TLK1-mediated RAD54 phosphorylation spatio-temporally regulates Homologous Recombination Repair. Nucleic Acids Research. [CrossRef]
- Segura-Bayona, S. and Stracker, T.H. [2019] The Tousled-like kinases regulate genome and epigenome stability: implications in development and disease. Cell Mol Life Sci, 76, 3827-3841. [CrossRef]
- Li, Z., Gourguechon, S.p. and Wang, C.C. [2007] Tousled-like kinase in a microbial eukaryote regulates spindle assembly and S-phase progression by interacting with Aurora kinase and chromatin assembly factors. Journal of Cell Science, 120, 3883-3894. [CrossRef]
- Awate, S. and De Benedetti, A. [2016] TLK1B mediated phosphorylation of Rad9 regulates its nuclear/cytoplasmic localization and cell cycle checkpoint. BMC Mol Biol, 17, 3. [CrossRef]
- Sunavala-Dossabhoy, G. and De Benedetti, A. [2009] Tousled homolog, TLK1, binds and phosphorylates Rad9; tlk1 acts as a molecular chaperone in DNA repair. DNA Repair, 8, 87-102. [CrossRef]
- Klimovskaia, I.M., Young, C., Stromme, C.B., Menard, P., Jasencakova, Z., Mejlvang, J., Ask, K., Ploug, M., Nielsen, M.L., Jensen, O.N. et al. [2014] Tousled-like kinases phosphorylate Asf1 to promote histone supply during DNA replication. Nat Commun, 5, 3394. [CrossRef]
- Sen, S. and De Benedetti, A. [2006] TLK1B promotes repair of UV-damaged DNA through chromatin remodeling by Asf1. BMC Mol Biol., 7, 37. [CrossRef]
- Klimovskaia, I.M., Young, C., Strømme, C.B., Menard, P., Jasencakova, Z., Mejlvang, J., Ask, K., Ploug, M., Nielsen, M.L., Jensen, O.N. et al. [2014] Tousled-like kinases phosphorylate Asf1 to promote histone supply during DNA replication. Nature Communications, 5, 3394. [CrossRef]
- Singh, V., Connelly, Z.M., Shen, X. and De Benedetti, A. [2017] Identification of the proteome complement of humanTLK1 reveals it binds and phosphorylates NEK1 regulating its activity. Cell Cycle, 16, 915-926. [CrossRef]
- Mengwasser, K.E., Adeyemi, R.O., Leng, Y., Choi, M.Y., Clairmont, C., D'Andrea, A.D. and Elledge, S.J. [2019] Genetic Screens Reveal FEN1 and APEX2 as BRCA2 Synthetic Lethal Targets. Mol Cell, 73, 885-899.e886. [CrossRef]
- Kiianitsa, K., Solinger Jachen, A. and Heyer, W.-D. [2006] Terminal association of Rad54 protein with the Rad51–dsDNA filament. Proceedings of the National Academy of Sciences, 103, 9767-9772. [CrossRef]
- Elisabeth, K., Reinhard, K., Hak, C. and Debabrata, S. [2008] Sustained Metaphase Arrest in Response to Ionizing Radiation in a Non-small Cell Lung Cancer Cell Line. Radiation Research, 169, 46-58. [CrossRef]
- Kelly, R. and Davey, S.K. [2013] Tousled-Like Kinase-Dependent Phosphorylation of Rad9 Plays a Role in Cell Cycle Progression and G2/M Checkpoint Exit. PLOS ONE, 8, e85859. [CrossRef]
- Kodym, R., Mayerhofer, T. and Ortmann, E. [2004] Purification and identification of a protein kinase activity modulated by ionizing radiation. Biochem Biophys Res Commun., 313, 97-103. [CrossRef]
- Singh, V., Bhoir, S., Chikhale, R.V., Hussain, J., Dwyer, D., Bryce, R.A., Kirubakaran, S. and De Benedetti, A. [2020] Generation of phenothiazine with potent anti-TLK1 activity for prostate cancer therapy. Iscience, 23, 101474. [CrossRef]
- Adkins, N.L., Niu, H., Sung, P. and Peterson, C.L. [2013] Nucleosome dynamics regulates DNA processing. Nat Struct Mol Biol, 20, 836-842. [CrossRef]
- Uhrig, M.E., Sharma, N., Maxwell, P., Selemenakis, P. and Wiese, C. [2023] RAD54L regulates replication fork progression and nascent strand degradation in BRCA1/2-deficient cells. bioRxiv. [CrossRef]
- Lisby, M., Barlow, J.H., Burgess, R.C. and Rothstein, R. [2004] Choreography of the DNA Damage Response: Spatiotemporal Relationships among Checkpoint and Repair Proteins. Cell, 118, 699-713. [CrossRef]
