Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction: Diabetes – A Global Epidemic
1.1. Glucose Homeostasis
1.2. Glucose-Stimulated Insulin Secretion
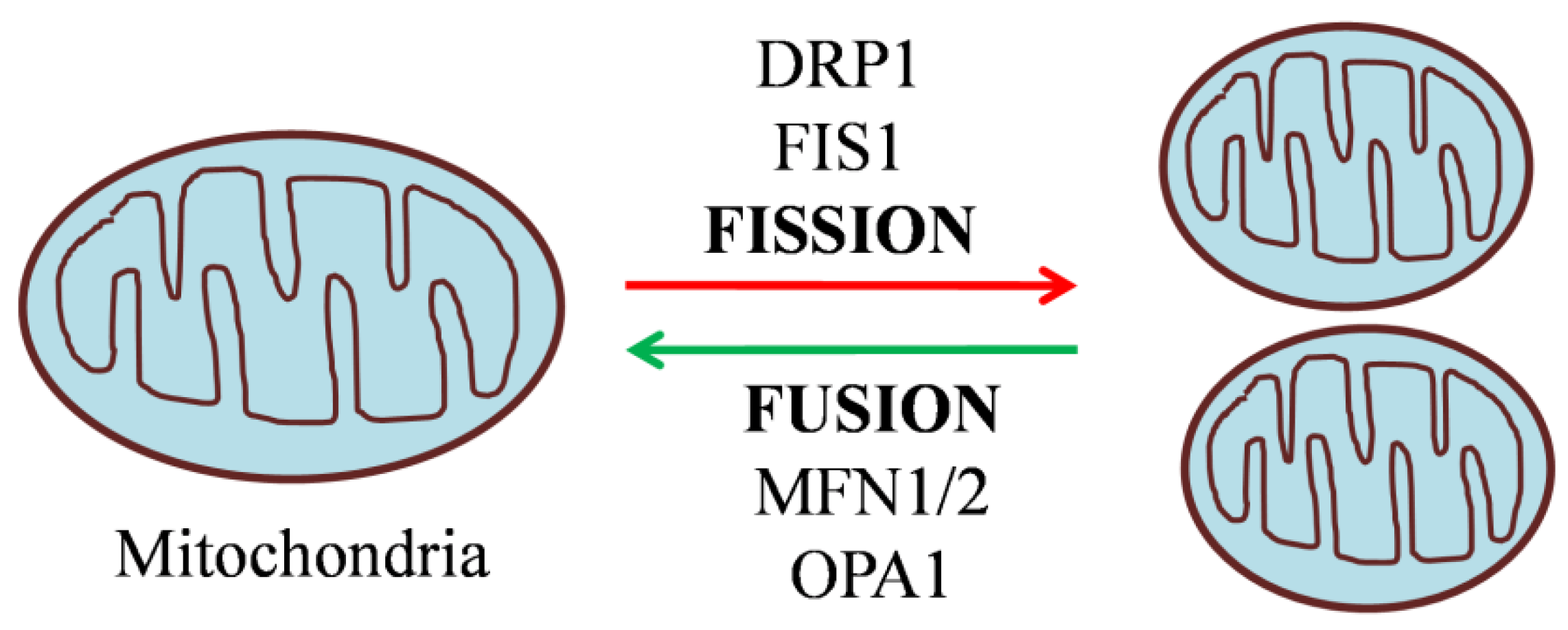
2. Mitochondrial Dynamics in Diabetes
2.1. Mitochondrial Fusion and its Machinery
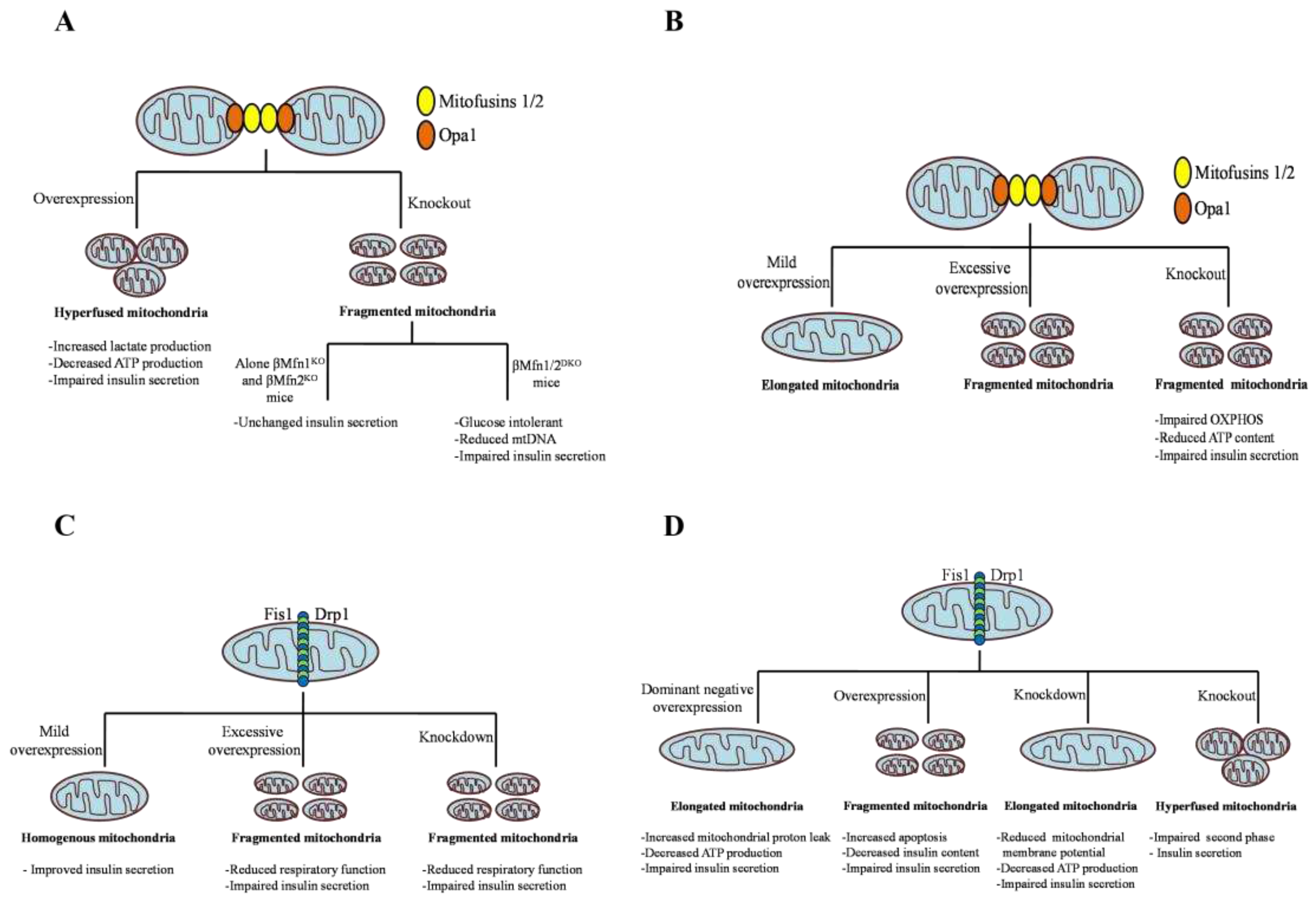
2.1.1. Outer Membrane Fusion Proteins: Mitofusins (Mfn1/2)
2.1.2. Inner Membrane Fusion Protein: Optic Atrophy 1 (Opa1)
2.2. Mitochondrial Fission and its Machinery
2.2.1. Mitochondrial Fission 1 Protein (Fis1)
2.2.2. Dynamin Related Protein 1 (Drp1)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debono, M.; Cachia, E. The impact of diabetes on psychological well being and quality of life. The role of patient education. Psychology, Health and Medicine. 2007, 12, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.Y.; Hsu, D.Y.; Chou, C.H. Predicting the Onset of Diabetes with Machine Learning Methods. Journal of Personalized Medicine. 2023, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Baynes, H.W. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J diabetes metab. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Medical clinics. 2004, 88, 787–835. [Google Scholar] [CrossRef]
- Weyer, C.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Clinical Diabetology. 2001, 2, 167–172. [Google Scholar] [CrossRef]
- Kaiser, N.; Leibowitz, G. Failure of beta-cell adaptation in type 2 diabetes: lessons from animal models. Frontiers in Bioscience-Landmark. 2009, 14, 1099–1115. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Migliorini, A.; Bade, E.; Lickert, H. Islet cell plasticity and regeneration. Molecular metabolism. 2014, 3, 268–274. [Google Scholar] [CrossRef]
- Donnelly, R.; Emslie-Smith, A.M.; Gardner, I.D.; Morris, A.D. Vascular complications of diabetes. Bmj. 2000, 320, 1062–1066. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019, 62, 3–16. [Google Scholar] [CrossRef]
- de Boer, P.; Giepmans, B.N. State-of-the-art microscopy to understand islets of Langerhans: what to expect next? Immunology and cell biology. 2021, 99, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Armanet, M.; Morel, P.; Niclauss, N.; Sgroi, A.; Muller, Y.D.; Giovannoni, L.; Parnaud, G.; Berney, T. Unique arrangement of α-and β-cells in human islets of Langerhans. Diabetes. 2010, 59, 1202–1210. [Google Scholar] [CrossRef]
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi. C.; Berggren, P.O.;Caicedo, A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences. 2006, 103, 2334–2339. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, J.W.; Shin, J.A.; Shin, J.; Yoon, K.H. β-cell mass in people with type 2 diabetes. Journal of diabetes investigation. 2011, 2, 6–17. [Google Scholar] [CrossRef]
- Tups, A.; Benzler, J.; Sergi, D.; Ladyman, S.R.; Williams, L.M. Central regulation of glucose homeostasis. Comprehensive physiology. 2011, 7, 741–764. [Google Scholar] [CrossRef]
- Marty, N.; Dallaporta, M.; Thorens, B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology. 2007, 22, 241–251. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Tschöp, M.H.; Woods, S.C.; Morton, G.J.; Myers, M.G.; D’Alessio, D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013, 503, 59–66. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Current diabetes reviews. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Seino, S. Cell signalling in insulin secretion: the molecular targets of ATP, cAMP and sulfonylurea. Diabetologia. 2012, 55, 2096–2108. [Google Scholar] [CrossRef]
