Submitted:
08 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Production of lentiviral vectors and transduction of Schwann cells
2.2. Animal surgery
2.3. Immunohistochemistry
2.4. Behavioural assessment
2.5. Image analysis
3. Results
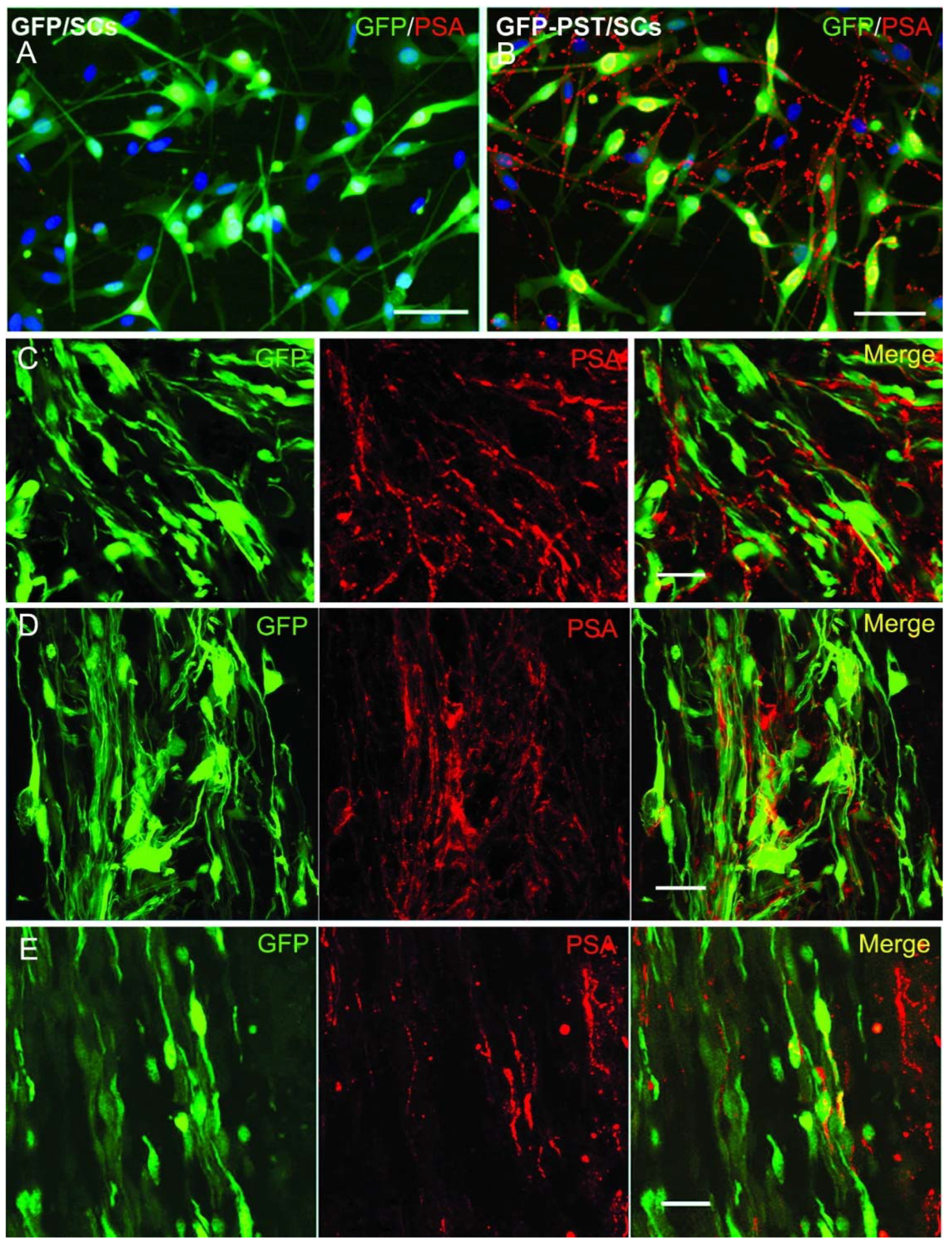
3.1. Lentiviral PST gene transfer induces PSA expression in vitro and in vivo
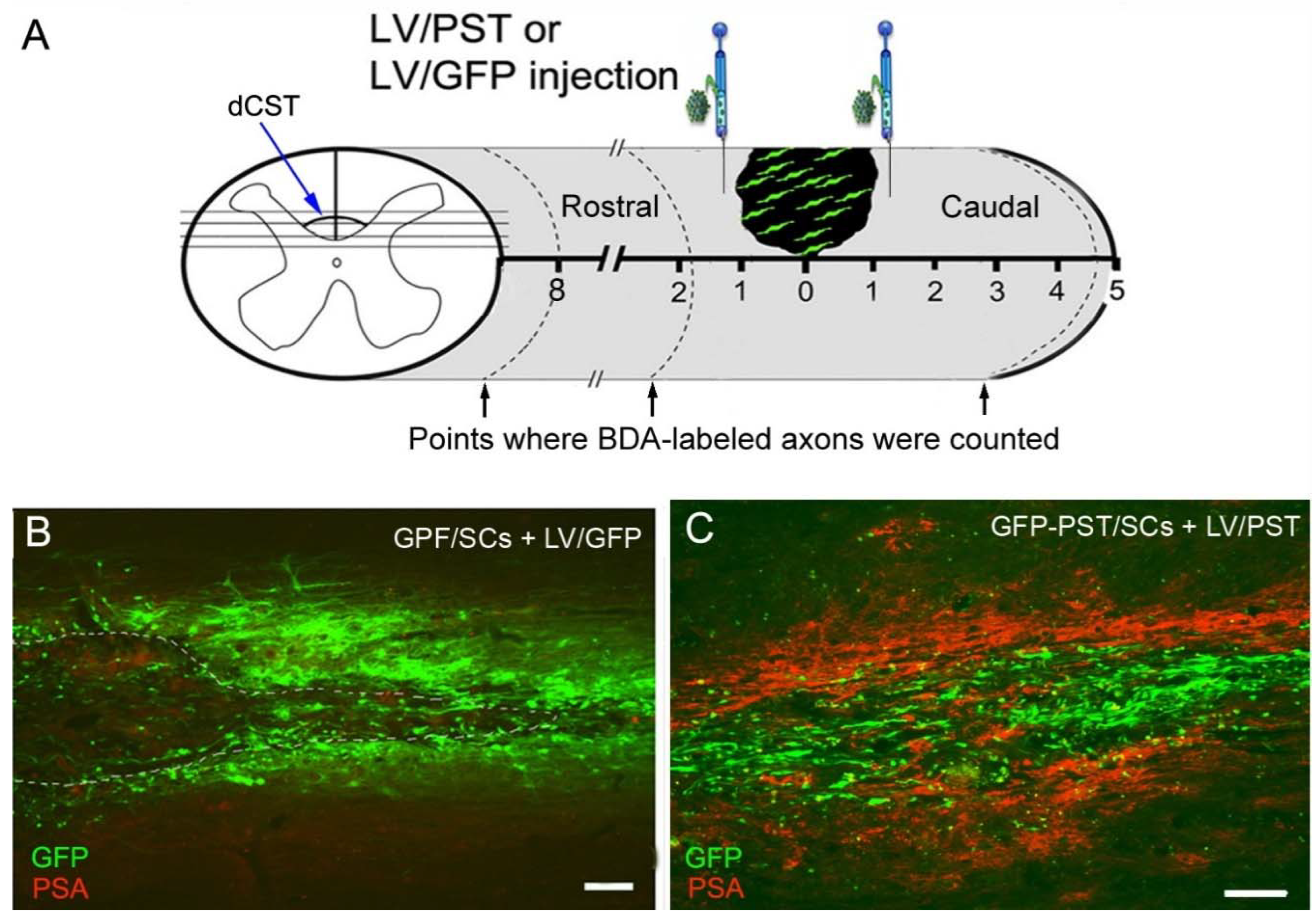
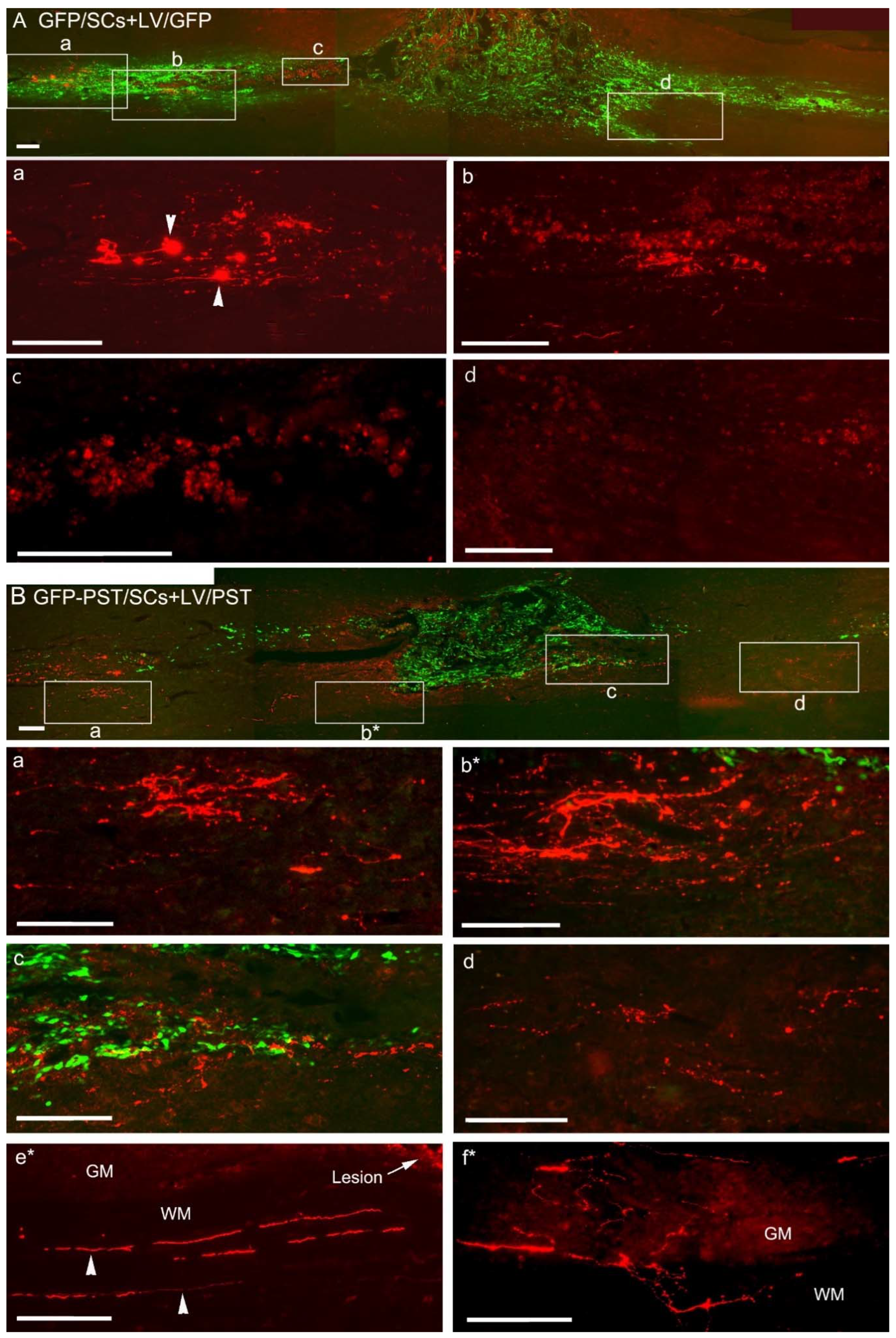
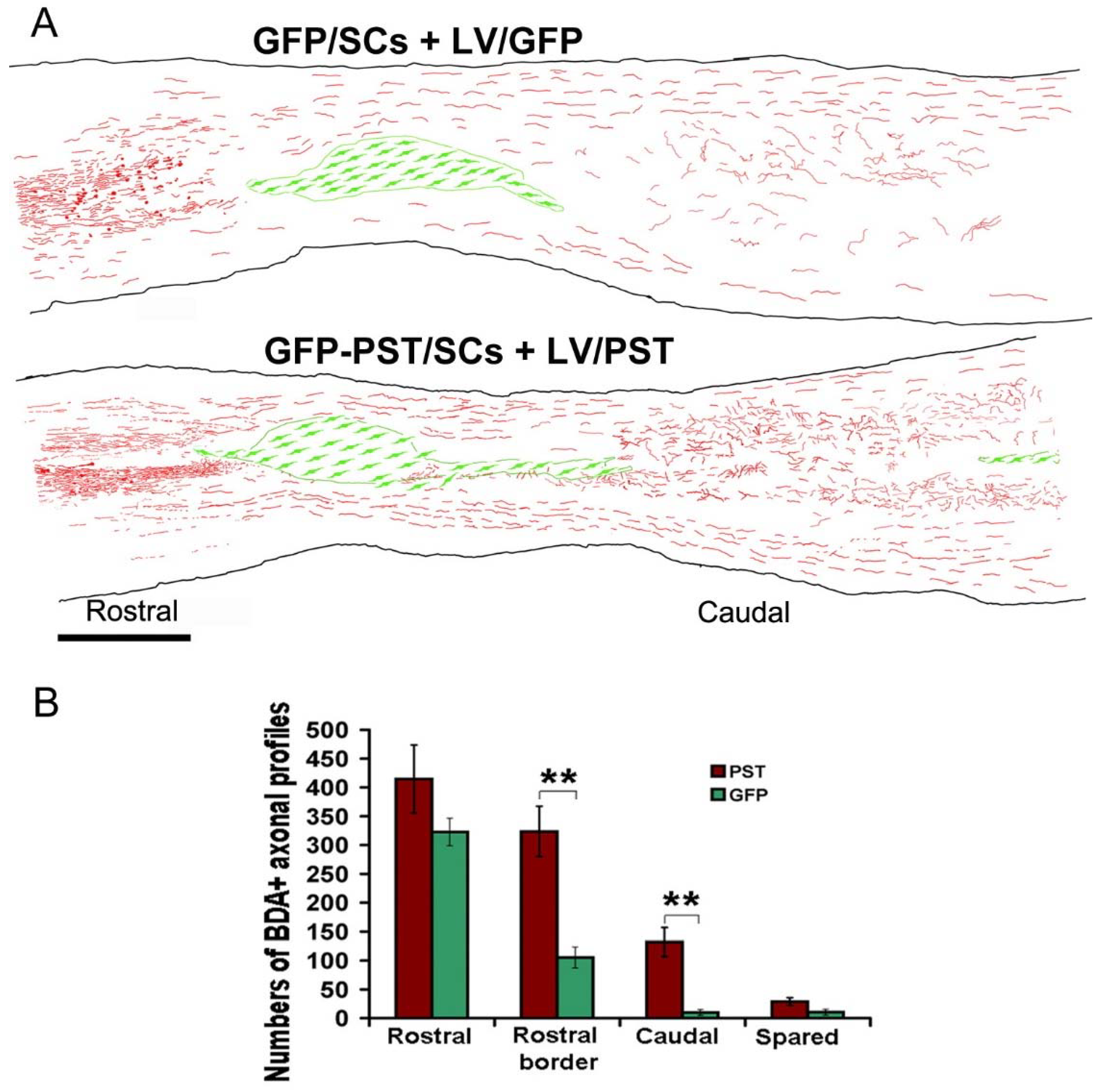
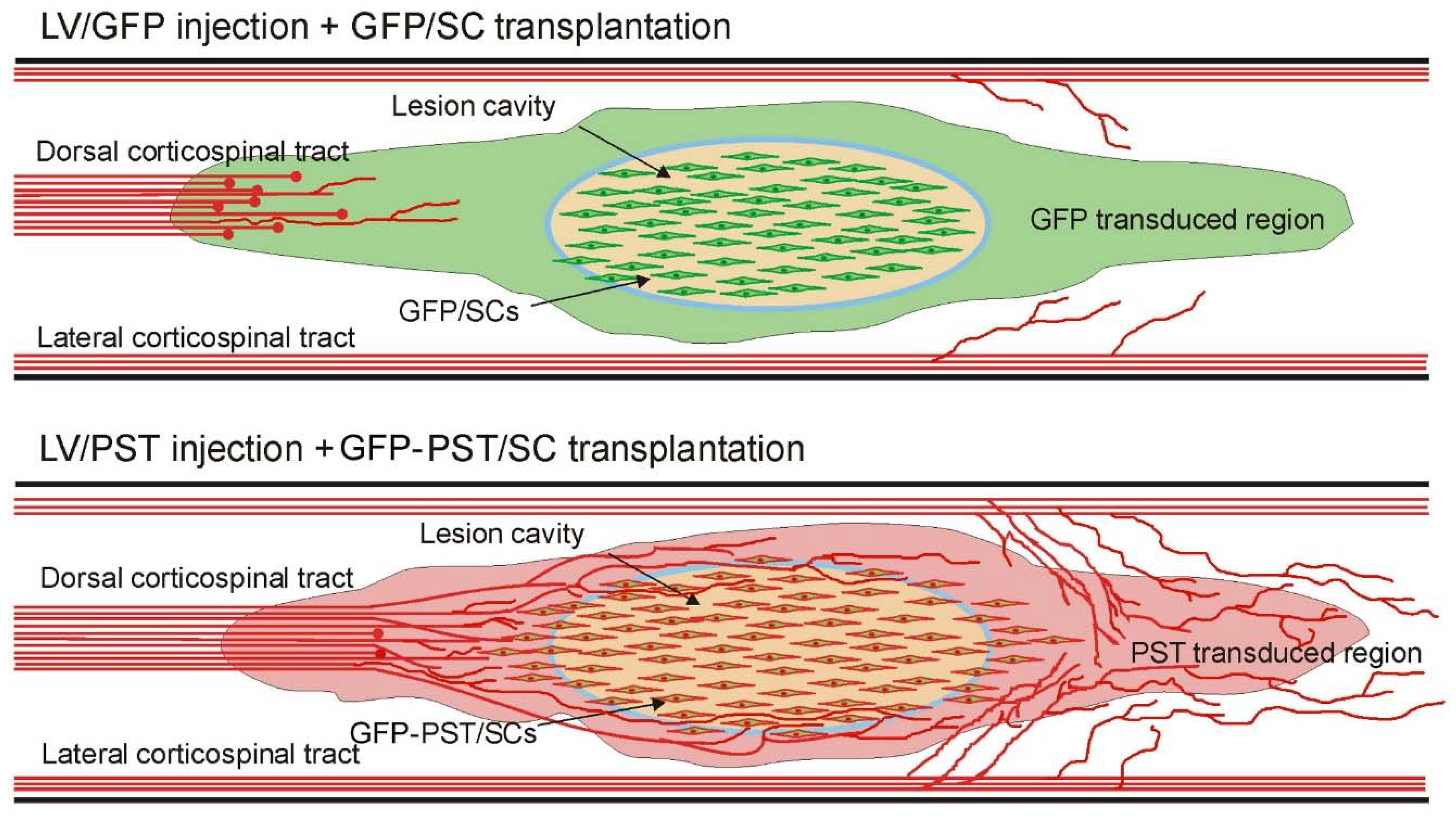
3.2. Combining PST/SC transplantation with LV/PST injection enhances the growth of corticospinal tract axons
3.3. Combining PST/SC transplantation with LV/PST injection promotes locomotor functional recovery
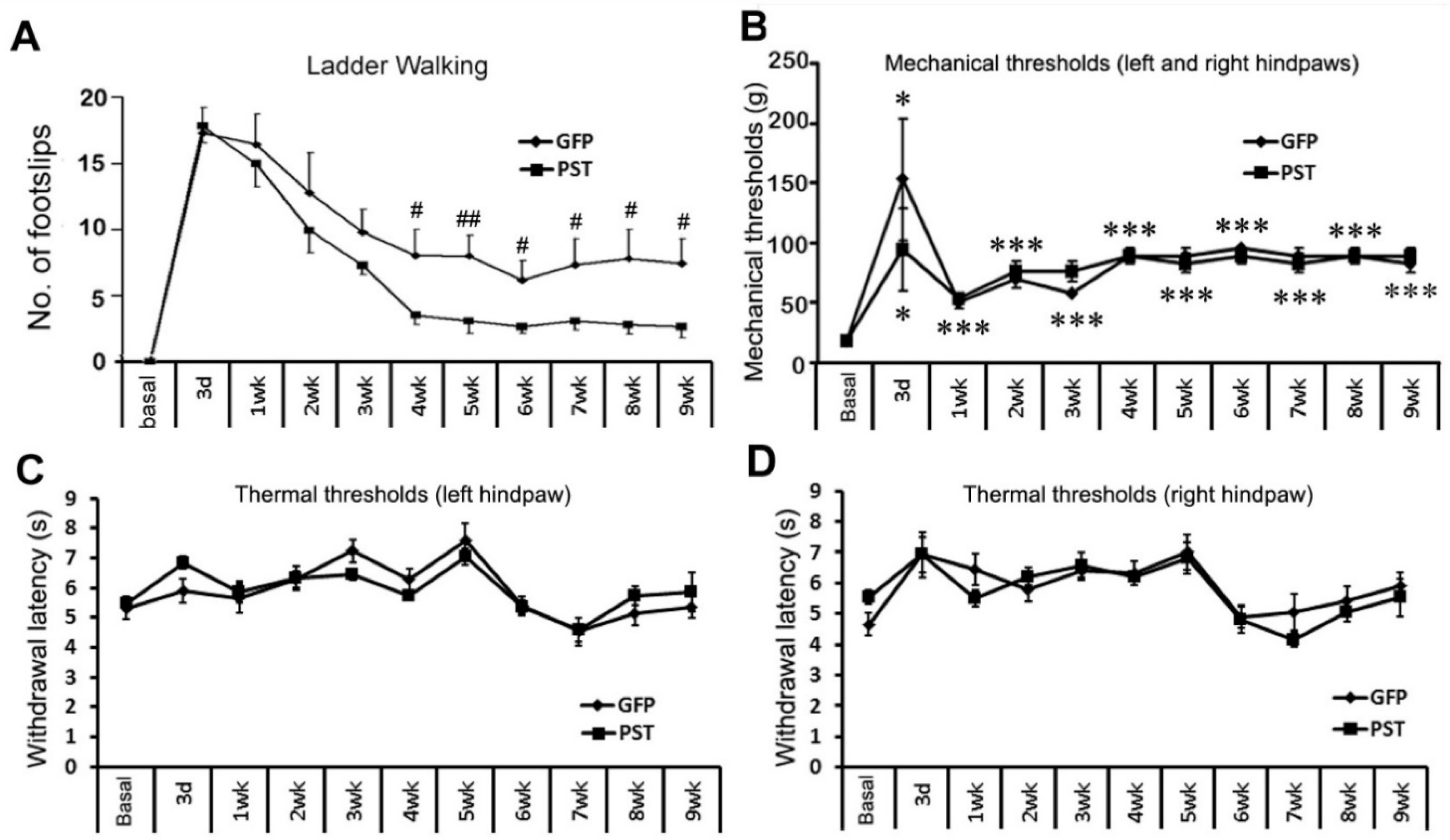
3.4. Combining PST/SC transplantation with LV/PST injection does not lead to elevated pain sensation
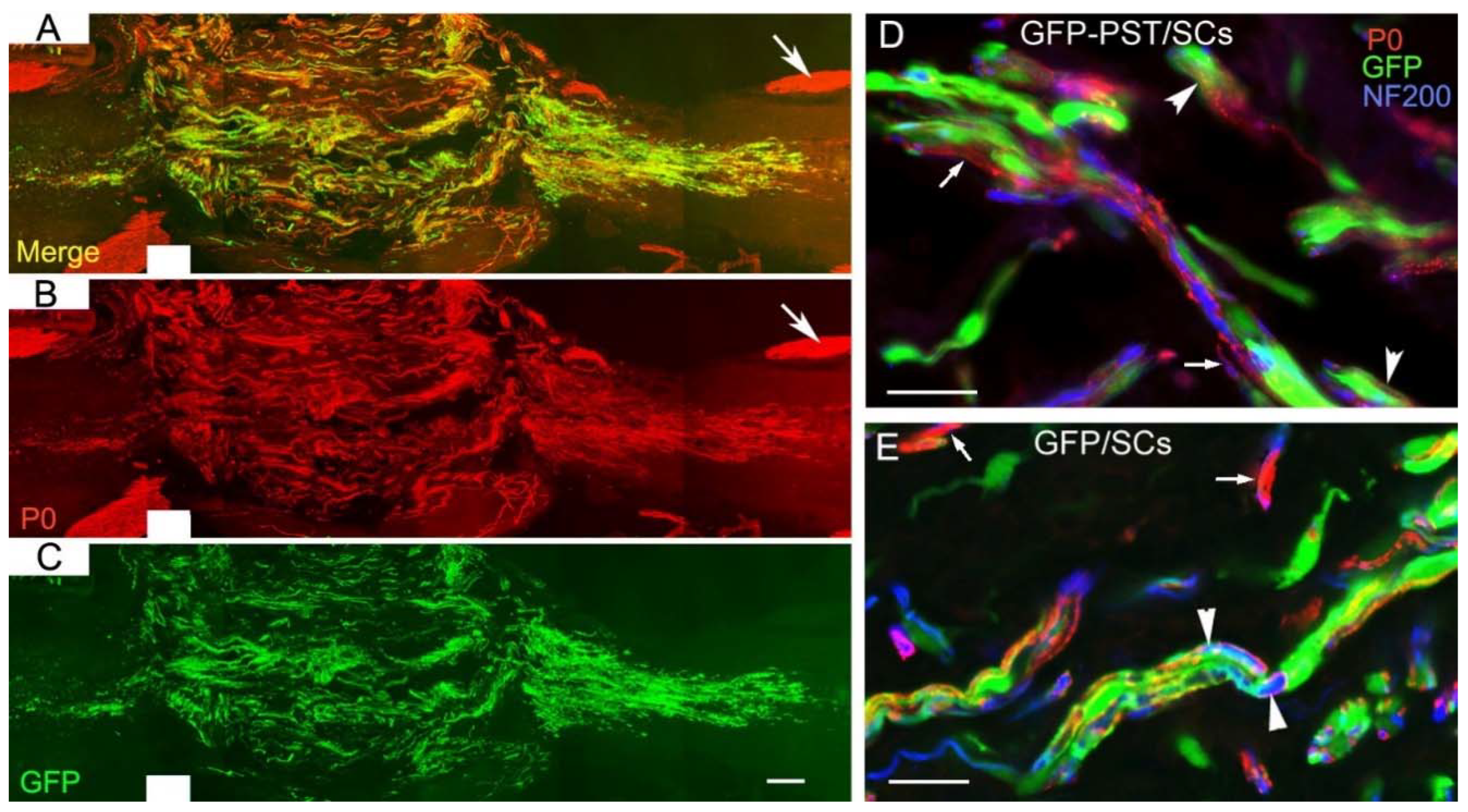
3.5. PST/SCs maintain their capacity to myelinate axons
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O'Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J Clin Invest. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, B.D.S.; de Almeida, F.M.; Martinez, A.M.B. Cell therapy and delivery strategies for spinal cord injury. Histol. Histopathol. 2021, 1. [Google Scholar] [CrossRef]
