Submitted:
09 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Background Information
3. Materials and Methods
4. Results
5. Discussion
5.1. Symmetry considerations
5.2. The relations between KB and WE
5.3. Structural complexity
5.4. On the mechanism of the Pb-Cu substitution in ‘oxypyromorphite’
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Kim, J.; Im, S.; An S., M.; Kwon, Y.-W.; Ho, A. Consideration for the Development of Room-Temperature Ambient-Pressure Superconductor (LK-99). J. Korean Crystal Growth Crystal Technol. 2023, 33, 61–70. [Google Scholar]
- Lee, S.; Kim, J.; Kim, H.-T.; Im, S.; An, S.; Auh, K.H. Superconductor Pb10-xCux(PO₄)₆O Showing Levitation at Room Temperature and Atmospheric Pressure and Mechanism. arXiv:2307. 1203. [Google Scholar]
- Lee, S.; Kim, J.-H.; Kwon, Y.-W. The First Room-Temperature Ambient-Pressure Superconductor. arXiv:2307. 1200. [Google Scholar] [CrossRef]
- Hou, Q.; Wei, W.; Zhou, X.; Sun, Y.; Shi, Z. Observation of Zero Resistance above 100 K in Pb10-xCux(PO₄)₆O. arXiv:2308. 0119. [Google Scholar] [CrossRef]
- Kurleto, R.; Lany, S.; Pashov, D.; Acharya, S.; Schilfgaarde, M. van; Dessau, D.S. Pb-Apatite Framework as a Generator of Novel Flat-Band CuO Based Physics, Including Possible Room Temperature Superconductivity. arXiv:2308. 0069. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Z.; Wang, X.; Chen, H.; Duan, Z.; Zhou, X.; Yan, H.; Qin, P.; Liu, Z. Semiconducting Transport in Pb10-xCux(PO₄)₆O Sintered from Pb₂SO₅ and Cu₃P. arXiv:2307. 1680. [Google Scholar] [CrossRef]
- Abramian, P.; Kuzanyan, A.; Nikoghosyan, V.; Teknowijoyo, S.; Gulian, A. Some Remarks on Possible Superconductivity of Composition Pb₉CuP₆O25. arXiv:2308. 0172. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Xiao, B.; Chang, H. Successful Growth and Room Temperature Ambient-Pressure Magnetic Levitation of LK-99. arXiv:2308. 0151. [Google Scholar] [CrossRef]
- Si, L.; Held, K. Electronic Structure of the Putative Room-Temperature Superconductor Pb₉Cu(PO₄)₆O. arXiv:2308. 0067. [Google Scholar] [CrossRef]
- Lai, J.; Li, J.; Liu, P.; Sun, Y.; Chen, X.-Q. First-Principles Study on the Electronic Structure of Pb10−xCux(PO4)6O (x = 0, 1). J. Mater. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Tavakol, O.; Scaffidi, T. Minimal Model for the Flat Bands in Copper-Substituted Lead Phosphate Apatite. arXiv:2308. 0131. [Google Scholar] [CrossRef]
- Griffin, S.M. Origin of Correlated Isolated Flat Bands in Copper-Substituted Lead Phosphate Apatite. arXiv:2307. 1689. [Google Scholar] [CrossRef]
- Cabezas-Escares, J.; Barrera, N.F.; Cardenas, C.; Munoz, F. Theoretical Insight on the LK-99 Material. arXiv:2308. 0113. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Crystal Chemistry of Lead Oxide Phosphates: Crystal Structures of Pb4O(PO4)2, Pb8O5(PO4)2 and Pb10(PO4)6O. Z. Kristallogr. 2003, 218, 357–365. [Google Scholar] [CrossRef]
- Brückner, S.; Lusvardi, G.; Menabue, L.; Saladini, M. Crystal Structure of Lead Hydroxyapatite from Powder X-Ray Diffraction Data. Inorganica Chimica Acta 1995, 236, 209–212. [Google Scholar] [CrossRef]
- Kim, Jean Y. ; Hunter, Brett A.; Fenton, R.R.; Kennedy, B.J. Neutron Powder Diffraction Study of Lead Hydroxyapatite. Aust. J. Chem. 1998, 50, 1061–1066. [Google Scholar]
- White, T.J.; ZhiLi, D. Structural Derivation and Crystal Chemistry of Apatites. Acta Crystallogr. 2003, B59, 1–16. [Google Scholar] [CrossRef]
- Rooksby, H.P. Identification by X-Ray Diffraction of Crystalline Inclusions in Glass. Analyst 1952, 77, 759–765. [Google Scholar] [CrossRef]
- Merker, L.; Wondratschek, H. Der Oxypromorphit Pb10(PO4)6O und der Ausschnitt Pb4P2O9—Pb3(PO4)2 des Systems PbO—P2O5. Z. Anorg. Allg. Chem. 1960, 306, 25–29. [Google Scholar] [CrossRef]
- Merker, L.; Engel, G.; Wondratschek, H.; Ito, J. Lead Ions and Empty Halide Sites in Apatites. Amer. Miner. 1970, 55, 1435–1437. [Google Scholar]
- Ito, J. Silicate Apatites and Oxyapatites. Amer. Miner. 1968, 53, 890–907. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Brown, I.D. Are the Compressive Effects of Encapsulation an Artifact of the Bond Valence Parameters? Z. Kristallogr. 2001, 216, 245–247. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive Derivation of Bond-Valence Parameters for Ion Pairs Involving Oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef]
- Müller, U. Symmetry Relationships between Crystal Structures: Applications of Crystallographic Group Theory in Crystal Chemistry; Oxford University Press, 2013; ISBN 978-0-19-966995-0.
