1. Introduction
Bisphenol-A (BPA) represents an important co-monomer for obtaining polycarbonate, epoxy resins, flame retardants, in paint and other chemicals products [
1,
2]. Products containing BPA are widely used, and thus, we can find it in the environment: in the air, in the soil, in natural surface water and sediments, underground water in landfills and in wastewater [
2]. BPA disturbes human and animals health [
3,
4,
5,
6]. This influence the enzymatic, androgenic, neurological, liver and reproductive systems at different stages of human life: like in foetal stage, children and adults’ stages [
7,
8]. BPA causes disorders of the reproductive system in both women and men that can cause diseases such as infertility, abortions, polycystic ovaries, endometrial hyperplasia, etc. [
9]. Taking these inconveniences into account, it is very important to remove BPA from water. Ultrasonic technology can be considered as a very sustainable and efficient method to remove BPA from water. The advantages of this method are: the method is easy to be implemented on existing water treatment and purification facilities, it does not produce residual compounds that produce sludge, the time required for degradation is of the order of 1-2 hours, and the level of degradation is very high [
2,
10]. Ultrasound (US) treatment of wastewater is considered an advanced oxidation process (AOP). Unlike other AOPs, ultrasound treatment does not require the addition of catalysts or oxidants [
11]. Among the various AOPs, the sonochemical process is used for the degradation of organic pollutants, because of its eco-friendly properties and because it does not generate hazardous by-products during the cavitation process. Its efficiency depends on the hydrophobicity of the pollutants, and BPA is a hydrophobic compound, being suitable for US treatment [
12].
The ultrasonic mechanism is mainly achieved by acoustic cavitation. It includes the formation of bubbles, the rapid growth and violent collapse of bubbles in the liquid. At this point, high temperatures and pressures rise to 5000 K and 100 Pa, are generated, fact which causes the decomposition of organic pollutants and water [
2]. Through the decomposition of water molecules, highly reactive radical species as hydroxyl, hydrogen and hydroperoxide radicals are formed, which can oxidize the organic matrix [
13], can destroy the molecular structure of pollutants, to achieve the degradation effect [
14]. Organic pollutants are attacked by radicals with a high oxidation potential, forming compounds with lower molecular weight [
15].
According to equations 1-5, the reactive radical species produced by the ultrasonic cavitation phenomenon are: [
16].
In addition to radicals, the implosion of collapsing bubbles generates strong pressure waves, which are used for physical and chemical processes in wastewater treatment [
15]. The diversification of free radical species can be done, for example, by adding a sonocatalyst [
14]. Previous studies against the use of US for wastewater treatment question the economic viability, based on energy consumption calculations [
12]. The effect of US wastewater treatment is limited by the disadvantage of energy consumption [
14]. The possibility and efficiency of US application for BPA degradation have been investigated in various studies, but most of the research have been carried out using the sonochemical process as an auxiliary technology, to assist the photocatalytic, Fenton, UV, electrochemical processes [
12]. Combining the US process with peroxydisulfate provided a promising advanced oxidation method for the removal of organic pollutants from aqueous solutions [
17].
An effective way to improve the US treatment method to increase the degradation rate and treatment efficiency is by combining it with materials with sonocatalytic activity. The principle of US treatment is based on the attack of free radicals on the pollutant to degrade it, while the input of active sonocatalytic materials can improve the generation and enrichment of free radical species, so that the degradation process is continuously enriched, in a natural way [
14]. A Fenton-like catalyst used in combination with US technology for BPA degradation is Schwertmannite (Sch), an iron (III) oxyhyroxysulfate mineral. BPA degradation was significantly enhanced (98.0%), when the catalytic system was coupled with US (US/Sch/H
2O
2), indicating that there is a synergistic effect between US and Sch on H
2O
2 activation [
16]. Due to the limited efficiency of BPA degradation by the single system with US or by oxidants without US assistance, the researchers investigated the activation of four different common oxidants by US. Following the analyses, they found that US and the investigated oxidants have a synergistic effect, obtaining the following synergy indices of the systems US-S
2O
82-, US-H
2O
2, US-HSO
5,
- and US-IO
4- 1.13, 1.16, 1.25, 2.22 [
13]. The study [
16] indicates that the degradation reaction of BPA in the presence of US and Sch occurs in two stages, consisting of an induction period, followed by a rapid degradation period, according to a pseudo first-order kinetic process. The degradation efficiency was affected by several operating parameters, especially pH, catalyst dosage, temperature, H
2O
2 concentration. The optimum pH was 3, lower or higher values leading to decreased degradation efficiency. Increasing the dosage of Sch from 0.1 to 1 g/L was effective, but an increase to 2g/L did not lead to the improvement of the process, the increase in temperature led to the increase in the reaction rate in stage II, and the concentration of H
2O
2 was maximally effective at 15mM, lower values were not effective, and higher values inhibited degradation.
From previous research, it was found that ultrasonic degradation was effective at a higher frequency. Investigating the factors affecting the ultrasonic degradation rate of BPA [
11], stated that dissolved oxygen, higher ultrasonic frequency in range 300-500 KHz is most efficiently [
2], higher power favored BPA degradation, while the presence of HCO
3-, HA prevented BPA degradation, and Cl
- a showed a minor influence. Investigating the effect of operating parameters on BPA degradation efficiency, it was found [
7] that degradation was stimulated by increasing ultrasound power (38.87-97.17W), temperature (30-70°C), and initial PDS concentration (peroxydisulfate) (1.71-17.11). Determining the significance of these operational parameters in BPA degradation, their importance was estimated in the following order: temperature 46.83%, initial PDS concentration 40.54%, ultrasound power 12.63%.
The researchers' studies demonstrate that US irradiation is a promising way to effectively treat BPA in aqueous solution under optimal operating conditions – US frequency, power intensity, addition of oxidants, impact of different additives, quickly and without negative side effects.
In this work, we presented the studies on the efficiency of ultrasonics under air atmosphere on the degradation of BPA. The influence of the frequency and of some additional compounds such as carbon tetrachloride, FeSO4 7H2O, and ethyl anthraquinone was studied. Three different frequencies were used: 1146 KHz, 864 KHz, 580 KHz, at a power of 50 W. It was identified the efficiency of BPA degradation over a period of 60 minutes, with sampling every 15 minutes.
Also, using the LC-MS/MS technique, there the degradation compounds that result from the application of ultrasound processes were identified. Pathways of BPA degradation were also proposed.
2. Materials and Methods
2.1. Chemicals and equipments
Chemical reagents used for experiments Bisphenol A (purity > 99.9 %),, ethanol (99%), carbon tetrachloride (purity > 99.9 %, CCl4,) ferous sulphate heptahidrated (FeSO4 7H2O), methanol (HPLC grade) and acetic acid, etil antrachinone, sulphuric acid were acquired by Sigma-Aldrich (Germany). All the rest of reagents used in the experiment were of analitique quality. High purity water was obtained in house, using an ultrapure water system, from Merk Millipore (Burlington, Massachusetts, US).
Ultrasonic system
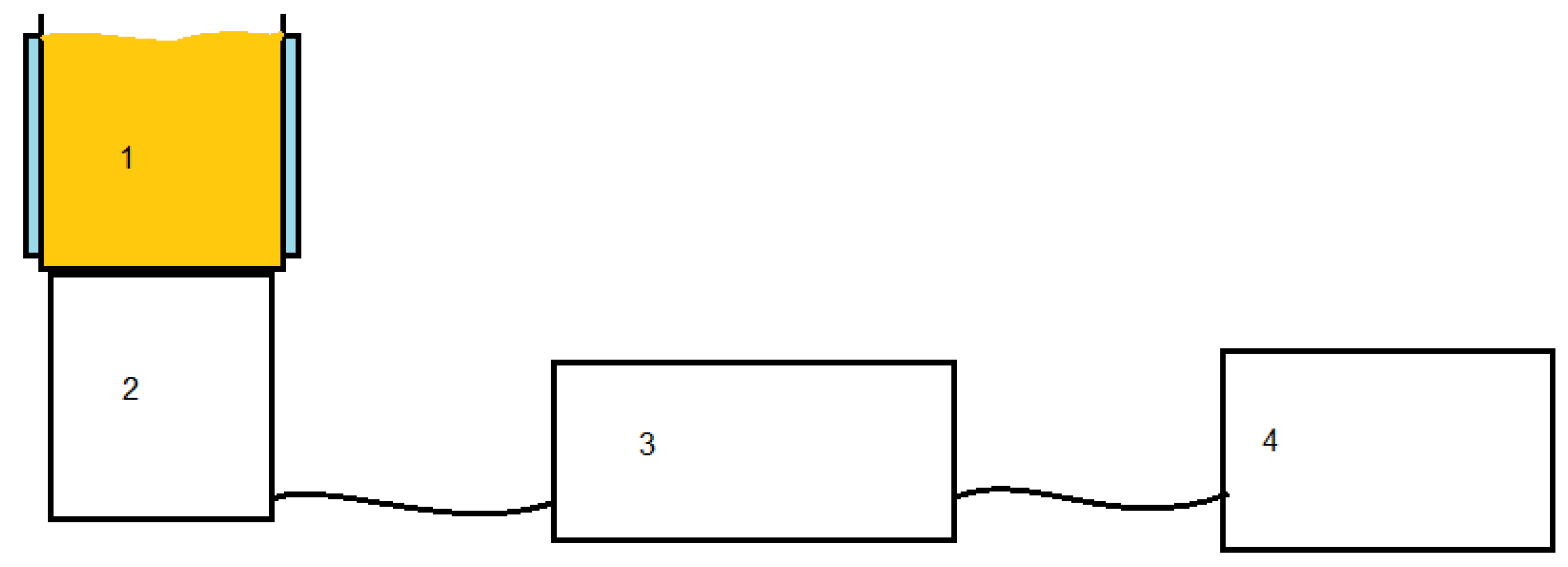
A Meinhardt multifrequency ultrasound system composed of a frequency generator (580, 684 and 1146 kHz), a power applicator and an ultrasonic transducer was used. The metal reactor is connected to the transducer through a flange and is provided with a jacket through which coolant from a thermostat circulates. The system diagram is presented in