- Deng, L.-X.; Walker, C.; Xu, X.-M. Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury. Brain Res. 2014, 1619, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V.; Deng, L.; Xu, X.-M. Human Schwann Cell Transplantation for Spinal Cord Injury: Prospects and Challenges in Translational Medicine. Front. Cell. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bunge, M.B.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Ning, G.-Z.; Feng, S.-Q.; Kong, X.-H.; Chen, J.-T.; Zheng, Y.-F.; Ban, D.-X.; Liu, T.; Li, H.; Wang, P. Transplantation of Autologous Activated Schwann Cells in the Treatment of Spinal Cord Injury: Six Cases, more than Five Years of Follow-up. Cell Transplant. 2012, 21, 39–47. [Google Scholar] [CrossRef]
- Saberi, H.; Moshayedi, P.; Aghayan, H.-R.; Arjmand, B.; Hosseini, S.-K.; Emami-Razavi, S.-H.; Rahimi-Movaghar, V.; Raza, M.; Firouzi, M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 2008, 443, 46–50. [Google Scholar] [CrossRef]
- Gant, K.L.; Guest, J.D.; Palermo, A.E.; Vedantam, A.; Jimsheleishvili, G.; Bunge, M.B.; Brooks, A.E.; Anderson, K.D.; Thomas, C.K.; Santamaria, A.J.; et al. Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury. J. Neurotrauma 2022, 39, 285–299. [Google Scholar] [CrossRef]
