1. Introduction
The need for sustainable and environmentally friendly energy cannot be ignored considering the rapid depletion of fossil fuel resources [
1,
2,
3]. Hydrogen energy-based systems are one of the leading technologies that can provide the requirements for such type of energy sources. Fuel cells are discussed as most promising technological approaches due to number of advantages such as:
Efficiency of fuel cells is higher than other energy generation systems due to the properties of the proton exchange membrane fuel cells (PEMFC) - high power density, great durability, low operating temperature, rapid response to changes in system conditions. [
5,
6,
7,
8,
9,
10,
11]
Fuel cells have found an application in nuclear facilities for separating and recovering the heavy and radioactive hydrogen isotope – tritium [
12,
13]. By electrolytically processing water, it can be enriched with the radioactive hydrogen isotope, tritium, and in such conditions the PEM is exposed to ionising radiation. As tritium is often a main fuel in nuclear fusion reactors, it is important for the PEM to have great radiation stability and be able to function properly under the influence of ionising radiation.[
14]
SPEEK polymer membranes have exhibited good chemical stability in fuel cell tests [
15,
16] and vanadium redox flow batteries [
16,
17]. The degree of sulfonation (DS) of this PEM determines how the membranes conducts protons, as well as the mechanical and chemical stability of the material [
18,
19]. By increasing the DS, proton conductivity is also increased, and that enhances its performance in PEMFCs. However, an increase in DS promotes quicker membrane deteoriation, both chemical and mechanical, which in turn decreases proton conductivity. [
20]
SPEEK polymers, the sulfonated poly(ether ether ketone) membranes have been investigated as polymer electrolyte materials due to relatively lower costs and advanced properties in contrast to other currently commercially available PEMs, such as Nafion™ [
21,
22,
23]. As the performance of PEMs depends highly on the sulfonation degree, a reliable and fast method for the determination of DS is required [
14,
22,
23,
24].
Due to its good radiation stability [
25] SPEEK can be used also in radiation environments, such as nuclear facilities, space applications and proton exchange membrane-based enrichment of hydrogen radioactive isotope tritium [
26]. Insufficient research has been done on the effects of ionizing radiation on the DS, as well as how electron beam radiation affects the structure of the membrane, which affects the determination of DS.
In this study, synthesis of SPEEK membranes with various degrees of sulfonation and the determination of the DS using various spectrometric and analytical methods was carried out for both non-irradiated and electron beam irradiated SPEEK membranes.
2. Materials and Methods
SPEEK synthesis
PEEK in granular form was purchased from Sigma-Aldrich. PEEK pellets were dried in a vacuum oven at 100 °C overnight. 10 g of the pellets were added slowly to 200 mL of concentrated sulfuric acid (95-97 %) with heating and vigorous stirring. After reaching the necessary sulfonation degree (see
Table 1), the reaction was terminated by pouring the sulfonated polymer directly into ice-water. The polymer precipitate was filtered and washed several times with deionized water until pH reached 7. The filtered polymer was then dried under vacuum at 60 °C for one week until constant weight [
27,
28,
29]. SPEEK membranes were produced by dissolving obtained highly sulfonated SPEEK materials in N,N-dimethylformamide (DMF) and pouring into Petri dishes, followed by drying for 48 h at 80 °C. The SPEEK membranes were removed from the Petri dishes and used for carrying out further experiments.
SPEEK irradiation
The dried cast membranes were irradiated by 10 MeV electron beam at the Institute of Nuclear Chemistry and Technology (Warsaw) with total absorbed dose of 500 kGy. Dosimetry was carried out using a graphite calorimeter according to ISO ISO/ASTM 51631:2013.
Impedance analysis
Impedance analysis of the membranes was performed in two electrode through-plane configuration (electrode diameter was 1 cm). Multichannel potentiostat/galvanostat BioLogic VMP3 was used, and measuring parameters frequency range was 50 kHz to 1 Hz; 10 frequencies per decade; signal amplitude 10 mV. The resistance with precision ± 1 Ohm was obtained from Nyquist plot extrapolating to the high frequencies. [
30]
Thermogravimetry analysis (TGA)
TGA measurements were performed using an MettlerToledo TGA1/SF (OH, U.S.) thermogravimetric instrument. Samples of SPEEK membranes (10 mg weight) were placed in alumina crucibles and thermally treated under air flux (50 ml/min) from 25 to 600 °C with a heating rate of 10 °C/min. For each membrane three samples were prepared and analysed as described above. The average TGA curves of the weight loss versus temperature and the derivative (DTG, %/°C) were analysed for each membrane.
TGA data of the sulfonate group decomposition were directly used to calculate DS for SPEEK membranes. The Equation 1 described in the literature [
28] was used:
where M(PEEK) and M(SO
3H) are the molecular masses of PEEK monomer (288.7 g/mol) and sulfonic acid groups (81 g/mol), m is the mass of SPEEK at the beginning of the desulfonation, and Δm is the mass loss due to the desulfonation. [
31]
Nuclear magnetic resonance spectroscopy
Degree of sulfonation was determined using
1H-NMR spectrum acquired with Bruker Fourier-300 spectrometer. 5 - 10 mg of the membranes was dissolved in deuterated dimethyl sulfoxide (DMSO-d
6) solution, and its spectrum acquired. The DS was calculated in the MestReNova program using the ratio of peak areas of the proton peaks near the keto-group of SPEEK to the ratio of the proton next to the -SO
3H group. A modified version of the formula presented by Parnian et Al was used.
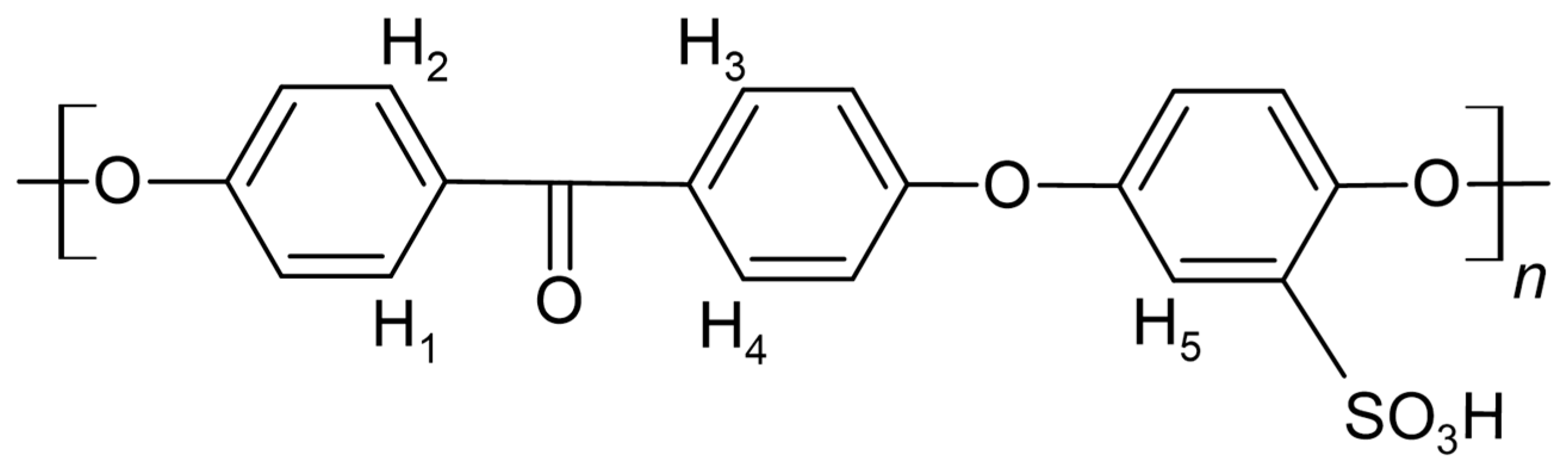
Figure 1.
SPEEK monomer marked with protons used for integration.
Figure 1.
SPEEK monomer marked with protons used for integration.
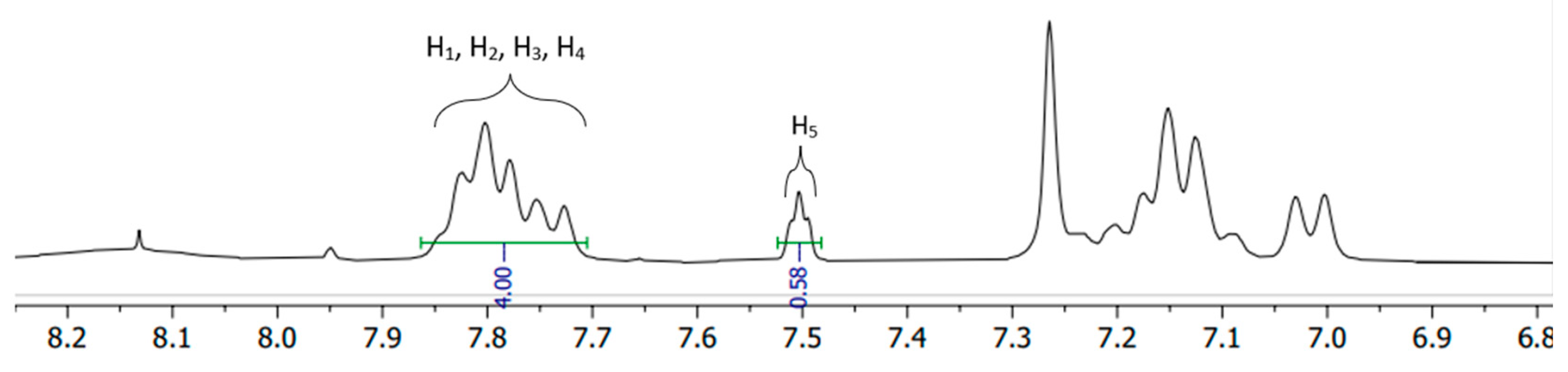
Figure 2.
The integrated 1H-NMR spectra example.
Figure 2.
The integrated 1H-NMR spectra example.
Spectrophotometry with Cr(III)
Metals such as Fe(III) and Cr(III) can form ionic bonds with sulfonic acid groups present in PEMs [
32,
33,
34]. Therefore, a novel method was developed to quickly and inexpensively determine the degree of sulfonation of proton exchange membranes (in this study – SPEEK) by photometric analysis of Cr(III).