Figure 1
2.2. Analytical methods used for BPA and intermediates resulted.
2.2.1. Analytical conditions used for BPA determination
The experiments for BPA quantification were carried out using Agilent 1260 series LC system (Agilent, Waldbronn, Germany) coupled with an Agilent 6410B triple-quadrupole mass spectrometer with electrospray ionization source (ESI). Luna C18 type (150 × 2.0 mm; 3.0-μm particle size) chromatographic column, maintained at 35°C was used. The mobile phase consisted of 0.01 % acetic acid in ultrapure water (A) and Methanol (B) 30/70 v/v, in isocratic mode, at a flow rate of 0.15 mL/min. The injection volume was 1 µL. BPA detection was performed in MRM (Multiple Reaction Monitoring) mode, monitoring two mass transitions: 227 → 212 and 227 → 133. MS parameters established for the BPA quantification were: cell accelerator voltage (1V), fragmentor voltage (150V), collision energy (15V), and dwell time (250msec). The BPA elution was performed in less than 6 min. ESI operational parameters: capillary voltage (6000V), drying gas temperature (300°C), drying gas flow (7 L/min) and nebulizer pressure (40 psi).
2.2.2. Analytical conditions used for the identification of the degradation products
Determination of unknown degradation products of BPA was executed using LC-MS/MS technique. The injection volume was 5 μL. The Luna C18 chromatographic column temperature was set at 20C°. The mobile phase consisted of 0.01% acetic acid in ultrapure water (A) and Methanol (B) 40/60 v/v, in isocratic mode, at a flow rate of 0.15 mL/min and a stop time of 15 min. Data were gathered in the enhanced mass spectra mode, between 50 and 350 Da. Full-scan chromatograms were registered, using the ESI source in negative mode. ESI operational parameters: capillary voltage (6000V), drying gas temperature (300°C), drying gas flow (7 L/min) and nebulizer pressure (40 psi).
2.2.3. Quality assurance and quality control
All samples were analyzed in duplicate. An etalon and a blank sample were injected at each test sequence. The method linearity was evaluated between 0.015 – 25 mg/L, using six calibration points (0.015, 0.01, 0.5, 5.0, 10 and 25 mg/L), high correlation coefficient value being obtained, R2 = 0.998. Recovery was not evaluated because sample preparation required only a dilution step. The RSD values for intra-day and inter-day precision were 3.2% and 5.7%, respectively. The method accuracy was 0.92%. Limit of detection (LOD) was 0.005 mg/L, and limit of quantitation (LOQ) was 0.015 mg/L, respectively. The measured uncertainty was determined to be 22.6%. Samples were diluted using ultra-purified water, to fit in the calibration domain. The degradation level of the BPA was determined using chemical oxygen demand analysis, COD according to the SR ISO 6060:19962.
2.4. Work Methods