- Siidra, O.I.; Krivovichev, S.V.; Filatov, S.K. Minerals and Synthetic Pb(II) Compounds with Oxocentered Tetrahedra: Review and Classification. Z. Kristallogr. 2008, 223, 114–125. [Google Scholar] [CrossRef]
- Barinova, A.; Bonin, M.; Pushcharovskii, D.; Rastsvetaeva, R.; Schenk, K.; Dimitrova, O. Crystal Structure of Synthetic Hydroxylpyromorphite Pb5(PO4)3(OH). Crystallogr. Rep. 1998, 43, 189–192. [Google Scholar]
- Ben Moussa, S.; Bachoua, H.; Brigui, N.; Badraoui, B. Synthèse, caractérisation et étude structurale des hydroxyapatites à base de plomb Pb10-xMx(PO4)6(OH)2 (M = Zn, Cu). J. Mater. Environ. Sci. 2016, 7, 4754–4766. [Google Scholar]
- Krivovichev, S.V. Structural Complexity of Minerals: Information Storage and Processing in the Mineral World. Miner. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S., V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I., V.; Kuporev, I., V.; et al. Structural and Chemical Complexity of Minerals: An Update. Miner. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Liu, R.S.; Hu, S.F.; Chen, D.H.; Shy, D.S.; Jefferson, D.A. Crystal Structure of the (Pb,Hg)Sr2(Ca,Y)Cu2O7-δ Superconductor. Physica C: Superconductivity 1994, 222, 13–18. [Google Scholar] [CrossRef]
- Wada, T.; Ichinose, A.; Izumi, F.; Nara, A.; Yamauchi, H.; Hajime-Asano; Tanaka, S. Neutron Powder Diffraction Study of the Pb-Based Copper Oxide Containing Thick Fluorite Blocks: (Pb,Cu)Sr2(Ho,Ce)3Cu2O11+z. Physica C: Superconductivity 1991, 179, 455–460. [Google Scholar] [CrossRef]
- Ishigaki, T.; Tokiwa-Yamamoto, A.; Izumi, F.; Kamiyama, T.; Asano, H.; Syono, Y. Structural Changes of PbBaSr(Y0.8Ca0.2)Cu3O7+z on Oxygen Introduction into Block Layers. Physica C: Superconductivity 1994, 231, 357–366. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states. Proc. R. Soc. Ser. A 1937, 161, 220–235. [Google Scholar]