Chromium(III) reacts with disodium ethylenediaminetetraacetic acid (EDTA) at temperatures ~ 373K readily to form a brightly colored purple complex that can be used to determine Cr(III) ion concentration in solution using spectrophotometric analysis. [
35]
To determine the DS, the membranes (0.005 - 0.01g) were submerged in chromium (III) nitrate solutions of known concentration and volume for 24h and light absorption measurements were performed with Jenway 6300 spectrophotometer using 540 nm (maximum of absorption for chromium (III) complexonate [
35]). Before the initial measurement of the various membrane samples, a calibration with standard solutions of Cr(III) ions was carried out. The Cr(III) standards were prepared in concentrations 0.04; 0.08; 0.12; 0.16 and 0.20 g L
-1. Acetate buffer solution (pKa = 4.7) and 5% EDTA solution was added. The obtained solutions were heated up to 373.15 K to obtain a purple colour and then diluted to a known volume using a volumetric flask. The light absorption of all standards and samples was measured in 1 cm plastic cuvettes. Each standard and sample was measured 3 times for 30 s and the values were recorded.
The decrease in concentration from the standard solution where the membranes were submerged was calculated from the calibration chart obtained from the standard solutions.
3. Results and discussion
Impendence analysis
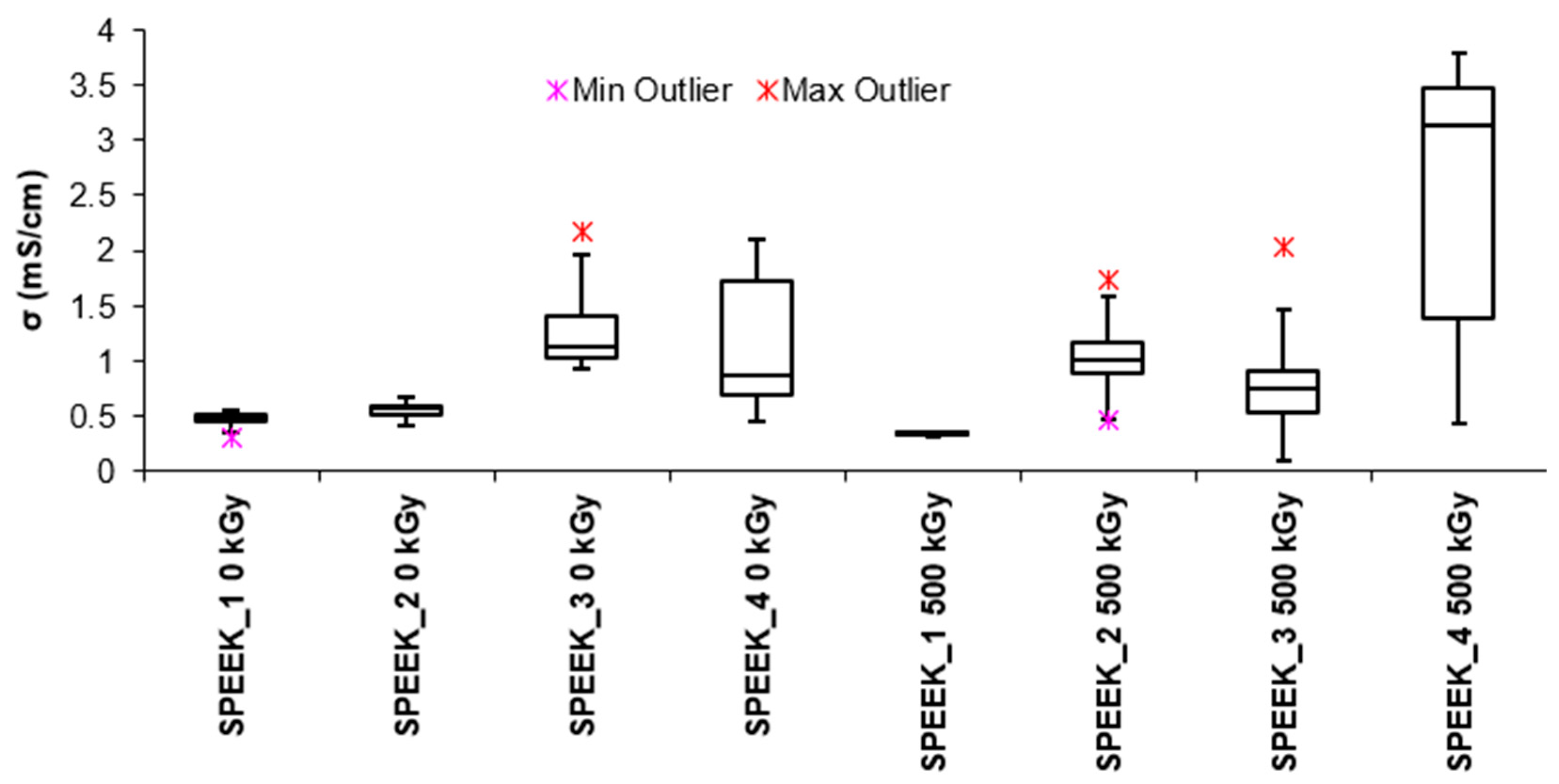
It can be seen in
Figure 3. that conductivity of the non-irradiated membranes are proportional to the DS. Increase in acidic groups promotes hydrophilic interactions, resulting in increased water absorption due to hydrogen bond formation. The absorbed water forms even more pathways for protons. By increasing the number of sulfonic acid groups in the polymer and by extension the membrane, hydrophilicity is increased, increasing water absorption and facilitating proton transport. [
16,
36,
37]
Figure.3. Conductivity of irradiated and non-irradiated SPEEK membranes depending on the DS of the SPEEK.
Thermogravimetry analysis (TGA)
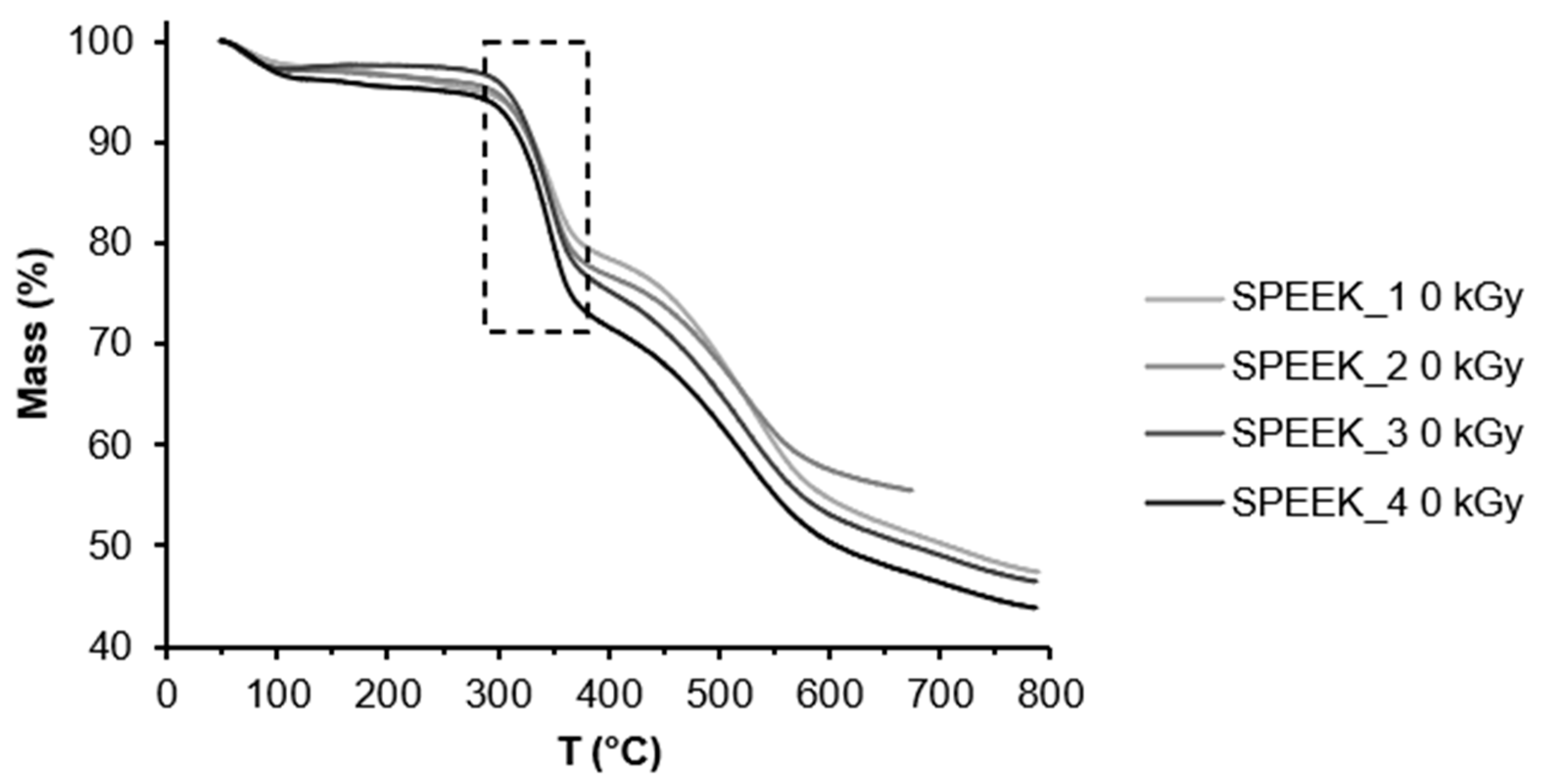
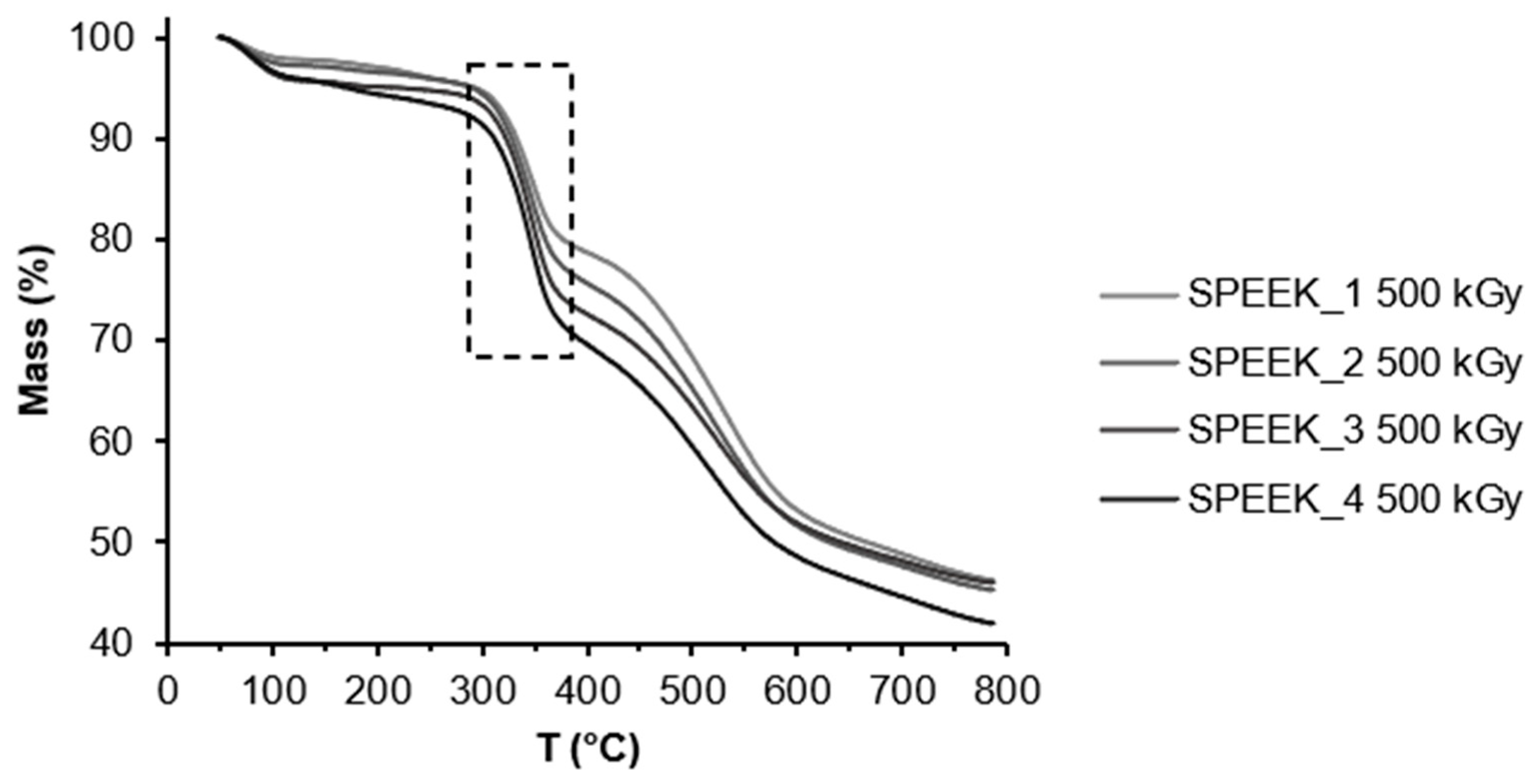
The TGA curves of non-irradiated and irradiated SPEEK membranes can be seen below in
Figure 4 and
Figure 5. The first mass loss can be attributed to water evaporation, the second one to the desulfonation reaction: 4SO
3H → 4SO
2 + 2H
2O + O
2, and the last one can be attributed to oxidative pyrolysis of the main polymer chain, forming H
2O and CO
2 upon decomposition. [
38] It is likely that due to varied distribution of the sulfonic acid groups in the polymer they decompose at slightly different temperatures. According to the TGA data the DS has slightly increased after irradiation. It might be related to the radiation induced sulfonation from the unreacted acid in the membrane structure.
