1. Introduction
Middle ear implant (MEI) and bone conduction implants (BCIs) are innovative and effective treatments for specific types of hearing loss. They represent a significant advancement in the field of audiology and otolaryngology, providing an alternative to traditional hearing aids for those who cannot use them or do not find them effective.
MEI are surgically placed in the middle ear and work by directly driving the structures of the middle ear. They bypass the outer ear and directly stimulate the ossicles, which are responsible for transmitting sound vibrations to the inner ear. MEI are primarily used in treating sensorineural, conductive, or mixed hearing loss, offering a wider range of amplification compared to conventional hearing aids.
On the other hand, BCI work on the principle of bone conduction, where sound vibrations are delivered directly to the inner ear through the skull, bypassing the outer and middle ear. This is especially beneficial for individuals with conductive or mixed hearing loss, or those with single-sided deafness or with an altered anatomy of the middle and/or outer ear. BCIs, therefore, are surgically implanted devices that effectively stimulate the cochlea by creating vibrations in the skull, which the cochlea interprets as sound.
Both types of implants offer significant benefits to patients with various types of hearing loss, bringing them a step closer to experiencing a more natural and clearer sound. Each type, however, has unique advantages and considerations.
These devices stand as a compelling alternative to conventional treatment, passive prosthetics, and traditional hearing aids for a significant number of patients. Active hearing implants work by bridging the air-bone gap in cases of conductive or mixed hearing loss and bolstering sensorineural hearing loss through amplifying sound energy. The decision to use these implants depends on both audiological and medical criteria [
1].
Choosing an active hearing implant involves a complex, multifaceted decision-making process, with audiological criteria just one of the considerations. Other factors include both objective considerations like surgical and anatomical feasibility, as well as subjective factors such as patient expectations. Often, the final choice reflects a balance between optimal audiological solutions and various other factors, but the patient’s expectations should not influence the physician’s decisions.
A recent consensus among ENT specialists, audiologists, health-policy researchers, and industry representatives has established the first procedural and technical characterization framework. This agreement is aimed at improving communication among different stakeholders and thereby enhancing healthcare services [
1]. The present review directs its attention to the range of devices currently available and their audiological usage indications. In addition, a broad consensus is being published that will make the indications for implantable prostheses even more precise [
