Submitted:
08 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Materials and reagents
2.2. Apparatus and measurements
2.3. Bacterial Cultivation
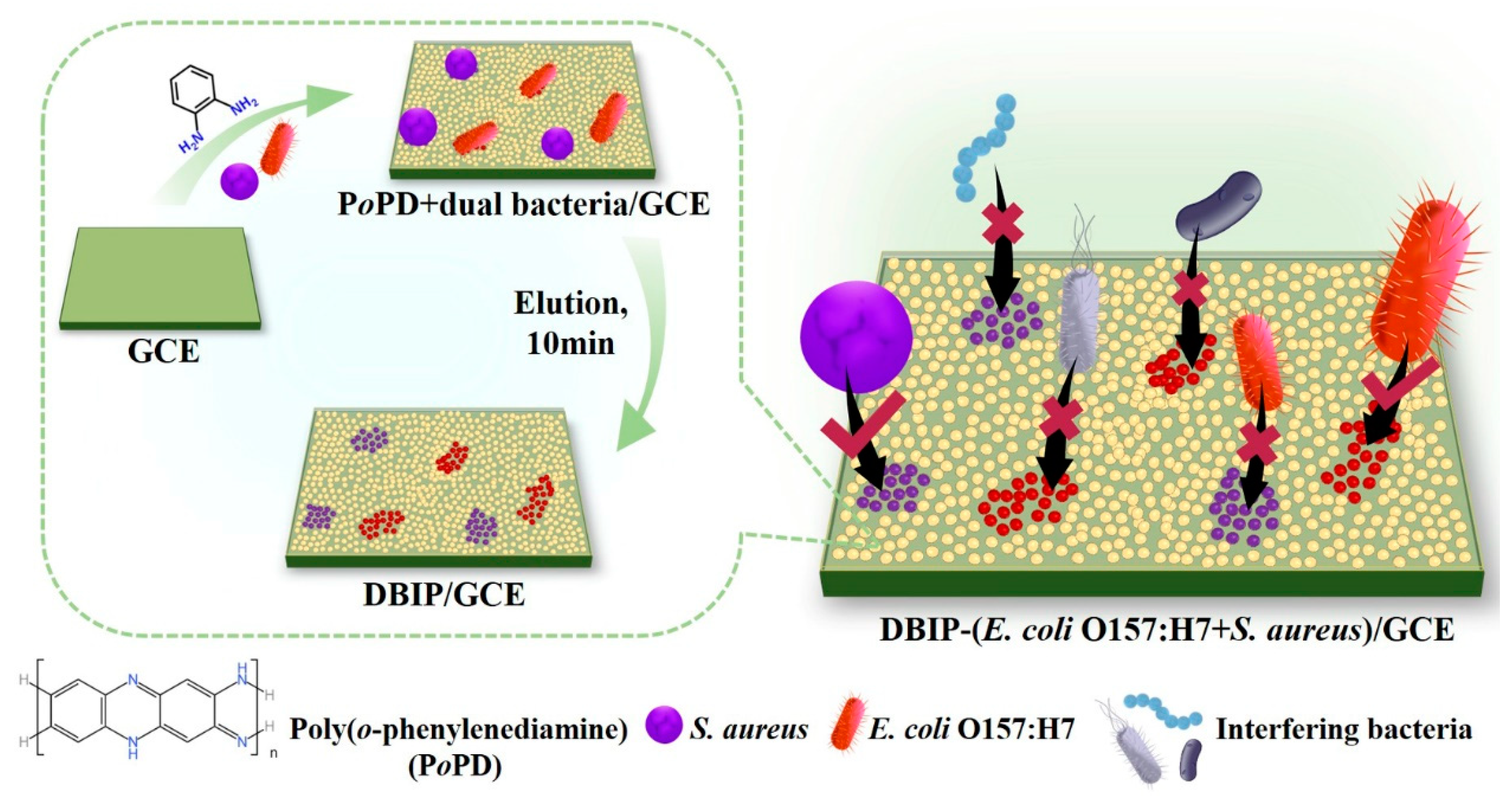
2.4. Preparation of the DBIP-modified electrode
2.5. Detection of E. coli O157:H7 and S. aureus
2.6. Optimization of experimental conditions
2.7. Real sample
3. Results and Discussion
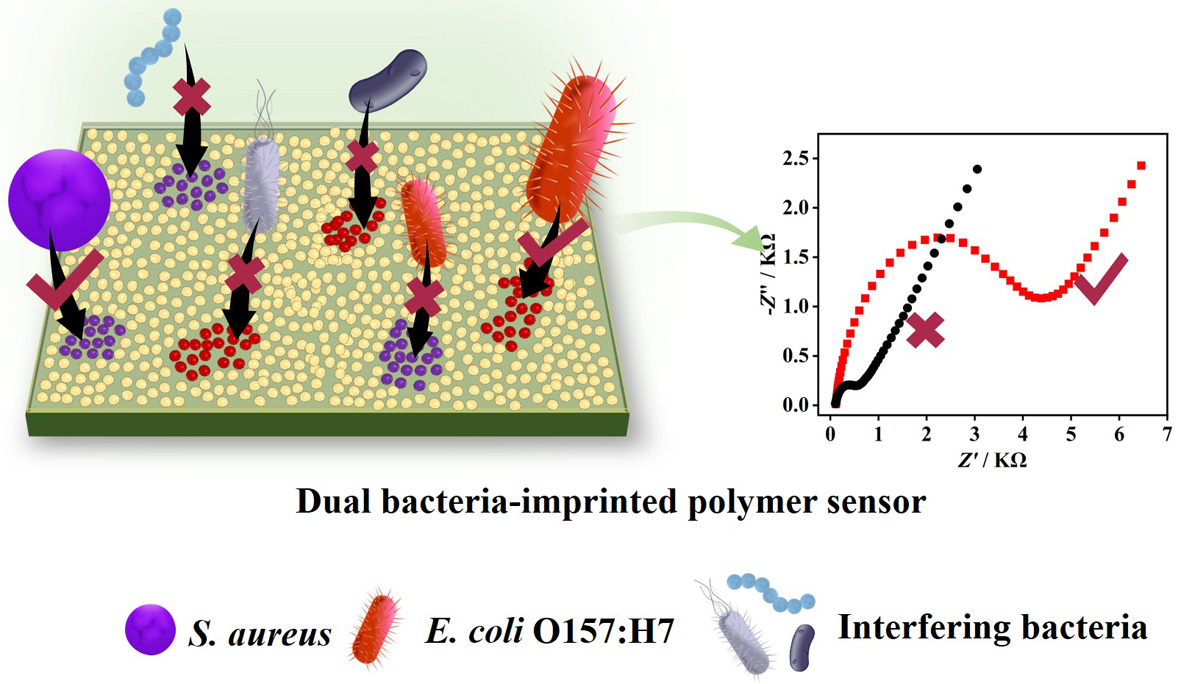
3.1. Fabrication of DBIP-modified electrode for bacterial capture and detection
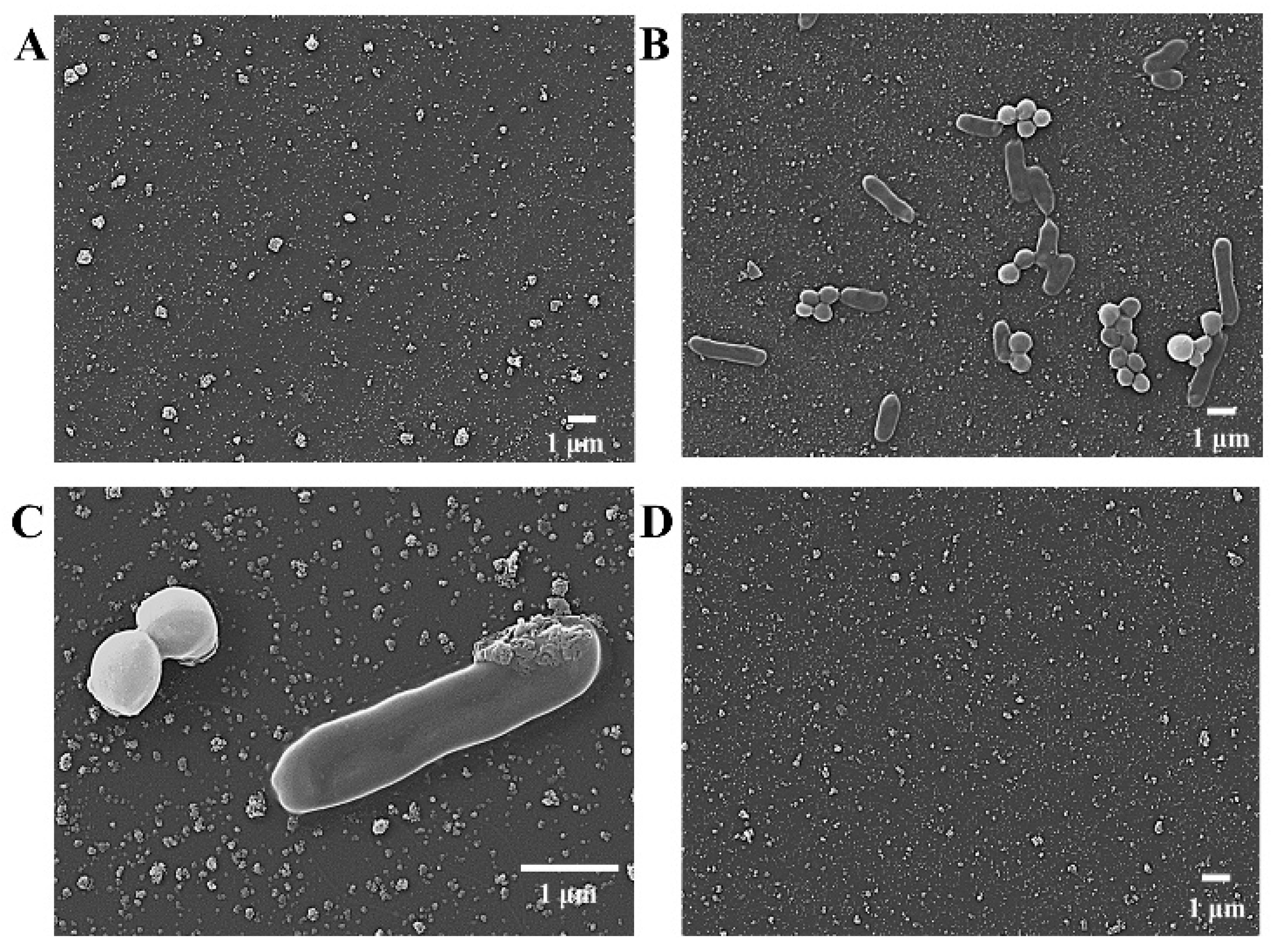
3.2. Characterization of the DBIP Fabrication
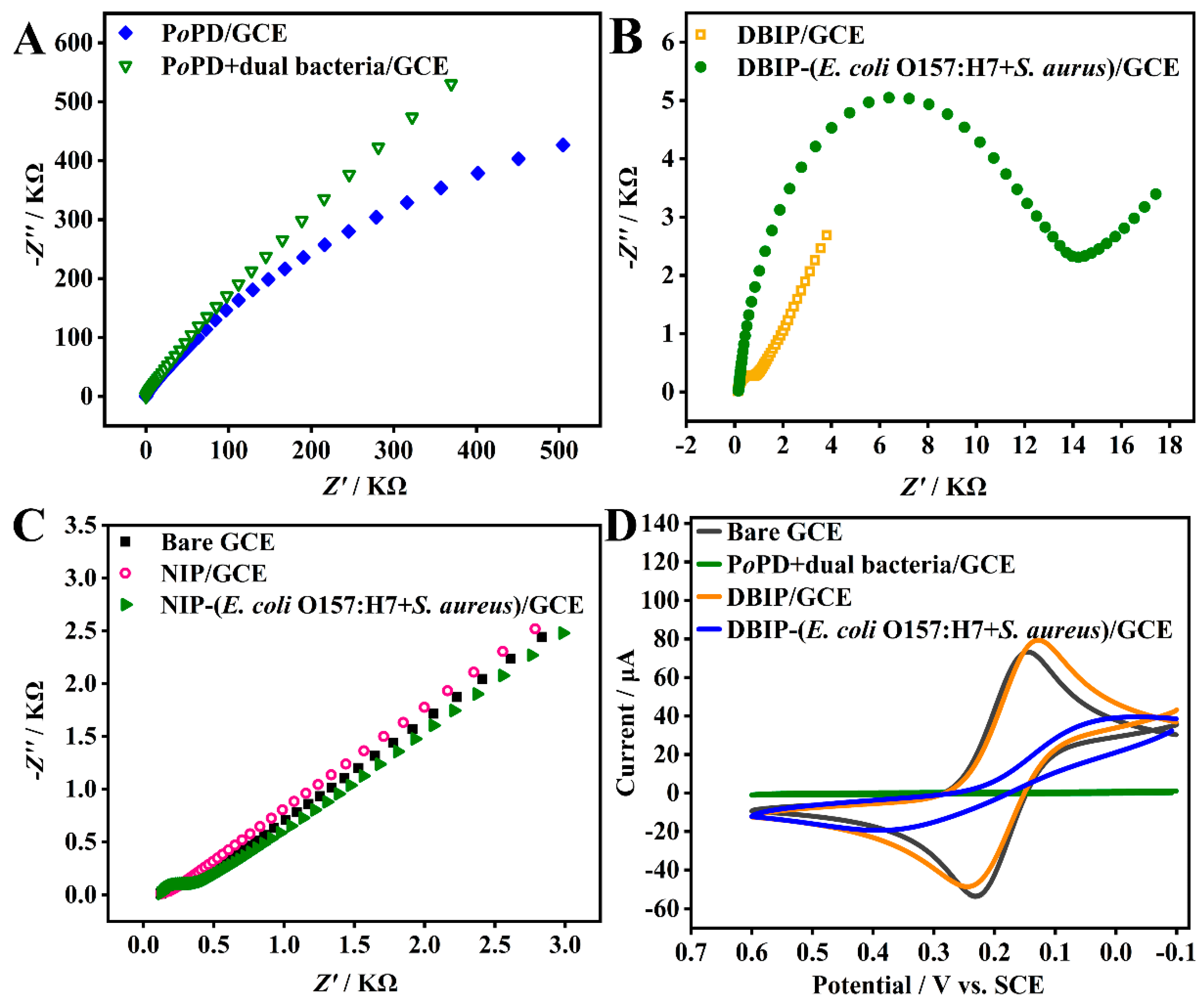
3.3. Electrochemical characterization of the DBIP-Based Sensor
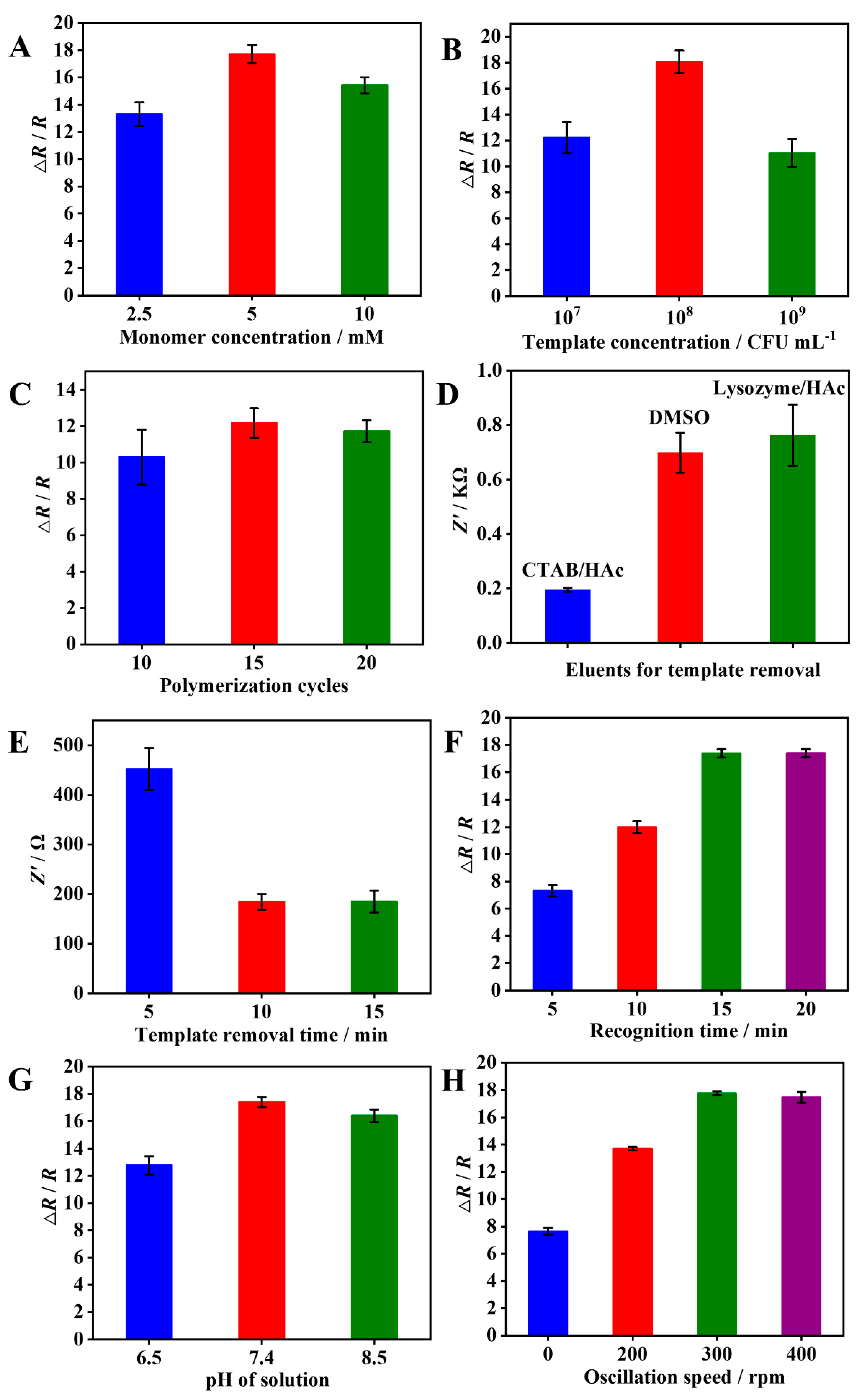
3.4. Optimization of experimental conditions
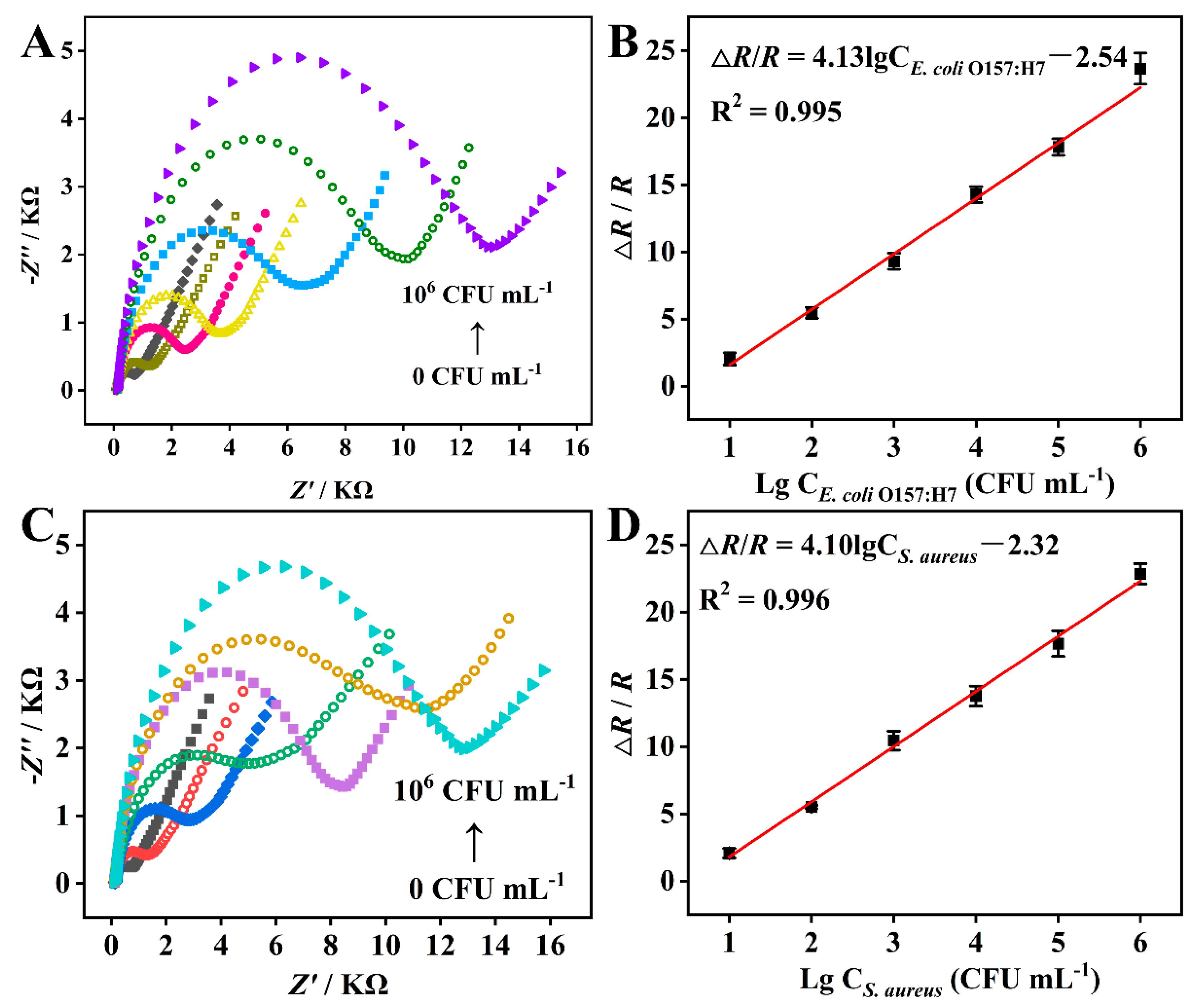
3.5. Quantitative Detection of E. coli O157:H7 and S. aureus
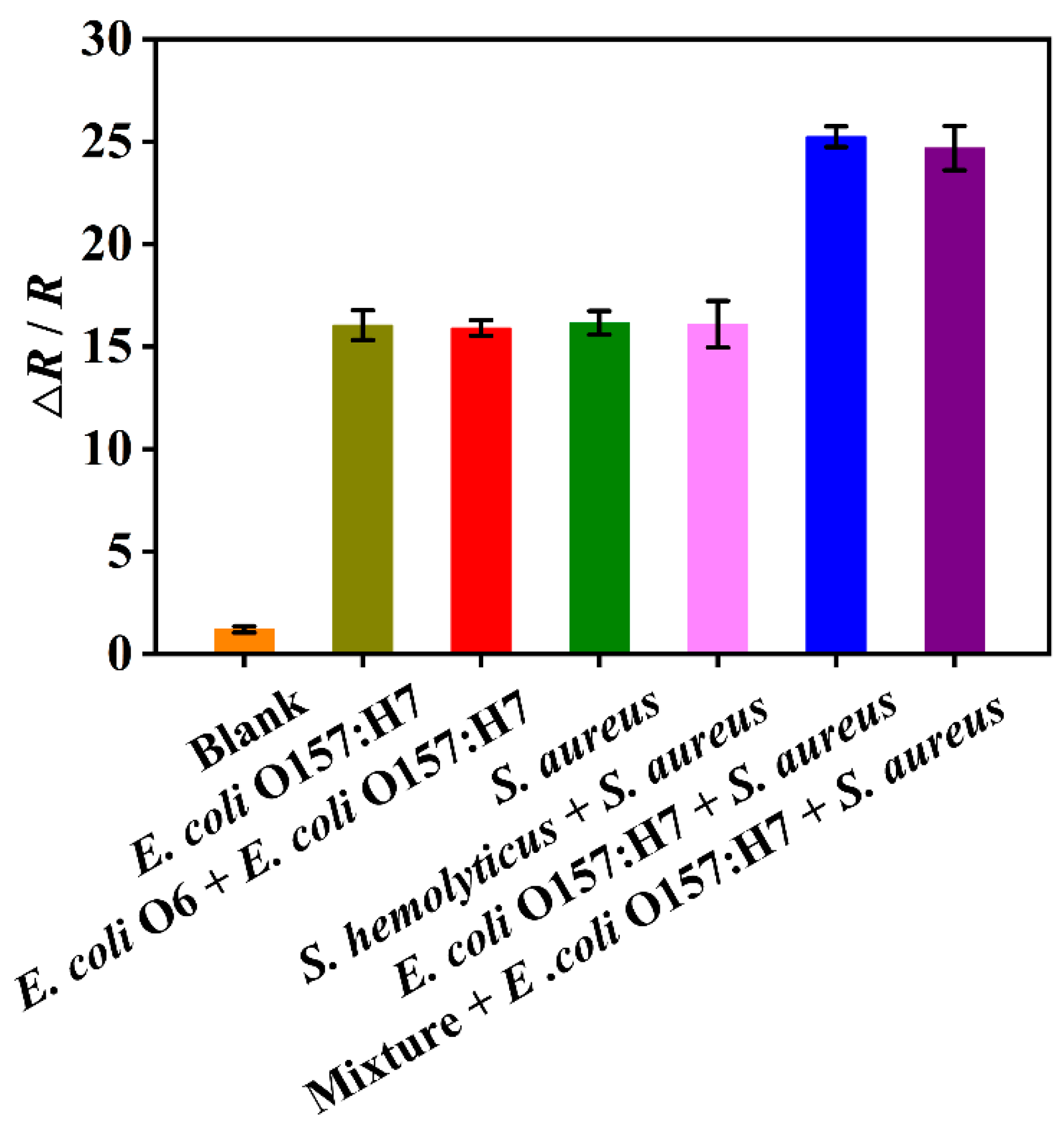
3.6. Selectivity of the DBIP Sensor
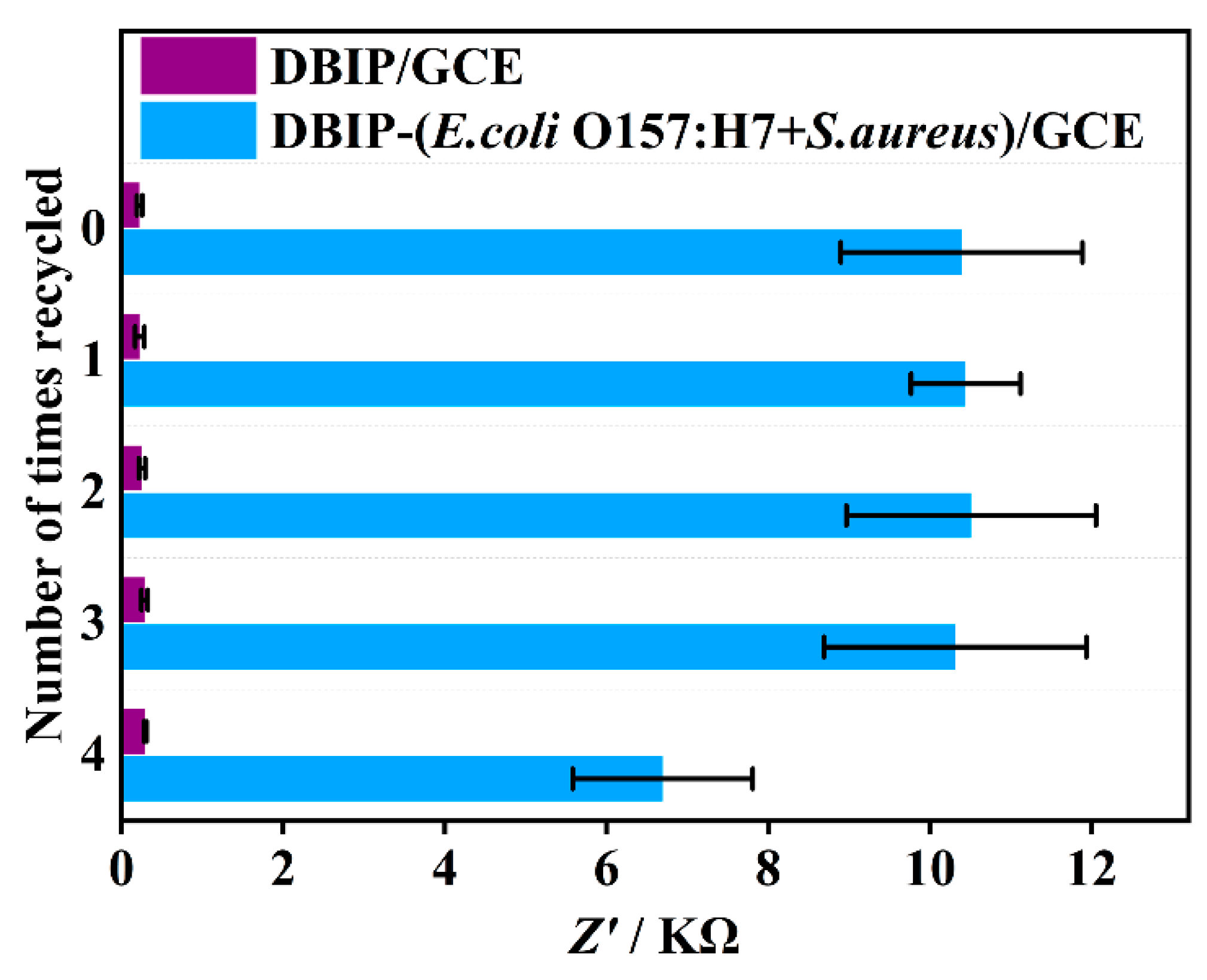
3.7. Reusability of the DBIP-based sensor
3.8. Detection of E. coli O157:H7 and S. aureus in Real Samples
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gu, R.H.; Duan, Y.X.; Li, Y.X.; Luo, Z.W. Fiber-Optic-Based Biosensor as an Innovative Technology for Point-of-Care Testing Detection of Foodborne Pathogenic Bacteria To Defend Food and Agricultural Product Safety. J Agr Food Chem 2023, 71, 10982–10988. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.Q.; Gu, M.B. An ultrasensitive electrochemical aptasensor using Tyramide-assisted enzyme multiplication for the detection of Staphylococcus aureus. Biosensors & Bioelectronics 2023, 228, 115199. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Li, Z.Y.; Huang, J.J.; Wu, Y.X.; Mao, X.Y.; Tan, Y.Z.; Liu, H.; Ma, D.X.; Li, X.; Wang, X.Y. Development of a phage-based electrochemical biosensor for detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.L.; Wang, X.W.; Yang, L.; Li, Q.H.; Zheng, X.T.; Bai, T.Y.; Wang, X. Antibacterial Activity of Juglone Revealed in a Wound Model of Staphylococcus aureus Infection. Int J Mol Sci 2023, 24, 3931. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Li, Y.; Li, Z.; Zhang, J.W.; Geng, X.; Zhang, F.; Wang, Q.J.; He, P.A. Zirconium-Based Metal-Organic Framework and Ti3C2Tx Nanosheet- Based Faraday Cage-Type Electrochemical Aptasensor for Escherichia coli Detection. Acs Appl Nano Mater 2022, 5, 9201–9208. [Google Scholar] [CrossRef]
- Li, S.; Konoval, H.M.; Marecek, S.; Lathrop, A.A.; Feng, S.; Pokharel, S. Control of Escherichia coli O157:H7 using lytic bacteriophage and lactic acid on marinated and tenderized raw pork loins. Meat Sci 2023, 196, 109030. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejek, A.M.; Minnich, S.A.; Hovde, C.J. Escherichia coli 0157:H7 virulence factors and the ruminant reservoir. Curr Opin Infect Dis 2022, 35, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Xiong, J.; Yue, H.; Fu, Z.F. Sandwich Fluorimetric Method for Specific Detection of Staphylococcus aureus Based on Antibiotic-Affinity Strategy. Analytical Chemistry 2015, 87, 9864–9868. [Google Scholar] [CrossRef]