- Herman, M.A.; Kahn, B.B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. The Journal of clinical investigation. 2006, 116, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Mertz, R.J.; Worley, J.F.; Spencer, B.; Johnson, J.H.; Dukes, I.D. Activation of Stimulus-Secretion Coupling in Pancreatic β-Cells by Specific Products of Glucose Metabolism: Evidence for privileged signaling by glycolysis. Journal of Biological Chemistry. 1996, 27, 4838–4845. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Current opinion in plant biology. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Liemburg-Apers, D.C.; Imamura, H.; Forkink, M.; Nooteboom, M.; Swarts, H.G.; Brock, R.; Smeitink, J.A.; Willems, P.H.; Koopman, W.J. Quantitative glucose and ATP sensing in mammalian cells. Pharmaceutical research. 2011, 28, 2745–2757. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M.; Proks, P.; Smith, P.A.; Ämmälä, C.; Bokvist, K.; Rorsman, P. Stimulus–secretion coupling in pancreatic β cells. Journal of cellular biochemistry. 1994, 55, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Pullen, T.J.; Hodson, D.J.; Martinez-Sanchez, A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochemical Journal. 2015, 466, 203–218. [Google Scholar] [CrossRef]
- Henquin, J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000, 49, 1751–1760. [Google Scholar] [CrossRef]
- Straub, S.G.; Sharp, G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes/metabolism research and reviews. 2002, 18, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.A.; Li, C.; Soleimanpour, S.A. Mitochondrial regulation of β-cell function: maintaining the momentum for insulin release. Molecular aspects of medicine. 2015, 42, 91–104. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006, 125, 1241–1252. [Google Scholar] [CrossRef]
- Wiederkehr, A.; Wollheim, C.B. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic β-cell. Cell calcium. 2008, 44, 64–76. [Google Scholar] [CrossRef]
- Kennedy, E.D.; Maechler, P.; Wollheim, C.B. Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes. 1998, 47, 374–380. [Google Scholar] [CrossRef]
- De Andrade, P.B.; Rubi, B.; Frigerio, F.; Van den Ouweland, J.M.; Maassen, J.A.; Maechler, P. Diabetes-associated mitochondrial DNA mutation A3243G impairs cellular metabolic pathways necessary for beta cell function. Diabetologia. 2006, 49, 1816–1826. [Google Scholar] [CrossRef]
- Silva, J.P.; Köhler, M.; Graff, C.; Oldfors, A.; Magnuson, M.A.; Berggren, P.O.; Larsson, N.G. Impaired insulin secretion and β-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nature genetics. 2000, 26, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Kytövuori, L.; Lipponen, J.; Rusanen, H.; Komulainen, T.; Martikainen, M.H.; Majamaa, K. A novel mutation m. 8561C> G in MT-ATP6/8 causing a mitochondrial syndrome with ataxia, peripheral neuropathy, diabetes mellitus, and hypergonadotropic hypogonadism. Journal of neurology. 2016, 263, 2188–2195. [Google Scholar] [CrossRef]