2].
2. Relevant Section - Implantable Devices
Over the years, a variety of devices with different technologies and capabilities have been created, allowing customized solutions for individual hearing problems.
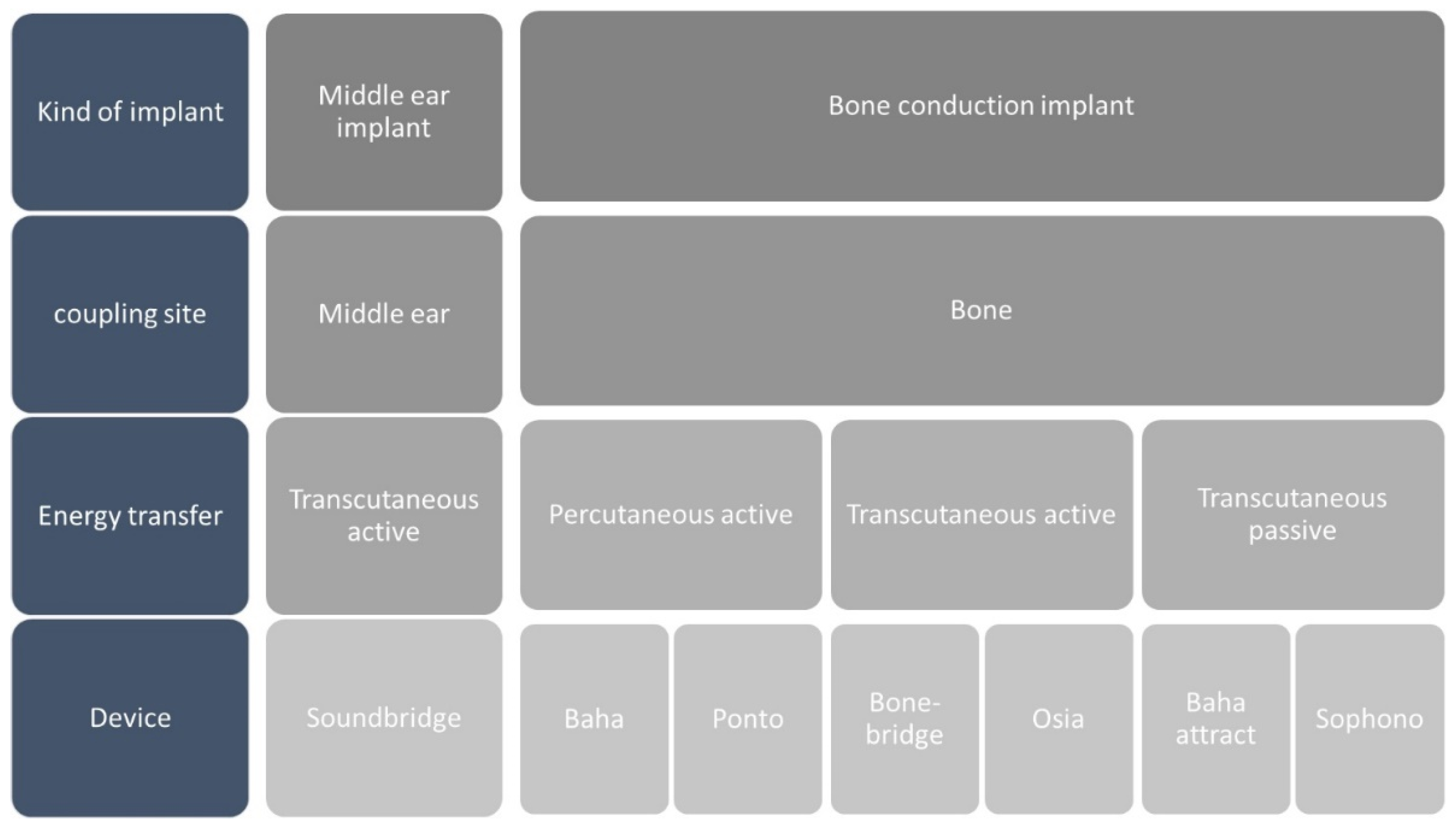
Figure 1 provides a snapshot of devices available across European markets.
Active hearing implants comprise an actuator that vibrates a specific anatomical structure; like ossicular chain, oval or round window; and an audio processor equipped with microphones, a signal processing unit, and an electric power supply. Bone conduction hearing devices transmit sound energy to the skull directly or via skin coupling.
In a broad sense, these prosthetic devices can be utilized to address any form of hearing impairment, including sensorineural, conductive, or mixed hearing loss; regardless of whether it’s bilateral symmetrical, asymmetrical, or unilateral. Of course, the essential requirement is that these devices must provide adequate prosthetic amplification for the specific type of hearing loss. These prostheses can be employed to manage almost any middle or outer ear condition.
Middle Ear Implant
Up today the only one MEI on the mark in the vibrant soundbridge (VSB). In 1994, the VSB semi-implantable MEI was introduced as new kind of acoustical prosthesis. It was consisting of two parts: the internal Vibrating Ossicular Replacement Prosthesis (VORP) with the terminal floating part, the floating mass transducer (FMT), and an external Audio Processor (AP). The system operates by converting sound into mechanical vibrations via the (FMT), bypassing the outer and middle ear to stimulate the inner ear directly. The FMT, secured on a middle ear structure, emulates the natural motion of the ossicular chain with small, virtually unobservable displacements. Depending on the patient’s hearing loss type and ear anatomy, the FMT can be placed in various positions within the middle ear. The VSB is designed to provide a broad frequency response in speech frequencies, with a band pass covering 100-10000 Hz and providing an average gain of 30-55 dB.
For this device, the most efficient energy transfer is realized when the actuator directly connects to the mobile components of the middle ear, such as the ossicles or the tympanic membrane, or to a cochlear window. This allows energy to be transmitted to the cochlea as either ‘forward stimulation’ (like the incus, stapes, or stapes footplate) or as ‘reverse stimulation’ through the round window membrane [
3,
4,
5,
6]. Given the minimal inertia of these structures, considerably less energy is needed compared to when the actuator is coupled to the skull or skin. In any case the skin resistance must be exceeded, and this results in a loss of about 1.5 dB for every 2 mm of tissue [
7].