- Ali, M.M.; Silva, R.; White, D.; Mohammadi, S.; Li, Y.F.; Capretta, A.; Brennan, J.D. A Lateral Flow Test for Staphylococcus aureus in Nasal Mucus Using a New DNAzyme as the Recognition Element. Angew Chem Int Edit 2022, 61, e202112346. [Google Scholar] [CrossRef]
- Foddai, A.C.G.; Grant, I.R. Methods for detection of viable foodborne pathogens: current state-of-art and future prospects. Appl Microbiol Biot 2020, 104, 4281–4288. [Google Scholar] [CrossRef]
- Wang, J.F.; Wu, X.Z.; Wang, C.W.; Shao, N.S.; Dong, P.T.; Xiao, R.; Wang, S.Q. Magnetically Assisted Surface-Enhanced Raman Spectroscopy for the Detection of Staphylococcus aureus Based on Aptamer Recognition. Acs Applied Materials & Interfaces 2015, 7, 20919–20929. [Google Scholar] [CrossRef]
- Macori, G.; McCarthy, S.C.; Burgess, C.M.; Fanning, S.; Duffy, G. A quantitative real time PCR assay to detect and enumerate Escherichia coli O157 and O26 serogroups in sheep recto-anal swabs. J Microbiol Meth 2019, 165, 105703. [Google Scholar] [CrossRef] [PubMed]
- Athamanolap, P.; Hsieh, K.; O'Keefe, C.M.; Zhang, Y.; Yang, S.; Wang, T.H. Nanoarray Digital Polymerase Chain Reaction with High-Resolution Melt for Enabling Broad Bacteria Identification and Pheno-Molecular Antimicrobial Susceptibility Test. Analytical Chemistry 2019, 91, 12784–12792. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.Y.; Hu, J.S.; Li, Y. Development of an ultra-sensitive single-tube nested PCR assay for rapid detection of Campylobacter jejuni in ground chicken. Food Microbiol 2022, 106, 104052. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.F.; Wang, H.W.; Guo, F.F.; Cao, X.Y.; Wang, X.; Zeng, X.M.; Cui, G.L.; Lin, J.; Xu, F.Z. Monoclonal antibody-based indirect competitive ELISA for quantitative detection of Enterobacteriaceae siderophore enterobactin. Food Chemistry 2022, 391, 133241. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhu, L.; Xa, H.; Tang, Q.; Ma, Y.X.; Chou, S.H.; He, J. Bio-hybrid nanoarchitectonics of nanoflower-based ELISA method for the detection of Staphylococcus aureus. Sensors and Actuators B-Chemical 2022, 366, 132005. [Google Scholar] [CrossRef]
- Wang, L.L.; Lin, X.H.; Liu, T.; Zhang, Z.H.; Kong, J.; Yu, H.; Yan, J.; Luan, D.L.; Zhao, Y.; Bian, X.J. Reusable and universal impedimetric sensing platform for the rapid and sensitive detection of pathogenic bacteria based on bacteria-imprinted polythiophene film. Analyst 2022, 147, 4433–4441. [Google Scholar] [CrossRef]
- Simoska, O.; Stevenson, K.J. Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst 2019, 144, 6461–6478. [Google Scholar] [CrossRef]
- Pankratov, D.; Bendixen, M.; Shipovskov, S.; Gosewinkel, U.; Ferapontova, E.E. Cellulase-Linked Immunomagnetic Microbial Assay on Electrodes: Specific and Sensitive Detection of a Single Bacterial Cell. Analytical Chemistry 2020, 92, 12451–12459. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhu, X.L.; Meng, Q.Y.; Zheng, P.M.; Zhang, J.; He, Z.W.; Jiang, H.Y. Gold interdigitated micro-immunosensor based on Mn-MOF-74 for the detection of Listeria monocytogens. Biosensors & Bioelectronics 2021, 183, 113186. [Google Scholar] [CrossRef]