- Luo, J.; Bo, X.; Wu, D.; Yeh, J.; Richardson, P.M.; Zhang, Y. Promoting survival, migration, and integration of transplanted Schwann cells by over-expressing polysialic acid. Glia 2010, 59, 424–434. [Google Scholar] [CrossRef]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research—Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Dusart I, Morel MP, Wehrle R, Sotelo C. Late axonal sprouting of injured Purkinje cells and its temporal correlation with permissive changes in the glial scar. J Comp Neurol. 1999, 408, 399–418. [CrossRef]
- Camand, E.; Morel, M.-P.; Faissner, A.; Sotelo, C.; Dusart, I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur. J. Neurosci. 2004, 20, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Seki, T.; Arai, Y. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci. Res. 1993, 17, 265–290. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Müller, D. Contribution of the Neural Cell Adhesion Molecule to Neuronal and Synaptic Plasticity. Rev. Neurosci. 2001, 12, 297–310. [Google Scholar] [CrossRef]
- Zhang, Y.; Yeh, J.; Richardson, P.M.; Bo, X. Cell adhesion molecules of the immunoglobulin superfamily in axonal regeneration and neural repair. Restor. Neurol. Neurosci. 2008, 26. [Google Scholar]
- El Maarouf A, Rutishauser U. Use of PSA-NCAM in repair of the central nervous system. ; Adv Exp Med Biol. 2010, 663, 137–147.
- Eckhardt, M.; Mühlenhoff, M.; Bethe, A.; Koopman, J.; Frosch, M.; Gerardy-Schahn, R. Molecular characterization of eukaryotic polysialyltransferase-1. Nature 1995, 373, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Angata, K.; Suzuki, M.; Fukuda, M. Differential and Cooperative Polysialylation of the Neural Cell Adhesion Molecule by Two Polysialyltransferases, PST and STX. PEDIATRICS 1998, 273, 28524–28532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Wu, D.; Verhaagen, J.; Richardson, P.M.; Yeh, J.; Bo, X. Lentiviral-mediated Expression of Polysialic Acid in Spinal Cord and Conditioning Lesion Promote Regeneration of Sensory Axons Into Spinal Cord. Mol. Ther. 2007, 15, 1796–1804. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghadiri-Sani, M.; Zhang, X.; Richardson, P.M.; Yeh, J.; Bo, X. Induced expression of polysialic acid in the spinal cord promotes regeneration of sensory axons. Mol. Cell. Neurosci. 2007, 35, 109–119. [Google Scholar] [CrossRef]
- El Maarouf, A.; Petridis, A.K.; Rutishauser, U. Use of polysialic acid in repair of the central nervous system. Proc. Natl. Acad. Sci. 2006, 103, 16989–16994. [Google Scholar] [CrossRef]