Volumes as 100 mL of synthetic solution with 25 mg/L initial BPA concentration were introduced into the ultrasonic reactor under air atmosphere. The ultrasonic reactor was inserted into a cooling bath that maintains a relatively constant temperature in the range between 20 and 32⁰C. The influence of frequency and doses of some additional compounds such as: carbon tetrachloride, ferrous sulphate heptahydrate and ethyl anthraquinone on the efficiency of BPA degradation were studied. Three different frequencies were used: 1146 KHz, 864 KHz, 580 KHz, at a power of 50 W, in the absence and in the presence of additional compounds.
Carbon tetrachloride, CCl4 was used in three different initial doses: 6 µL, 12 µL and 25 µL to 100 mL initial solution. The degradation efficiency is studied for 864 KHz, 580 KHZ, at a power of 50 W, after 15 minutes of exposure. Another additional compound, FeSO4 7H2O, FS, with 0.1 mg/L concentration, was used in two doses: 5mL /95ml initial solution and 20 mL /80mL of initial solution. The variation of efficiencies of BPA degradation in time, for each 15 minutes, time of 60 minutes, at a frequency of 580 KHz was studied. In the end, the BPA degradation efficiency was studied for a time of 60 minutes at 580 KHz for Ethyl anthraquinone with doses of 0.1, 0.2, 0.3 mL/100 mL initial solution, respectively. All the experiments have been done in duplicate; the values used in the paper represent the average value between the two runs. For optimization of the process, a comparison between all methods, all doses used for a frequency of 580 KHz, and after 15 minutes, were made.
For determined the effects of the process applied were determined the:
- efficiency of BPA degradation using the equation:
were:
is the initial concentration of BPA in initial solution, mg/L,
is the concentration of BPA at the t moment in the system, mg/L
-efficiency of BPA mineralization using equation:
where:
is the initial Chemical oxygen demand, COD, concentration for BPA in initial solution, mg/L,
is the COD concentration of BPA solution at the
t moment in the system, mg/L
4. Discussions
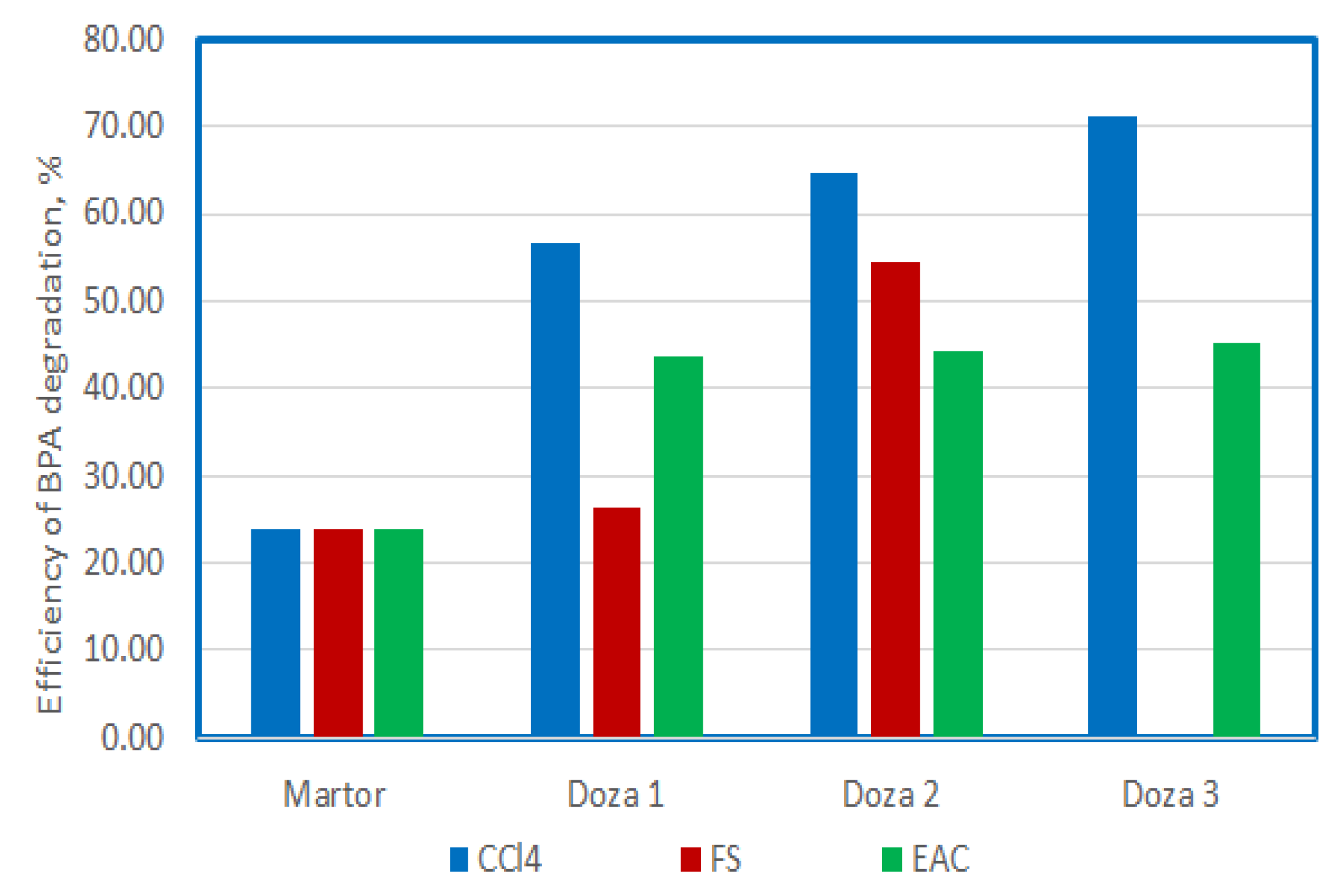
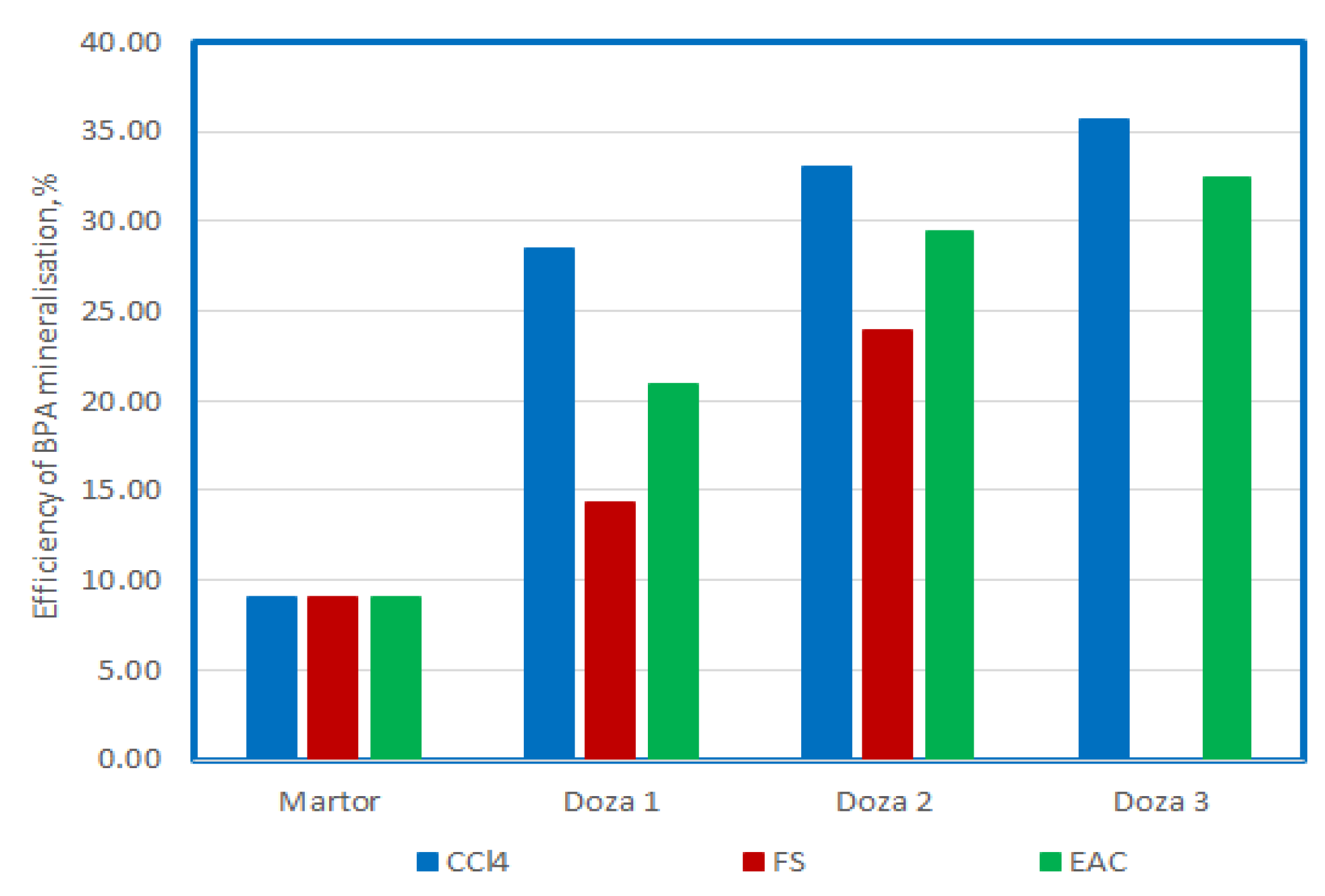
To optimize the process, the use of US with a frequency of 580 KHz, after a 15-minute exposure was chosen, as a comparison term.
Figure 14 and
Figure 15 show the comparative values of the degradation efficiency and the mineralization efficiency for all the situations presented previously.
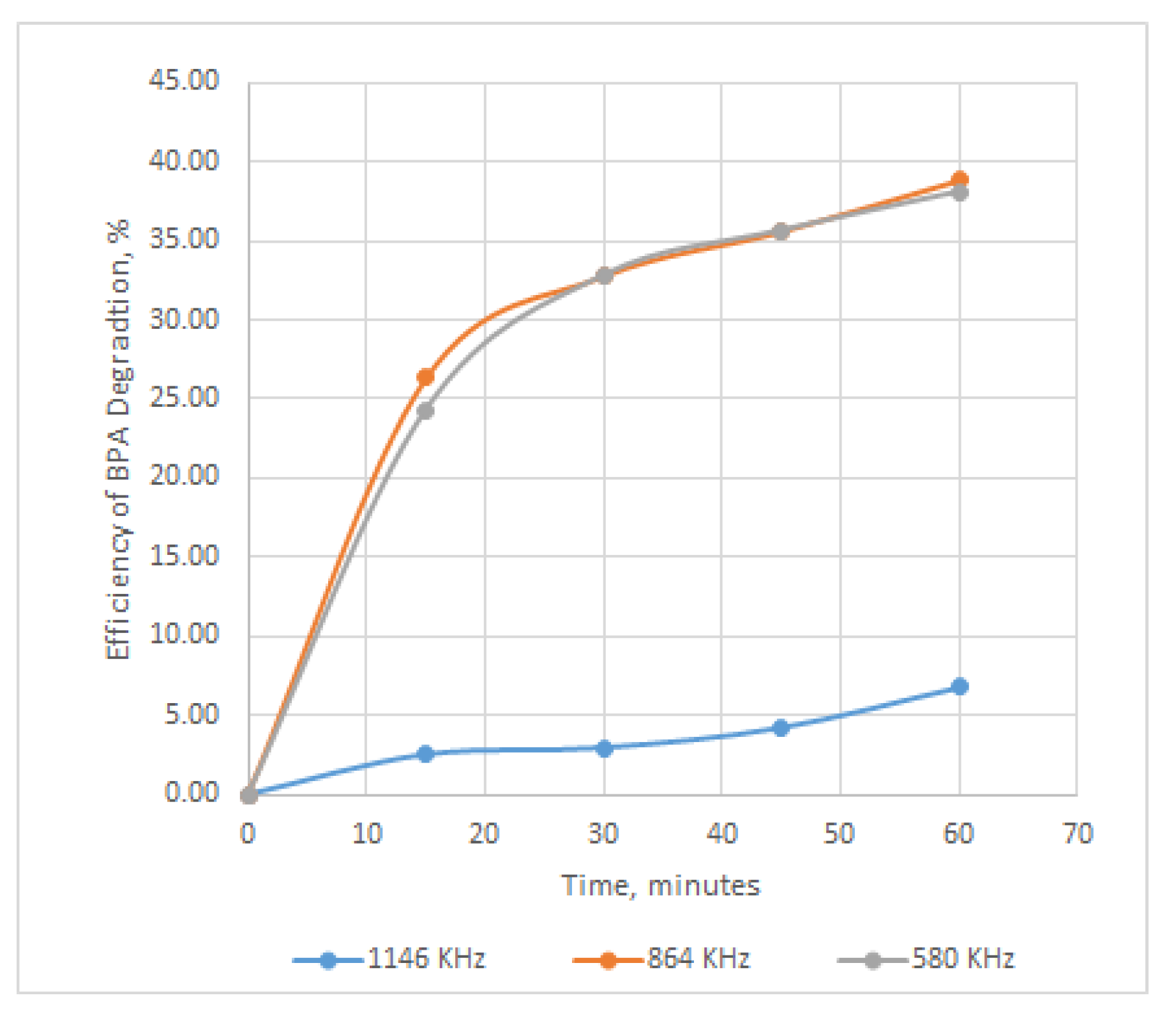
The use of ultrasound with high frequencies of 580 and 864 KHz and P 50 W determines degradation in proportion of 23%, and mineralization in proportion of 9% for BPA present in solutions with the initial concentration of 25 mg/L. The best degradation efficiency is obtained when using carbon tetrachloride, and the efficiency increases with the increase of the doses used, in the order of 58%, 64 and 70%. In this situation, the demineralization capacity increases from 28%, to 33% and 36%, which means that almost half of the amount that degrades reaches mineralization.
