Submitted:
08 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
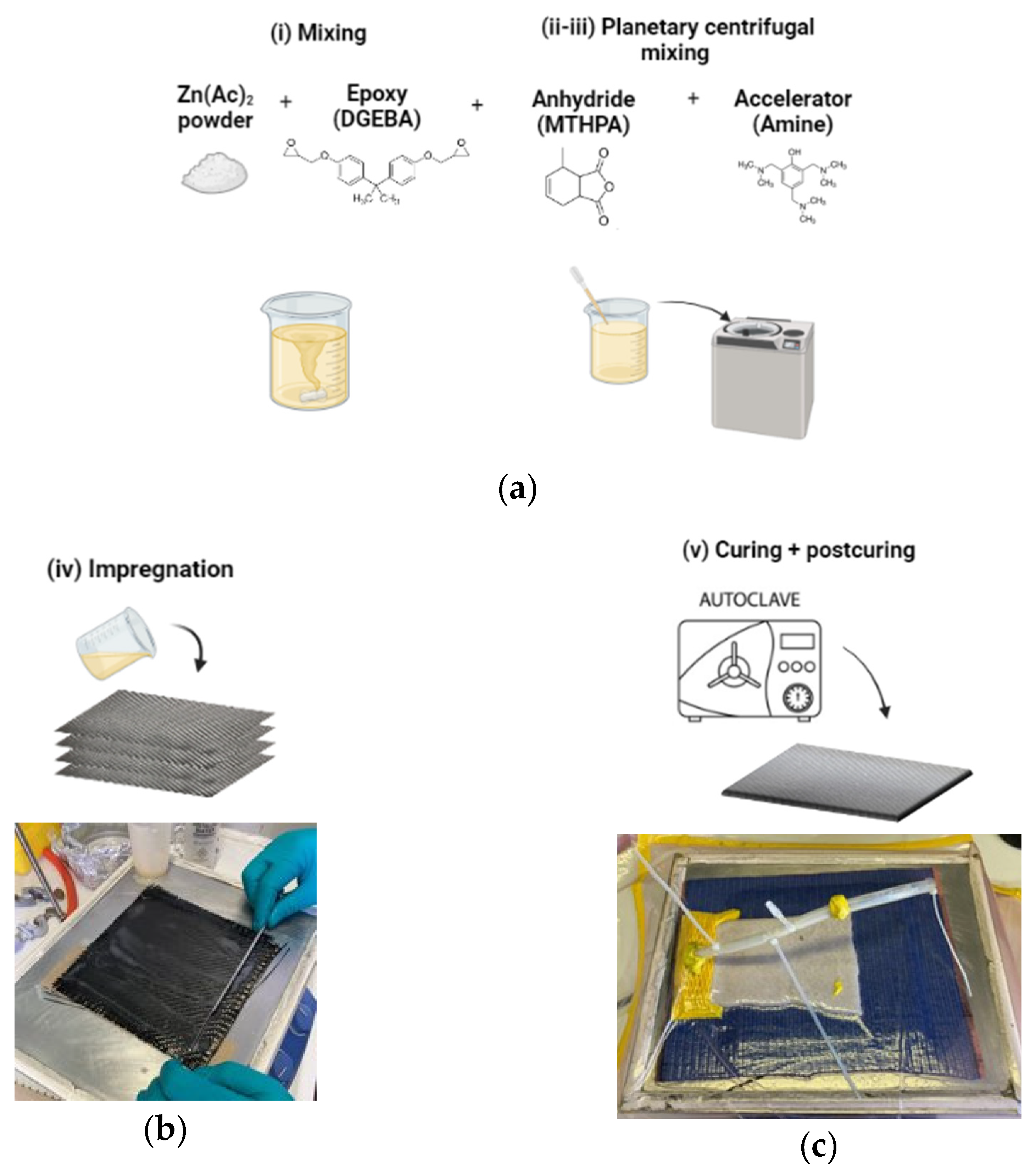
2.1. Epoxy Vitrimer formulation and CFRP manufacturing
2.2. Experimental Characterization
3. Results
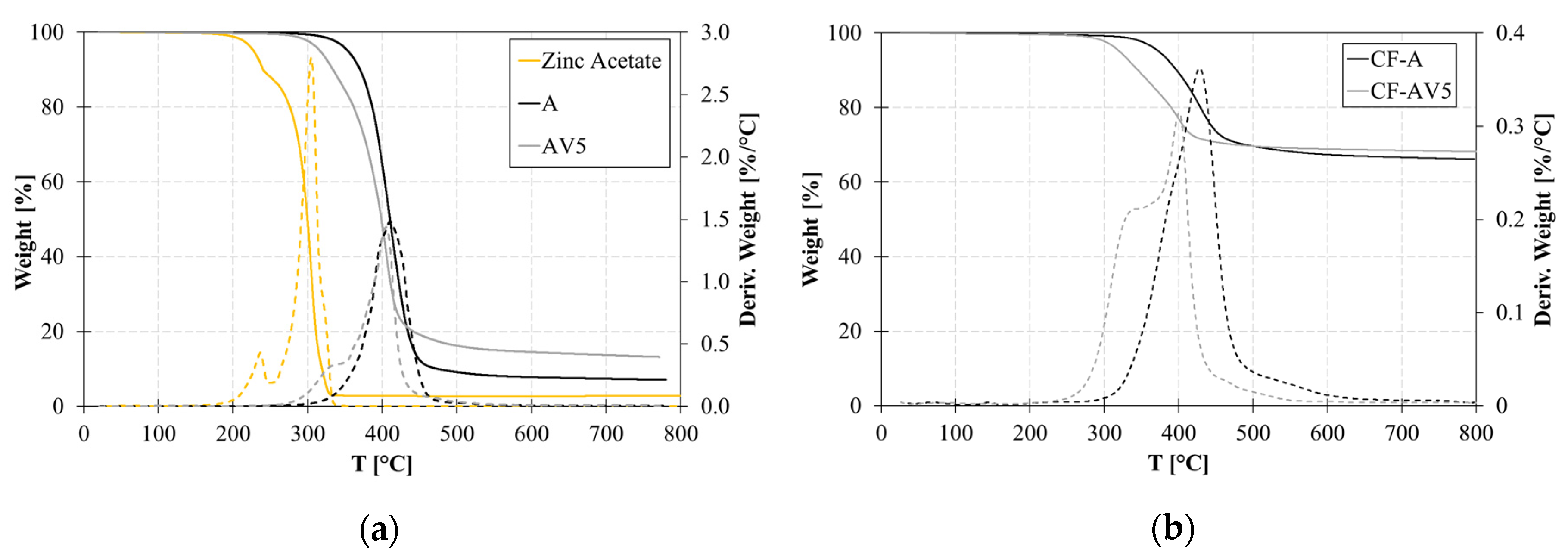
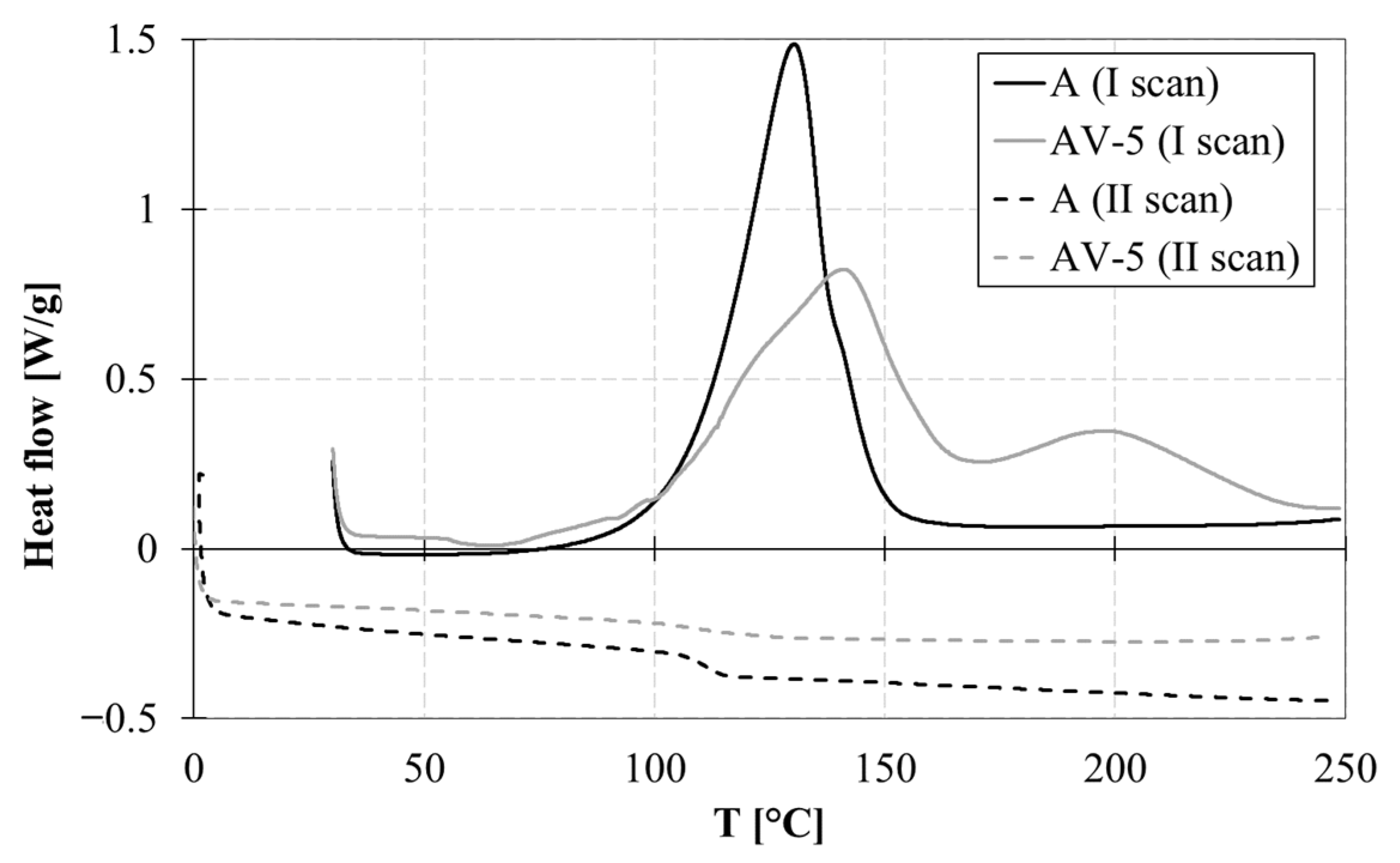
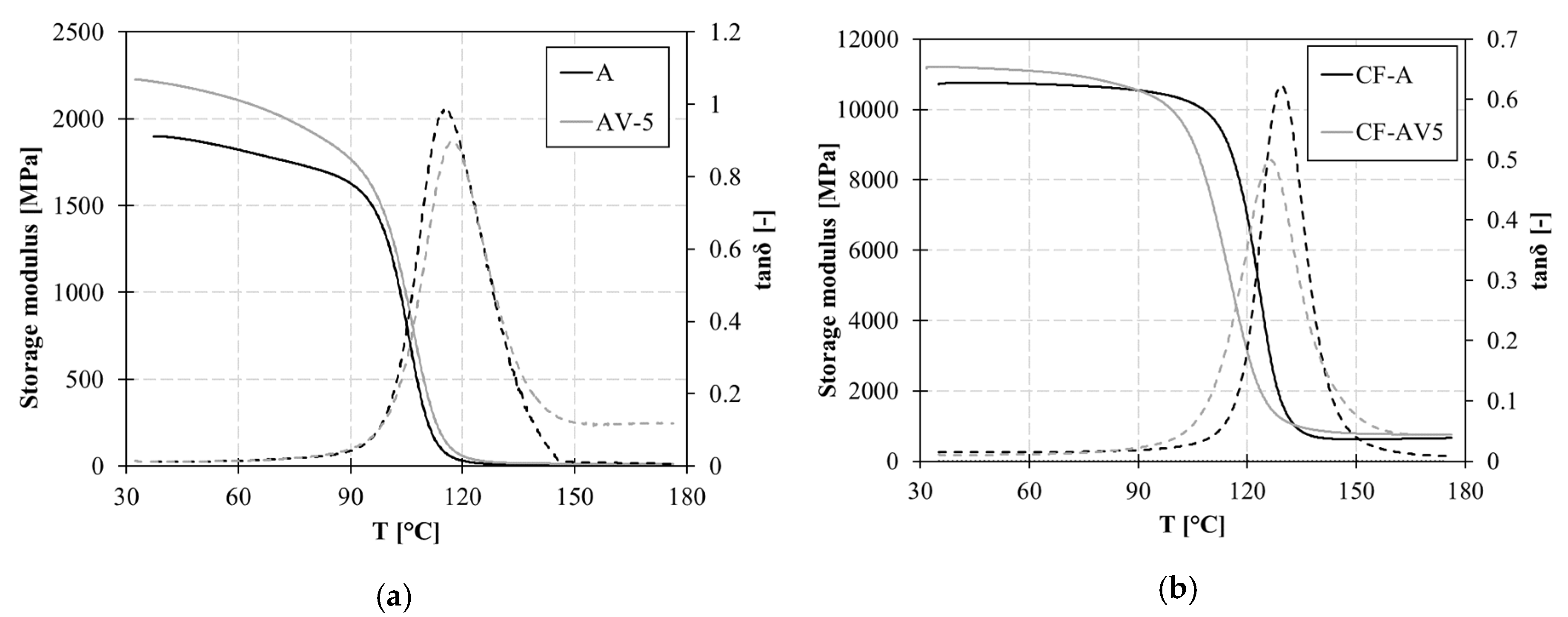
3.1. Thermal Characterization
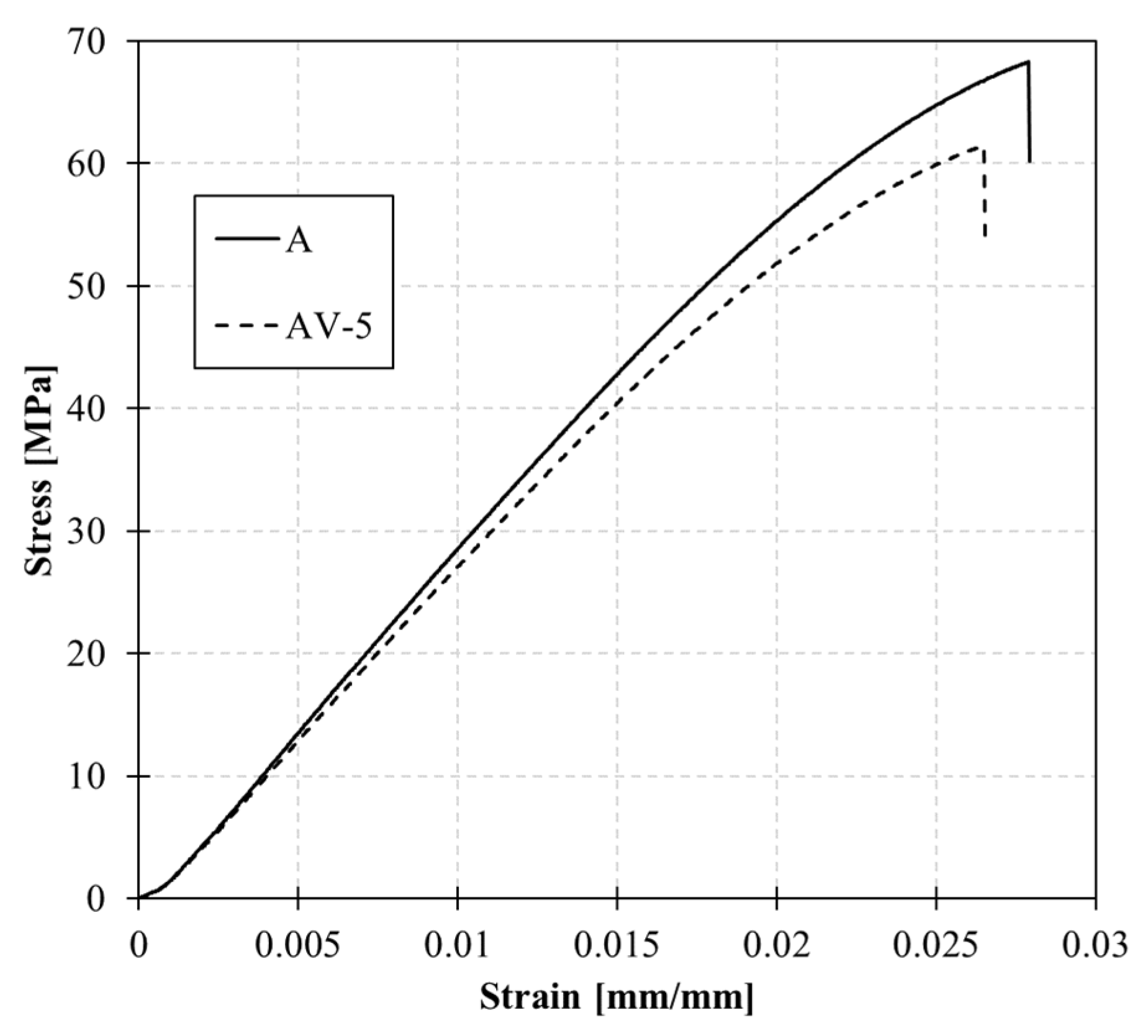
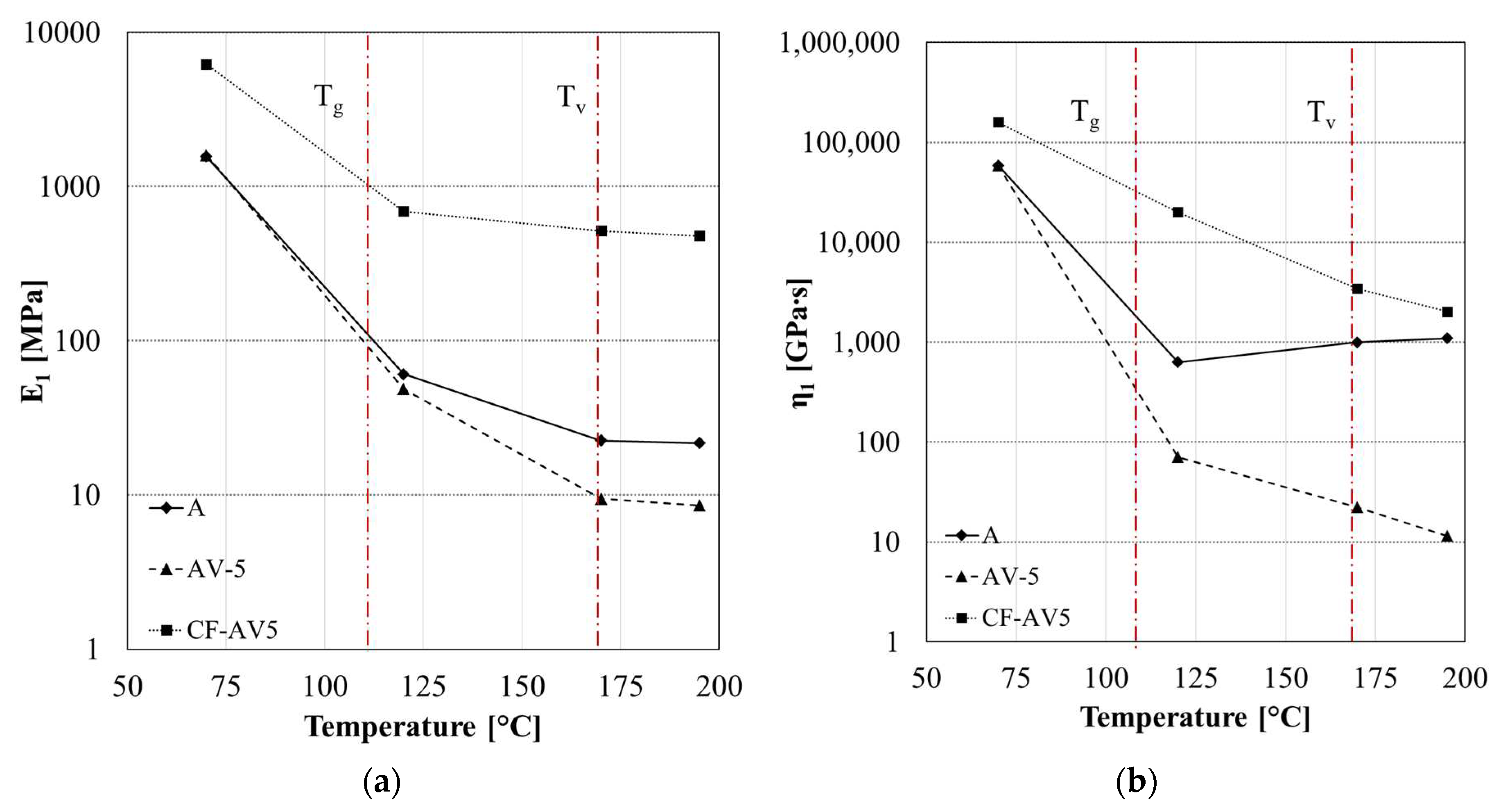
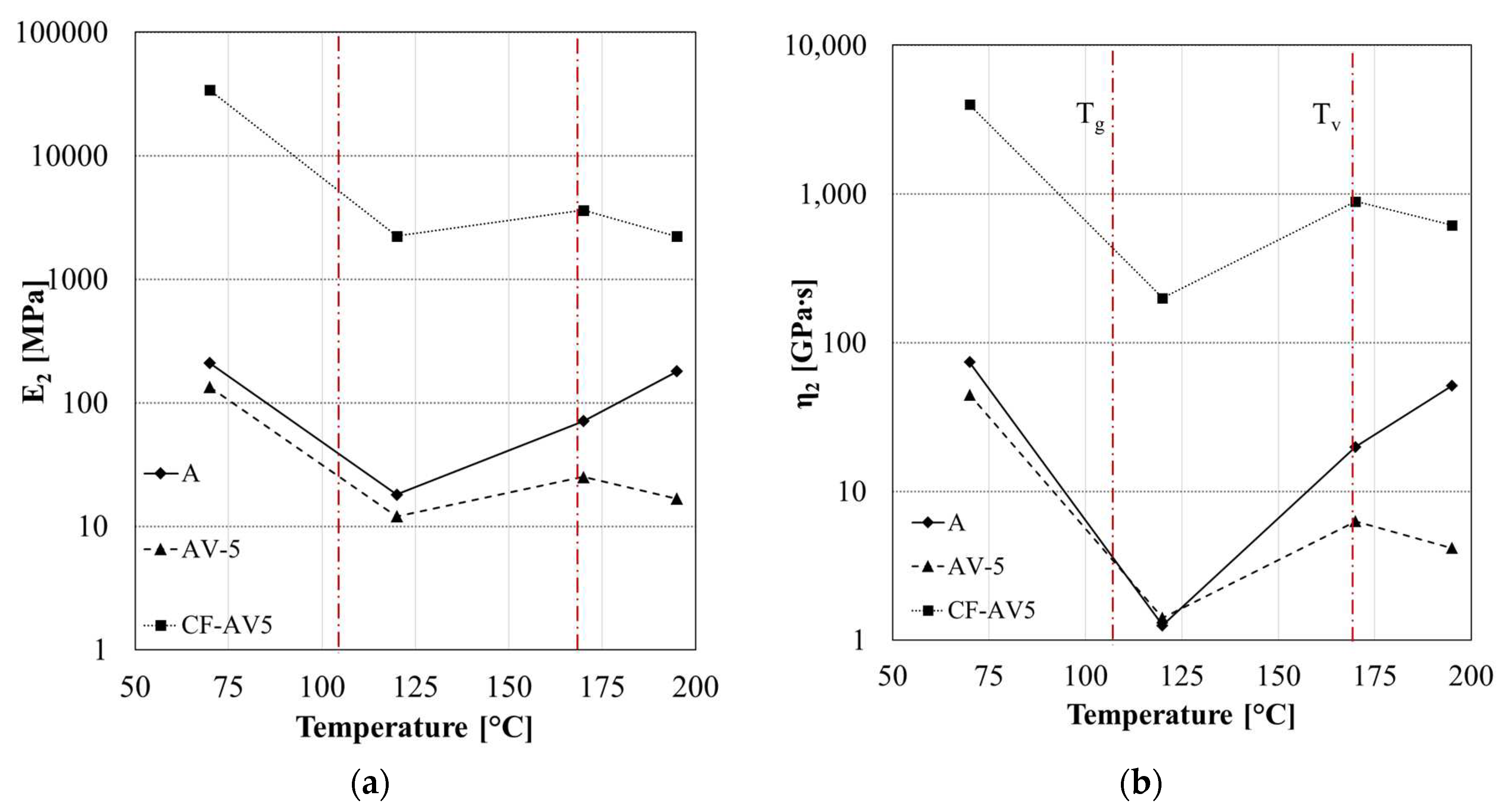
3.2. Static and Dynamic Mechanical Characterization
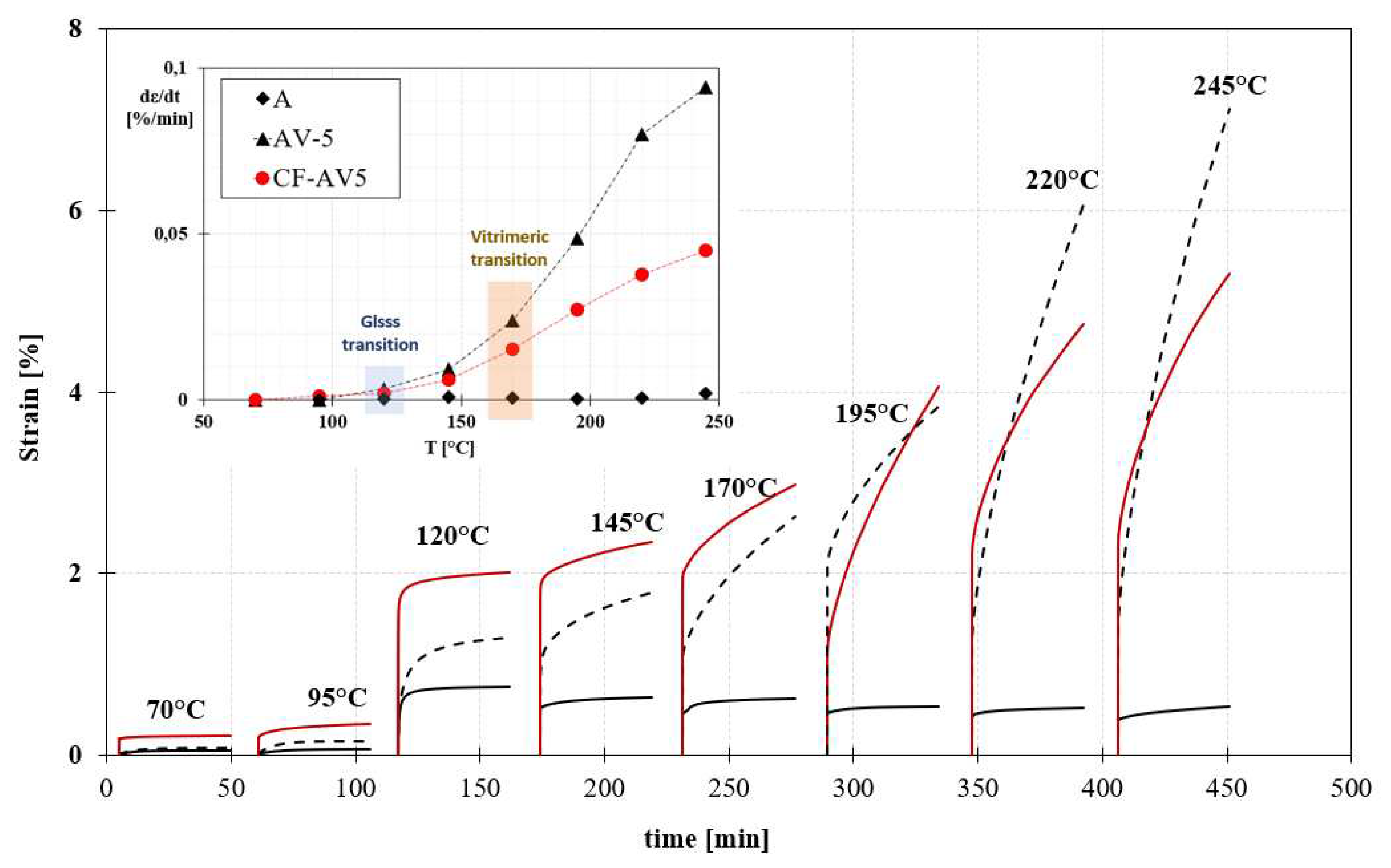
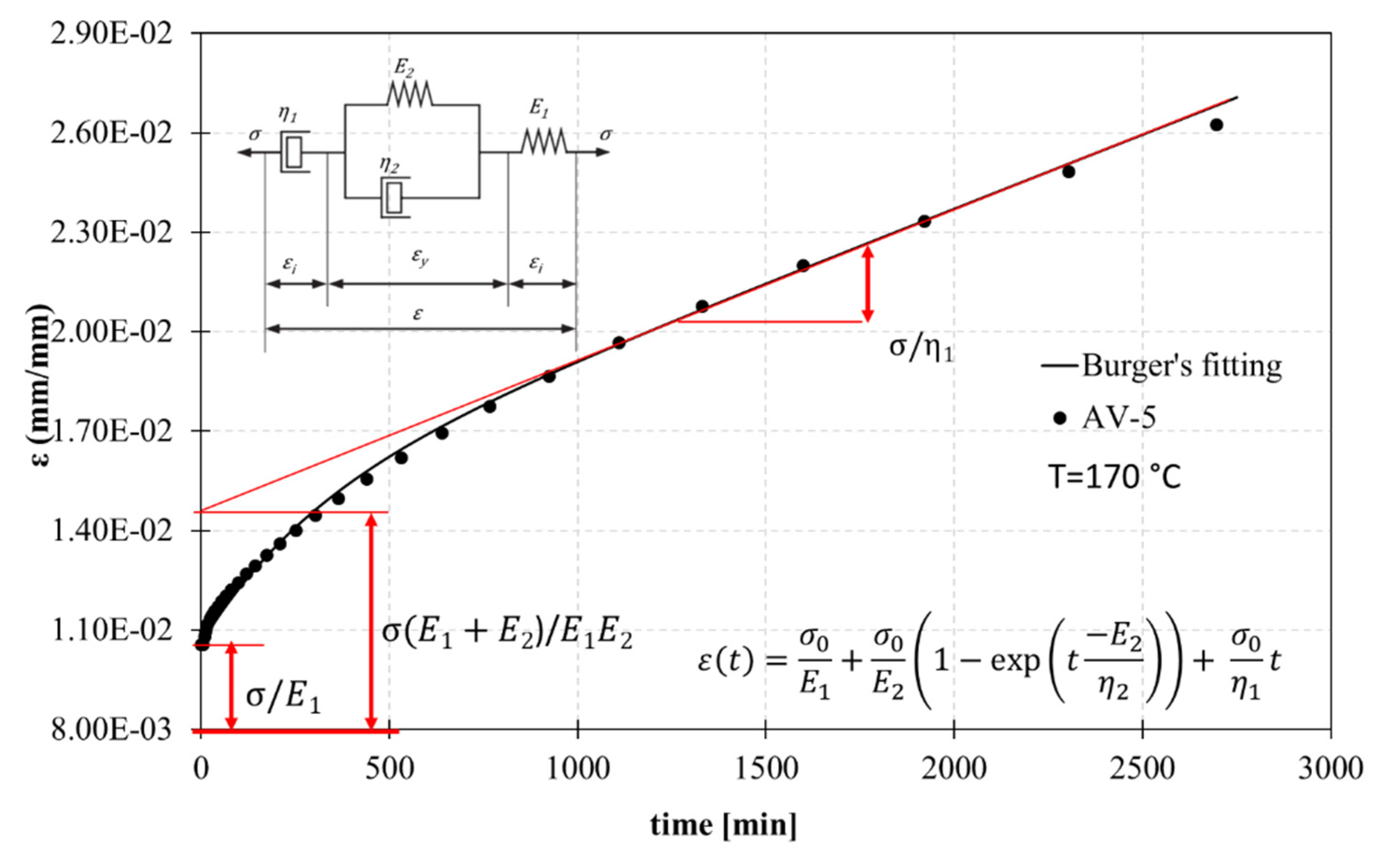
3.3. Isothermal Creep Test
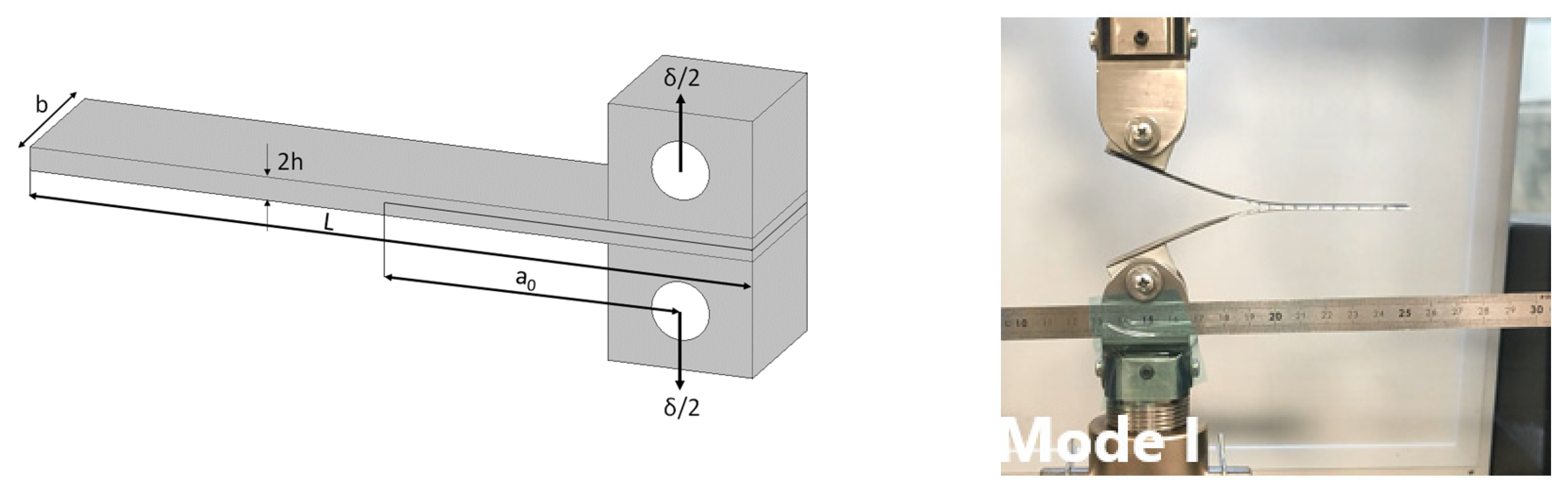
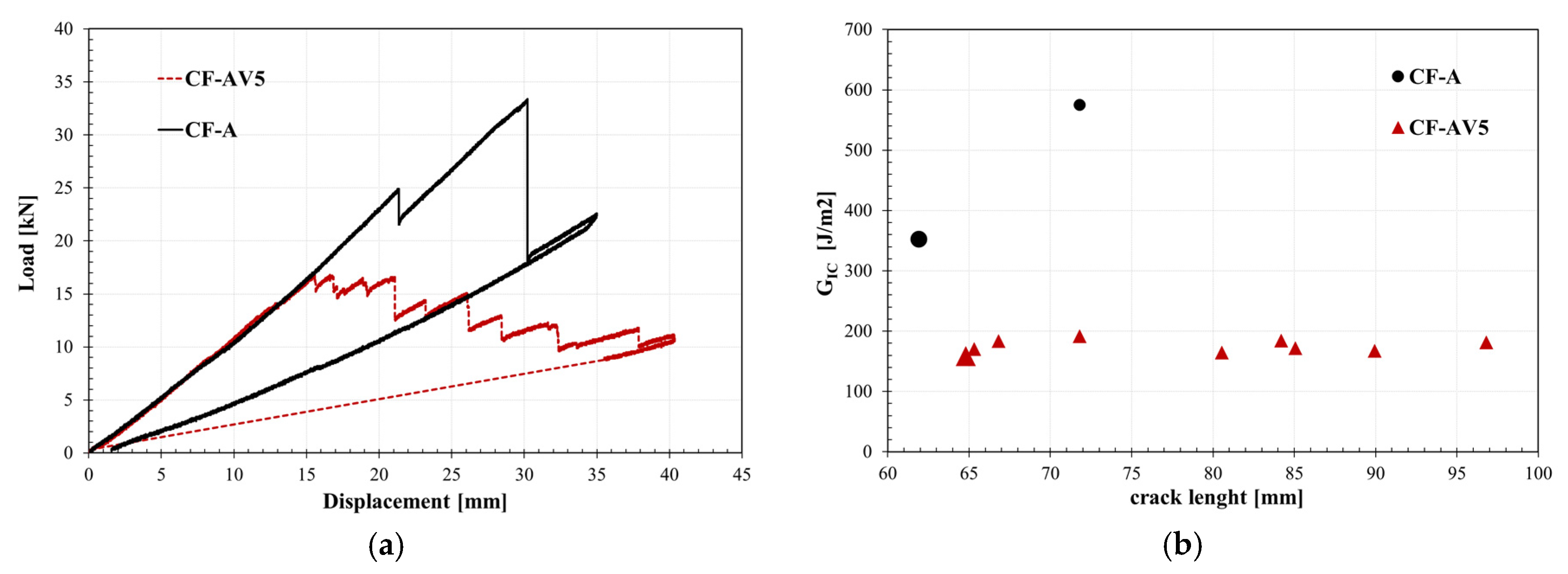
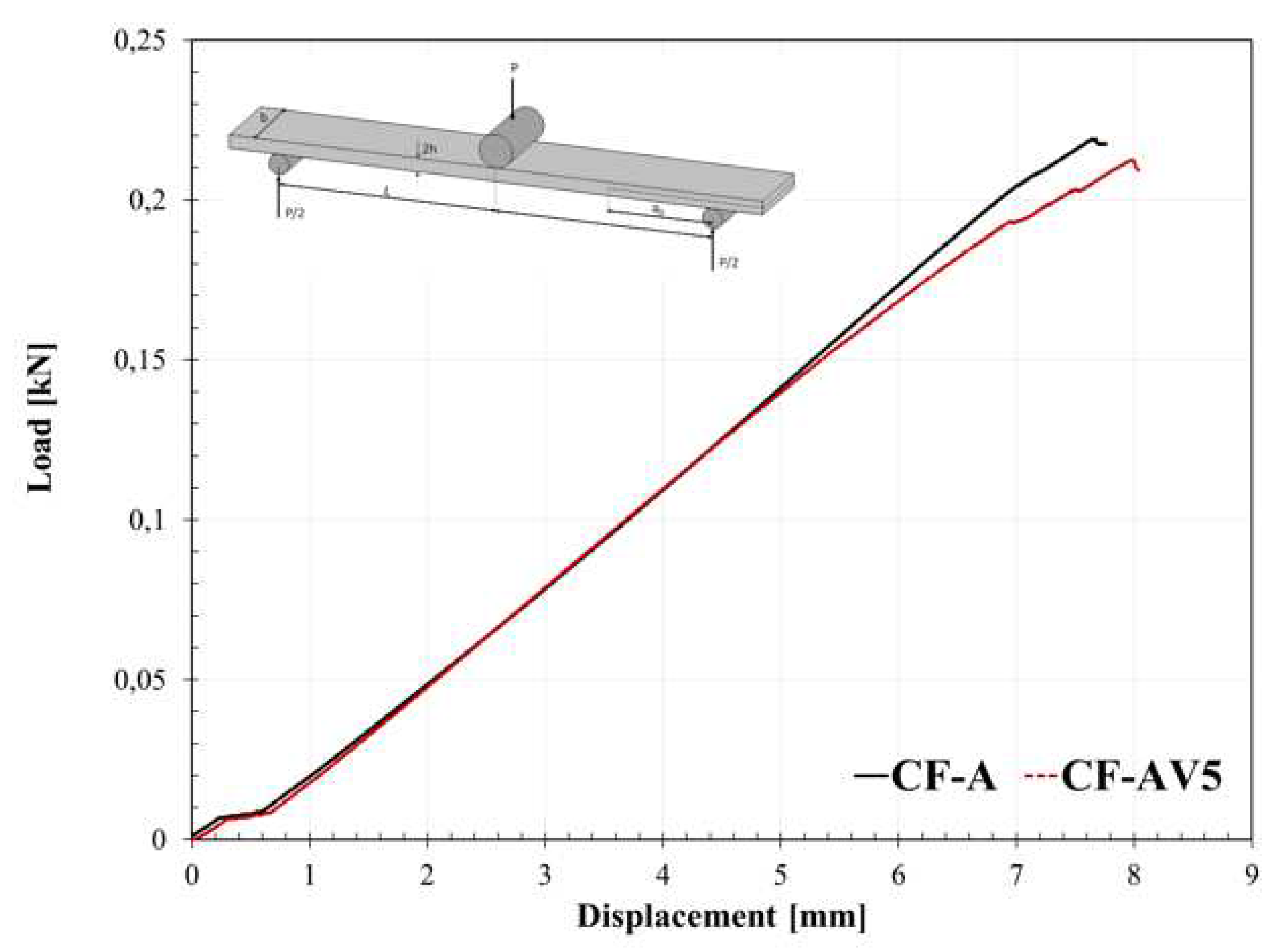
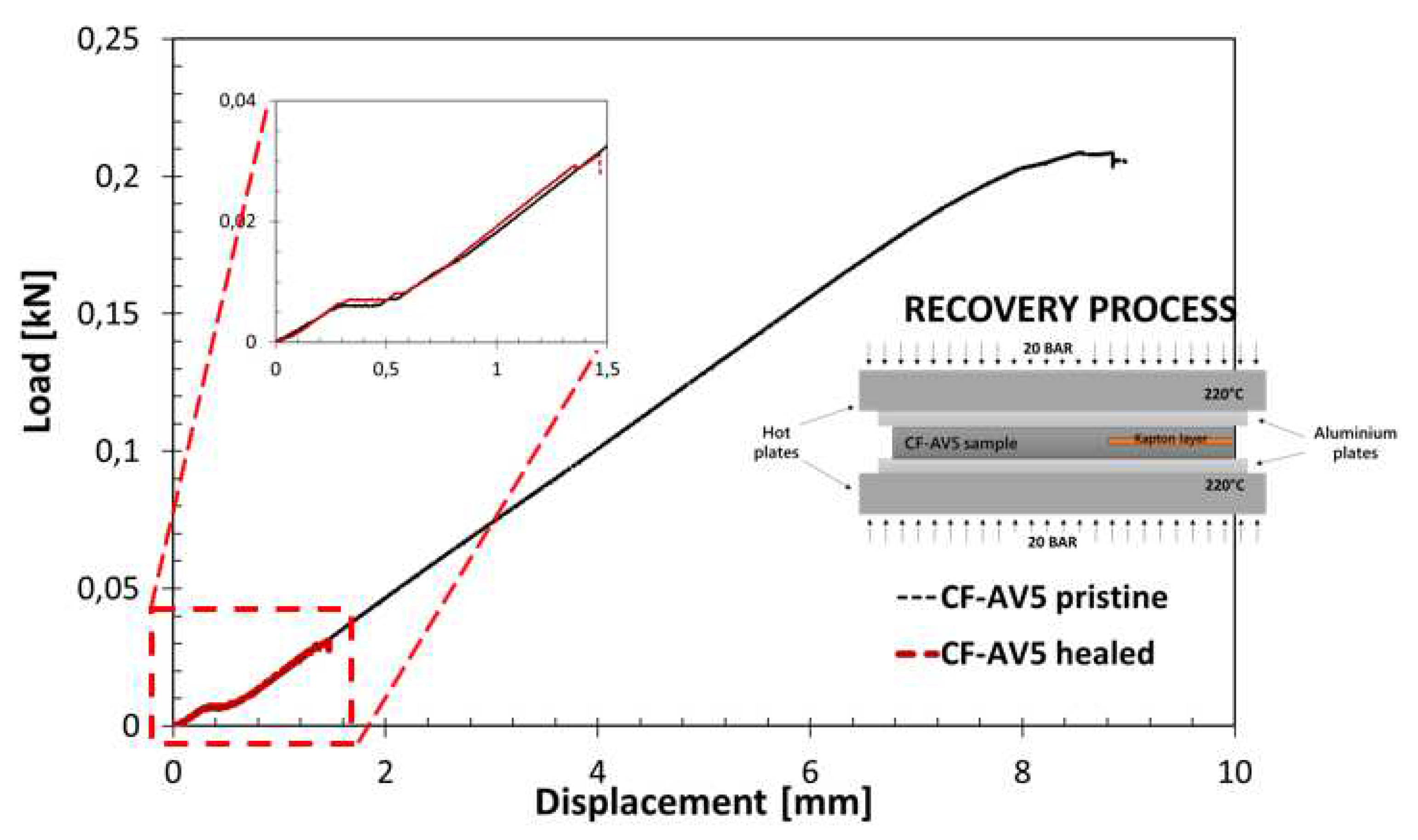
3.4. Fracture behaviour
4. Discussion
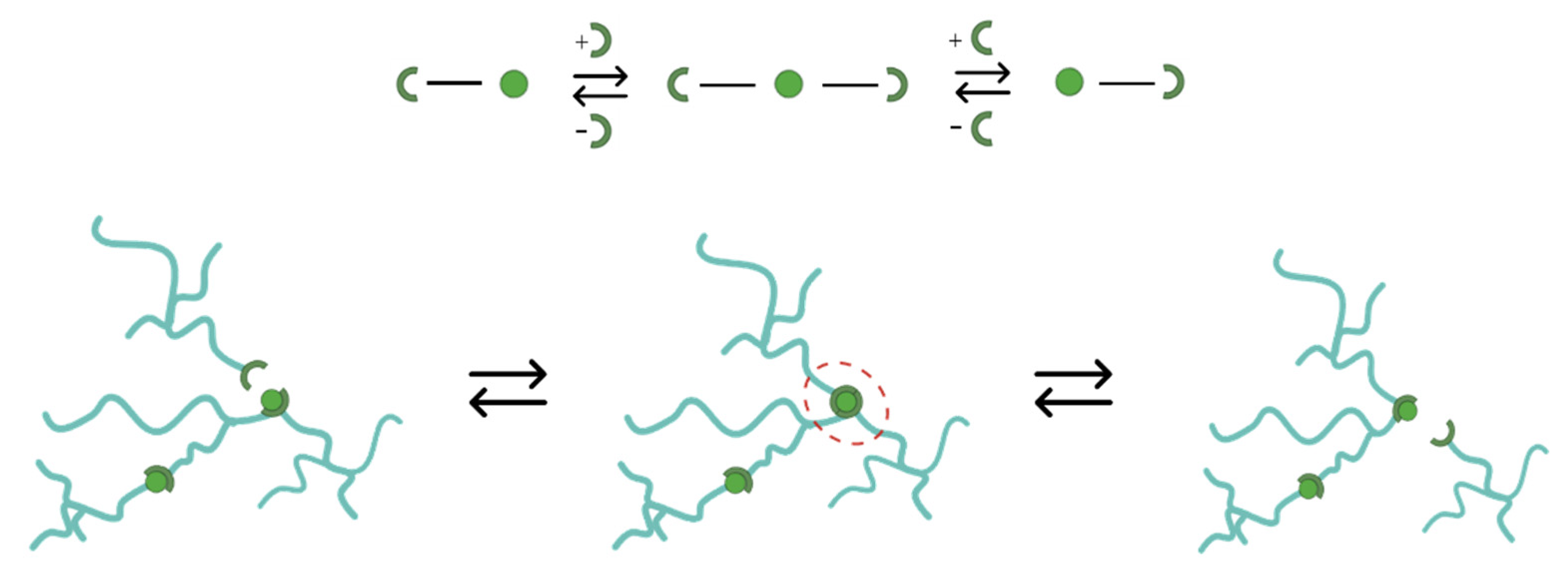
4.1. Epoxy vitrimer structure and its effect on the dynamic properties
4.2. Influence of reinforcement on the repairability of CFRP vitrimers
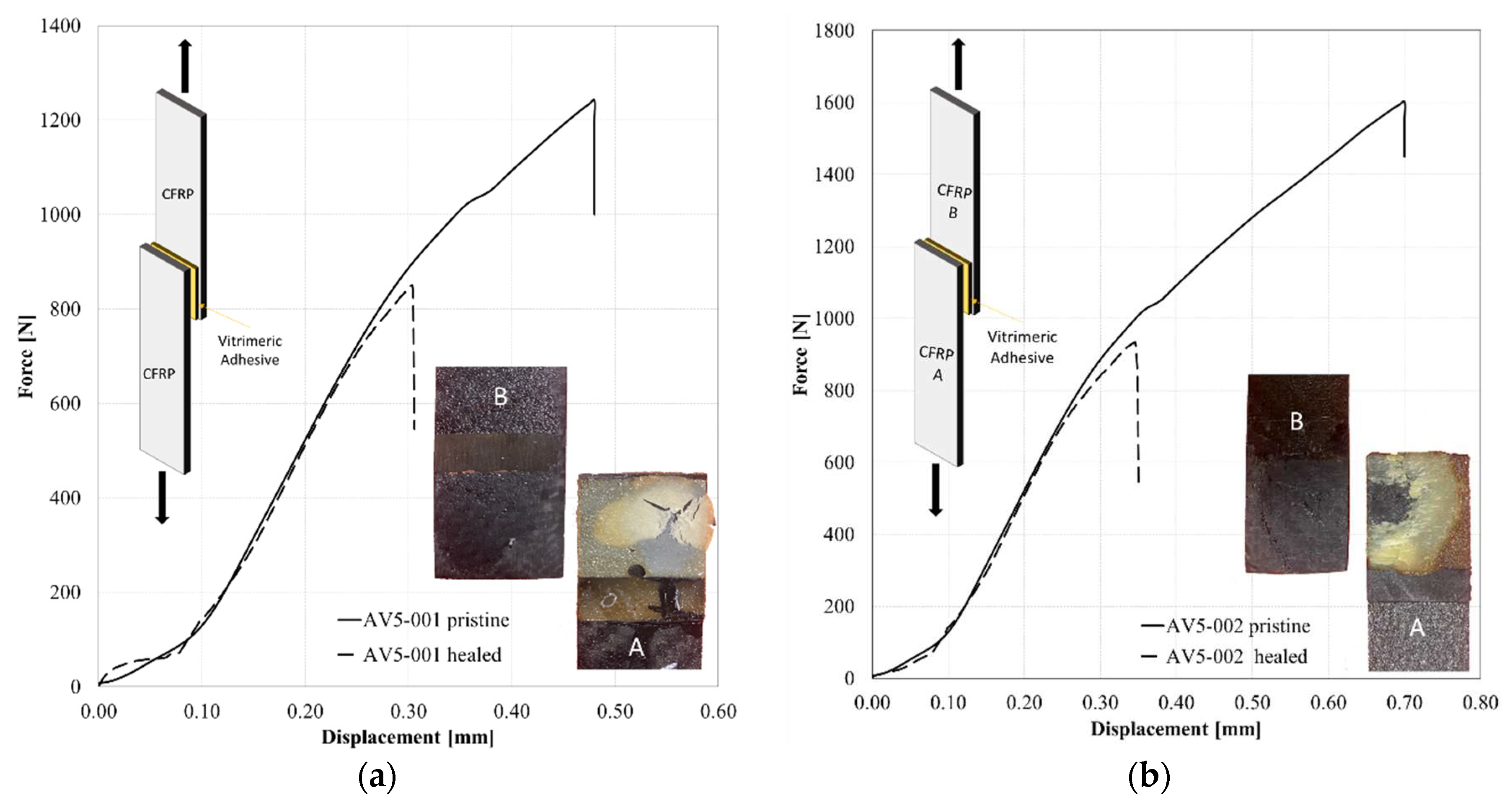
4.3. Use of vitrimer as adhesive layer: reassembly of lap shear joints
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgements
Conflicts of Interest
References
- Witik, R.A.; Teuscher, R.; Michaud, V.; Ludwig, C.; Månson, J.-A.E. Carbon Fibre Reinforced Composite Waste: An Environmental Assessment of Recycling, Energy Recovery and Landfilling. Compos. Part A Appl. Sci. Manuf. 2013, 49, 89–99. [Google Scholar] [CrossRef]