- Farooq, U.; Yang, Q.L.; Ullah, M.W.; Wang, S.Q. Bacterial biosensing: Recent advances in phage-based bioassays and biosensors. Biosensors & Bioelectronics 2018, 118, 204–216. [Google Scholar] [CrossRef]
- Bulard, E.; Bouchet-Spinelli, A.; Chaud, P.; Roget, A.; Calemczuk, R.; Fort, S.; Livache, T. Carbohydrates as New Probes for the Identification of Closely Related Escherichia coli Strains Using Surface Plasmon Resonance Imaging. Analytical Chemistry 2015, 87, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.Y.; Peng, H.S.; Zhang, F.X.; Zhang, L.; Liu, Y.F.; Jia, R.; Song, Y.X.; Wang, B.N. An ultrasensitive sandwich-type electrochemical aptasensor using silver nanoparticle/titanium carbide nanocomposites for the determination of Staphylococcus aureus in milk. Microchim Acta 2022, 189, 276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, T.W.; Hwang, I.J.; Kim, H.I.; Jeon, S.J.; Yim, D.; Choi, C.; Son, W.; Kim, H.; Yang, C.S.; et al. Transition-Metal Dichalcogenide Artificial Antibodies with Multivalent Polymeric Recognition Phases for Rapid Detection and Inactivation of Pathogens. J Am Chem Soc 2021, 143, 14635–14645. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Miao, H.H.; Wang, J.X.; Pan, G.Q. Molecularly Imprinted Synthetic Antibodies: From Chemical Design to Biomedical Applications. Small 2020, 16, 6644. [Google Scholar] [CrossRef]