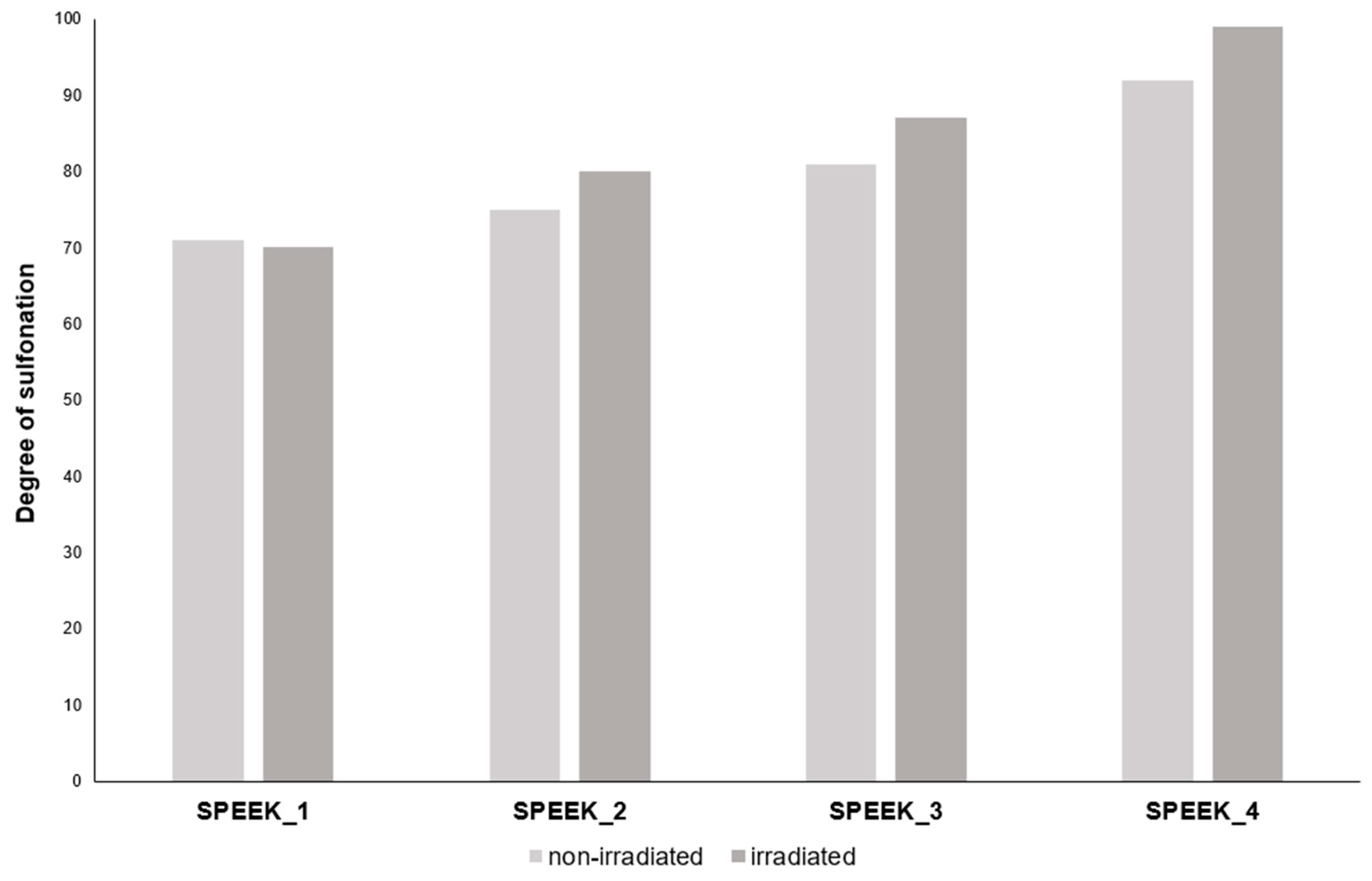
The DS was calculated using the TGA curves and Equation 1. The calculated DS can be seen in the table below.
Table 2.
Calculated DS by TGA.
Table 2.
Calculated DS by TGA.
| Sample |
SPEEK_1 |
SPEEK_2 |
SPEEK_3 |
SPEEK_4 |
| Expected DS, % |
60 |
70 |
80 |
90 |
| Non-irradiated DS, % |
71 |
75 |
81 |
92 |
| Irradiated DS, % |
70 |
80 |
87 |
99 |
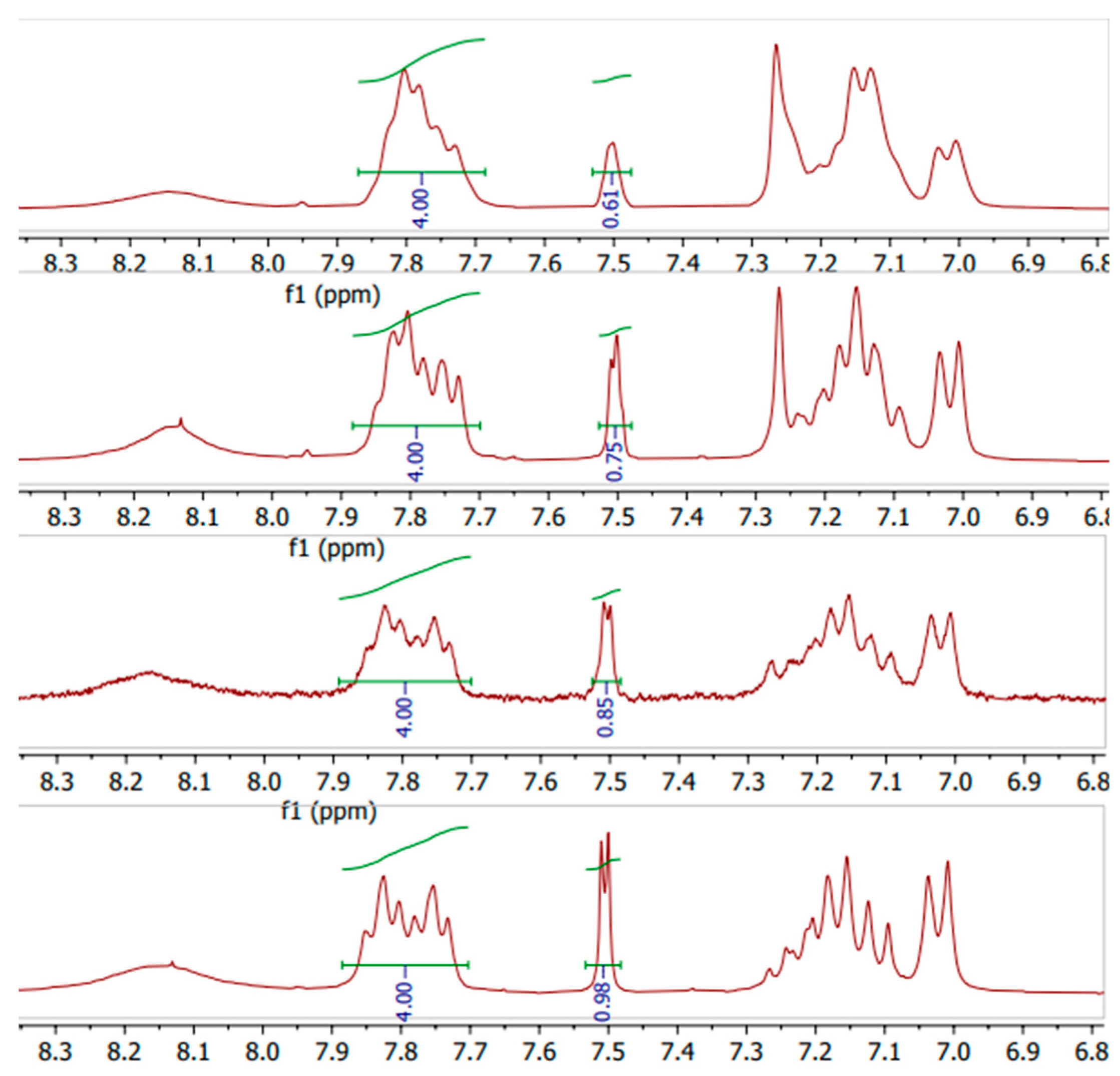
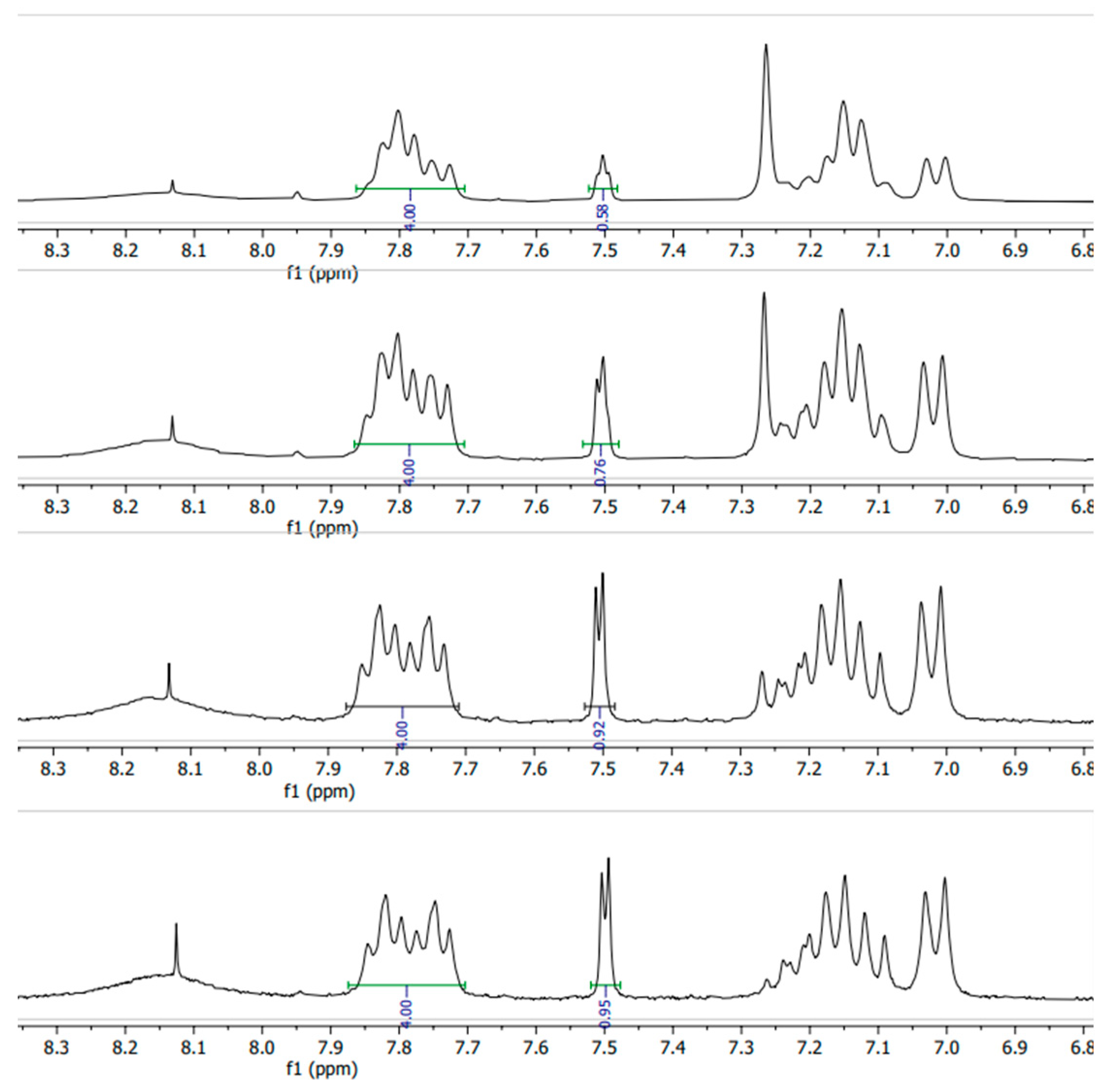
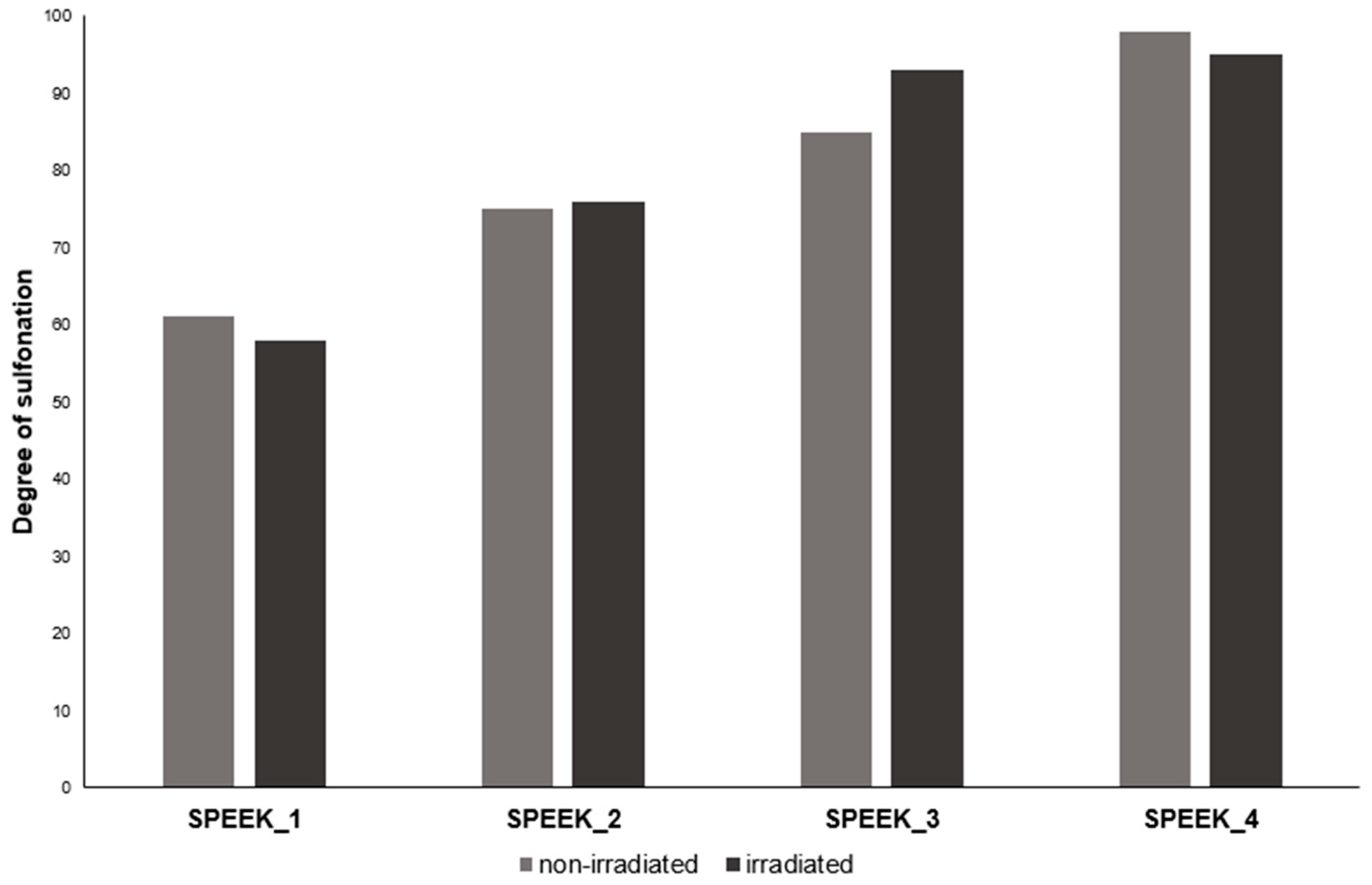
1. H-NMR
The close-up of the integrated
1H-NMR spectra can be seen in
Figure 11 and
Figure 12. The DS was determined by setting the integral of the H
1, H
2, H
3 and H
4 protons to 4 (shown in
Figure 1.), as their peak intensities are not affected by DS, and the peak integral of the H
5 proton directly correspond to the DS of the SPEEK membrane [
40].
Figure 11.
1H-NMR spectra of various DS (60%-90%) non-irradiated SPEEK membranes.
Figure 11.
1H-NMR spectra of various DS (60%-90%) non-irradiated SPEEK membranes.
Figure 12.
1H-NMR spectra of various DS (60%-90%) irradiated SPEEK membranes.
Figure 12.
1H-NMR spectra of various DS (60%-90%) irradiated SPEEK membranes.
Table 3.
Calculated DS by 1H-NMR.
Table 3.