- de Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy Resin with Exchangeable Disulfide Crosslinks to Obtain Reprocessable, Repairable and Recyclable Fiber-Reinforced Thermoset Composites. Mater. Horizons 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S. Recycling of Carbon Fiber with Epoxy Composites by Chemical Recycling for Future Perspective: A Review. Chem. Pap. 2020, 74, 3785–3807. [Google Scholar] [CrossRef]
- Palmieri, B.; Borriello, C.; Rametta, G.; Iovane, P.; Portofino, S.; Tammaro, L.; Galvagno, S.; Giordano, M.; Ambrosio, L.; Martone, A. Investigation on Stress Relaxation of Discontinuous Recycled Carbon Fiber Composites. J. Mater. Eng. Perform. 2023, 32, 3938–3945. [Google Scholar] [CrossRef]
- Prinçaud, M.; Aymonier, C.; Loppinet-Serani, A.; Perry, N.; Sonnemann, G. Environmental Feasibility of the Recycling of Carbon Fibers from CFRPs by Solvolysis Using Supercritical Water. ACS Sustain. Chem. Eng. 2014, 2, 1498–1502. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Spencer, N. Waste Prevention and Technologies in the Context of the EU Waste Framework Directive: Lost in Translation? Eur. Energy Environ. Law Rev. 2017, 26. [Google Scholar]
- Memon, H.; Wei, Y.; Zhu, C. Recyclable and Reformable Epoxy Resins Based on Dynamic Covalent Bonds – Present, Past, and Future. Polym. Test. 2022, 105, 107420. [Google Scholar] [CrossRef]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789. [Google Scholar] [CrossRef]