- Dlasková, A.; Špaček, T.; Šantorová, J.; Plecitá-Hlavatá, L.; Berková, Z.; Saudek, F.; Lessard, M.; Bewersdorf, J.; Ježek, P. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet β-cells, an experimental model of type-2 diabetes. Biochimica et Biophysica Acta -Bioenergetics. 2010, 1797, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Fusion and fission: interlinked processes critical for mitochondrial health. Annual review of genetics. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Higa, M.; Zhou, Y.T.; Ravazzola, M.; Baetens, D.; Orci, L.; Unger, R.H. Troglitazone prevents mitochondrial alterations, β cell destruction, and diabetes in obese prediabetic rats. Proceedings of the National Academy of Sciences. 1999, 96, 11513–11518. [Google Scholar] [CrossRef]
- Mizukami, H.; Wada, R.; Koyama, M.; Takeo, T.; Suga, S.; Wakui, M.; Yagihashi, S. Augmented β cell loss and mitochondrial abnormalities in sucrose-fed GK rats. VirchowsArchiv. 2008, 452, 383–392. [Google Scholar] [CrossRef]
- Anello, M.; Lupi, R.; Spampinato, D.; Piro, S.; Masini, M.; Boggi, U.; Del Prato, S.; Rabuazzo, A.M.; Purrello, F.; Marchetti, P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005, 48, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Vatamaniuk, M.; Huang, X.; Doliba, N.; Lian, M.M.; Frank, A.; Velidedeoglu, E.; Desai, N.M.; Koeberlein, B.; Wolf, B.; Barker, C.F. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004, 53, 624–632. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006, 22, 79–99. [Google Scholar] [CrossRef]
- Hoppins, S.; Lackner, L.; Nunnari, J. The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 2007, 76, 751–780. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Eura, Y.; Mihara, K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. Journal of cell science. 2004, 117, 6535–6546. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. The Journal of cell biology. 2003, 160, 189–200. [Google Scholar] [CrossRef]
- Chen, H.; Vermulst, M.; Wang, Y.E.; Chomyn, A.; Prolla, T.A.; McCaffery, J.M.; Chan, D.C. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010, 141, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Frank, S.; Gaume, B.; Herrler, M.; Youle, R.J.; Fuller, M.T. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. Journal of cell science. 2003, 116, 2763–2774. [Google Scholar] [CrossRef]
- Züchner, S.; Mersiyanova, I.V.; Muglia, M.; Bissar-Tadmouri, N.; Rochelle, J.; Dadali, E.L.; Zappia, M.; Nelis, E.; Patitucci, A.; Senderek, J.; Parman, Y. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nature genetics. 2004, 36, 449–451. [Google Scholar] [CrossRef]
- Park, K.S.; Wiederkehr, A.; Kirkpatrick, C.; Mattenberger, Y.; Martinou, J.C.; Marchetti, P.; Demaurex, N.; Wollheim, C.B. Selective Actions of Mitochondrial Fission/Fusion Genes on Metabolism-Secretion Coupling in Insulin-releasing Cells. Journal of Biological Chemistry. 2008, 283, 33347–33356. [Google Scholar] [CrossRef]
- Park, K.S.; Wiederkehr, A.; Wollheim, C.B. Defective mitochondrial function and motility due to mitofusin 1 overexpression in insulin secreting cells. The Korean Journal of Physiology & Pharmacology. [CrossRef]
- Sidarala, V.; Zhu, J.; Levi-D’Ancona, E.; Pearson, G.L.; Reck, E.C.; Walker, E.M.; Kaufman, B.A.; Soleimanpour, S.A. Mitofusin 1 and 2 regulation of mitochondrial DNA content is a critical determinant of glucose homeostasis. Nature Communications. 2022, 13, 2340. [Google Scholar] [CrossRef]
- Georgiadou, E.; Muralidharan, C.; Martinez, M.; Chabosseau, P.; Tomas, A.; Wern, F.Y.; Stylianides, T.; Rothery, S.M.; Di Gregorio, A.; Leclerc, I.; Ali, Y. Pancreatic beta cell selective deletion of mitofusins 1 and 2 (Mfn1 and Mfn2) disrupts mitochondrial architecture and abrogates glucose-stimulated insulin secretion in vivo. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L. Dysregulated mitochondrial dynamics and metabolism in obesity, diabetes, and cancer. Frontiers in endocrinology. 2019, 10, 570. [Google Scholar] [CrossRef]