Calculated DS by 1H-NMR.
| Sample |
SPEEK_1 |
SPEEK_2 |
SPEEK_3 |
SPEEK_4 |
| Expected DS, % |
60 |
70 |
80 |
90 |
| Non-irradiated DS, % |
61 |
75 |
85 |
98 |
| Irradiated DS, % |
58 |
76 |
92 |
95 |
Spectrophotometry with Cr(III)
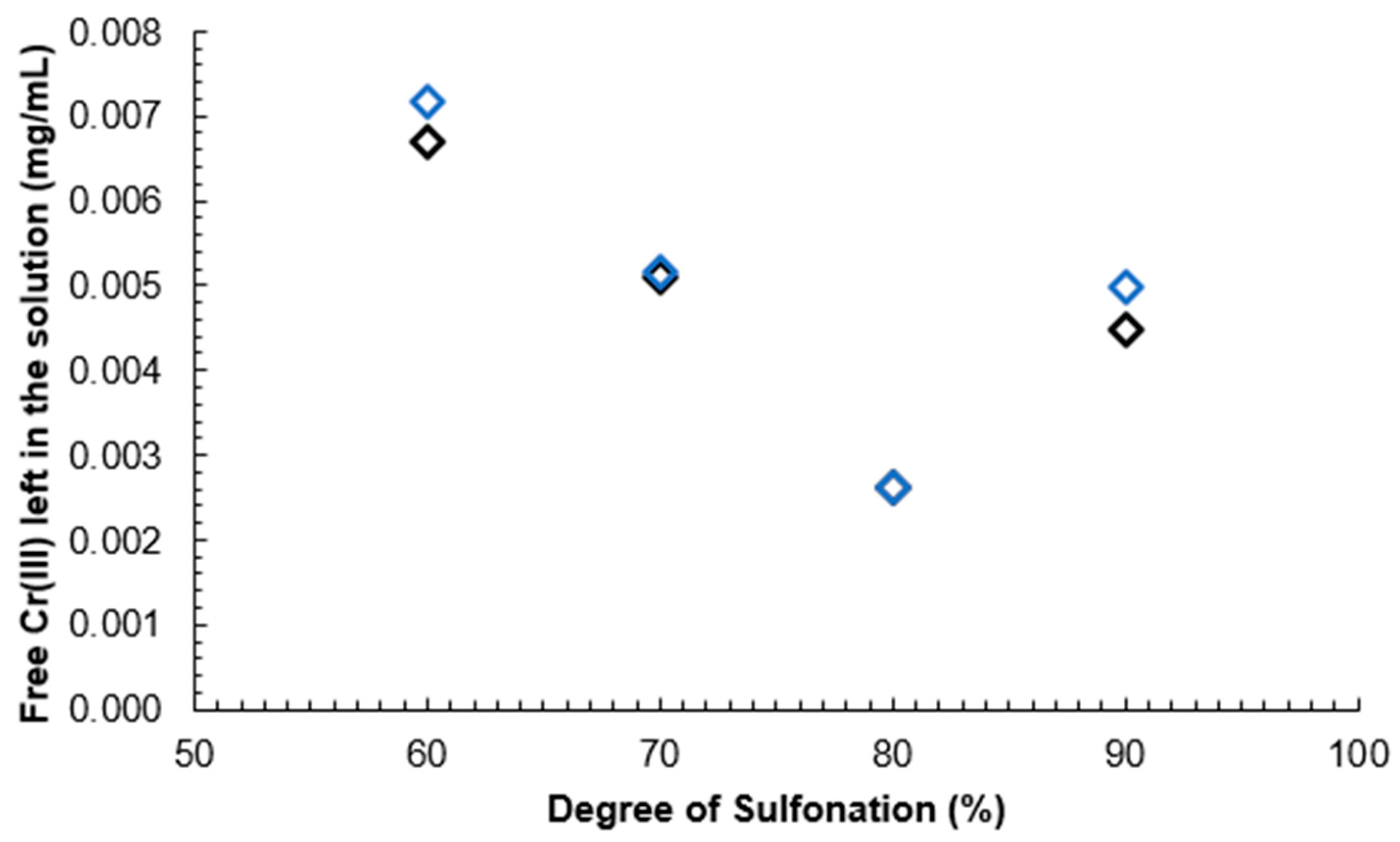
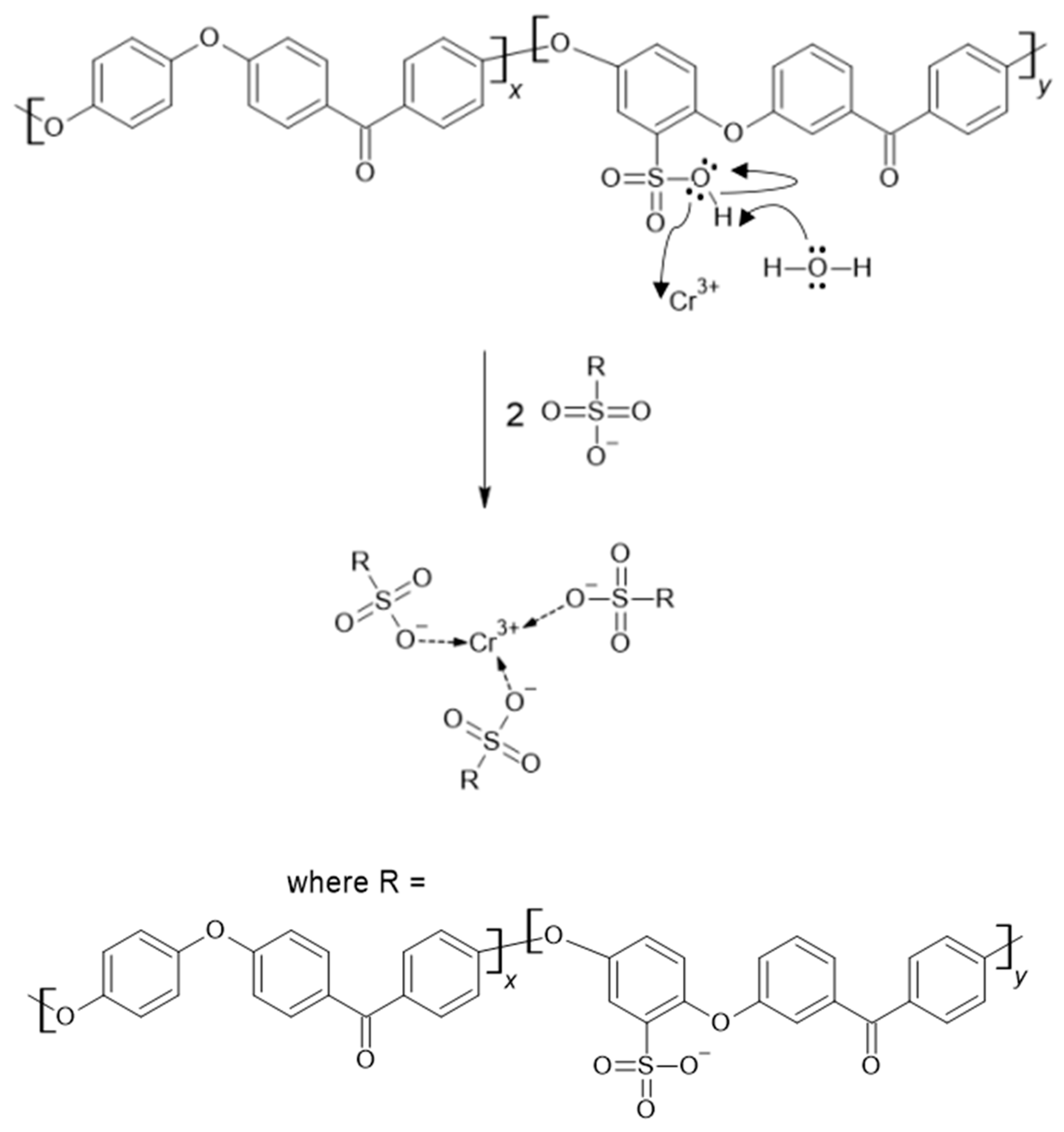
It can be seen that a higher DS correlates with a lower leftover Cr(III) mass concentration compared to the original solution due to the reaction of Cr(III) ions with -SO
3H groups. The pKa of SPEEK is reported as 1.58 for SPEEK with DS 85% in [
41], meaning that above pH 1.58 SPEEK -SO
3H groups will have already displaced the protons creating an ionic bond between the -SO
3- group and Cr(III) atoms. The obtained pH values for SPEEK samples with DS 60%, 70%, 80% and 90% were as follows: 2.63, 2.84, 1.94, 2.32. As Cr(III) ions carry a 3+ charge, 3 sulfonic acid groups are expected to be attached to 1 Cr atom.