- Imbernon, L.; Norvez, S. From Landfilling to Vitrimer Chemistry in Rubber Life Cycle. Eur. Polym. J. 2016, 82, 347–376. [Google Scholar] [CrossRef]
- Dello Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Thermally Activated Multiple Self-Healing Diels-Alder Epoxy System. Polym. Eng. Sci. 2017, 57, 674–679. [Google Scholar] [CrossRef]
- van den Tempel, P.; van der Boon, E.O.; Winkelman, J.G.M.; Krasnikova, A. V.; Parisi, D.; Deuss, P.J.; Picchioni, F.; Bose, R.K. Beyond Diels-Alder: Domino Reactions in Furan-Maleimide Click Networks. Polymer (Guildf). 2023, 274, 125884. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, J.; Hu, G.; Zhang, B. Recycling Strategies for Vitrimers. Int. J. Smart Nano Mater. 2022, 13, 367–390. [Google Scholar] [CrossRef]
- Wang, S.; Fu, D.; Wang, X.; Pu, W.; Martone, A.; Lu, X.; Lavorgna, M.; Wang, Z.; Amendola, E.; Xia, H. High Performance Dynamic Covalent Crosslinked Polyacylsemicarbazide Composites with Self-Healing and Recycling Capabilities. J. Mater. Chem. A 2021, 9, 4055–4065. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Wang, D. Triple-Shape Memory Epoxy Based on Diels–Alder Adduct Molecular Switch. Polymer (Guildf). 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Amendola, E.; Palmieri, B.; Iacono, S. Dello; Martone, A. Thermally Mendable Self-Healing Epoxy Coating for Corrosion Protection in Marine Environments. 2023, 1–11.