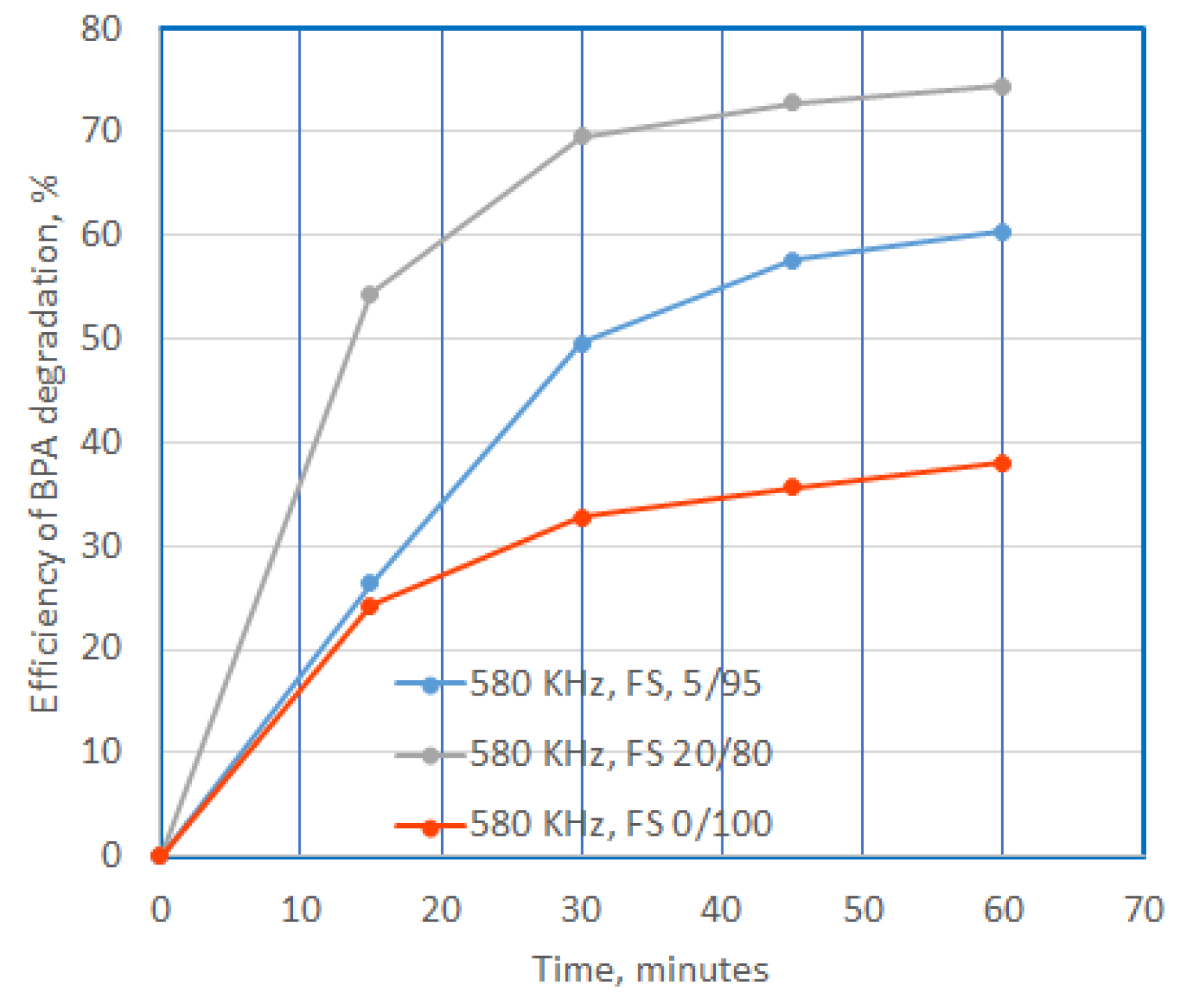
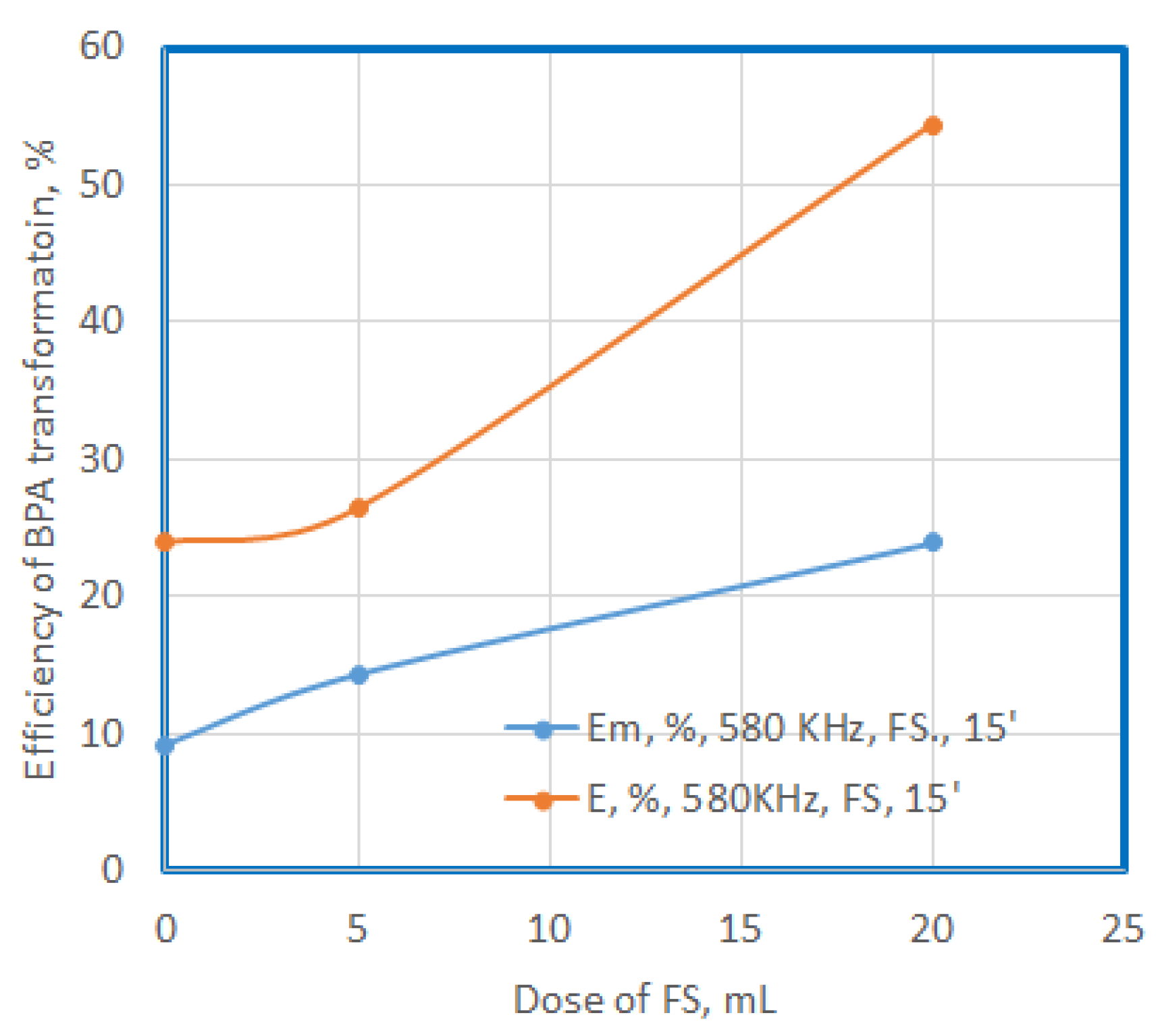
The use of FS increases the efficiency of degradation and mineralization as the dose used increases, reaching values of 28% and 54%; and 14% and 24%, respectively. The mineralization capacity is also almost half of the degradation capacity.