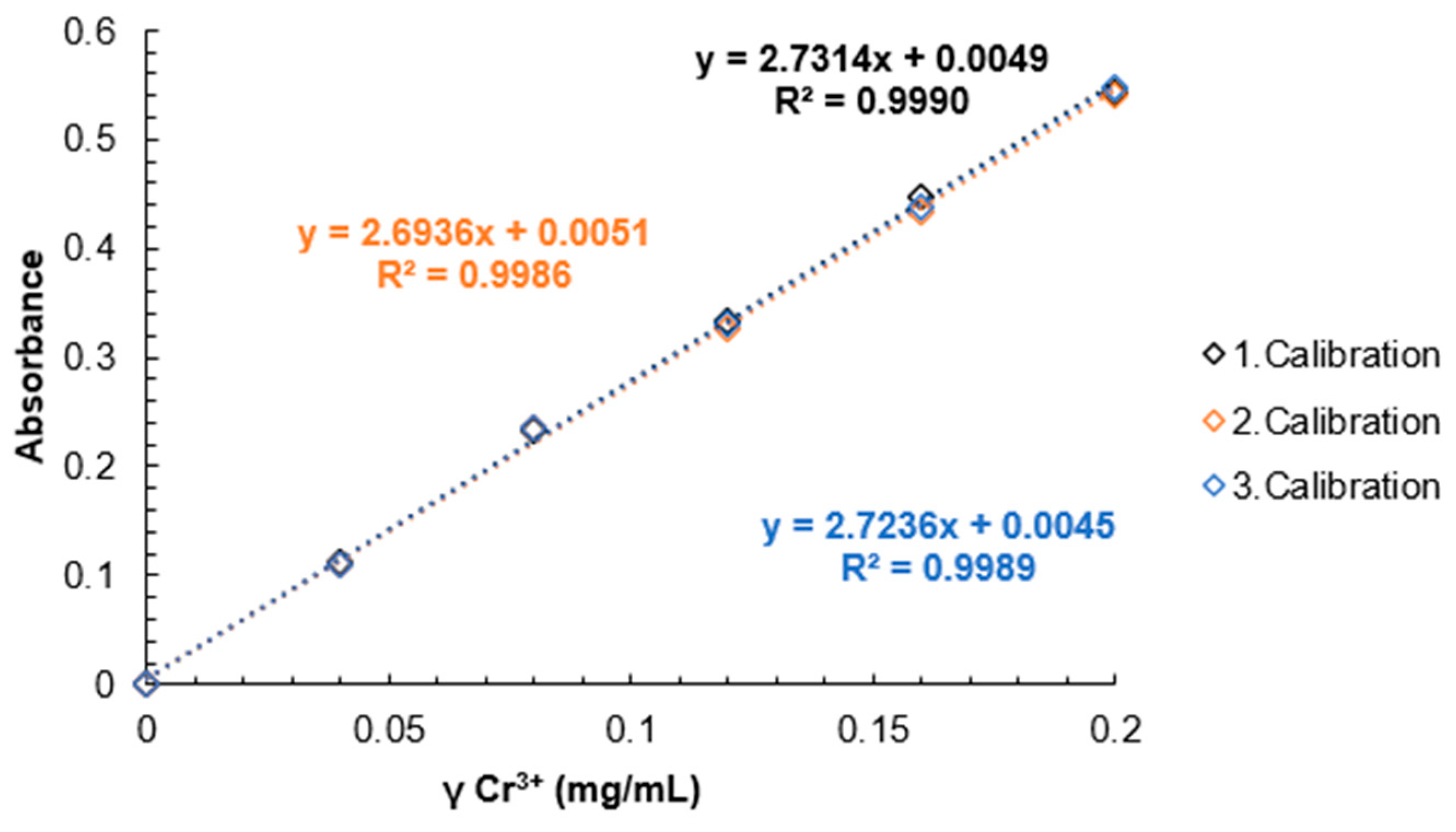
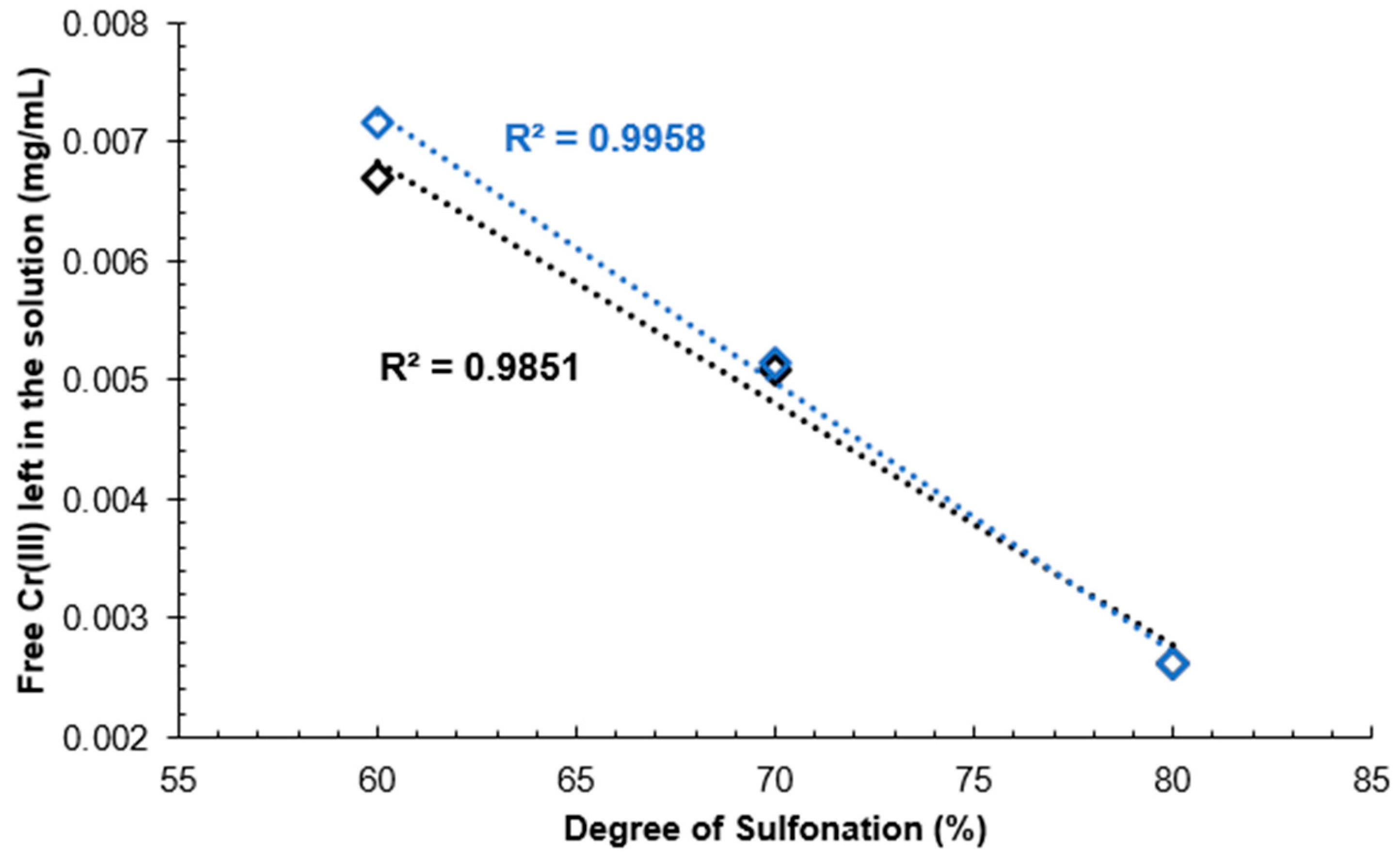
Calibration curve results can be seen in
Figure 13. A decreasing linear correlation can be observed in Figure 24 and
Figure 15 from DS 60% to 80%, indicating that the method is valid for the determination of the DS for SPEEK membranes with DS < 90%.
Figure 16. illustrates one of the possible bond creation mechanisms for SPEEK and Cr(III).
Figure 13.
Calibration curves for Cr(III) standard solutions.
Figure 13.
Calibration curves for Cr(III) standard solutions.
Figure 14.
Unreacted Cr(III) mass concentration depending on the DS of the membrane.
Figure 14.
Unreacted Cr(III) mass concentration depending on the DS of the membrane.
Figure 15.
Linear range of unreacted Cr(III) mass concentration depending on the DS of the membrane.
Figure 15.
Linear range of unreacted Cr(III) mass concentration depending on the DS of the membrane.
Figure 16.
A theoretical mechanism for the binding of Cr(III) to SPEEK.
Figure 16.
A theoretical mechanism for the binding of Cr(III) to SPEEK.
One explanation for deviation from the linear correlation with DS 90% could be that the polymer starts dissolving in the aqueous medium inhibiting the reaction with Cr(III) ions.
Summary of the results