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder Based, Thermo-Reversible Cross-Linked Epoxies for Use in Self-Healing Composites. Polymer (Guildf). 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Fierro, G.-P.M.; Pinto, F.; Iacono, S. Dello; Martone, A.; Amendola, E.; Meo, M. Monitoring of Self-Healing Composites: A Nonlinear Ultrasound Approach. Smart Mater. Struct. 2017, 26, 115015. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like Malleable Materials from Permanent Organic Networks. Science (80-. ). 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Yue, L.; Guo, H.; Kennedy, A.; Patel, A.; Gong, X.; Ju, T.; Gray, T.; Manas-Zloczower, I. Vitrimerization: Converting Thermoset Polymers into Vitrimers. ACS Macro Lett. 2020, 9, 836–842. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile: In Situ Preparation of High-Performance Epoxy Vitrimer from Renewable Resources and Its Application in Nondestructive Recyclable Carbon Fiber Composite. Green Chem. 2019, 21, 1484–1497. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent Organic Networks with Glass-like Fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Y.; Ji, Y.; Wei, Y. Functional Epoxy Vitrimers and Composites. Prog. Mater. Sci. 2021, 120. [Google Scholar] [CrossRef]
- Liu, W.; Schmidt, D.F.; Reynaud, E. Catalyst Selection, Creep, and Stress Relaxation in High-Performance Epoxy Vitrimers. Ind. Eng. Chem. Res. 2017, 56, 2667–2672. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy Vitrimers with a Covalently Bonded Tertiary Amine as Catalyst of the Transesterification Reaction. Eur. Polym. J. 2019, 113, 297–304. [Google Scholar] [CrossRef]
- Yue, L.; Amirkhosravi, M.; Gong, X.; Gray, T.G.; Manas-Zloczower, I. Recycling Epoxy by Vitrimerization: Influence of an Initial Thermoset Chemical Structure. ACS Sustain. Chem. Eng. 2020, 8, 12706–12712. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, G.; Wu, S.; Hao, W. A Repairable Anhydride-Epoxy System with High Mechanical Properties Inspired by Vitrimers. Polymer (Guildf). 2018, 159, 162–168. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.; Qi, H.J. Solvent Assisted Pressure-Free Surface Welding and Reprocessing of Malleable Epoxy Polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]
- Demongeot, A.; Mougnier, S.J.; Okada, S.; Soulié-Ziakovic, C.; Tournilhac, F. Coordination and Catalysis of Zn2+ in Epoxy-Based Vitrimers. Polym. Chem. 2016, 7, 4486–4493. [Google Scholar] [CrossRef]
- Kamble, M.; Vashisth, A.; Yang, H.; Pranompont, S.; Picu, C.R.; Wang, D.; Koratkar, N. Reversing Fatigue in Carbon-Fiber Reinforced Vitrimer Composites. Carbon N. Y. 2022, 187, 108–114. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, Y.; Ji, Y. Carbon Material/Vitrimer Composites: Towards Sustainable, Functional, and High-Performance Crosslinked Polymeric Materials. Giant 2023, 13, 100136. [Google Scholar] [CrossRef]