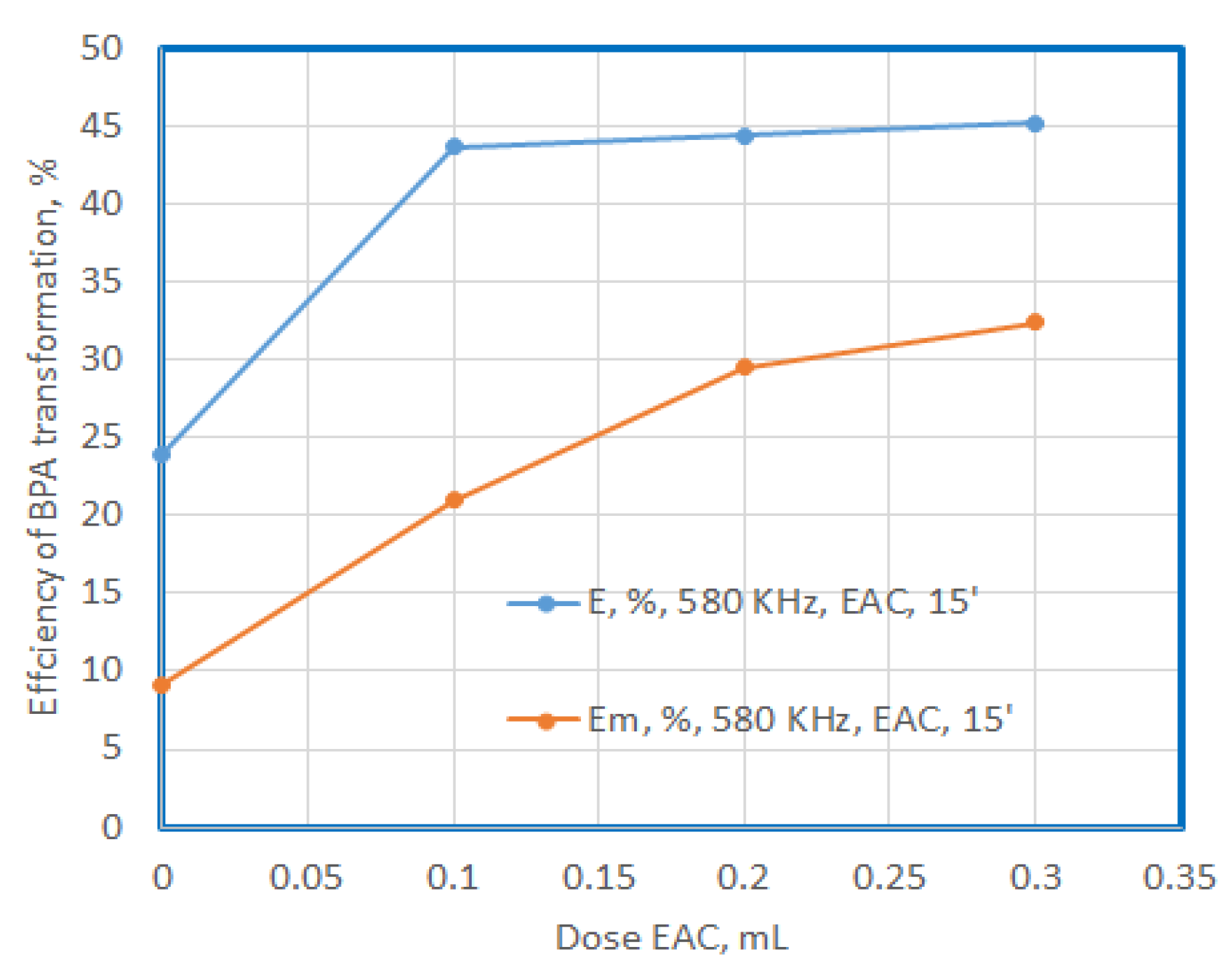
In the case of the use of EAC, it can be observed that the increase in the dose does not cause a sharp increase in the degree of degradation, it remains at values around 45%, while the mineralization capacity increases at values of 20%, 29% and 35% respectively as the dose increases. It seems that the use of doses of 0.3 mL of EAC causes an increase in the degree of mineralization to approximately 75% of the degradation capacity.
Figure 1.
Schematic diagram of the experimental apparatus (Meinhardt Multifrequency system): 1 – US reactor – water jacketed; 2 – ultrasonic transducer;.3 – power amplifier; 4 – frequency generator 580; 684 and 1146 KHz.
Figure 1.
Schematic diagram of the experimental apparatus (Meinhardt Multifrequency system): 1 – US reactor – water jacketed; 2 – ultrasonic transducer;.3 – power amplifier; 4 – frequency generator 580; 684 and 1146 KHz.
Figure 2.
Influence of the US frequencies on the BPA degradation efficiency.
Figure 2.
Influence of the US frequencies on the BPA degradation efficiency.
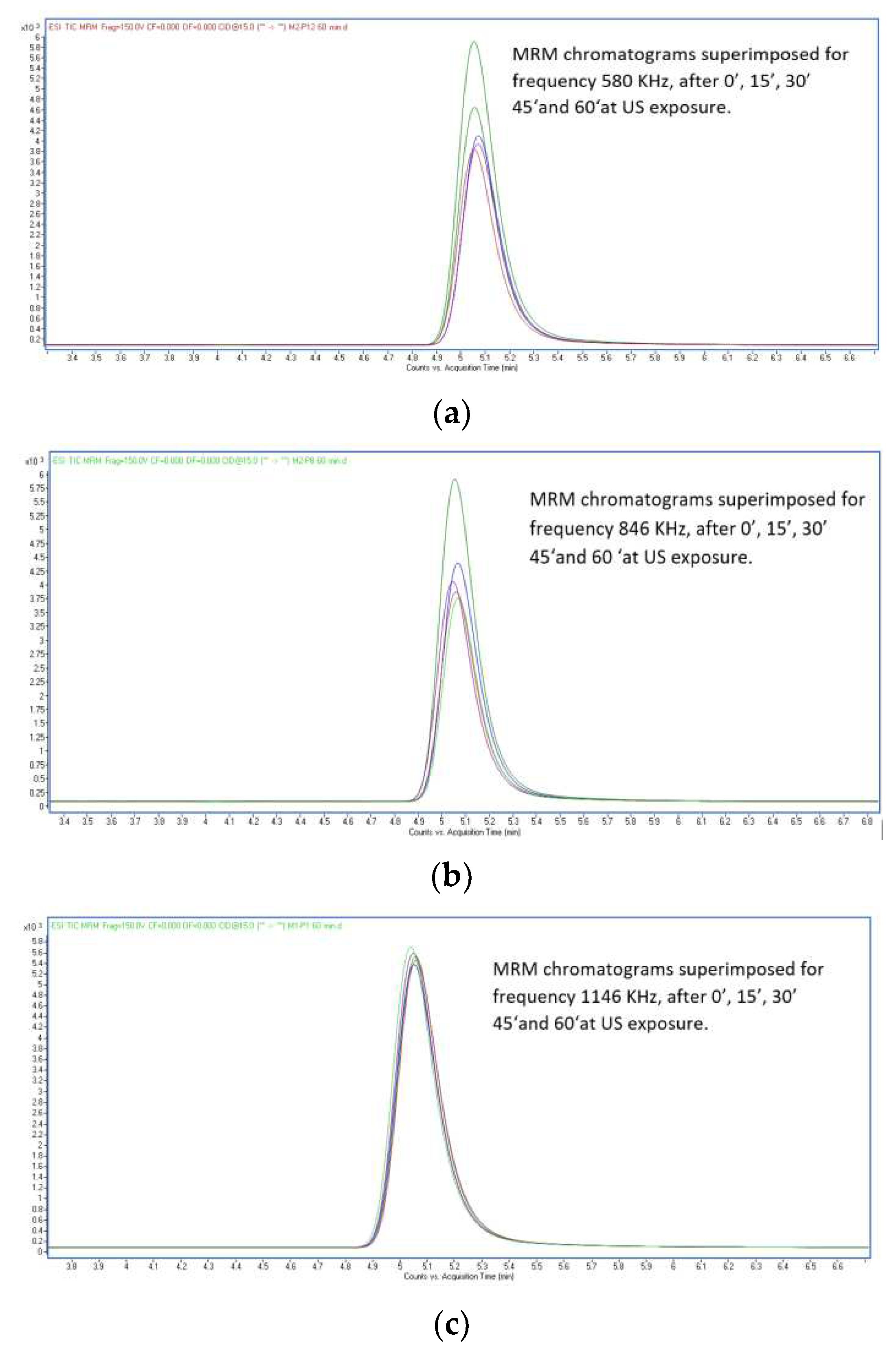
Figure 3.
Concentration chromatograms superimposed at US exposure for frequencies 580 KHz, 864 KHz and 1146 KHz after 0’, 15’, 30’ 45‘and 60‘.
Figure 3.
Concentration chromatograms superimposed at US exposure for frequencies 580 KHz, 864 KHz and 1146 KHz after 0’, 15’, 30’ 45‘and 60‘.