- Georgiadou, E.; Muralidharan, C.; Martinez, M.; Chabosseau, P.; Akalestou, E.; Tomas, A.; Wern, F.Y.; Stylianides, T.; Wretlind, A.; Legido-Quigley, C.; Jones, B. Mitofusins Mfn1 and Mfn2 Are Required to Preserve Glucose-but Not Incretin-Stimulated β-Cell Connectivity and Insulin Secretion. Diabetes. 2022, 71, 1472–1489. [Google Scholar] [CrossRef]
- Ramırez, S.; Gomez-Valades, A.G.; Schneeberger, M.; Varela, L.; Haddad-Tovolli, R.; Altirriba, J.; Noguera, E.; Drougard, A.; Flores-Martınez, A.; Imbernon, M.; Chivite, I. Mitochondrial dynamics mediated by mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab. 2017, 25, 1390–1399. [Google Scholar] [CrossRef]
- Benard, G.; Karbowski, M. Mitochondria fusion and fission. Cell Death. 2010, 22, 97. [Google Scholar]
- Cipolat, S.; de Brito, O.M.; Dal Zilio, B.; Scorrano, L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proceedings of the National Academy of Sciences. 2004, 101, 5927–5932. [Google Scholar] [CrossRef]
- Alexander, C.; Votruba, M.; Pesch, U.E.; Thiselton, D.L.; Mayer, S.; Moore, A.; Rodriguez, M.; Kellner, U.; Leo-Kottler, B.; Auburger, G.; Bhattacharya, S.S. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nature genetics. 2000, 26, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Olichon, A.; Baricault, L.; Gas, N.; Guillou, E.; Valette, A.; Belenguer, P.; Lenaers, G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. Journal of Biological Chemistry. 2003, 278, 7743–7746. [Google Scholar] [CrossRef] [PubMed]
- Olichon, A.; Landes, T.; Arnauné-Pelloquin, L.; Emorine, L.J.; Mils, V.; Guichet, A.; Delettre, C.; Hamel, C.; Amati-Bonneau, P.; Bonneau, D.; Reynier, P. Effects of OPA1 mutations on mitochondrial morphology and apoptosis: relevance to ADOA pathogenesis. Journal of cellular physiology. 2007, 211, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Cipolat, S.; De Brito, O.M.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; Scorrano, L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006, 126, 177–189. [Google Scholar] [CrossRef]
- Hudson, G.; Amati-Bonneau, P.; Blakely, E.L. , Stewart, J.D.; He, L.; Schaefer, A.M.; Griffiths, P.G.;Ahlqvist, K.;Suomalainen, A.;Reynier, P.; McFarland, R. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain. 2008, 131, 329–337. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Physiological functions of mitochondrial fusion. Annals of the New York Academy of Sciences. 2010, 1201, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wakabayashi, N.; Wakabayashi, J.; Tamura, Y.; Song, W.J.; Sereda, S. Clerc, P.Polster, B.M.; Aja, S.M.;Pletnikov, M.V.;Kensler, T.W. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Molecular biology of the cell. 2011, 22, 2235–2245. [Google Scholar] [CrossRef]
- Molina, A.J.; Wikstrom, J.D.; Stiles, L.; Las, G.; Mohamed, H.; Elorza, A.; Walzer, G.; Twig, G.; Katz, S.; Corkey, B.E.; Shirihai, O.S. Mitochondrial networking protects β-cells from nutrient-induced apoptosis. Diabetes. 2009, 58, 2303–2315. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L. , Haigh, S.E.; Katz, S.; Las, G.;Alroy, J. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Shurland, D.L.; Ryazantsev, S.N.; van der Bliek, A.M. A human dynamin-related protein controls the distribution of mitochondria. The Journal of cell biology. 1998, 143, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Van Der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science. 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Yu, R.; Jin, S.B.; Lendahl, U.; Nistér, M.; Zhao, J. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. The EMBO journal. 2019, 38, e99748. [Google Scholar] [CrossRef]