Bone Conduction Implant
Transmitting sound to the skull bone is highly efficient as it circumvents compromised middle ear function. Vibrations of the skull travel through various pathways to the cochlear capsule, causing movement relative to the inner ear fluids and stimulating sensory hair cells [
8]. The actuator, which typically accomplishes this energy transmission, is attached to the skull using screws, with or without osseointegration.
Owing to its inertia, large forces are necessary to accelerate the skull bone. This is optimally achieved when the actuator’s moving mass is substantial enough to match the resonance behaviour of the skull bone. Thus, bone conduction stimulators are generally large, and their peak performance is somewhat constrained. To maximize energy transfer, the optimal coupling site is near the cochlea to be stimulated, resulting in a maximum transcranial attenuation to the contralateral cochlear of approximately 10 dB [
9,
10,
11,
12]. However, in patients with asymmetrical sensorineural hearing loss, this could compromise source separation and consequently impact hearing in noise and directional hearing.
The systems can also be distinguished by the route of energy transfer from the audio processor to the implant. For instance, in percutaneous bone-anchored devices, the audio processor and actuator are rigidly connected via an abutment that penetrates the bone, through the skin, to maintain mechanical energy transfer, earning them the title of direct-drive bone-conduction devices. In percutaneous, mechanical energy transfer scenarios, all available energy is directly converted into the skull bone’s vibrational energy. Despite its effectiveness, this technology necessitates direct skin penetration, which could lead to skin irritation, inflammation, or infection [
13,
14].
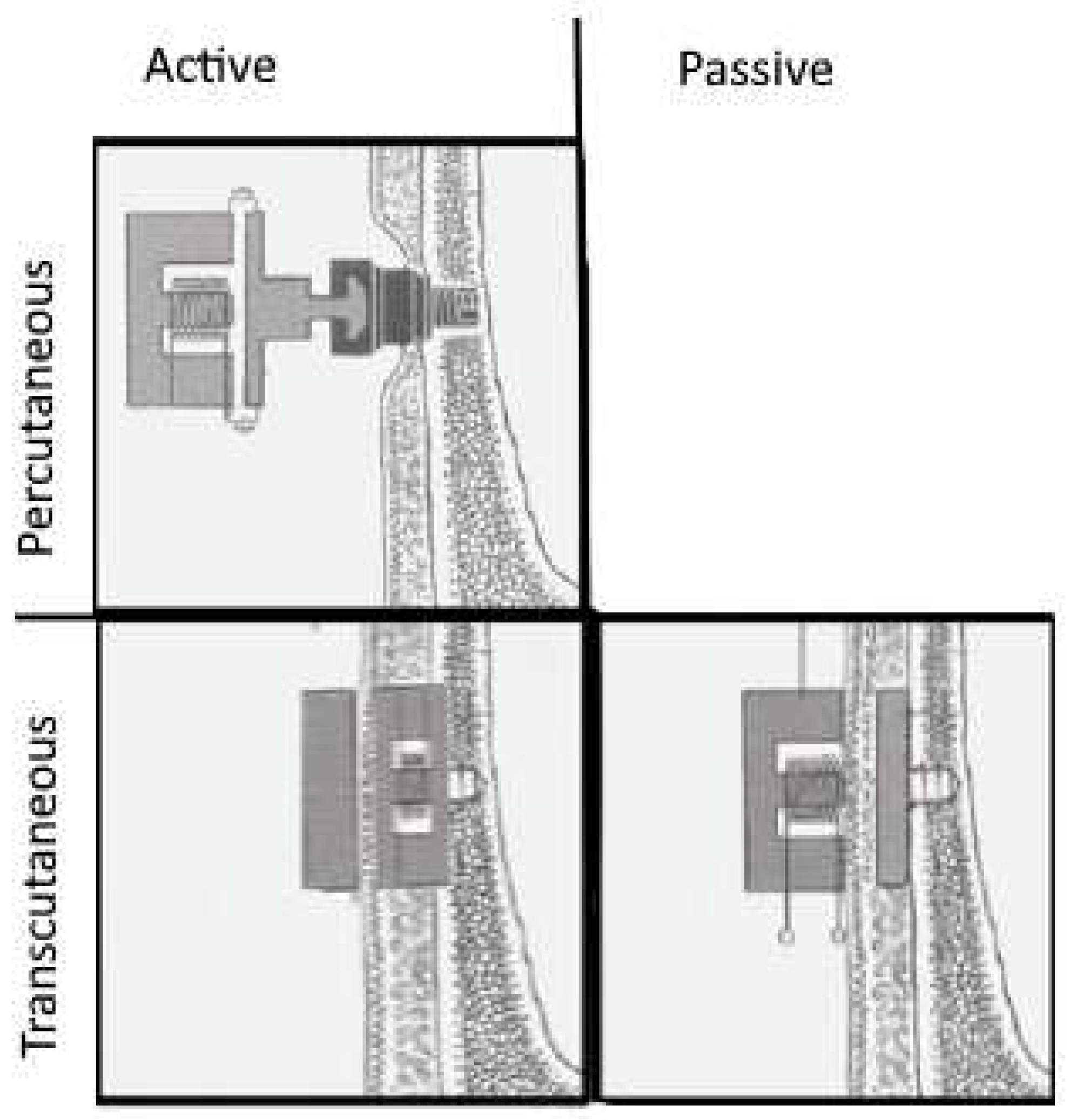
When there’s a need to circumvent direct attachment to the skull, certain bone-conduction devices can be affixed through the skin. These devices employ transcutaneous, magnetostatic energy transfer to connect to an implant placed under the skin and attached to the skull. Transcutaneous devices are distinguished into active and passive based on the position of the vibrating actuator (
Figure 2).