- Delacroix, S., Wagner, J.M., Kobayashi, M., Yamamoto, K.-i. and Karnitz, L.M. [2007] The Rad9–Hus1–Rad1 (9–1–1) clamp activates checkpoint signaling via TopBP1. Genes & Development, 21, 1472-1477. [CrossRef]
- Zhu, A., Zhang, C.X. and Lieberman, H.B. [2008] Rad9 Has a Functional Role in Human Prostate Carcinogenesis. Cancer Research, 68, 1267-1274. [CrossRef]
- Post, S.M., Tomkinson, A.E. and Lee, E.Y.H.P. [2003] The human checkpoint Rad protein Rad17 is chromatin-associated throughout the cell cycle, localizes to DNA replication sites, and interacts with DNA polymerase ϵ. Nucleic Acids Research, 31, 5568-5575. [CrossRef]
- Lin, C.-Y., Chang, H.-H., Wu, K.-J., Tseng, S.-F., Lin, C.-C., Lin, C.-P. and Teng, S.-C. [2005] Extrachromosomal Telomeric Circles Contribute to Rad52-, Rad50-, and Polymerase δ-Mediated Telomere-Telomere Recombination in Saccharomyces cerevisiae. Eukaryotic Cell, 4, 327-336. [CrossRef]
- Canfield, C., Rains, J. and De Benedetti, A. [2009] TLK1B promotes repair of DSBs via its interaction with Rad9 and Asf1. BMC Mol Biol, 10, 110. [CrossRef]
- Brandsma, I. and Gent, D.C. [2012] Pathway choice in DNA double strand break repair: observations of a balancing act. Genome Integr, 3, 9. [CrossRef]
- Melo, J. and Toczyski, D. [2002] A unified view of the DNA-damage checkpoint. Curr Opin Cell Biol, 14, 237-245. [CrossRef]
- Liu, S., Ho, C.K., Ouyang, J. and Zou, L. [2013] Nek1 kinase associates with ATR-ATRIP and primes ATR for efficient DNA damage signaling. Proc Natl Acad Sci U S A, 110, 2175-2180. [CrossRef]
- Day, M., Rappas, M., Ptasinska, K., Boos, D., Oliver, A.W. and Pearl, L.H. [2018] BRCT domains of the DNA damage checkpoint proteins TOPBP1/Rad4 display distinct specificities for phosphopeptide ligands. eLife, 7, e39979. [CrossRef]
- Hammet, A., Magill, C., Heierhorst, J. and Jackson, S.P. [2007] Rad9 BRCT domain interaction with phosphorylated H2AX regulates the G1 checkpoint in budding yeast. EMBO Rep, 8, 851-857. [CrossRef]
- Goyal, N., Rossi, M.J., Mazina, O.M., Chi, Y., Moritz, R.L., Clurman, B.E. and Mazin, A.V. [2018] RAD54 N-terminal domain is a DNA sensor that couples ATP hydrolysis with branch migration of Holliday junctions. Nature Communications, 9, 34. [CrossRef]
- Maranon, D.G., Sharma, N., Huang, Y., Selemenakis, P., Wang, M., Altina, N., Zhao, W. and Wiese, C. [2020] NUCKS1 promotes RAD54 activity in homologous recombination DNA repair. Journal of Cell Biology, 219. [CrossRef]
- Selemenakis, P., Sharma, N., Uhrig, M.E., Katz, J., Kwon, Y., Sung, P. and Wiese, C. [2022] RAD51AP1 and RAD54L Can Underpin Two Distinct RAD51-Dependent Routes of DNA Damage Repair via Homologous Recombination. Frontiers in Cell and Developmental Biology, 10. [CrossRef]
- Sunavala-Dossabhoy, G., Li, Y., Williams, B. and De Benedetti, A. [2003] A dominant negative mutant of TLK1 causes chromosome missegregation and aneuploidy in normal breast epithelial cells. BMC Cell Biology, 4, 16. [CrossRef]
- Lee, S.-B., Segura-Bayona, S., Villamor-Payà, M., Saredi, G., Todd, M.A.M., Attolini, C.S.-O., Chang, T.-Y., Stracker, T.H. and Groth, A. Tousled-like kinases stabilize replication forks and show synthetic lethality with checkpoint and PARP inhibitors. Science Advances, 4, eaat4985. [CrossRef]
- Timiri Shanmugam, P.S., Nair, R.P., De Benedetti, A., Caldito, G., Abreo, F. and Sunavala-Dossabhoy, G. Tousled kinase activator, gallic acid, promotes homologous recombinational repair and suppresses radiation cytotoxicity in salivary gland cells. [CrossRef]