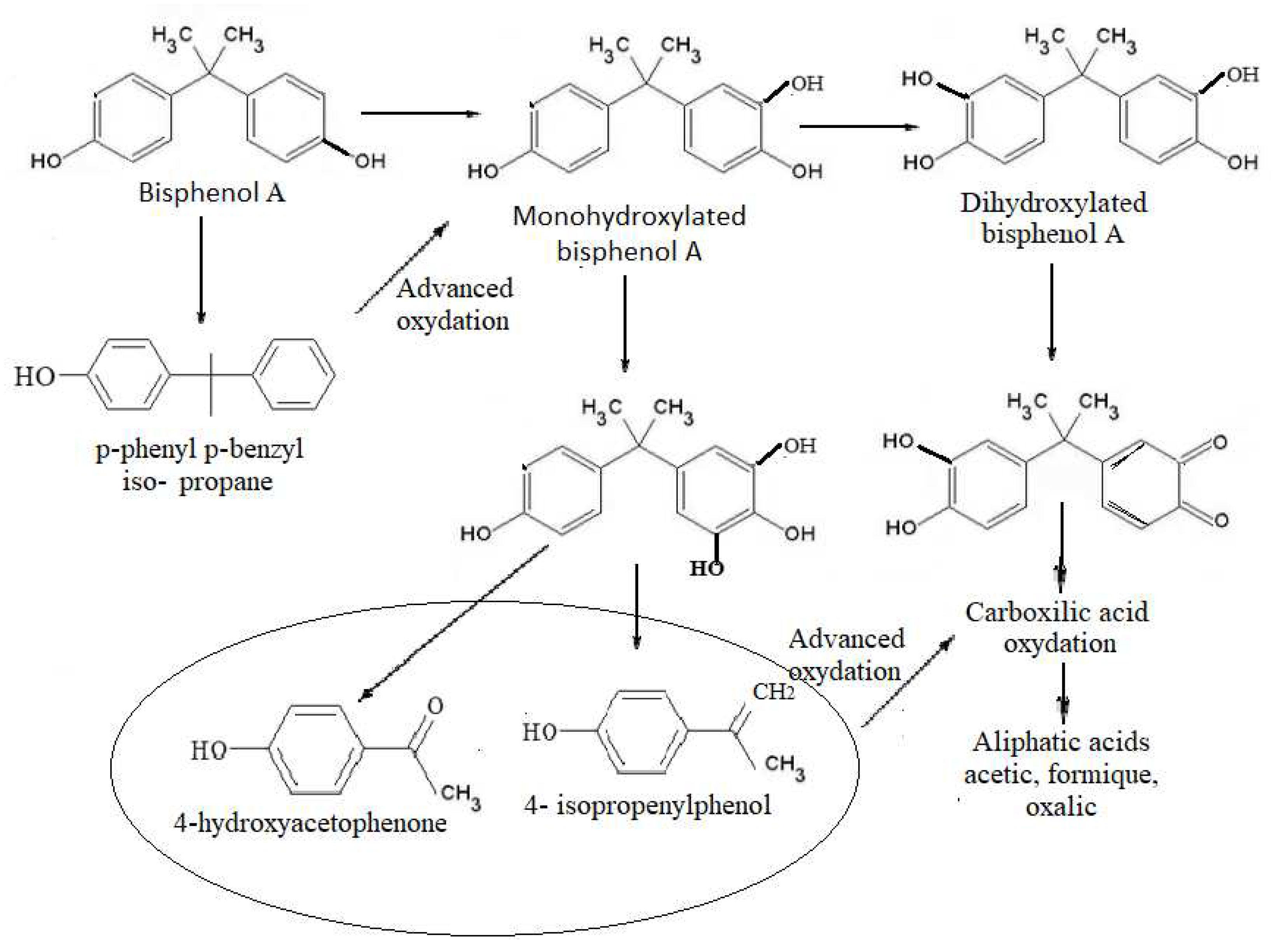
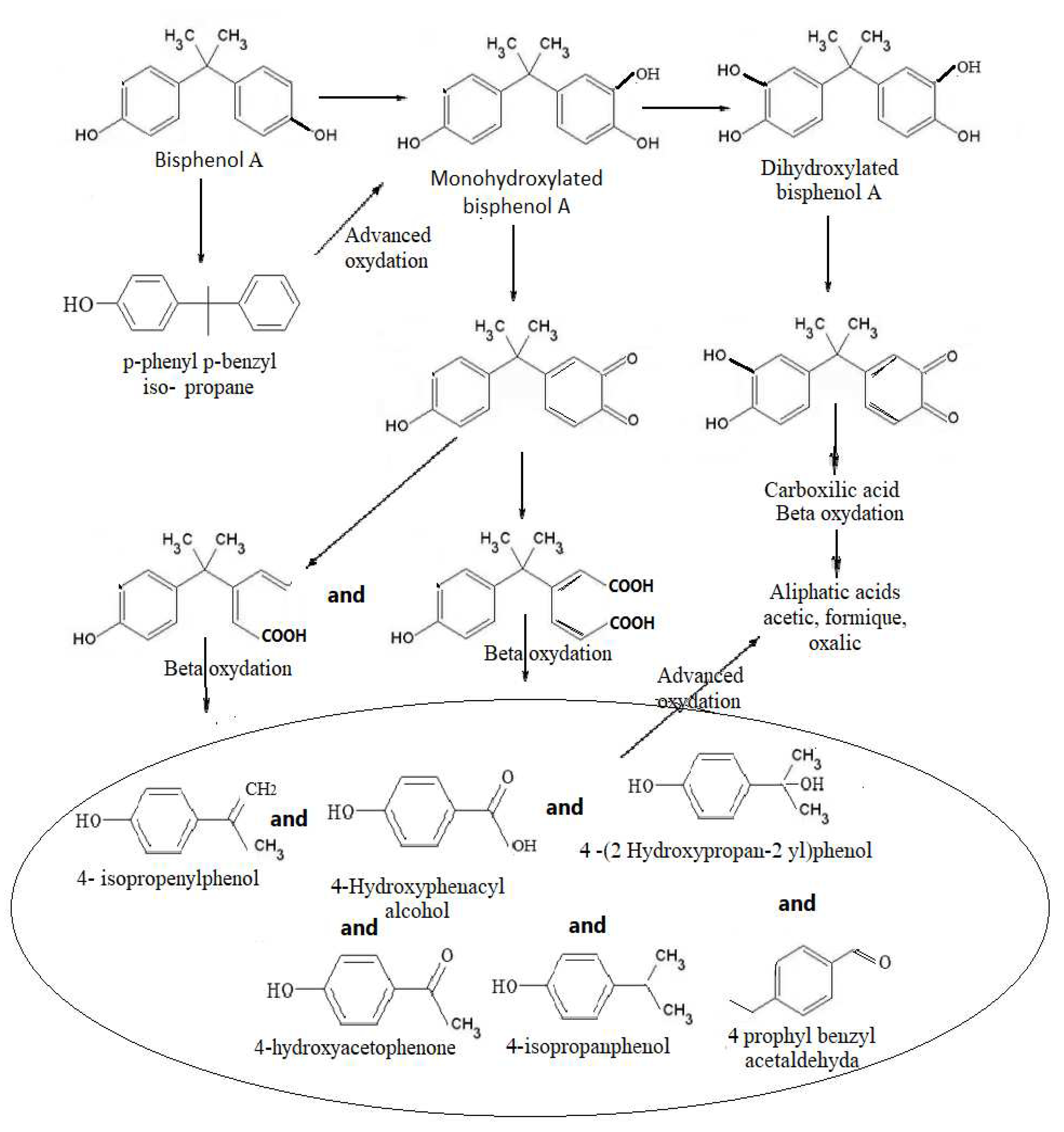
Figure 4.
The proposal pathway to BPA degradation using US at frequencies of 580 KHz and 864 KHz, for an exposure of 60 minutes.
Figure 4.
The proposal pathway to BPA degradation using US at frequencies of 580 KHz and 864 KHz, for an exposure of 60 minutes.