There is a tendency for the proton conductivity of SPEEK membranes to increase with both DS of and irradiation, indicating that there is a possibility that electron beam irradiated membranes undergo structural changes after irradiation that are significant enough to improve the conductivity of protons.
Electron beam irradiated SPEEK membranes showed a different degree of sulfonation than non-irradiated SPEEK membranes with TGA, which indicates that the irradiated membranes might have either undergone electron-beam induced crosslinking or radiation induced sulfur addition from the remaining acid.
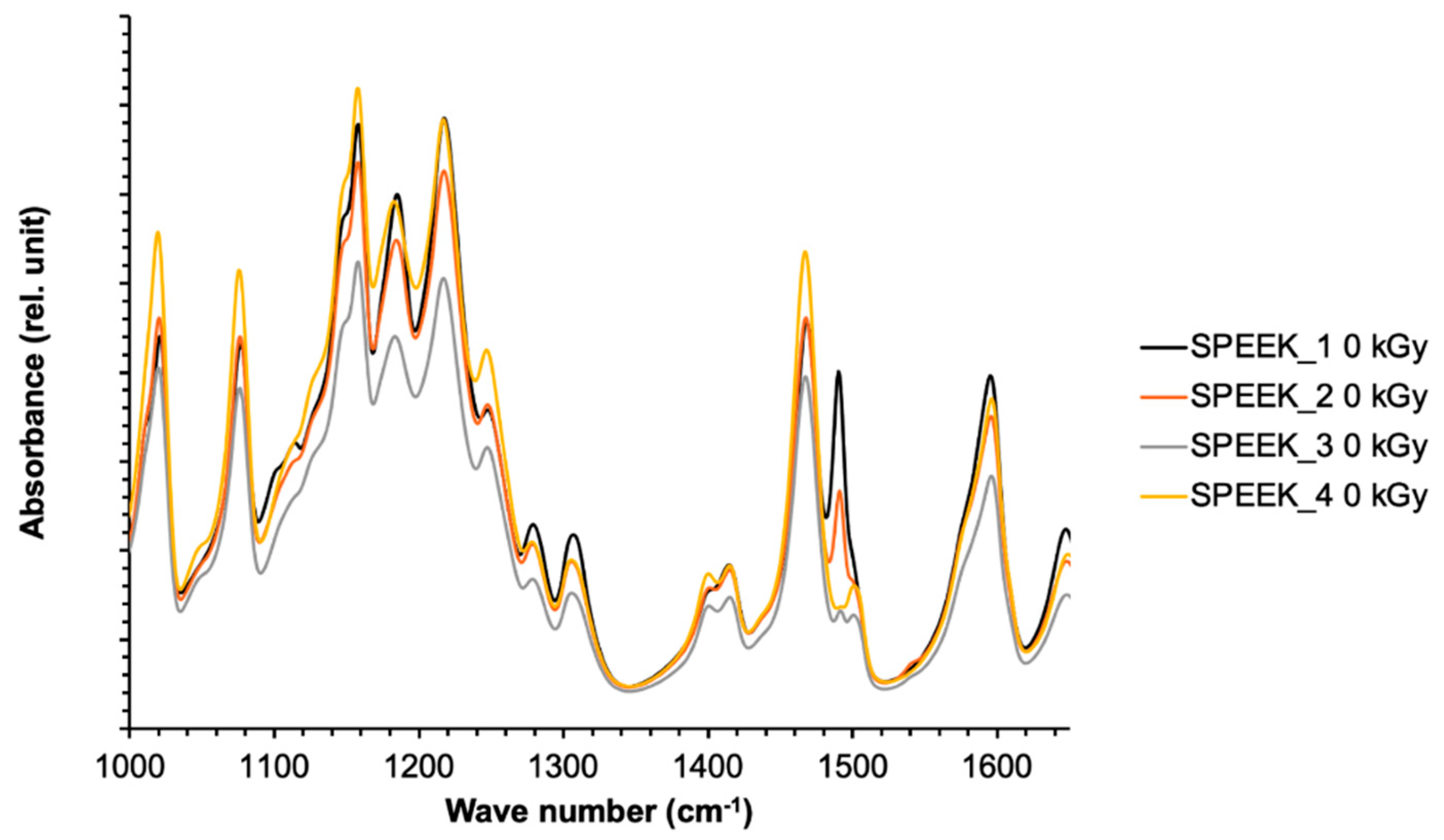
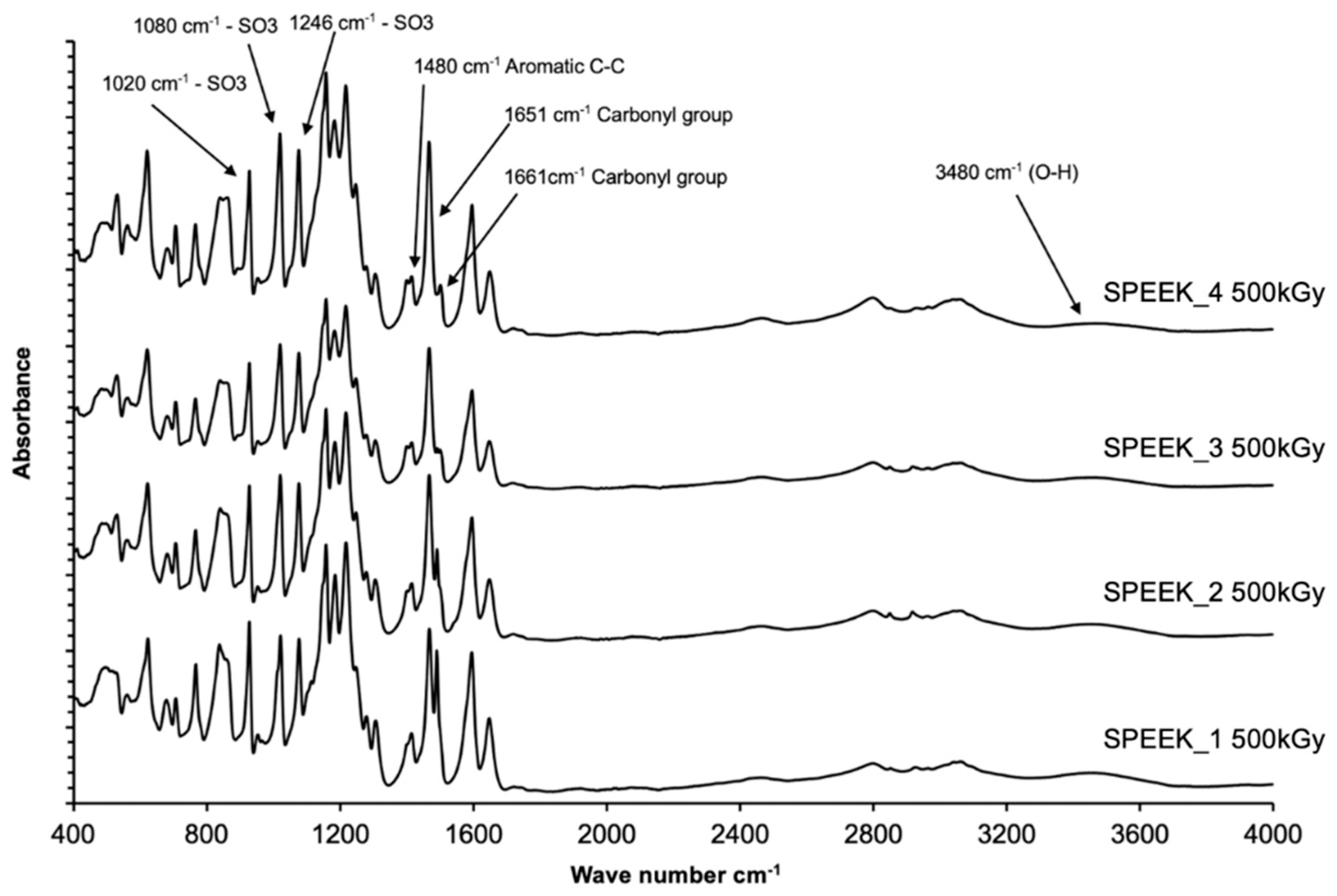
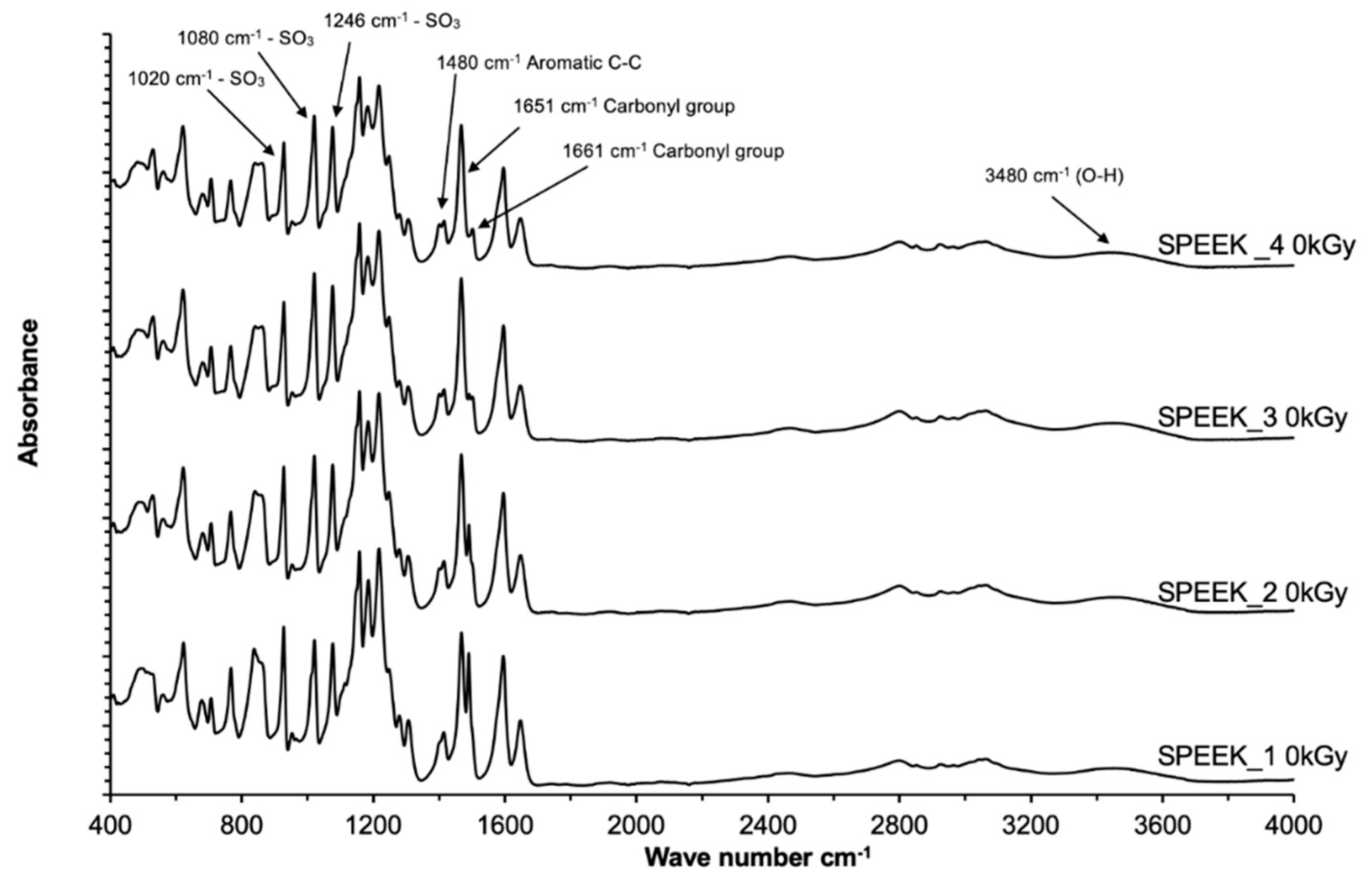
The abovementioned correlation between the sulfonation degree and FT-IR spectra peak intensities can be considered for application of the FT-IR ATR method not only for the qualitative determination of the presence of functional groups in the SPEEK membranes, but as well as for quantification. In order to correctly determine the DS with FT-IR, a calibration graph using multiple reference membranes with known DS should be obtained, and then normalized based on the most intense peak, which was determined to be at 1158 cm-1 for this experiment series. The DS can be obtained by comparing the intensities of the normalized signals in the calibration graph.
1H-NMR integration results showed a higher DS on average than TGA, and because of the manual integration aspect, determining the DS with
1H-NMR can lead to more inaccuracies.