- Hathaway, B.J. A new look at the stereochemistry and electronic properties of complexes of the copper(II) ion. In Complex Chemistry. Structure and Bonding; Emsley, J., Ernst, R.D., Hathaway, B.J., Warren, K.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 57, pp. 55–118. [Google Scholar]
- Burns, P.C.; Hawthorne, F.C. Static and dynamic Jahn-Teller effects in Cu2+ oxysalt minerals. Can. Mineral. 1996, 34, 1089–1105. [Google Scholar]
- Shuvalov, R.R.; Vergasova, L.P.; Semenova, T.F.; Filatov, S.K.; Krivovichev, S.V.; Siidra, O.I.; Rudashevsky, N.S. Prewittite, KPb1.5Cu6Zn(SeO3)2O2Cl10, a New Mineral from Tolbachik Fumaroles, Kamchatka Peninsula, Russia: Description and Crystal Structure. Amer. Miner. 2013, 98, 463–469. [Google Scholar] [CrossRef]
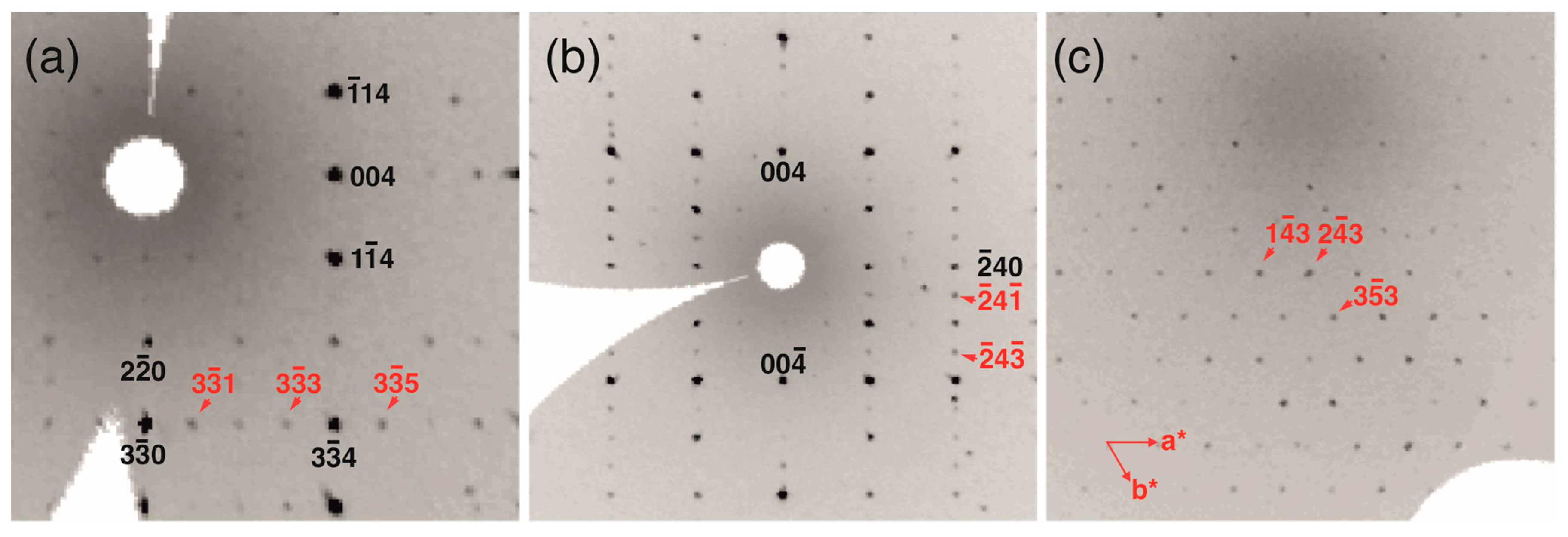
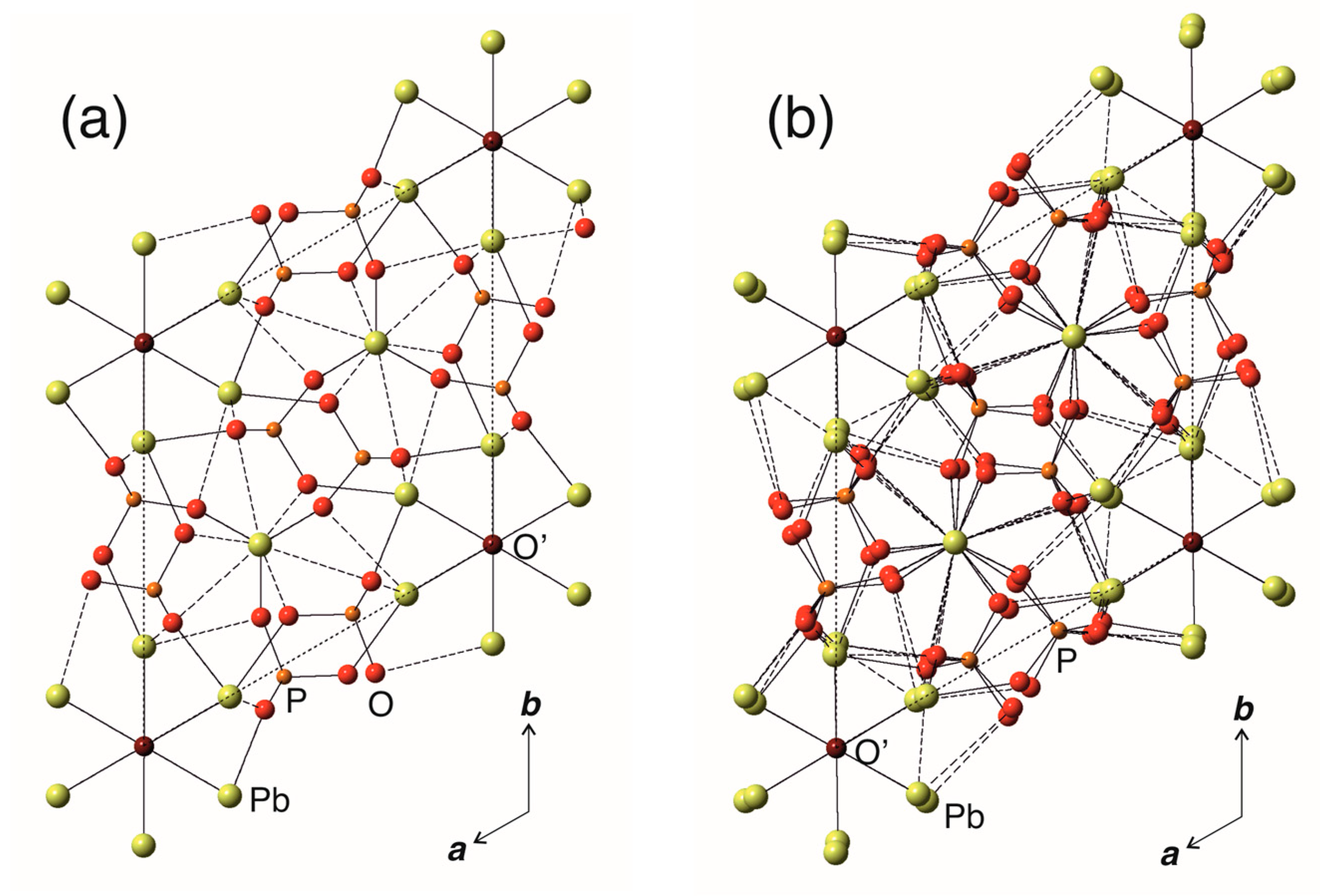
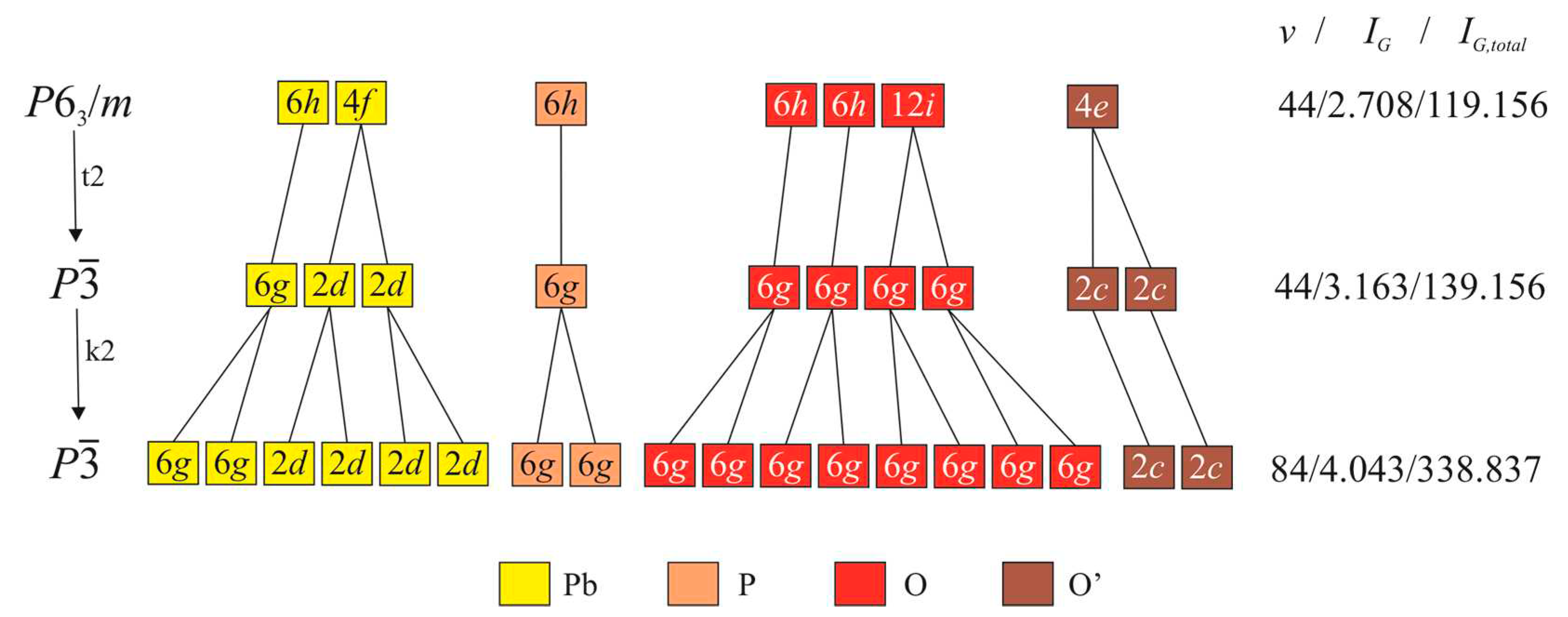
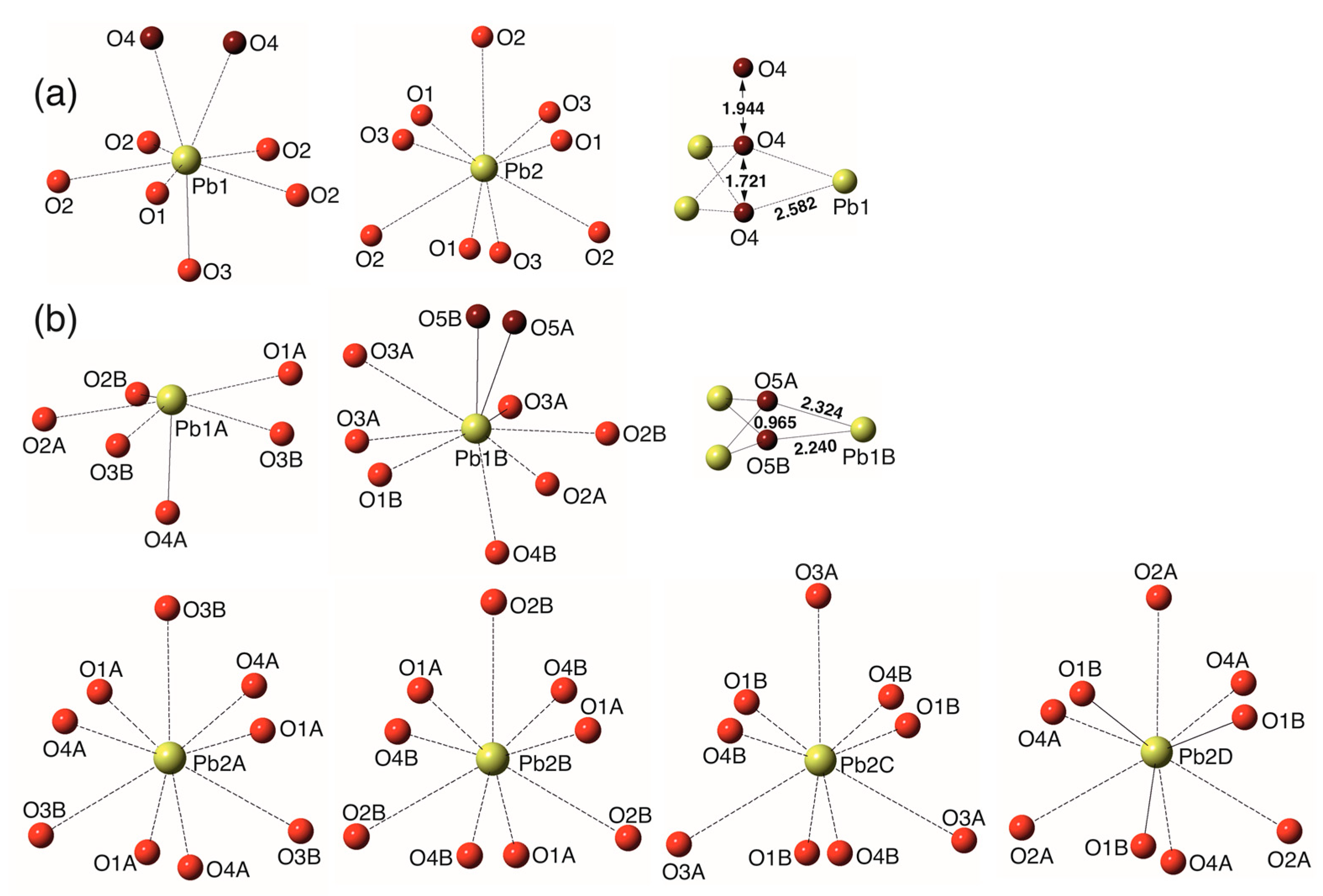
| Temperature/K | 293(2) |
|---|---|
| Crystal system | trigonal |
| Space group | P |
| a/Å | 9.8109(6) |
| c/Å | 14.8403(12) |