- Ronald, S., Awate, S., Rath, A., Carroll, J., Galiano, F., Dwyer, D., Kleiner-Hancock, H., Mathis, J.M., Vigod, S. and De Benedetti, A. [2013] Phenothiazine Inhibitors of TLKs Affect Double-Strand Break Repair and DNA Damage Response Recovery and Potentiate Tumor Killing with Radiomimetic Therapy. Genes Cancer, 4, 39-53. [CrossRef]
- Segura-Bayona, S., Villamor-Payà, M., Attolini, C.S., Koenig, L.M., Sanchiz-Calvo, M., Boulton, S.J. and Stracker, T.H. [2020] Tousled-Like Kinases Suppress Innate Immune Signaling Triggered by Alternative Lengthening of Telomeres. Cell Rep, 32, 107983. [CrossRef]
- Sukackaite, R., Cornacchia, D., Jensen, M.R., Mas, P.J., Blackledge, M., Enervald, E., Duan, G., Auchynnikava, T., Köhn, M., Hart, D.J. et al. [2017] Mouse Rif1 is a regulatory subunit of protein phosphatase 1 (PP1). Sci Rep, 7, 2119. [CrossRef]
- Yang, S., Liu, L., Cao, C., Song, N., Wang, Y., Ma, S., Zhang, Q., Yu, N., Ding, X., Yang, F. et al. [2018] USP52 acts as a deubiquitinase and promotes histone chaperone ASF1A stabilization. Nat Commun, 9, 1285. [CrossRef]
- Escribano-Díaz, C., Orthwein, A., Fradet-Turcotte, A., Xing, M., Young, Jordan T.F., Tkáč, J., Cook, Michael A., Rosebrock, Adam P., Munro, M., Canny, Marella D. et al. [2013] A Cell Cycle-Dependent Regulatory Circuit Composed of 53BP1-RIF1 and BRCA1-CtIP Controls DNA Repair Pathway Choice. Molecular Cell, 49, 872-883. [CrossRef]
- Batenburg, N.L., Walker, J.R., Noordermeer, S.M., Moatti, N., Durocher, D. and Zhu, X.-D. [2017] ATM and CDK2 control chromatin remodeler CSB to inhibit RIF1 in DSB repair pathway choice. Nature Communications, 8, 1921. [CrossRef]
- Tang, M., Chen, Z., Wang, C., Feng, X., Lee, N., Huang, M., Zhang, H., Li, S., Xiong, Y. and Chen, J. [2022] Histone chaperone ASF1 acts with RIF1 to promote DNA end-joining in BRCA1-deficient cells. Journal of Biological Chemistry, 101979. [CrossRef]
- Xu, L. and Blackburn, E.H. [2004] Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. Journal of Cell Biology, 167, 819-830. [CrossRef]
- Carrera, P., Moshkin, Y.M., Grönke, S., Silljé, H.H.W., Nigg, E.A., Jäckle, H. and Karch, F. [2003] Tousled-like kinase functions with the chromatin assembly pathway regulating nuclear divisions. Genes & Development, 17, 2578-2590. [CrossRef]
- Han, Z., Riefler, G.M., Saam, J.R., Mango, S.E. and Schumacher, J.M. [2005] The C. elegans Tousled-like Kinase Contributes to Chromosome Segregation as a Substrate and Regulator of the Aurora B Kinase. Current Biology, 15, 894-904. [CrossRef]
- Korsholm, L.M., Gál, Z., Nieto, B., Quevedo, O., Boukoura, S., Lund, C.C. and Larsen, D.H. [2020] Recent advances in the nucleolar responses to DNA double-strand breaks. Nucleic Acids Research, 48, 9449-9461. [CrossRef]
- Takayama, Y., Kokuryo, T., Yokoyama, Y., Ito, S., Nagino, M., Hamaguchi, M. and Senga, T. [2010] Silencing of Tousled-like kinase 1 sensitizes cholangiocarcinoma cells to cisplatin-induced apoptosis. Cancer Lett, 296, 27-34. [CrossRef]
- Lairmore, T.C., Abdulsattar, J., De Benedetti, A., Shi, R., Huang, S., Khalil, M.I. and Witt, S.N. [2023] Loss of tumor suppressor menin expression in high grade cholangiocarcinomas. BMC Research Notes, 16, 15. [CrossRef]
- Singh, V., Jaiswal, P.K., Ghosh, I., Koul, H.K., Yu, X. and De Benedetti, A. [2019] The TLK1-Nek1 axis promotes prostate cancer progression. Cancer Letters, 453, 131-141. [CrossRef]
- Singh, V., Jaiswal, P.K., Ghosh, I., Koul, H.K., Yu, X. and De Benedetti, A. [2019] Targeting the TLK1/NEK1 DDR axis with Thioridazine suppresses outgrowth of androgen independent prostate tumors. International Journal of Cancer, 145, 1055-1067.