Active implants connect actuators transcutaneously using a radio frequency electromagnetic link to the sound processor. The implant decodes the acoustically encoded electromagnetically transmitted signal so electromechanical or piezoelectric actuators can generate vibrations. Transcutaneous energy transfer to the implant addresses limitations of percutaneous (skin penetrating) implants. Systems like the active transcutaneous implants use an analogue electromagnetic signal transmission to the implant. The voltage induced in the receiver coil diminishes with the distance between the induction coils (or the thickness of the skin), dropping about 1.5 dB per 2 mm [
7]. This method significantly reduces the risk of feedback loops compared to percutaneous signal transmission methods [
15]. However, some actuators are relatively large, prompting recommendations for preoperative radiological planning, especially in cases of small mastoids, malformations, children, or reduced bone volume after canal wall down mastoidectomy [
16]. This need has also spurred companies to develop smaller actuators [
17].
Alternatively, a passive bone implant attached to the skull can be operated transcutaneously by magnetic forces from the actuator co-housed with the audio processor on the skin. These are referred to as skin-driven bone-conduction devices. Transcutaneous energy transfer can also be facilitated as electromagnetic energy transfer (induction) from the audio processor coil to a receiver coil beneath the skin. The device’s two components are connected via the magnetostatic forces of two permanent magnets. The skin’s elasticity lessens the force exerted by around 10-20 dB according to Gründer et al., 2008 [
18].
3. Discussion
In addition to traditional hearing aids and cochlear implants, there are also implantable prostheses that contribute to the spectrum of hearing solutions available for individuals who are deaf. Audiologists and otolaryngologists have these three options at their disposal to manage various kinds of hearing loss. While BCI and, particularly, MEI can be utilized to address all types of hearing loss, they are mostly indicated for treating mixed and conductive hearing losses. Indeed, when considering patients with conductive hearing loss or mixed hearing loss, both MEI and BCIs are viable options. These devices can be particularly beneficial for patients who struggle with regular hearing aids, such as those with skin issues, recurring or chronic otitis externa, external ear aplasia, or exceptionally narrow ear canals.
While the MEI supports a wider audiometric range, up to about 55 dB of bone conduction hearing threshold, compared to the BCIs’s about 45 dB, the selection between the two can be complex especially for patients with an air conduction threshold up to 45 dB. This scenario always necessitates detailed preoperative audiometric analysis, counselling, and patient education. Additionally, BCIs can be tested with a softband to gauge a patient’s preoperative preference or comfort with the device. In contrast, this method is not applicable with MEI.
The decision to implant an MEI or BCIs requires comprehensive patient counselling and an evaluation of the potential impact of the device on hearing. Implanting an MEI can be a complex process depending on the existing middle ear structures. Other considerations like scarring and postoperative migration can also influence the device’s functional results. For a BCIs, careful preoperative planning is required to determine optimal placement. In terms of explantation, revision, and complication rates, the BCIs seems to perform better than the MEI [
19,
20].
The postoperative performance of patients using either an MEI or a BCIs is generally equivalent [
19]. However, the MEI does offer an advantage by avoiding cross hearing in the opposite ear through direct stimulation via remaining ossicles or the round or oval window. With a BCIs, cross hearing is more likely because of the limited attenuation of bone-conducted sound.