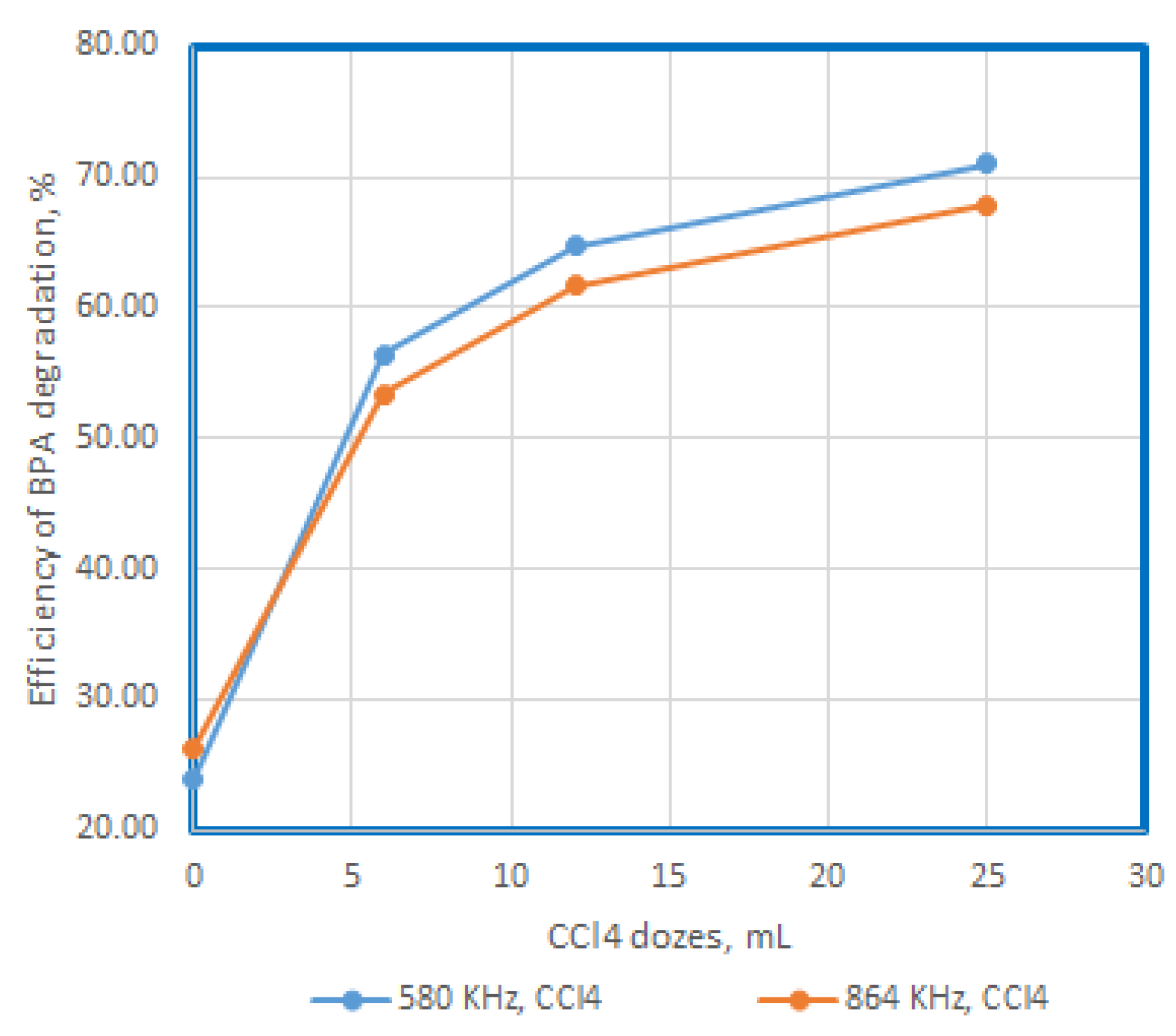
Figure 5.
Efficiency of BPA degradation at US exposure at 580 KHz and 864 KHz, in presence of CCL4,, for 15 minutes.
Figure 5.
Efficiency of BPA degradation at US exposure at 580 KHz and 864 KHz, in presence of CCL4,, for 15 minutes.
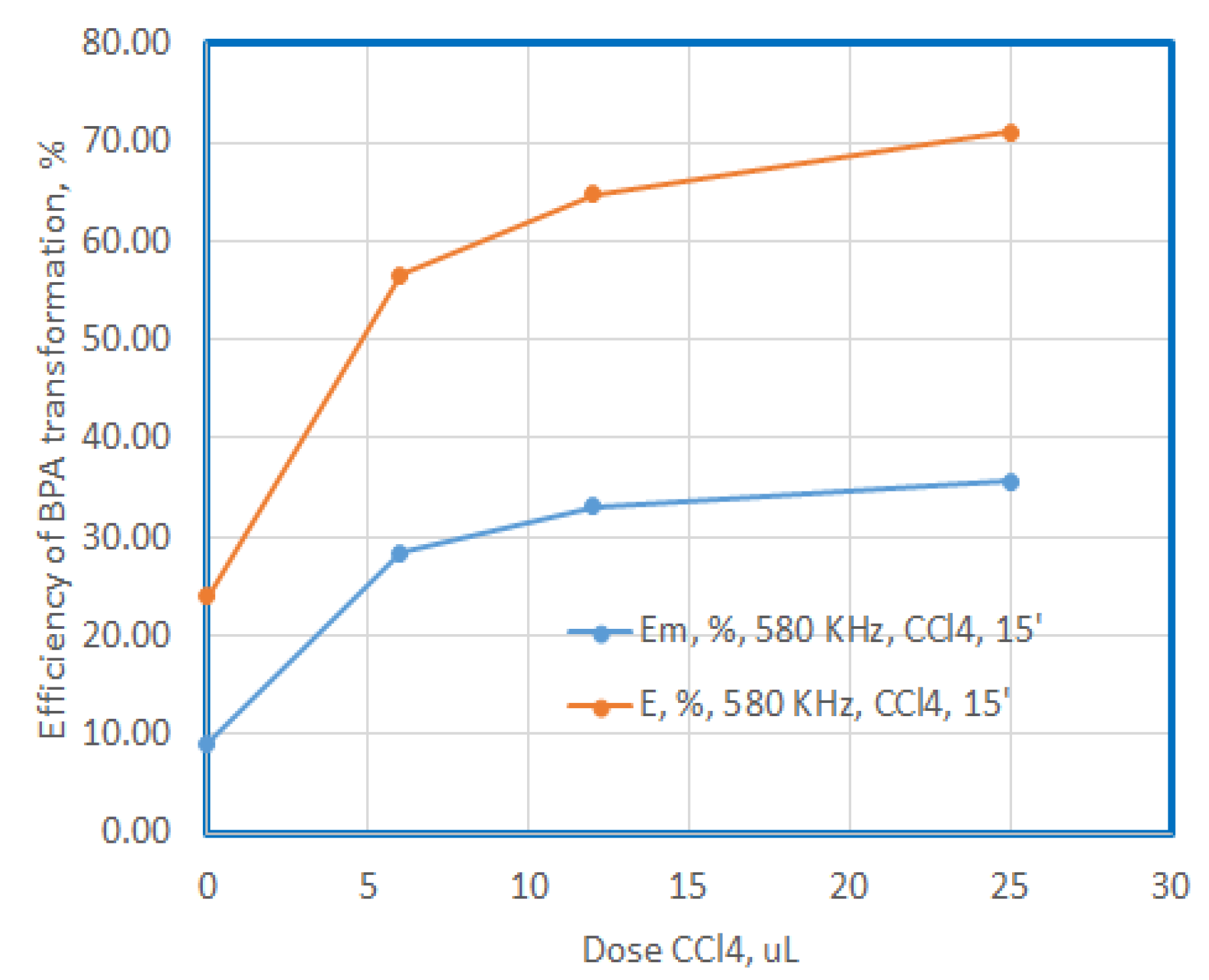
Figure 6.
Efficiency of BPA degradation and mineralization at US exposure with 580 KHz, in presence of CCL4, for 15 minutes .
Figure 6.
Efficiency of BPA degradation and mineralization at US exposure with 580 KHz, in presence of CCL4, for 15 minutes .
