Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Generation of Igf-I KO mice
2.2. MicroCT evaluation
2.3. Immunohistochemistry
2.4. Statistical Analysis
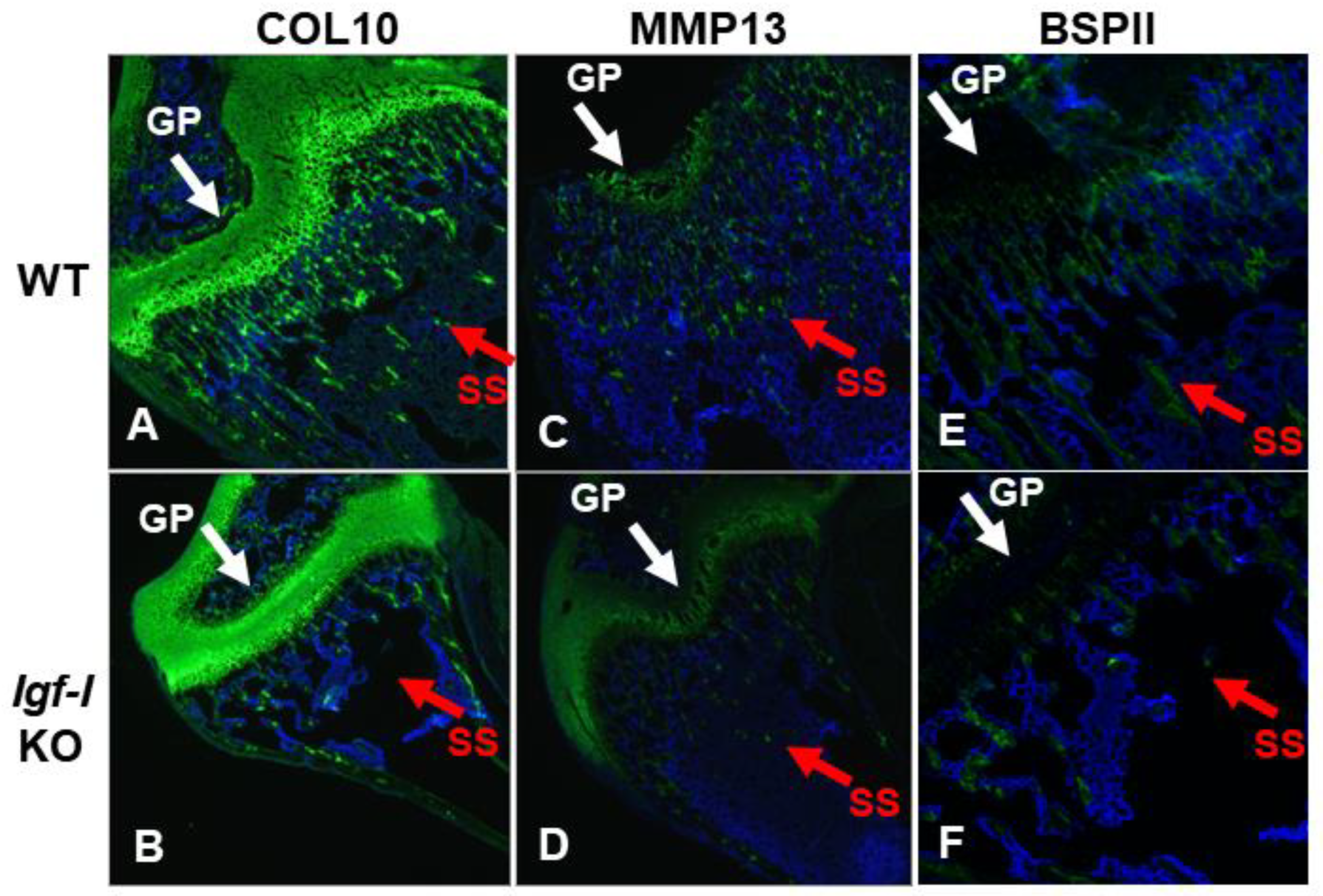
3. Results
3.1. Bone size at the epiphysis is reduced in mice with global and osteoblastic specific disruption of the Igf-I gene but not in chondrocyte-specific conditional KO mice
3.2. Trabecular bone volume and vBMD are reduced at the secondary spongiosa of the distal femur in mice with disruption of Igf-I gene
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ: Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology 2003, 144(3):929-936. [CrossRef]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH et al: Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 2000, 141(7):2674-2682. [CrossRef]
- Bagi CM, Brommage R, Deleon L, Adams S, Rosen D, Sommer A: Benefit of systemically administered rhIGF-I and rhIGF-I/IGFBP-3 on cancellous bone in ovariectomized rats. J Bone Miner Res 1994, 9(8):1301-1312. [CrossRef]
- Libanati C, Baylink DJ, Lois-Wenzel E, Srinvasan N, Mohan S: Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab 1999, 84(8):2807-2814. [CrossRef]