- A Lavdas, A.; Franceschini, I.; Dubois-Dalcq, M.; Matsas, R. Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia 2006, 53, 868–878. [Google Scholar] [CrossRef]
- Bachelin, C.; Zujovic, V.; Buchet, D.; Mallet, J.; Evercooren, A.B.-V. Ectopic expression of polysialylated neural cell adhesion molecule in adult macaque Schwann cells promotes their migration and remyelination potential in the central nervous system. Brain 2009, 133, 406–420. [Google Scholar] [CrossRef]
- Ghosh, M.; Tuesta, L.M.; Puentes, R.; Patel, S.; Melendez, K.; El Maarouf, A.; Rutishauser, U.; Pearse, D.D. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. Glia 2012, 60, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Chen, J.; Lavdas, A.A.; Thomaidou, D.; Schachner, M.; Matsas, R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 2007, 130, 2159–2174. [Google Scholar] [CrossRef]
- Angata, K.; Yen, T.-Y.; El-Battari, A.; Macher, B.A.; Fukuda, M. Unique Disulfide Bond Structures Found in ST8Sia IV Polysialyltransferase Are Required for Its Activity. J. Biol. Chem. 2001, 276, 15369–15377. [Google Scholar] [CrossRef]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef] [PubMed]
- Brockes, J.; Fields, K.; Raff, M. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979, 165, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, E.J.; Bold, E.L.; Haas, G.; Hurley-Gius, K.M.; Quirk, G.; Sievert, C.F.; Terreberry, R.R. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986, 396. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994, 53, 55–63. [CrossRef]
- Enomoto, M.; Bunge, M.B.; Tsoulfas, P. A multifunctional neurotrophin with reduced affinity to p75NTR enhances transplanted Schwann cell survival and axon growth after spinal cord injury. Exp. Neurol. 2013, 248, 170–182. [Google Scholar] [CrossRef]
- Lavdas, A.A.; Chen, J.; Papastefanaki, F.; Chen, S.; Schachner, M.; Matsas, R.; Thomaidou, D. Schwann cells engineered to express the cell adhesion molecule L1 accelerate myelination and motor recovery after spinal cord injury. Exp. Neurol. 2010, 221, 206–216. [Google Scholar] [CrossRef]
- Steward, O.; Zheng, B.; Tessier-Lavigne, M.; Hofstadter, M.; Sharp, K.; Yee, K.M. Regenerative Growth of Corticospinal Tract Axons via the Ventral Column after Spinal Cord Injury in Mice. J. Neurosci. 2008, 28, 6836–6847. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. The role of the serotonergic system in locomotor recovery after spinal cord injury. Front. Neural Circuits 2015, 8, 151. [Google Scholar] [CrossRef]