The challenge is that the clinical or audiological guidelines for selecting the appropriate device often intersect, causing confusion in determining the optimal solution for those with hearing impairments. Sometimes, the decision is guided more by the preferences of the specialist or patient rather than concrete, evidence-based clinical data.
Over time, many researchers have tried to clarify the proper application of these devices, but their guidelines can be vague or ambiguous. This led to the creation of a consensus statement on implantable prostheses that offers precise directions. Recently, a group of experts developed and approved twenty-nine consensus statements, which outline the best practices for diagnosis, clinical and audiological indications, and surgical procedures [
2]. These statements signify an important progression toward accurately identifying suitable candidates for these devices.
The consensus statements have helped to clarify various uncertain aspects found in the global scientific literature. For instance, while the existing literature does not detail the necessary preoperative exams for implantable device candidates, the expert panel has emphatically defined that a complete evaluation, including all audiological tests, imaging, and questionnaires, is essential prior to surgery [
2].
The panel also firmly stated that implantable hearing devices are not a substitute for conventional surgery. BCIs are only advised when treatment for chronic otitis or otosclerosis has failed, and MEI can be utilized exclusively for specific cases like dry ear without cholesteatoma or with advanced otosclerosis alongside a stapedial prosthesis. According to the experts, implantable hearing aids are not the preferred treatment for SSD and asymmetrical hearing loss [
2,
21]. However, for conductive or mixed hearing loss, they are helpful for adults and essential for children’s cognitive development. In instances of bilateral symmetrical hearing loss, fitting implantable hearing aids on both sides is recommended [
2].
The consensus statement also tackled some surgical considerations. The expert panel concluded that there are no strict age restrictions for recommending an implantable device, as age limitations are solely tied to surgical feasibility, anaesthesia type, or anatomical contraindications. Lastly, the panel asserts that the operating room is always the most appropriate setting for implants, and only percutaneous BCIs may be inserted using local anaesthesia in adults [
2].
4. Conclusions
In conclusion, the range of hearing solutions for deaf individuals is broad, with options including traditional hearing aids, cochlear implants, and implantable prostheses like BCIs and MEIs. While both BCIs and MEIs are beneficial in treating mixed and conductive hearing loss, their selection requires careful consideration and patient consultation. BCIs have the advantage of being testable with a softband preoperatively, unlike MEIs. The decision-making process is complex and requires an evaluation of multiple factors, including the patient’s specific condition and the audiometric range supported by the devices. Consensus statements from experts have recently offered clearer guidelines for practitioners, emphasizing the importance of thorough preoperative evaluation and the limitations and considerations regarding different devices. Overall, these insights reflect a significant step towards improved clarity and precision in the selection and application of hearing aids, aiming for patient-centric and evidence-based solutions.
5. Future Directions
Looking to the future, continued research, and development in implantable hearing devices, including BCIs and MEIs, could further expand the options for addressing diverse hearing loss challenges. Emphasis should be placed on developing more refined patient selection guidelines, minimizing surgical complexity, and creating personalized solutions that cater to individual preferences and medical conditions. Collaborative efforts among audiologists, otolaryngologists, and medical device manufacturers could facilitate more precise treatment plans, ultimately enhancing the quality of life for those with hearing impairments.
Author Contributions
Conceptualization, L.B.; methodology, L.B..; validation, L.B., F.F., F.L., S.B. and P.C..; formal analysis, F.L..; investigation, L.B., P.C..; resources, O.M.; data curation, O.M..; writing—original draft preparation, L.B, F.L, O.M..; writing—review and editing, L.B., F.L..; supervision, S.B..; project administration, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Consensus Statement on Bone Conduction Devices and Active Middle Ear Implants in Conductive and Mixed Hearing Loss. Otol Neurotol 2022, 43, 513–529. [CrossRef] [PubMed]
- Bruschini, L.; Canzi, P.; Canale, A.; Covelli, E.; Laborai, A.; Monteforte, M.; Cinquini, M.; Barbara, M.; Beltrame, M.A.; Bovo, R.; et al. Implantable Hearing Devices in Clinical Practise. Systematic Review and Consensus Statements. Acta Otorhinolaryngol Ital submitted.