- 5Gao ST, Lv ZT, Zhou CK, Mao C, Sheng WB: Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population: a meta-analysis. BMC Musculoskelet Disord 2018, 19(1):141. [CrossRef]
- Kineman RD, Del Rio-Moreno M, Sarmento-Cabral A: 40 YEARS of IGF1: Understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J Mol Endocrinol 2018, 61(1):T187-T198. [CrossRef]
- Wang Y, Bikle DD, Chang W: Autocrine and Paracrine Actions of IGF-I Signaling in Skeletal Development. Bone research 2013, 1(3):249-259. [CrossRef]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D: Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A 1999, 96(13):7324-7329. [CrossRef]
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson OG, Jansson JO et al: Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A 1999, 96(12):7088-7092. [CrossRef]
- Sjogren K, Jansson JO, Isaksson OG, Ohlsson C: A model for tissue-specific inducible insulin-like growth factor-I (IGF-I) inactivation to determine the physiological role of liver-derived IGF-I. Endocrine 2002, 19(3):249-256. [CrossRef]
- Lazowski DA, Fraher LJ, Hodsman A, Steer B, Modrowski D, Han VK: Regional variation of insulin-like growth factor-I gene expression in mature rat bone and cartilage. Bone 1994, 15(5):563-576. [CrossRef]
- Wang E, Wang J, Chin E, Zhou J, Bondy CA: Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrinology 1995, 136(6):2741-2751. [CrossRef]
- Xing W, Govoni K, Donahue LR, Kesavan C, Wergedal J, Long C, Bassett JH, Gogakos A, Wojcicka A, Williams GR et al: Genetic evidence that thyroid hormone is indispensable for prepubertal IGF-I expression and bone acquisition in mice. J Bone Miner Res 2012, 27:1067-1079. [CrossRef]
- Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S: Conditional deletion of IGF-I in collagen type 1{alpha}2 (Col1{alpha}2) expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 2007, 148(12):5706-5715. [CrossRef]
- Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S: Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics 2007, 30(3):354-362. [CrossRef]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R: Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 2010, 25(7):1468-1486. [CrossRef]