- Losón, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Molecular biology of the cell. 2013, 24, 659–667. [Google Scholar] [CrossRef]
- Ihenacho, U.K.; Meacham, K.A.; Harwig, M.C.; Widlansky, M.E.; Hill, R.B. ; Mitochondrial fission protein 1: emerging roles in organellar form and function in health and disease. Frontiers in Endocrinology. 2021, 12, 660095. [Google Scholar] [CrossRef]
- Maechler, P. Mitochondrial function and insulin secretion. Molecular and cellular endocrinology. 2013, 379, 2–8. [Google Scholar] [CrossRef]
- Schultz, J.; Waterstradt, R.; Kantowski, T.; Rickmann, A.; Reinhardt, F.; Sharoyko, V.; Mulder, H.; Tiedge, M.; Baltrusch, S. Precise expression of Fis1 is important for glucose responsiveness of beta cells. J Endocrinol. 2016, 230, 81–91. [Google Scholar] [CrossRef]
- Smirnova, E.; Shurland, D.L.; Ryazantsev, S.N.; van der Bliek, A.M. A human dynamin-related protein controls the distribution of mitochondria. The Journal of cell biology. 1998, 143, 51–58. [Google Scholar] [CrossRef]
- Hoppins, S.; Lackne,r L. ; Nunnari, J. The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 2007, 76, 751–780. [Google Scholar] [CrossRef]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; Van Der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Molecular biology of the cell. 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Macdonald, P.J.; Francy, C.A.; Stepanyants, N.; Lehman, L.; Baglio, A.; Mears, J.A.; Qi, X.; Ramachandran, R. Distinct splice variants of dynamin-related protein 1 differentially utilize mitochondrial fission factor as an effector of cooperative GTPase activity. Journal of Biological Chemistry. 2016, 291, 493–507. [Google Scholar] [CrossRef]
- Strack, S.; Wilson, T.J.; Cribbs, J.T. Cyclin-dependent kinases regulate splice-specific targeting of dynamin-related protein 1 to microtubules. Journal of Cell Biology. 2013, 201, 1037–1051. [Google Scholar] [CrossRef]
- Uo, T.; Dworzak, J.; Kinoshita, C.; Inman, D.M.; Kinoshita, Y.; Horner, P.J.; Morrison, R.S. Drp1 levels constitutively regulate mitochondrial dynamics and cell survival in cortical neurons. Experimental neurology. 2009, 218, 274–285. [Google Scholar] [CrossRef]
- Mears, J.A.; Lackner, L.L.; Fang, S.; Ingerman, E.; Nunnari, J.; Hinshaw, J.E. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nature structural & molecular biology. 2011, 18, 20–26. [Google Scholar] [CrossRef]
- Yapa, N.M.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS letters. 2021, 595, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. Journal of Cell Biology. 2010, 191, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Richter, V.; Palmer, C.S.; Osellame, L.D.; Singh, A.P.; Elgass, K.; Stroud, D.A.; Sesaki, H.; Kvansakul, M.; Ryan, M.T. Structural and functional analysis of MiD51, a dynamin receptor required for mitochondrial fission. Journal of Cell Biology. 2014, 204, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Elgass, K.D.; Parton, R.G.; Osellame, L.D.; Stojanovski, D.; Ryan, M.T. Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. Journal of Biological Chemistry. 2013, 288, 27584–27593. [Google Scholar] [CrossRef]