| Volume/Å3 | 1237.06(15) |
| Z | 2 |
| Dcalc, g/cm3 | 7.135 |
| μ/mm-1 | 68.270 |
| F(000) | 2220 |
| Crystal size/mm3 | 0.06 × 0.05 × 0.01 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 5.50 to 69.06 |
| Index ranges | -6 ≤ h ≤ 15, -15 ≤ k ≤ 11, -23 ≤ l ≤ 22 |
| Reflections collected | 11777 |
| Independent reflections | 3456 [Rint = 0.0885, Rsigma = 0.0955] |
| Data/restraints/parameters | 3456/0/105 |
| Goodness-of-fit on F2 | 0.866 |
| Final R indices [I ≥ 2σ(I)] | R1 = 0.0413, wR2 = 0.0749 |
| Final R indices [all data] | R1 = 0.0544, wR2 = 0.0771 |
| Largest diff. peak/hole / e Å-3 | 4.010/-4.537 |
| Site | BVS* | BVS** | x/a | y/b | z/c | Uiso |
|---|---|---|---|---|---|---|
| Pb1A | 1.90 | 1.98 | 0.25369(5) | -0.00015(5) | -0.12920(4) | 0.01239(10) |
| Pb1B | 1.93 | 1.97 | -0.00445(6) | 0.22423(5) | 0.37576(6) | 0.02123(12) |
| Pb2A | 2.05 | 2.06 | 1/3 | 2/3 | 0.00402(8) | 0.0123(3) |
| Pb2B | 2.07 | 2.07 | 1/3 | 2/3 | 0.25395(7) | 0.0132(3) |