- Xing W, Pourteymoor S, Mohan S: Ascorbic acid regulates osterix expression in osteoblasts by activation of prolyl hydroxylase and ubiquitination-mediated proteosomal degradation pathway. Physiol Genomics 2011, 43(12):749-757. [CrossRef]
- Xing W, Kim J, Wergedal J, Chen ST, Mohan S: Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Mol Cell Biol 2010, 30(3):711-721. [CrossRef]
- Hjortebjerg R, Flyvbjerg A, Frystyk J: Insulin growth factor binding proteins as therapeutic targets in type 2 diabetes. Expert Opin Ther Targets 2014, 18(2):209-224. [CrossRef]
- Rotwein P: Mapping the growth hormone--Stat5b--IGF-I transcriptional circuit. Trends Endocrinol Metab 2012, 23(4):186-193. [CrossRef]
- Rodriguez-Arnao J, Miell JP, Ross RJ: Influence of thyroid hormones on the GH-IGF-I axis. Trends Endocrinol Metab 1993, 4(5):169-173. [CrossRef]
- Boot AM, Engels MA, Boerma GJ, Krenning EP, De Muinck Keizer-Schrama SM: Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab 1997, 82(8):2423-2428. [CrossRef]
- Barrett-Connor E, Goodman-Gruen D: Gender differences in insulin-like growth factor and bone mineral density association in old age: the Rancho Bernardo Study. J Bone Miner Res 1998, 13(8):1343-1349. [CrossRef]