- Kamerkar, S.C.; Kraus, F.; Sharpe, A.J.; Pucadyil, T.J.; Ryan, M.T. Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nature communications. 2018, 9, 5239. [Google Scholar] [CrossRef] [PubMed]
- Santel, A. Frank, S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB life. 2008, 60, 448–455. [Google Scholar] [CrossRef]
- Taguchi, N.; Ishihara, N.; Jofuku, A.; Oka, T.; Mihara, K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. Journal of Biological Chemistry. 2007, 282, 11521–11529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, B.O.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. Journal of neuroscience. 2009, 29, 9090–9103. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Disatnik, M.H.; Shen, N.; Sobel, R.A.; Mochly-Rosen, D. Aberrant mitochondrial fission in neurons induced by protein kinase Cδ under oxidative stress conditions in vivo. Molecular biology of the cell. 2011, 22, 256–265. [Google Scholar] [CrossRef]
- Chang, C.R.; Blackstone, C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. Journal of Biological Chemistry. 2007, 282, 21583–21587. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Benedetto, G.D.; Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nature cell biology. 2011, 13, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Cereghetti, G.M.; Stangherlin, A.; De Brito, O.M.; Chang, C.R.; Blackstone, C.; Bernardi, P.; Scorrano, L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proceedings of the National Academy of Sciences. 2008, 105, 15803–15808. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Iñiguez-Lluhí, J.A.; Stadler, J.; Chang, C.R.; Arnoult, D.; Keller, P.J.; Hong, Y.; Blackstone, C.; Feldman, E.L. SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. The FASEB Journal. 2009, 23, 3917. [Google Scholar] [CrossRef] [PubMed]
- Harder, Z.; Zunino, R.; McBride, H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Current Biology. 2004, 14, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Zunino, R.; Schauss, A.; Rippstein, P.; Andrade-Navarro, M.; McBride, H.M. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. Journal of cell science. 2007, 120, 1178–1188. [Google Scholar] [CrossRef]
- Karbowski, M.; Neutzner, A.; Youle, R.J. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. The Journal of cell biology. 2007, 178, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, P.; Du, L.; Tian, W.; Yue, W.; Liu, M.; Li, D.; Wang, B.; Zhu, Y.; Cao, C.; Zhou, J. Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. Journal of Biological Chemistry. 2011, 286, 11649–11658. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science. 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Gawlowski, T.; Suarez, J.; Scott, B.; Torres-Gonzalez, M.; Wang, H.; Schwappacher, R.; Han, X.; Yates, J.R.; Hoshijima, M.; Dillmann, W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. Journal of Biological Chemistry. 2012, 287, 30024–30034. [Google Scholar] [CrossRef]
- Chang, C.R.; Manlandro, C.M.; Arnoult, D.; Stadler, J.; Posey, A.E.; Hill, R.B.; Blackstone, C. A lethal de novo mutation in the middle domain of the dynamin-related GTPase Drp1 impairs higher order assembly and mitochondrial division. Journal of Biological Chemistry. 2010, 285, 32494–32503. [Google Scholar] [CrossRef]