| Pb2C | 2.04 | 2.06 | 1/3 | 2/3 | 0.49982(8) | 0.0187(3) |
| Pb2D | 2.05 | 2.07 | 1/3 | 2/3 | -0.25587(7) | 0.0125(3) |
| P1A | 4.94 | 4.94 | 0.3740(3) | 0.4021(3) | 0.3698(3) | 0.0078(5) |
| P1B | 4.90 | 4.90 | 0.0230(3) | -0.3765(3) | -0.1215(3) | 0.0084(5) |
| O1A | 1.99 | 2.00 | -0.1578(9) | -0.4786(9) | -0.1223(9) | 0.0131(11) |
| O1B | 2.00 | 2.02 | 0.4953(10) | 0.3468(9) | 0.3692(9) | 0.0131(11) |
| O2A | 1.90 | 1.90 | 0.0807(14) | -0.2676(14) | -0.2034(6) | 0.0121(19) |
| O2B | 1.99 | 1.99 | 0.2777(14) | 0.3560(13) | 0.2826(6) | 0.0121(19) |
| O3A | 1.94 | 1.93 | 0.2533(14) | 0.3211(14) | 0.4471(6) | 0.021(2) |
| O3B | 1.85 | 1.85 | 0.0869(16) | -0.2716(17) | -0.0366(6) | 0.021(2) |
| O4A | 2.11 | 2.15 | 0.0949(9) | -0.4866(10) | -0.1233(9) | 0.0166(12) |
| O4B | 2.06 | 2.07 | 0.4563(10) | 0.5820(10) | 0.3809(9) | 0.0166(12) |
| O5A*** | 1.43 | 1.55 | 0 | 0 | 0.330(3) | 0.031(7) |
| O5B**** | 1.70 | 1.87 | 0 | 0 | 0.395(3) | 0.031(7) |
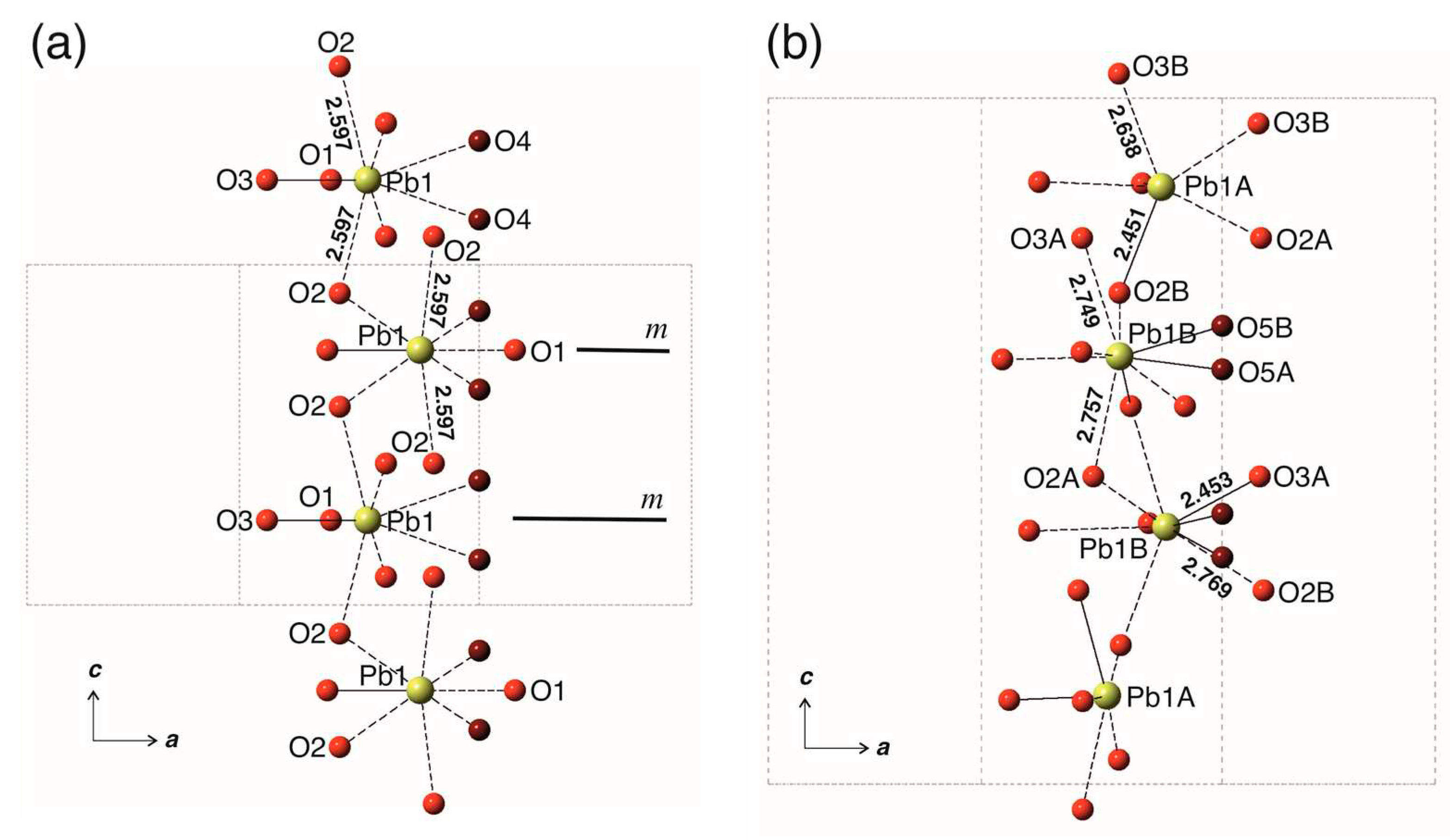