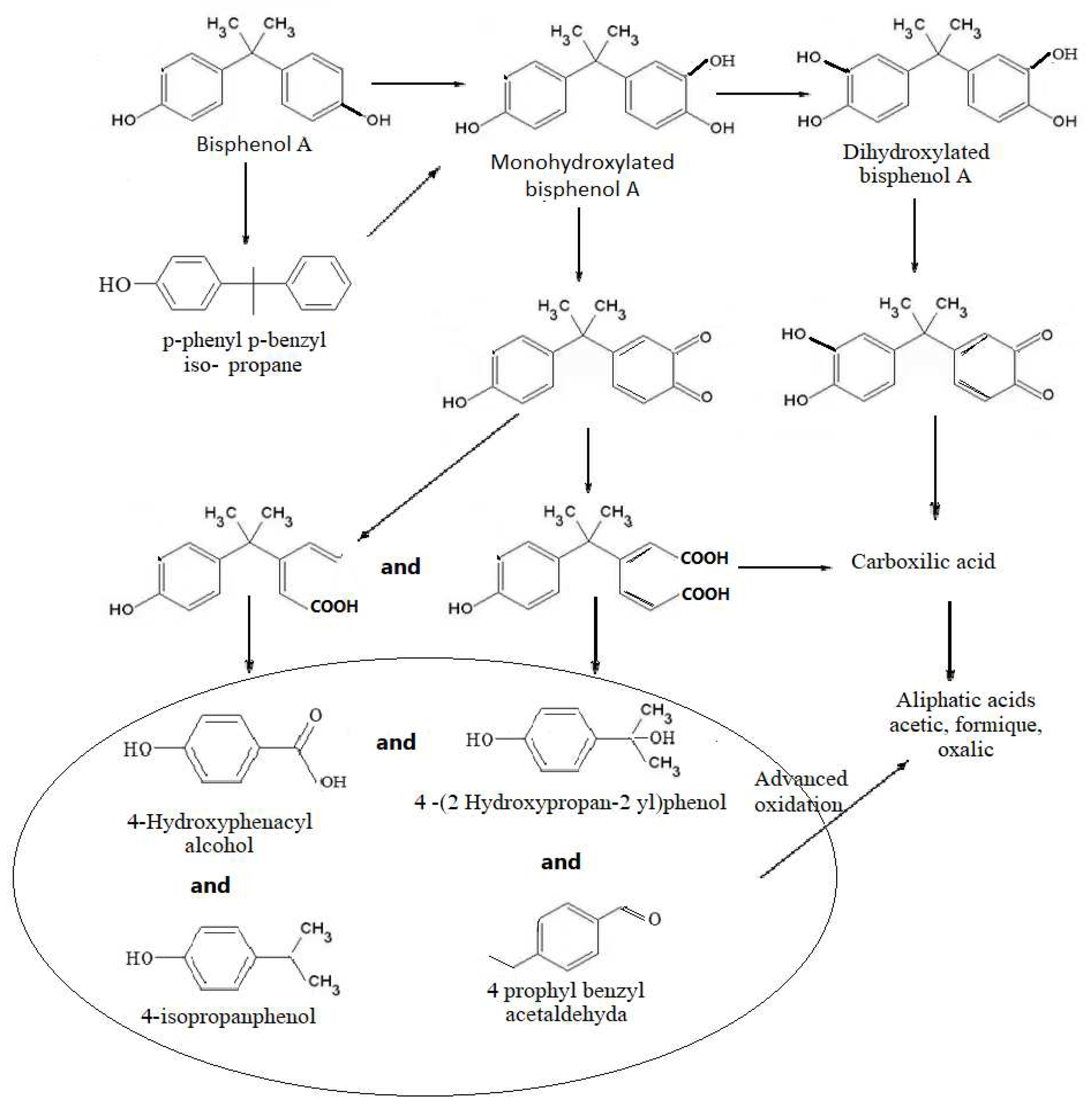
Figure 7.
The proposal pathway to BPA degradation using US at frequencies of 580 KHz and 864 KHz, for an exposure of 15 minutes in presence of CCl4.
Figure 7.
The proposal pathway to BPA degradation using US at frequencies of 580 KHz and 864 KHz, for an exposure of 15 minutes in presence of CCl4.
Figure 8.
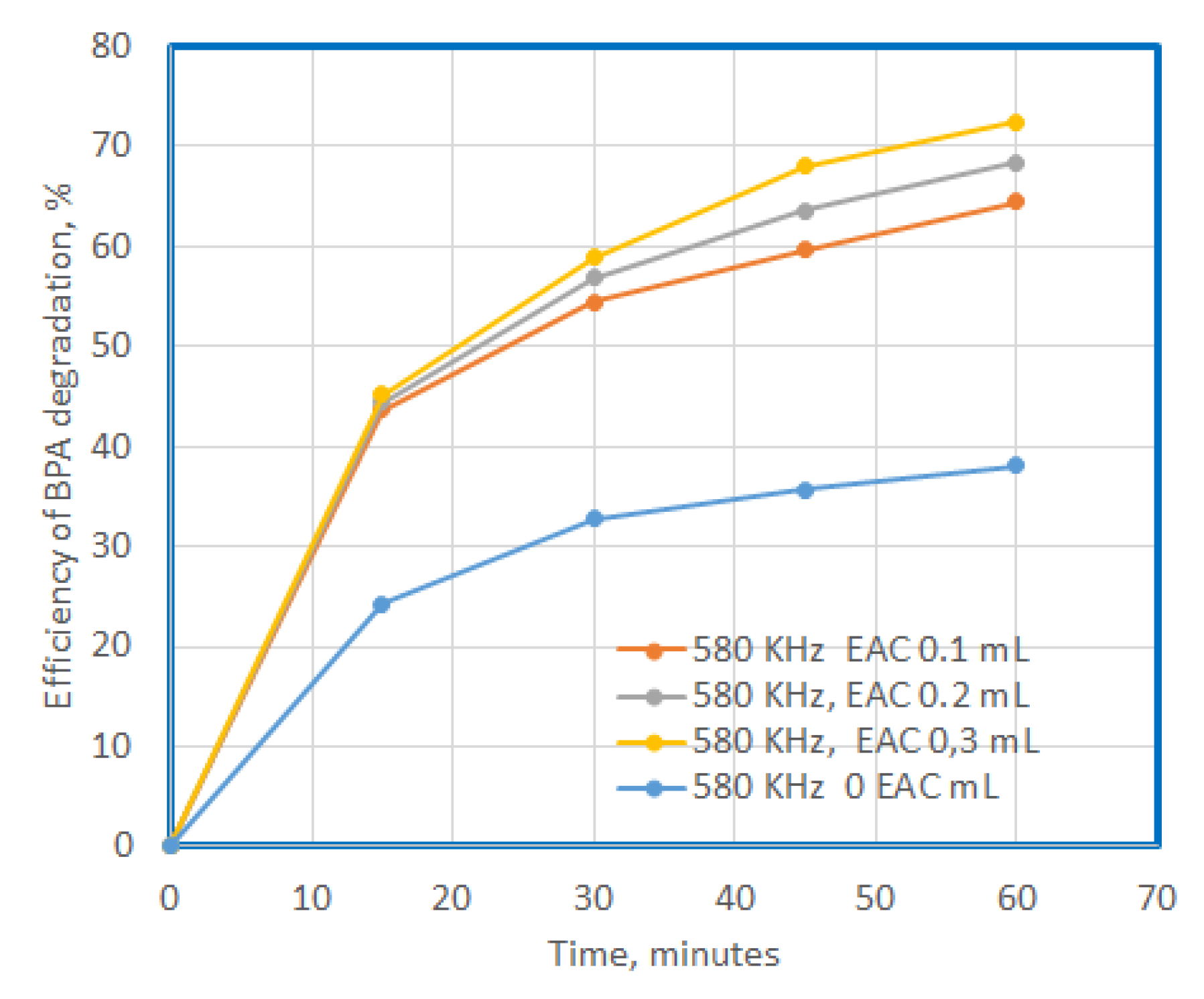
Efficiency of BPA degradation at US exposure in presence of SF, additional compunds, with a concentration of 0.1 mg/L, in time.
Figure 8.
Efficiency of BPA degradation at US exposure in presence of SF, additional compunds, with a concentration of 0.1 mg/L, in time.
Figure 9.
Efficiency of BPA transformation at US exposure in presence of SF, additional compunds, after 15’.
Figure 9.
Efficiency of BPA transformation at US exposure in presence of SF, additional compunds, after 15’.
Figure 10.
The proposal pathway to BPA degradation, using US and FeSO4 7H2O with concentration 0.1 mg/L, as additional compunds at different doses, and at frequency of 580 KHz for an exposure of 60 minutes.
Figure 10.
The proposal pathway to BPA degradation, using US and FeSO4 7H2O with concentration 0.1 mg/L, as additional compunds at different doses, and at frequency of 580 KHz for an exposure of 60 minutes.
Figure 11.
Efficiency of BPA degradation at US exposure in presence of EAC, in time.
Figure 11.
Efficiency of BPA degradation at US exposure in presence of EAC, in time.
Figure 12.
Efficiency of BPA transformation at US exposure in presence of EAC after 15’ exposure.
Figure 12.
Efficiency of BPA transformation at US exposure in presence of EAC after 15’ exposure.
Figure 13.
The proposal pathway to BPA degradation using US and EAC, as additional compunds at different doses and at frequency of 580 KHz for an exposure of 60 minutes.
Figure 13.
The proposal pathway to BPA degradation using US and EAC, as additional compunds at different doses and at frequency of 580 KHz for an exposure of 60 minutes.
Figure 14.
The comparison between the degradation efficency of BPA at 15 minutes of US exposure and different adittional compounds.
Figure 14.
The comparison between the degradation efficency of BPA at 15 minutes of US exposure and different adittional compounds.
Figure 15.
The comparison between the mineralisation efficency of BPA at 15 minutes of US exposure and different adittional compounds.
Figure 15.
The comparison between the mineralisation efficency of BPA at 15 minutes of US exposure and different adittional compounds.