- Parone, P.A.; Da Cruz, S.; Tondera, D.; Mattenberger, Y.; James, D.I.; Maechler, P.; Barja, F.; Martinou, J.C. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PloS one. 2008, 3, e3257. [Google Scholar] [CrossRef]
- Singh, M.; Denny, H.; Smith, C.; Granados, J.; Renden, R. Presynaptic loss of dynamin-related protein 1 impairs synaptic vesicle release and recycling at the mouse calyx of Held. The Journal of Physiology. 2018, 596, 6263–6287. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Y.; Gan, X.; Fang, D.; Zhong, C.; Wu, L.; Hu, G.; Sosunov, A.A.; McKhann, G.M.; Yu, H.; Yan, S.S. Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes. 2015, 64, 1728–1742. [Google Scholar] [CrossRef]
- Jhun, B.S.; Lee, H.; Jin, Z.G.; Yoon, Y. Glucose stimulation induces dynamic change of mitochondrial morphology to promote insulin secretion in the insulinoma cell line INS-1E. PloS one. 2013, 8, e60810. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, F.; Schultz, J.; Waterstradt, R.; Baltrusch, S. Drp1 guarding of the mitochondrial network is important for glucose-stimulated insulin secretion in pancreatic beta cells. Biochemical and biophysical research communications. 2016, 474, 646–651. [Google Scholar] [CrossRef]
- Nan, J.; Lee, J.S.; Moon, J.H.; Lee, S.A.; Park, Y.J.; Lee, D.S.; Chung, S.S.; Park, K.S. SENP2 regulates mitochondrial function and insulin secretion in pancreatic β cells. Experimental & Molecular Medicine. 2022, 54, 72–80. [Google Scholar] [CrossRef]
- Kabra, U.D.; Pfuhlmann, K.; Migliorini, A.; Keipert, S.; Lamp, D.; Korsgren, O.; Gegg, M.; Woods, S.C.; Pfluger, P.T.; Lickert, H.; Affourtit, C. Direct Substrate Delivery Into Mitochondrial Fission–Deficient Pancreatic Islets Rescues Insulin Secretion. Diabetes. 2017, 66, 1247–1257. [Google Scholar] [CrossRef]
- Bordt, E.A.; Clerc, P.; Roelofs, B.A.; Saladino, A.J.; Tretter, L.; Adam-Vizi, V.; Cherok, E.; Khalil, A.; Yadava, N.; Shealinna, X.G.; Francis, T.C. The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Developmental cell. 2017, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Kabra, U.D.; Moruzzi, N.; Berggren, P.O.; Jastroch, M. Drp1 Overexpression Decreases Insulin Content in Pancreatic MIN6 Cells. International Journal of Molecular Sciences. 2022, 23, 12338. [Google Scholar] [CrossRef] [PubMed]
- Hennings, T.G.; Chopra, D.G.; DeLeon, E.R.; VanDeusen, H.R.; Sesaki, H.; Merrins, M.J.; Ku, G.M. In vivo deletion of β-cell Drp1 impairs insulin secretion without affecting islet oxygen consumption. Endocrinology. 2018, 159, 3245–3256. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Wang, H.; Li, M.; Cai, H.; Xu, S.; Zhang, W.; Xu, Y.; Ye, L.; Yang, W.; Wollheim, C.B.; Lou, J. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. The international journal of biochemistry & cell biology. 2009, 41, 879–890. [Google Scholar] [CrossRef]
- Peng, L.; Men, X.; Zhang, W.; Wang, H.; Xu, S.; Xu, M.; Xu, Y.; Yang, W.; Lou, J. Dynamin-related protein 1 is implicated in endoplasmic reticulum stress-induced pancreatic β-cell apoptosis. International journal of molecular medicine. 2011, 28, 161–169. [Google Scholar] [CrossRef] [PubMed]

| Dynamin GTPase | Genetic intervention | Effect on mitochondrial morphology | Effect on β cell function | References |
|---|---|---|---|---|
| Mfn1/2 | Mfn1 overexpression in Ins1e cells and primary β cells | Hyperfused and aggregated | Increased lactate production, decreased cellular ATP levels, and impaired GSIS | [48] |