- Nakayama, J.; Fukuda, M.N.; Fredette, B.; Ranscht, B. Expression cloning of a human polysialyltransferase that forms the polysialylated neural cell adhesion molecule present in embryonic brain. Proc. Natl. Acad. Sci. 1995, 92, 7031–7035. [Google Scholar] [CrossRef]
- Angata, K.; Nakayama, J.; Fredette, B.; Chong, K.; Ranscht, B.; Fukuda, M. Human STX Polysialyltransferase Forms the Embryonic Form of the Neural Cell Adhesion Molecule. J. Biol. Chem. 1997, 272, 7182–7190. [Google Scholar] [CrossRef] [PubMed]
- El Maarouf, A.; Kolesnikov, Y.; Pasternak, G.; Rutishauser, U. Polysialic acid-induced plasticity reduces neuropathic insult to the central nervous system. Proc. Natl. Acad. Sci. 2005, 102, 11516–11520. [Google Scholar] [CrossRef] [PubMed]
- Fewou, S.N.; Ramakrishnan, H.; Büssow, H.; Gieselmann, V.; Eckhardt, M. Down-regulation of Polysialic Acid Is Required for Efficient Myelin Formation. J. Biol. Chem. 2007, 282, 16700–16711. [Google Scholar] [CrossRef] [PubMed]
- Zhang Y, Dijkhuizen PA, Anderson PN, Lieberman AR, Verhaagen J. NT-3 delivered by an adenoviral vector induces injured dorsal root axons to regenerate into the spinal cord of adult rats. J Neurosci Res. 1998, 54, 554–562. [CrossRef]
- Rougon, G.; K, K.; S, T.; P, L.; G, C.; D, G.; Mt, F.; A, B.; Mh, T. Faculty Opinions recommendation of Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. . 2009, 64. [Google Scholar] [CrossRef]