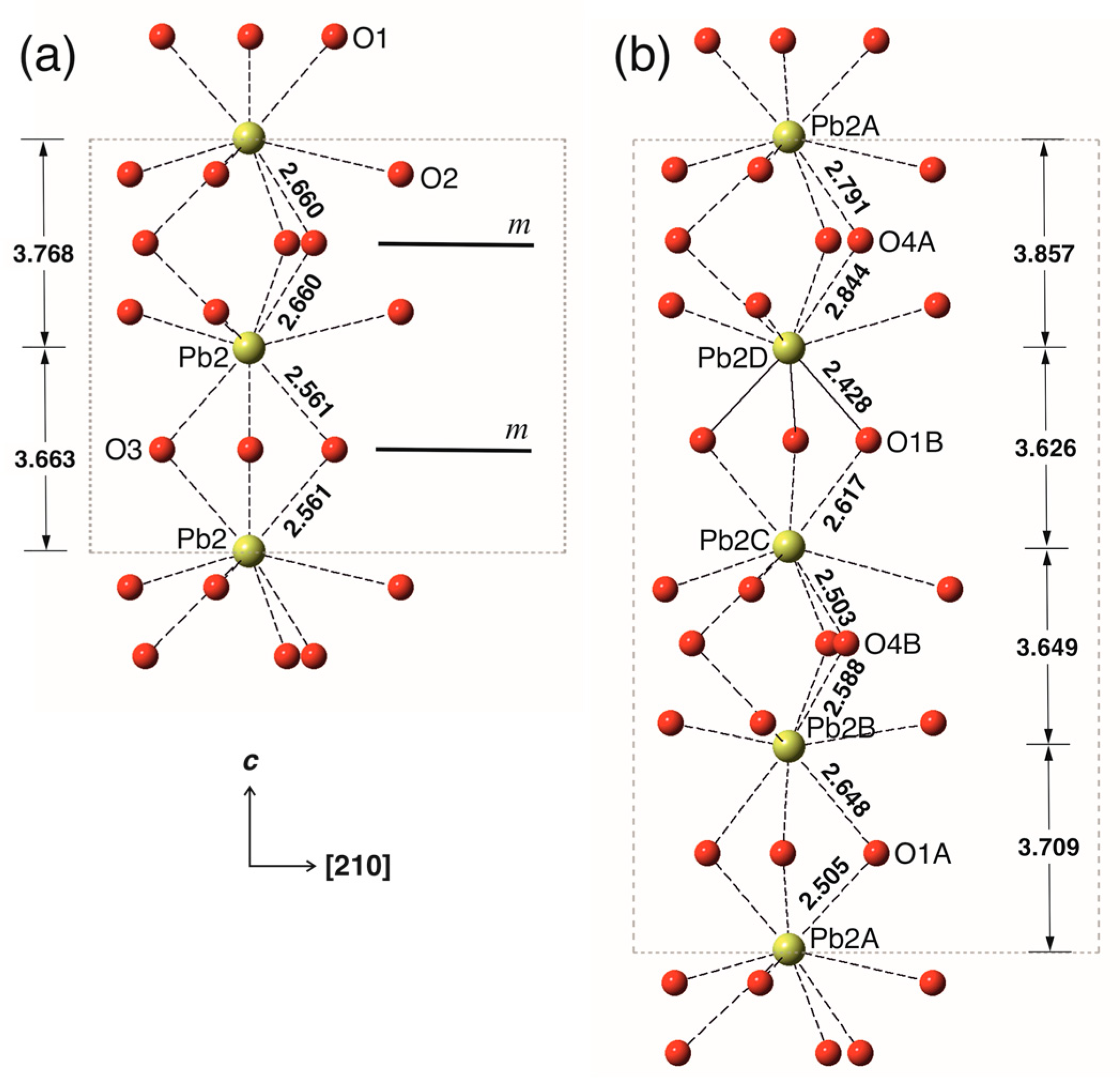
| Pb1A-O4A | 2.234(8) | Pb2A-O1A | 2.505(11) 3x |
| Pb1A-O2B | 2.451(10) | Pb2A-O4A | 2.791(11) 3x |
| Pb1A-O2A | 2.554(12) | Pb2A-O3B | 2.835(13) 3x |
| Pb1A-O3B | 2.638(10) | <Pb2A-O> | 2.710 |
| Pb1A-O3B | 2.703(13) | ||
| Pb1A-O1A | 2.801(8) | Pb2B-O4B | 2.588(11) 3x |
| <Pb1A-O> | 2.564 | Pb2B-O1A | 2.647(11) 3x |
| Pb2B-O2B | 2.847(11) 3x | ||
| Pb1B-O5B | 2.240(6) | <Pb2B-O> | 2.694 |
| Pb1B-O5A | 2.325(14) | ||
| Pb1B-O3A | 2.453(12) | Pb2C-O4B | 2.503(11) 3x |
| Pb1B-O4B | 2.731(8) | Pb2C-O1B | 2.616(11) 3x |
| Pb1B-O3A | 2.749(10) | Pb2C-O3A | 3.172(12) 3x |
| Pb1B-O2A | 2.757(10) | <Pb2C-O> | 2.764 |
| Pb1B-O2B | 2.769(12) | ||
| Pb1B-O1B | 3.057(8) | Pb2D-O1B | 2.428(11) 3x |
| Pb1B-O3A | 3.175(12) | Pb2D-O4A | 2.843(11) 3x |
| <Pb1B-O> | 2.695 | Pb2D-O2A | 2.961(11) 3x |
| <Pb2D-O> | 2.744 | ||
| P1A-O2B | 1.532(11) | P1B-O2A | 1.527(11) |
| P1A-O1B | 1.536(9) | P1B-O1A | 1.541(8) |