- Wu, P.; Liu, L.; Wu, Z. A Transesterification-Based Epoxy Vitrimer Synthesis Enabled High Crack Self-Healing Efficiency to Fibrous Composites. Compos. Part A Appl. Sci. Manuf. 2022, 162, 107170. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Wang, A.; Chang, Z.; Wang, Z.; Zhang, K. Experimental Study on the Mode Ι Interlaminar Properties of Self-Healable Vitrimeric CFRP with Various Interfaces. Compos. Part B Eng. 2023, 261, 110806. [Google Scholar] [CrossRef]
- ASTM INTERNATIONAL Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. D790. Annu. B. ASTM Stand. 2002, 1–12. [Google Scholar] [CrossRef]
- Pini, T.; Briatico-Vangosa, F.; Frassine, R.; Rink, M. Matrix Toughness Transfer and Fibre Bridging Laws in Acrylic Resin Based CF Composites. Eng. Fract. Mech. 2018, 203, 115–125. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Sanka, R.V.S.P.; Binder, W.H.; Parthasarthy, V.; Rana, S.; Karak, N. Vitrimers: Associative Dynamic Covalent Adaptive Networks in Thermoset Polymers. Chem. Eng. J. 2020, 385, 123820. [Google Scholar] [CrossRef]
- Zhao, W.; An, L.; Wang, S. Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions. Polymers (Basel). 2021, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liu, X.; Feng, Y.; Lu, B.; Huang, M.; Liu, C.; Shen, C. Influence of Zn2+ Catalyst Stoichiometry on Curing Dynamics and Stress Relaxation of Polyester-Based Epoxy Vitrimer. Polymer (Guildf). 2023, 278. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast Fourier Transform IR Characterization of Epoxy GY Systems Crosslinked with Aliphatic and Cycloaliphatic EH Polyamine Adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Xiao, F. Applications of Epoxy Materials in Pavement Engineering. Constr. Build. Mater. 2020, 235, 117529. [Google Scholar] [CrossRef]
- Daelemans, L.; van der Heijden, S.; De Baere, I.; Muhammad, I.; Van Paepegem, W.; Rahier, H.; De Clerck, K. Bisphenol A Based Polyester Binder as an Effective Interlaminar Toughener. Compos. Part B Eng. 2015, 80, 145–153. [Google Scholar] [CrossRef]
- Chekanov, Y.; Arrington, D.; Brust, G.; Pojman, J.A. Frontal Curing of Epoxy Resins: Comparison of Mechanical and Thermal Properties to Batch-Cured Materials. J. Appl. Polym. Sci. 1997, 66, 1209–1216. [Google Scholar] [CrossRef]
- Suslick, B.A.; Hemmer, J.; Groce, B.R.; Stawiasz, K.J.; Geubelle, P.H.; Malucelli, G.; Mariani, A.; Moore, J.S.; Pojman, J.A.; Sottos, N.R. Frontal Polymerizations: From Chemical Perspectives to Macroscopic Properties and Applications. Chem. Rev. 2023, 123, 3237–3298. [Google Scholar] [CrossRef] [PubMed]
- Ward, I.M.; Sweeney, J. Mechanical Properties of Solid Polymers; Wiley, 2012. ISBN 9781444319507.