| Mfn1 overexpression in Ins1e cells | Hyperfused and aggregated | Loss of mitophagy, hypomotility, and impaired mitochondrial function and insulin secretion | [49] | |
| DN-Mfn1 overexpression in INS1e cells | Discrete |
No significant changes in apoptosis, mitochondrial hyperpolarisation, and metabolism–secretion coupling | [55,56] | |
| βMfn1/2KO mice |
Fragmented mitochondria, disrupted cristae shape and structures | Reduced Ca++ accumulation, mitochondrial membrane potential, β cell connectivity, and GSIS | [51,52,53] | |
| Alone βMfn1KO mice and βMfn2KO mice | Fragmented | Normal glucose homeostasis and no change in insulin secretion | [50] | |
| βMfn1/2DKO mice |
Fragmented | Glucose intolerant, reduced mtDNA content, and reduced insulin secretion | [50] | |
| Opa1 | RIP2-Opa1 KO β cells |
Fragmented mitochondria and abnormal cristae structures | Decreased complex IV level, impaired OXPHOS, decreased ATP content, reduced insulin secretion and cell proliferation | [63] |
| Mild overexpression in INS1e cell | Elongated | NA | [64,65] | |
| High overexpression in INS1e cell | Fragmented | NA | [64,65] | |
| Overexpression in primary β cell | Fragmented | NA | [64] | |
| Fis1 | Downregulation by RNAi in INS1e cells | Elongated |
Reduced mitophagy, respiratory functions, and GSIS |
[65] |
| Knockdown by shRNA INS832/13 and primary mouse β cells | Elongated |
Impaired GSIS but no changes in expression of OXPHOS complexes and ATP synthase activity | [72] |
|
| Overexpression in INS 1e cells | Fragmented, reduced volume, and swollen mitochondria | Increased lactate production, reduced mitochondrial energy metabolism, and impaired insulin secretion | [48] |
|
| Overexpression in primary mouse β cells | Fragmented | Reduced insulin secretion |
[72] |
|
| Overexpression in INS832/12 cells | Homogenous mitochondrial network | Enhanced insulin secretion |
[72] |
|
| Moderate overexpression in RINm5F cells | Homogenous mitochondrial network | Improved insulin secretion |
[72] |
|
| High overexpression in RINm5F cells | Clusters |
Reduced insulin secretion |
[72] |
|
| Drp1 | Drp1 DN (DLP1-K38A) overexpression in INS1e cells | Hyperfused | No significant change in GSIS; prevented apoptosis | [110] |
| Drp1 DN (DLP1-K38A) overexpression in INS1e cells | Swollen and elongated | Decreased mitochondrial autophagy, respiratory functions, and GSIS |
[64,65] | |
| Drp1 DN (DLP1-K38A) overexpression in INS1e cells | Elongated | Increased mitochondrial proton leak, decreased ATP production and GSIS |
[103] |
|
| Downregulation by shRNA in INS1e cells |
Elongated | Decreased mitochondrial membrane potential, reduced ATP production and GSIS |
[104] | |
| Knockdown in NIT-1cells |
Elongated | Impaired GSIS |
[105] | |
| Knockdown genetically or pharmacological inactivation by Mdivi1 in MIN6 cells and islets | Elongated |
Reduced mitochondrial ATP synthesis, and impaired GSIS due to compromised substrate delivery upstream of mitochondria | [106] | |
| β Drp1b-KO mice |
Hyperfused |
Normal oxygen consumption rate and calcium concentration but significantly impaired second-phase insulin secretion | [109] |
|
| Overexpression in INS1e cells |
Round and short | Increased cytochrome C release, and ROS production, no significant change in GSIS but tends towards decreased GSIS | [110,111] | |
| Overexpression in MIN6 cells | Fragmented | No effect on mitochondrial metabolism, impaired GSIS due to reduced insulin content | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