- Leibinger, M.; Zeitler, C.; Gobrecht, P.; Andreadaki, A.; Gisselmann, G.; Fischer, D. Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Wu, D.; Lee, S.; Luo, J.; Xia, H.; Gushchina, S.; Richardson, P.M.; Yeh, J.; Krügel, U.; Franke, H.; Zhang, Y.; et al. Intraneural Injection of ATP Stimulates Regeneration of Primary Sensory Axons in the Spinal Cord. J. Neurosci. 2017, 38, 1351–1365. [Google Scholar] [CrossRef]
- Wu, D.; Yang, P.; Zhang, X.; Luo, J.; E Haque, M.; Yeh, J.; Richardson, P.M.; Zhang, Y.; Bo, X. Targeting a Dominant Negative Rho Kinase to Neurons Promotes Axonal Outgrowth and Partial Functional Recovery After Rat Rubrospinal Tract Lesion. Mol. Ther. 2009, 17, 2020–2030. [Google Scholar] [CrossRef]
- Hannila, S.S.; Filbin, M.T. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol. 2008, 209, 321–332. [Google Scholar] [CrossRef]
- Kanno, H.; Pressman, Y.; Moody, A.; Berg, R.; Muir, E.M.; Rogers, J.H.; Ozawa, H.; Itoi, E.; Pearse, D.D.; Bunge, M.B. Combination of Engineered Schwann Cell Grafts to Secrete Neurotrophin and Chondroitinase Promotes Axonal Regeneration and Locomotion after Spinal Cord Injury. J. Neurosci. 2014, 34, 1838–1855. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