- Janssen JA, Burger H, Stolk RP, Grobbee DE, de Jong FH, Lamberts SW, Pols HA: Gender-specific relationship between serum free and total IGF-I and bone mineral density in elderly men and women. Eur J Endocrinol 1998, 138(6):627-632. [CrossRef]
- Sugimoto T, Nishiyama K, Kuribayashi F, Chihara K: Serum levels of insulin-like growth factor (IGF) I, IGF-binding protein (IGFBP)-2, and IGFBP-3 in osteoporotic patients with and without spinal fractures. J Bone Miner Res 1997, 12(8):1272-1279. [CrossRef]
- Bozic D, Grgurevic L, Erjavec I, Razdorov G, Brkljacic J, Orlic I, Plancak D: Effect of bone morphogenetic protein-7 on gene expression of bone morphogenetic protein-4, dentin matrix protein-1, insulin-like growth factor-I and -II in cementoblasts in vitro. Coll Antropol 2012, 36(4):1265-1271. [CrossRef]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A: Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75(1):59-72. [CrossRef]
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA: IGF-I is required for normal embryonic growth in mice. Genes Dev 1993, 7(12B):2609-2617. [CrossRef]
- Aghajanian P, Mohan S: The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone research 2018, 6:19. [CrossRef]
- Chaqour B, Han JS, Tamura I, Macarak E: Mechanical regulation of IGF-I and IGF-binding protein gene transcription in bladder smooth muscle cells. J Cell Biochem 2002, 84(2):264-277. [CrossRef]
- Bulbring E, Kuriyama H: The action of catecholamines on guinea-pig taenia coli. Philos Trans R Soc Lond B Biol Sci 1973, 265(867):115-121. [CrossRef]
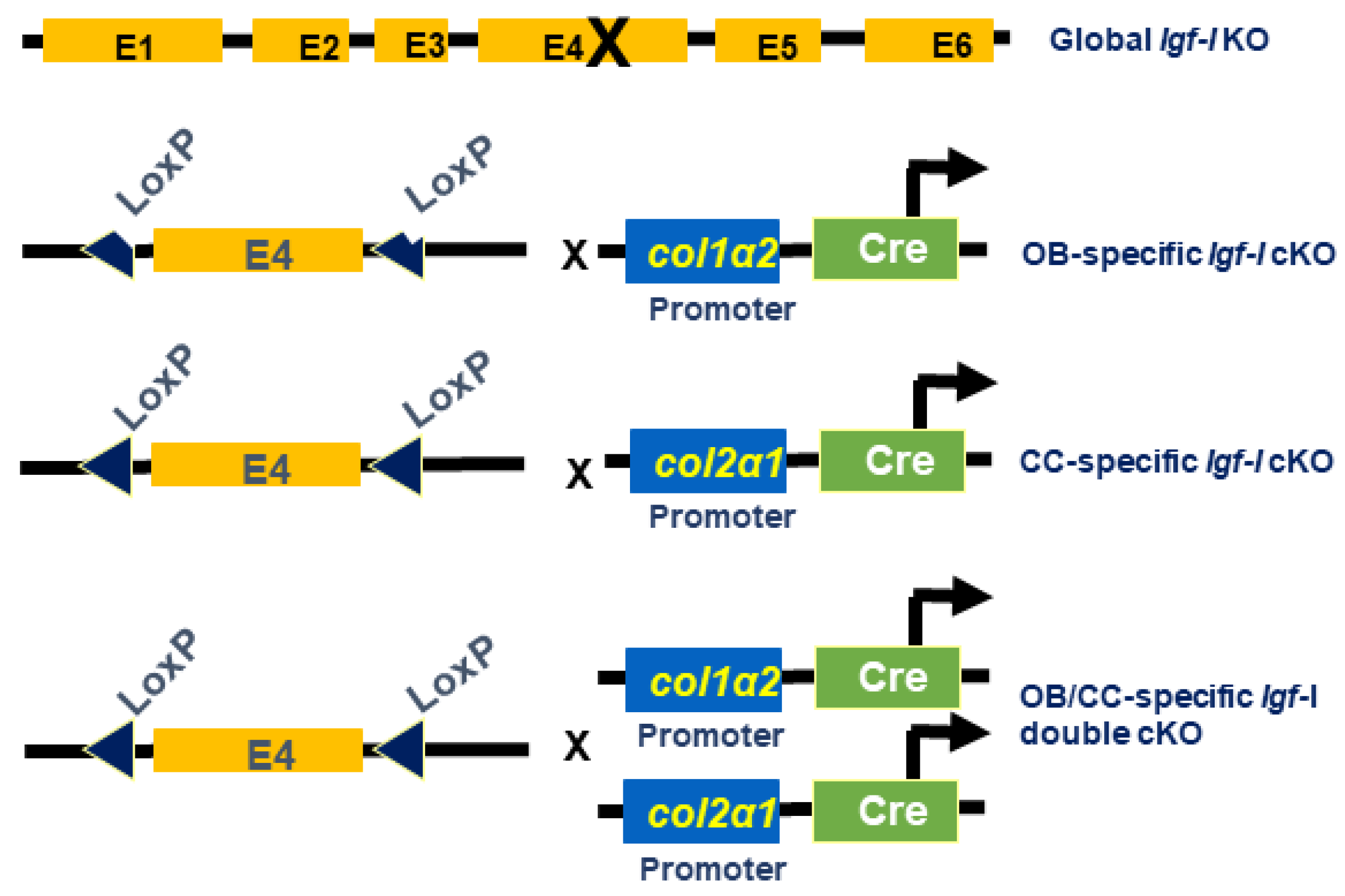
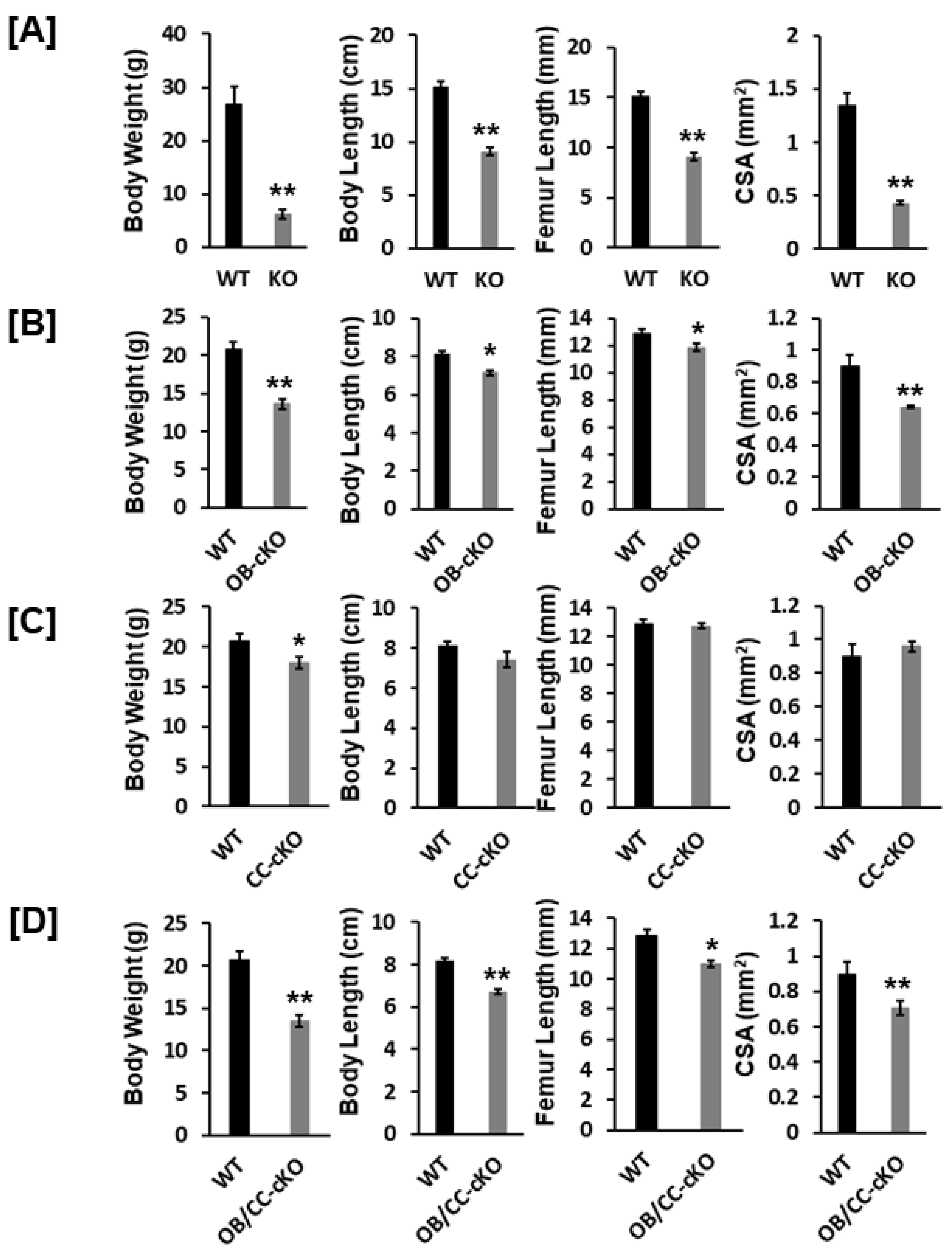
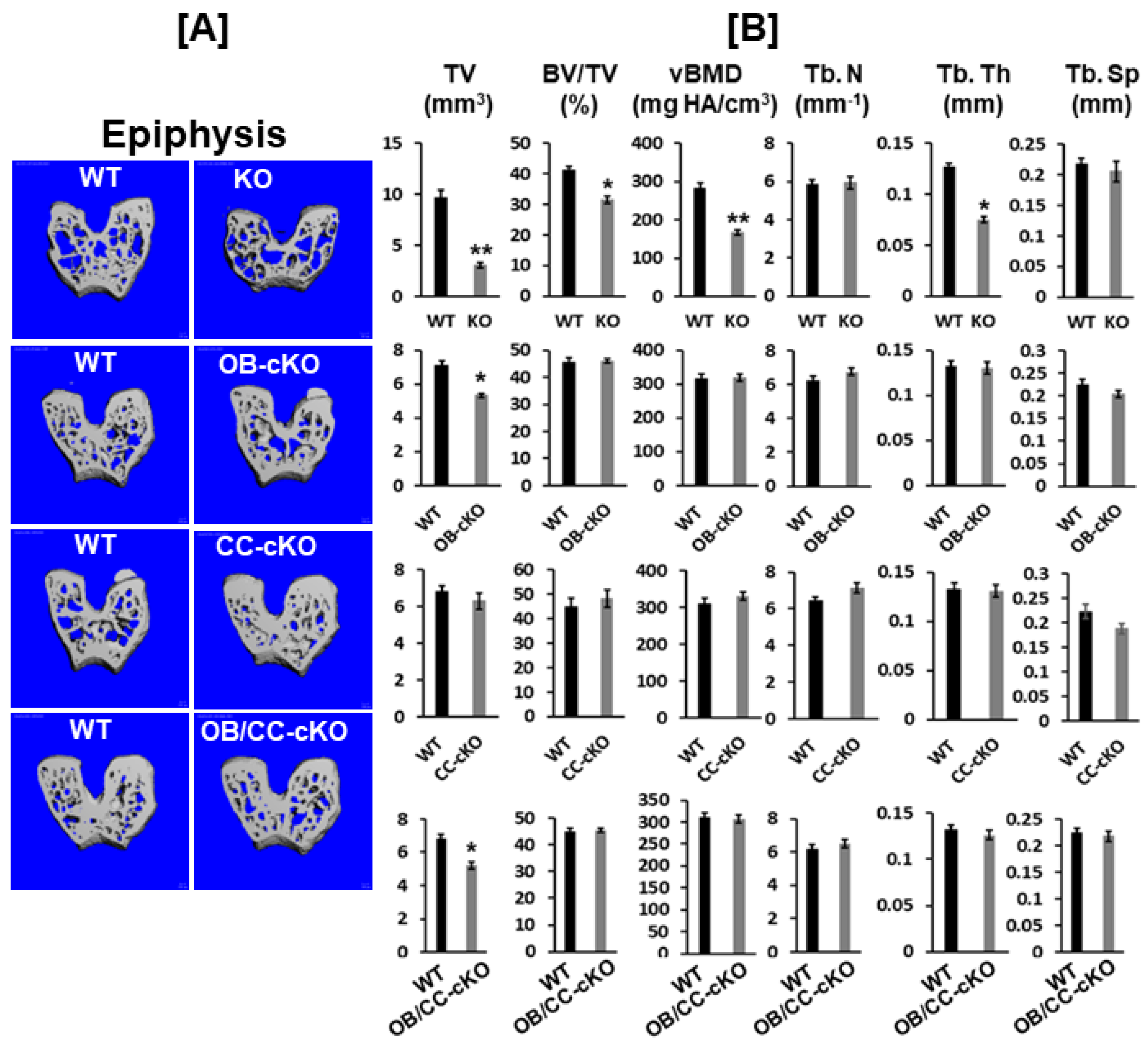
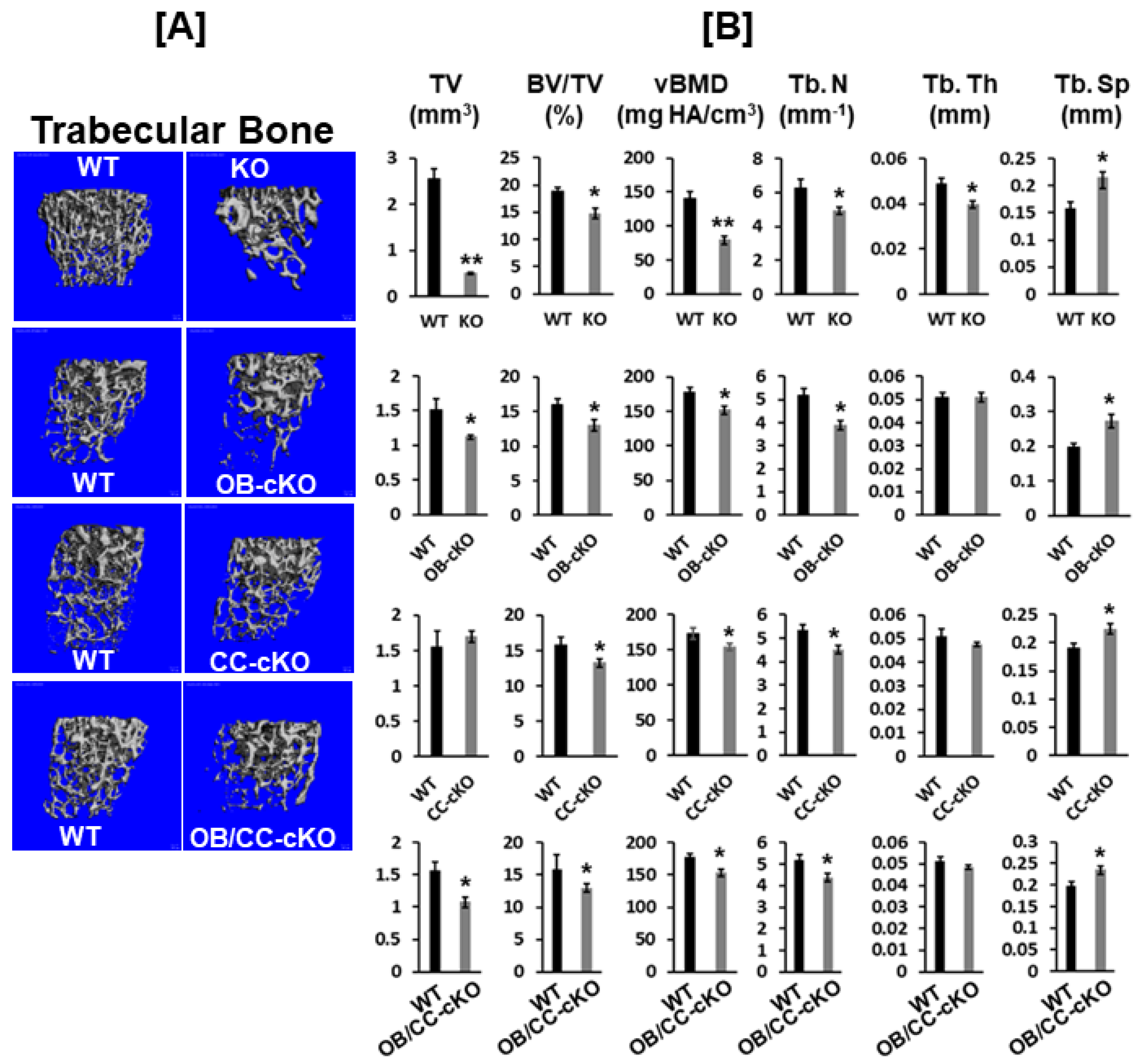
| KO Cell Type | Mechanism | Bone Size | Trabecular Bone |
|---|---|---|---|
| Every cell type (global) | Endocrine and local action | Reduced | Reduced |
| Osteoblasts | Local action | Reduced | Reduced |
| Chondrocytes | Local action | No change | Reduced |
| Osteoblasts/Chondrocytes | Local action | Reduced | Reduced |
| Hepatocytes | Local action | Reduced | No change |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