- Ibrahim, K., Abdul Murad, N.A., Harun, R., Jamal, R., Ibrahim, K., Abdul Murad, N.A., Harun, R., Jamal, R., Ibrahim, K., Abdul Murad, N.A. et al. [2020] Knockdown of Tousled-like kinase 1 inhibits survival of glioblastoma multiforme cells. Int J Mol Med, 46, 685-699. [CrossRef]
- Lee, S.-B., Segura-Bayona, S., Villamor-Payà, M., Saredi, G., Todd, M.A.M., Attolini, C.S.-O., Chang, T.-Y., Stracker, T.H. and Groth, A. [2018] Tousled-like kinases stabilize replication forks and show synthetic lethality with checkpoint and PARP inhibitors. Science Advances, 4, eaat4985. [CrossRef]
- Kim, J.-A., Anurag, M., Veeraraghavan, J., Schiff, R., Li, K. and Wang, X.-S. [2016] Amplification of TLK2 Induces Genomic Instability via Impairing the G2–M Checkpoint. Molecular Cancer Research, 14, 920-927.
- Lin, M., Yao, Z., Zhao, N. and Zhang, C. [2019] TLK2 enhances aggressive phenotypes of glioblastoma cells through the activation of SRC signaling pathway. Cancer Biol Ther, 20, 101-108. [CrossRef]
- Van Roy, N., Vandesompele, J., Berx, G., Staes, K., Van Gele, M., De Smet, E., De Paepe, A., Laureys, G., van der Drift, P., Versteeg, R. et al. [2002] Localization of the 17q breakpoint of a constitutional 1;17 translocation in a patient with neuroblastoma within a 25-kb segment located between the ACCN1 and TLK2 genes and near the distal breakpoints of two microdeletions in neurofibromatosis type 1 patients. Genes Chromosomes Cancer, 35, 113-120. [CrossRef]
- Shaaban, M., Othman, H., Ibrahim, T., Ali, M., Abdelmoaty, M., Abdel-Kawi, A.R., Mostafa, A., El Nakeeb, A., Emam, H. and Refaat, A. [2020] Immune Checkpoint Regulators: A New Era Toward Promising Cancer Therapy. Curr Cancer Drug Targets, 20, 429-460. [CrossRef]
- Lentz, R.W., Colton, M.D., Mitra, S.S. and Messersmith, W.A. [2021] Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol Cancer Ther, 20, 961-974. [CrossRef]
- Villamor-Paya, M., Sanchiz-Calvo, M., Smak, J., Pais, L., Sud, M., Shankavaram, U., Lovgren, A.K., Austin-Tse, C., Ganesh, V.S., Gay, M. et al. [2023] Identification of a de novo mutation in TLK1 associated with a neurodevelopmental disorder and immunodeficiency. medRxiv, 2023.2008.2022.23294267. [CrossRef]
- Mortuza, G.B., Hermida, D., Pedersen, A.-K., Segura-Bayona, S., López-Méndez, B., Redondo, P., Rüther, P., Pozdnyakova, I., Garrote, A.M., Muñoz, I.G. et al. [2018] Molecular basis of Tousled-Like Kinase 2 activation. Nature Communications, 9, 2535. [CrossRef]
- Bhoir, S. and De Benedetti, A. [2023] Targeting Prostate Cancer, the ‘Tousled Way&rsquo. International Journal of Molecular Sciences. [CrossRef]
- Khalil, M.I. and De Benedetti, A. [2022] Tousled-like kinase 1: a novel factor with multifaceted role in mCRPC progression and development of therapy resistance. Cancer Drug Resistance, 5, 93-101. [CrossRef]
- Singh, V., Khalil, M.I. and De Benedetti, A. [2020] The TLK1/Nek1 axis contributes to mitochondrial integrity and apoptosis prevention via phosphorylation of VDAC1. Cell Cycle, 19, 363-375. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