- Beltrame, A.M.; Todt, I.; Sprinzl, G.; Profant, M.; Schwab, B. Consensus Statement on Round Window Vibroplasty. Ann Otol Rhinol Laryngol 2014, 123, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Colletti, V.; Soli, S.D.; Carner, M.; Colletti, L. Treatment of Mixed Hearing Losses via Implantation of a Vibratory Transducer on the Round Window. Int J Audiol 2006, 45, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, G.M.; Schoerg, P.; Muck, S.; Jesenko, M.; Speiser, S.; Ploder, M.; Edlinger, S.H.; Magele, A. Long-Term Stability and Safety of the Soundbridge Coupled to the Round Window. Laryngoscope 2021, 131, E1434–E1442. [Google Scholar] [CrossRef] [PubMed]
- Bruschini, L.; Forli, F.; Giannarelli, M.; Bruschini, P.; Berrettini, S. Exclusive Transcanal Surgical Approach for Vibrant Soundbridge Implantation: Surgical and Functional Results. Otol Neurotol 2009, 30, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, H.; Håkansson, B.; Reinfeldt, S. Analysis and Design of RF Power and Data Link Using Amplitude Modulation of Class-E for a Novel Bone Conduction Implant. IEEE Trans Biomed Eng 2012, 59, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, S. Acoustic and Physiologic Aspects of Bone Conduction Hearing. Adv Otorhinolaryngol 2011, 71, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Claes, A.; Bouzegta, R.; Van Rompaey, V.; Vanderveken, O.; Van de Heyning, P.; Mertens, G. Bone Conduction Trial Device to Eliminate the Effect of Transcranial Attenuation: A Prospective Observational Study in Single-Sided Deaf Subjects. Audiol Neurootol 2020, 25, 231–236. [Google Scholar] [CrossRef] [PubMed]
- I, D.; Jh, S.; F, P.; Am, H.; C, R. Experimental Investigation of Promontory Motion and Intracranial Pressure Following Bone Conduction: Stimulation Site and Coupling Type Dependence. Hearing research 2019, 378. [Google Scholar] [CrossRef]
- Rigato, C.; Reinfeldt, S.; Håkansson, B.; Fredén Jansson, K.-J.; Renvall, E.; Eeg-Olofsson, M. Effect of Transducer Attachment on Vibration Transmission and Transcranial Attenuation for Direct Drive Bone Conduction Stimulation. Hear Res 2019, 381, 107763. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, S. Transcranial Attenuation of Bone-Conducted Sound When Stimulation Is at the Mastoid and at the Bone Conduction Hearing Aid Position. Otol Neurotol 2012, 33, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Surgical Explantation of Bone-Anchored Hearing Devices: A 10-Year Single Institution Review - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31570059/ (accessed on 5 August 2023).
- Shapiro, S.; Ramadan, J.; Cassis, A. BAHA Skin Complications in the Pediatric Population: Systematic Review With Meta-Analysis. Otol Neurotol 2018, 39, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Rahne, T. [Physical audiological principles of implantable hearing systems : About power transmission, coupling and power output]. HNO 2021, 69, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, I.; Schilde, S.; Wenzel, C.; Rahne, T.; Plontke, S.K. Planning Tools and Indications for “Virtual Surgery” for the Bonebridge Bone Conduction System. HNO 2021, 69, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, C.; Schilde, S.; Plontke, S.K.; Rahne, T. Changes in Bone Conduction Implant Geometry Improve the Bone Fit in Mastoids of Children and Young Adults. Otol Neurotol 2020, 41, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Gründer, I.; Seidl, R.O.; Ernst, A.; Todt, I. [Relative value of BAHA testing for the postoperative audiological outcome]. HNO 2008, 56, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.L.; Weiss, B.G.; Bertlich, M.; Stoycheva, I.; Canis, M.; Ihler, F. Functional Results with Active Middle Ear Implant or Semi-Implantable Bone Conduction Device in Patients with Comparable Hearing Loss. Int J Audiol 2022, 61, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Bone-Anchored Hearing Aids Fitted According to NAL and DSL Procedures in Adults with Mixed Hearing Loss - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35894526/ (accessed on 5 August 2023).
- L, B.; R, C.; A, M.; C, C.; G, F.; S, B.; F, F. Bone Anchored Hearing Aids for the Treatment of Asymmetric Hearing Loss. The journal of international advanced otology 2020, 16. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).