- Lorandi, N.P.; Cioffi, M.O.H.; Shigue, C.; Ornaghi Jr., H. L. On the Creep Behavior of Carbon/Epoxy Non-Crimp Fabric Composites. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]

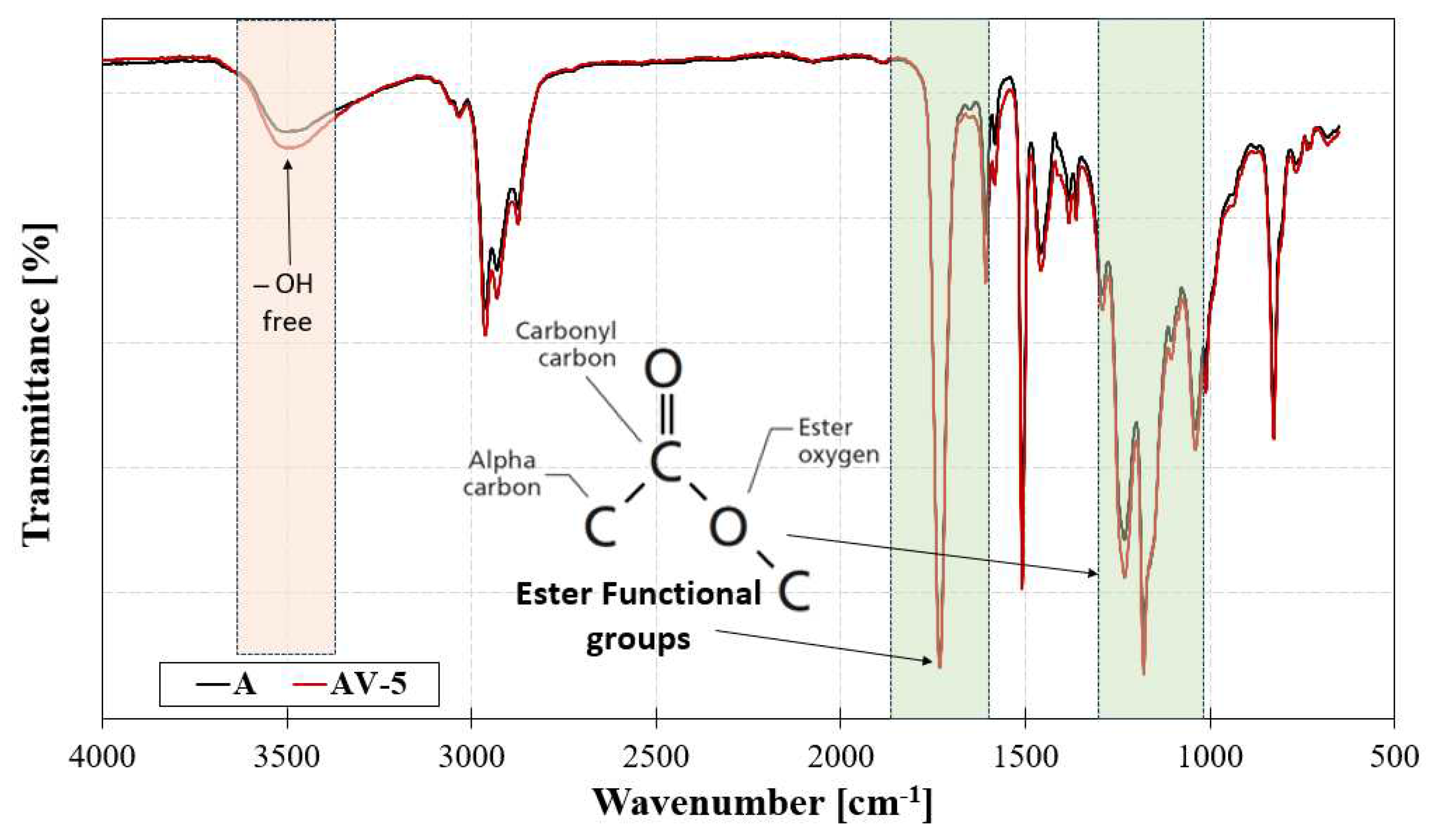
| Description | Residue at 600 °C | CF actual content | Tg, DSC |
|---|---|---|---|
| [wt%] | [wt%] | [°C] | |
| A | 7.8 | - | 111.2 |
| AV5 | 14.5 | - | 105.1 |
| CF-A | 67.2 | 64.4 | 116.2 |
| CF-AV5 | 68.9 | 62.6 | 107.3 |
| Elastic modulus | Ultimate strain | Ultimate stress | |
|---|---|---|---|
| [MPa] | [mm/mm] | [MPa] | |
| A | 2704±5 | 0.028±0.002 | 68.3±0.7 |
| AV5 | 2596±8 | 0.027±0.003 | 61.5±0.6 |
| @35°C | @170°C | ||||||
|---|---|---|---|---|---|---|---|
| Tg, DMA | E' | E'' | tanδ | E' | E'' | tanδ | |
| [°C] | [MPa] | [MPa] | [-] | [MPa] | [MPa] | [-] | |
| A | 105.6±1.1 | 1899 | 22.1 | 0.0116 | 5.14 | 0.02 | 0.0459 |
| AV5 | 108.8±0.9 | 2221 | 28.5 | 0.0128 | 12.08 | 1.43 | 0.1180 |
| CF-A | 123.6±0.8 | 10717 | 166.5 | 0.0155 | 649.4 | 6.33 | 0.0097 |
| CF-AV5 | 115.3±1.2 | 11210 | 111.2 | 0.0099 | 762.6 | 33.46 | 0.0439 |
| Description | GQ, | KIc, |
|---|---|---|
| [kJ/m2] | [MPa √m] | |
| A | 3.13±0.7 | 2.24±0.3 |
| AV5 | 2.85±0.8 | 2.01±0.4 |
| Description | GIc, initiation [J/m2] |
GIc, propagation [J/m2] |
GIIc [J/m2] |
|---|---|---|---|
| CF-A | 352±22 | 574±30 | 463±35 |
| CF-AV5 | 158±10 | 184±15 | 459±16 |
| Description | LSS | Healing Efficiency |
|---|---|---|
| [MPa] | [%] | |
| AV5_001 | 2.31 | - |
| AV5_001_Healed | 1.94 | 84% |
| AV5_002 | 2.41 | - |
| AV5_002_ Healed | 1.48 | 61% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