- Amiri, M.; Bezaatpour, A.; Jafari, H.; Boukherroub, R.; Szunerits, S. Electrochemical Methodologies for the Detection of Pathogens. Acs Sensors 2018, 3, 1069–1086. [Google Scholar] [CrossRef]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef]
- Yu, L.; Shen, Y.; Chen, L.; Zhang, Q.; Hu, X.; Xu, Q. Molecularly imprinted ultrasensitive cholesterol photoelectrochemical sensor based on perfluorinated organics functionalization and hollow carbon spheres anchored organic-inorganic perovskite. Biosensors & bioelectronics 2023, 237, 115496–115496. [Google Scholar] [CrossRef]
- Xing, R.R.; Wen, Y.R.; Dong, Y.R.; Wang, Y.J.; Zhang, Q.; Liu, Z. Dual Molecularly Imprinted Polymer-Based Plasmonic Immunosandwich Assay for the Specific and Sensitive Detection of Protein Biomarkers. Analytical Chemistry 2019, 91, 9993–10000. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Ning, K.-P.; Wang, Z.; Yao, Y.; Xu, Q.; Hu, X.-Y. Flexible Electrochemical Platform Coupled with In Situ Prepared Synthetic Receptors for Sensitive Detection of Bisphenol A. Biosensors-Basel 2022, 12. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Lu, Y.; Jia, M.; Yan, J.; Bian, X. Facile preparation of a bacteria imprinted artificial receptor for highly selective bacterial recognition and label-free impedimetric detection. Anal. Chem. 2019, 91, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, P.P.; Yan, J.; Luan, D.; Sun, T.; Bian, X. Dual Synthetic Receptor-Based Sandwich Electrochemical Sensor for Highly Selective and Ultrasensitive Detection of Pathogenic Bacteria at the Single-Cell Level. Anal Chem 2023, 95, 5561–5567. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Liu, X.B.; Luo, Y.G.; Pearlstein, A.J.; Wang, S.L.; Dillow, H.; Reed, K.; Jia, Z.; Sharma, A.; Zhou, B.; et al. Machine learning-enabled non-destructive paper chromogenic array detection of multiplexed viable pathogens on food. Nat Food 2021, 2, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hao, T.T.; Wang, Z.L.; Lin, H.; Wei, W.T.; Hu, Y.F.; Wang, S.; Shi, X.Z.; Guo, Z.Y. Machine learning-assisted cell-imprinted electrochemical impedance sensor for qualitative and quantitative analysis of three bacteria. Sensors and Actuators B-Chemical 2023, 384, 133672. [Google Scholar] [CrossRef]
- Tokonami, S.; Shimizu, E.; Tamura, M.; Iida, T. Mechanism in External Field-mediated Trapping of Bacteria Sensitive to Nanoscale Surface Chemical Structure. Sci Rep-Uk 2017, 7, 16651. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, R.; Potts, E.I.; Dick, J.E. Quantifying Interferent Effects on Molecularly Imprinted Polymer Sensors for Per- and Polyfluoroalkyl Substances (PFAS). Analytical Chemistry 2020, 92, 10597–10605. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.L.; Yamauchi, T.; Yamamoto, Y.; Niyomdecha, S.; Ishiki, K.; Le, D.Q.; Shiigi, H.; Nagaoka, T. Spontaneous and specific binding of enterohemorrhagic Escherichia coli to overoxidized polypyrrole-coated microspheres. Chem Commun 2017, 53, 3890–3893. [Google Scholar] [CrossRef]