| P1A-O4B | 1.539(9) | P1B-O3B | 1.548(12) |
| P1A-O3A | 1.552(12) | P1B-O4A | 1.558(9) |
| <P1A-O> | 1.540 | <P1B-O> | 1.544 |
| Phase | a, Å | c, Å | V/Z, Å3 | O’ coordination | O’-Pb, Å | Ref. |
|---|---|---|---|---|---|---|
| Pb10(PO4)6O-WE | 9.84 | 14.86 | 623.0 | - | - | 19 |
| Pb10(PO4)6O-WE | 9.811 | 14.840 | 618.5 | trigonal | 2.240/2.325 3x | this work |
| Pb10(PO4)6O-KB | 9.865 | 7.431 | 626.3 | trigonal | 2.582 3x | 14 |
| Pb10(PO4)6(OH)2 | 9.866 | 7.426 | 626.0 | trigonal | 2.896 3x | 15 |
| Pb10(PO4)6(OH)2 | 9.883 | 7.441 | 629.4 | trigonal | 2.588 3x | 16 |
| Pb10(PO4)6(OH)2 | 9.774 | 7.291 | 603.2 | octahedral | 2.926 6x | 27 |
| Pb10(PO4)6(OH)2 | 9.871 | 7.427 | 626.7 | - | - | 28 |
| LK-99 | 9.843 | 7.428 | 623.2 | - | - | 3 |
| Pb8Cu2(PO4)6(OH)2 | 9.870 | 7.398 | 624.1 | - | - | 28 |
| Pb6Cu4(PO4)6(OH)2 | 9.868 | 7.392 | 623.4 | - | - | 28 |
| Pb4Cu6(PO4)6(OH)2 | 9.866 | 7.383 | 622.3 | - | - | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