- Lin, X.H.; Liu, P.P.; Yan, J.; Luan, D.L.; Sun, T.; Bian, X.J. Dual Synthetic Receptor-Based Sandwich Electrochemical Sensor for Highly Selective and Ultrasensitive Detection of Pathogenic Bacteria at the Single-Cell Level. Analytical Chemistry 2023. [Google Scholar] [CrossRef]
- Haupt, K. Molecularly imprinted polymers: the next generation. Anal. Chem. 2003, 377A–383A. [Google Scholar]
- Dong, S.B.; Zhao, R.T.; Zhu, J.G.; Lu, X.; Li, Y.; Qiu, S.F.; Jia, L.L.; Jiao, X.; Song, S.P.; Fan, C.H.; et al. Electrochemical DNA Biosensor Based on a Tetrahedral Nanostructure Probe for the Detection of Avian Influenza A (H7N9) Virus. Acs Applied Materials & Interfaces 2015, 7, 8834–8842. [Google Scholar] [CrossRef]
- Wu, J.K.; Wang, R.N.; Lu, Y.F.; Jia, M.; Yan, J.; Bian, X.J. Facile Preparation of a Bacteria Imprinted Artificial Receptor for Highly Selective Bacterial Recognition and Label-Free Impedimetric Detection. Analytical Chemistry 2019, 91, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
| Sample | Bacteria | Original (CFU mL-1) |
Added (CFU mL-1) |
Found (Mean±SD/CFU mL-1) |
Recovery (%) |
|---|---|---|---|---|---|
| Apple juice | E. coli O157:H7 | Not found |
102 | 98.40±8.2 | 98.40 |
| 103 | 980.47±135.3 | 98.05 | |||
| 104 | 8686.27±1163.2 | 86.86 | |||
| S. aureus | 102 | 93.65±9.8 | 93.65 | ||
| 103 | 813.56±80.3 | 81.36 | |||
| 104 | 10057.69±202.9 | 100.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