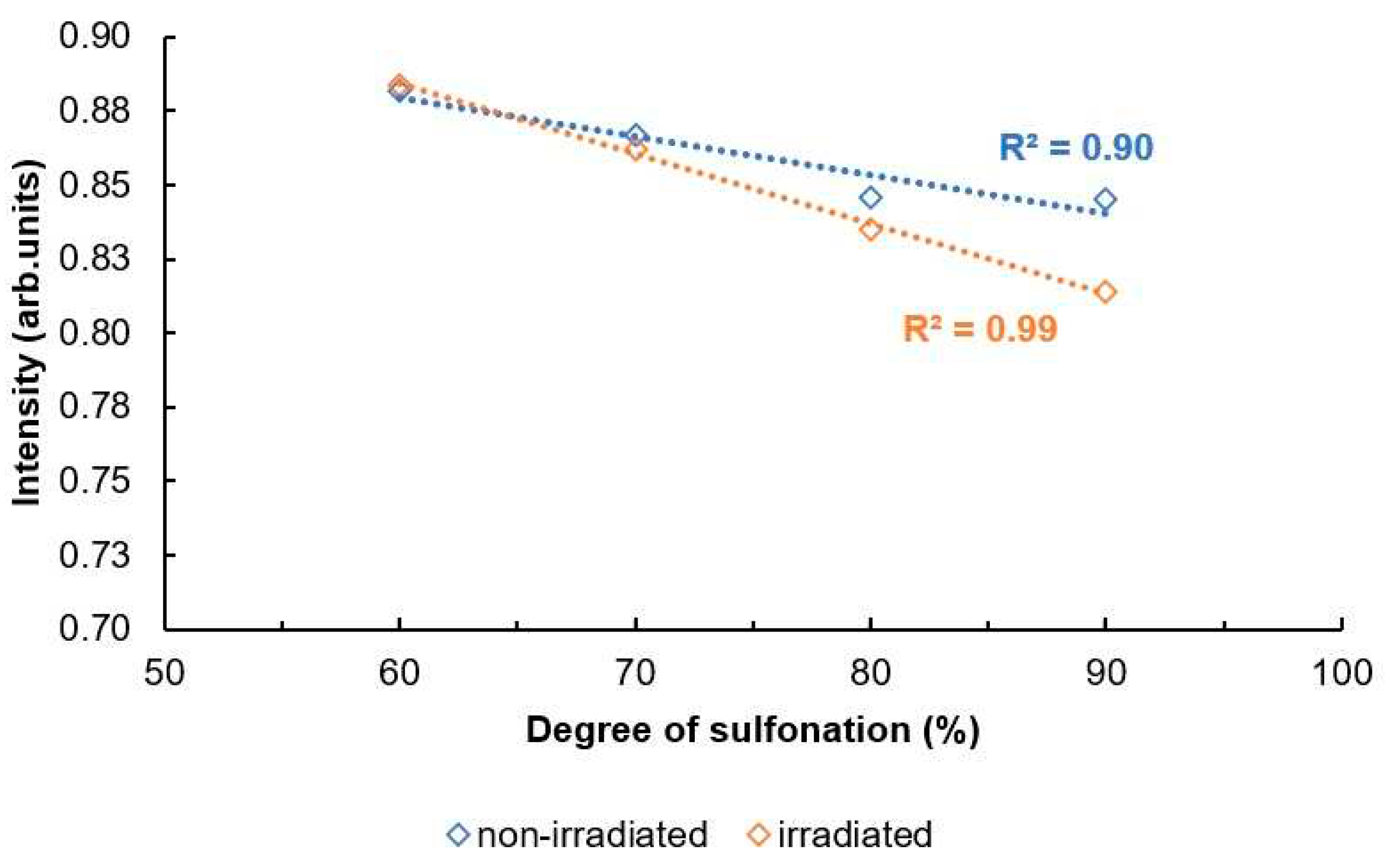
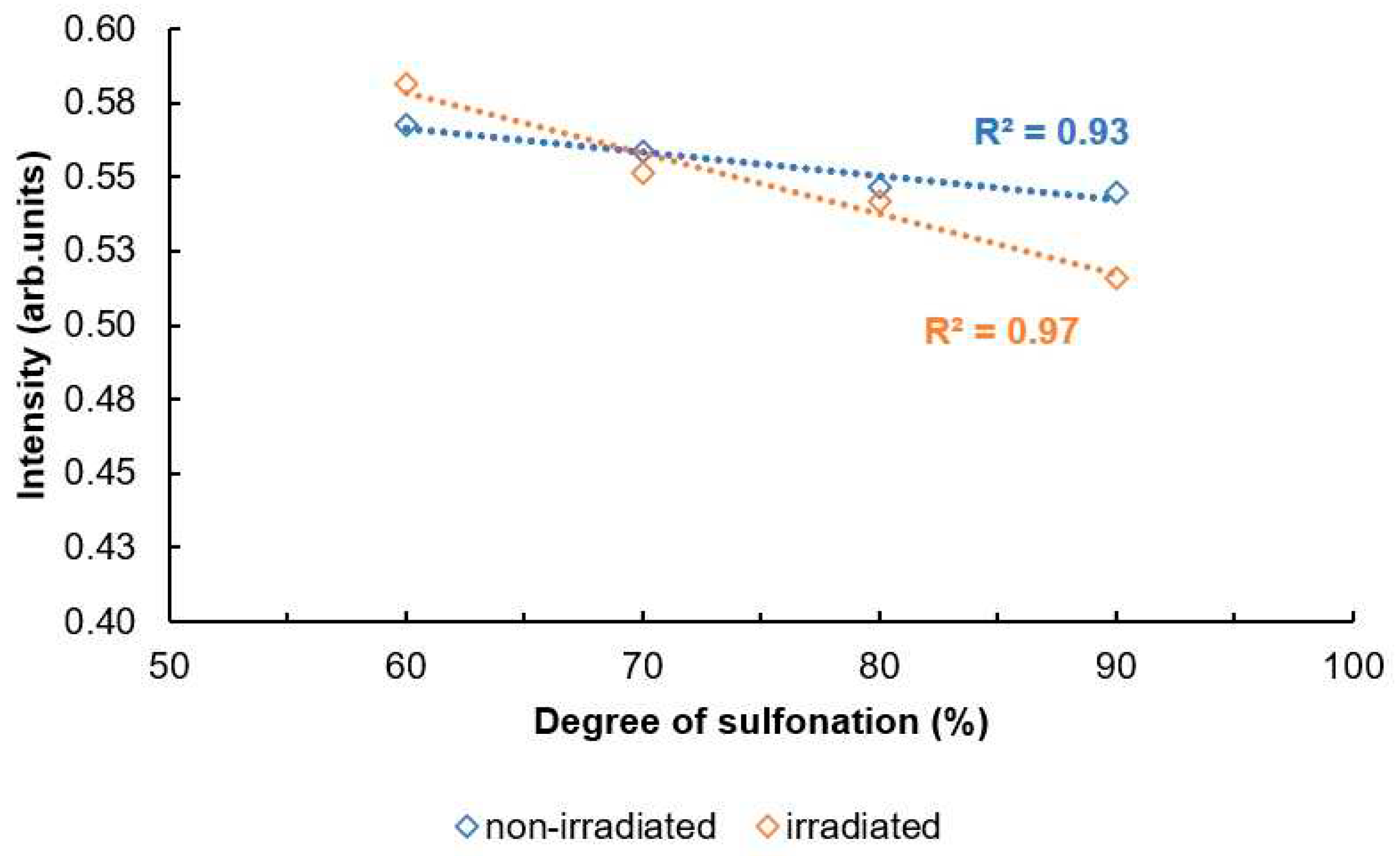
Figure 17 and
Figure 18. both show an overview for non-irradiated and irradiated SPEEK membrane DS results by method.
A calibration curve was obtained with Cr(III) standard solutions and it was determined that this method can be used to determine the DS of SPEEK with values above 60% and up to 80%, as that is where the linear range ends.
Table 4.
An overview of various DS determination methods and their advantages, disadvantages.
Table 4.
An overview of various DS determination methods and their advantages, disadvantages.
| |
TGA |
1H NMR |
FT-IR |
Spectrophotometry |
| Absolute value |
+ |
+ |
- |
- |
| Quick analysis time |
- |
+ |
+ |
+ |
| Inexpensive equipment |
- |
- |
- |
+ |
| No calibration curve required |
+ |
+ |
- |
- |
| Easily accessible |
- |
- |
- |
+ |
| Non-degenerative sample analysis |
- |
- |
+ |
- |
5. Conclusions
In this work, various methods for determining the degree of sulfonation for non-iradiated SPEEK membranes were applied and compared.
Direct measurement is possible with TGA and 1H-NMR methods, however, both require sophisticated equipment and are destructive. FT-IR method requires calibration with the known DS samples, it also requires sophisticated equipment, but it is a comparably fast method and non-destructive.
Spectrophotometric determination of the DS by using Cr(III) can be done for a DS that is lower than 90%, as the linear region was observed to end at 80% DS, but a calibration curve with known concentrations of Cr(III) in a solution should be obtained first.
It was observed that the DS values after irradiation in most cases were elevated up by 8%, showing that electron beam radiation influences the structure of the membrane. However, for absorbed doses up to 500 kGy SPEEK remains stable and is suitable for beta negative radiation environments. The biggest change was observed in SPEEK_4 with DS 90%, while the smallest in SPEEK_1 with DS 60%, indicating that after irradiation the higher the DS, the larger the change in the membrane’s structure possibly due to higher sulfonic acid group counts giving more opportunities for irradiation-induced polymer structure change.
Author Contributions
Conceptualization, E.Pajuste, E.Maskova and L.D.Pakalniete.; methodology, E.Maskova, L.D.Pakalniete, L.Avotina, R.J.Zabolockis, E.Sprugis, validation, L.Avotina, I.Reinholds and E.Pajuste.; investigation, E.Maskova, L.D.Pakalniete, L.Avotina.; resources, G.Vaivars, M.Rzepna.; data curation, R.J.Zabolockis.; writing—original draft preparation, E.Maskova, L.D.Pakalniete, L.Avotina.; writing—review and editing, E.Pajuste.; visualization, E.Maskova, L.D.Pakalniete, L.Avotina.; supervision, E.Pajuste.; project administration, E.Pajuste.; funding acquisition, E.Pajuste. All authors have read and agreed to the published version of the manuscript.”
Funding
“This research was funded by by the European Regional Development Funds (Project No.1.1.1.1/19/137 “Graphene-based electrochemical pumping system for radioactive hydrogen isotope separation”.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The support of the Faculty of Chemistry in NMR analysis is gratefully acknowledged.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Midilli, A.; Dincer, I. Hydrogen as a renewable and sustainable solution in reducing global fossil fuel consumption. Int. J. Hydrogen Energy 2008, 33, 4209–4222. [Google Scholar] [CrossRef]
- Stephens, J.C. Time to stop investing in carbon capture and storage and reduce government subsidies of fossil-fuels. WIREs Clim. Chang. 2013, 5, 169–173. [Google Scholar] [CrossRef]
- Likhanov, V.A.; Lopatin, O.P. Use of natural gas, methanol, and ethanol fuel emulsions as environmentally friendly energy carriers for mobile heat power plants. Therm. Eng. 2017, 64, 935–944. [Google Scholar] [CrossRef]
- Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Peighambardoust, S.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Şengül, E.; Erdener, H.; Akay, R.G.; Yücel, H.; Baç, N.; Eroğlu, İ. Effects of sulfonated polyether-etherketone (SPEEK) and composite membranes on the proton exchange membrane fuel cell (PEMFC) performance. Int. J. Hydrogen Energy 2009, 34, 4645–4652. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, H.; Shao, K.; Li, X.; Ni, H.; Wang, Z.; Na, H. Block sulfonated poly(ether ether ketone)s (SPEEK) ionomers with high ion-exchange capacities for proton exchange membranes. J. Power Sources 2006, 162, 1003–1009. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N.; Zaiman, N.F.H.N. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly(ether ether ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- Gil, M.; Ji, X.; Li, X.; Na, H.; Hampsey, J.E.; Lu, Y. Direct synthesis of sulfonated aromatic poly(ether ether ketone) proton exchange membranes for fuel cell applications. J. Membr. Sci. 2004, 234, 75–81. [Google Scholar] [CrossRef]
- Hamrock, S.J.; Yandrasits, M.A. Proton Exchange Membranes for Fuel Cell Applications. J. Macromol. Sci. Part C: Polym. Rev. 2006, 46, 219–244. [Google Scholar] [CrossRef]
- Cao, N.; Zhou, C.; Wang, Y.; Ju, H.; Tan, D.; Li, J. Synthesis and Characterization of Sulfonated Graphene Oxide Reinforced Sulfonated Poly (Ether Ether Ketone) (SPEEK) Composites for Proton Exchange Membrane Materials. Materials 2018, 11, 516. [Google Scholar] [CrossRef]
- Takata, H.; Nishikawa, M.; Egawa, T.; Mizuno, N. HTO electrolysis method by using proton exchange membrane fuel cell. J. Nucl. Mater. 2007, 367–370, 1102–1106. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Han, L.-F.; Schiefer, T.; Kainz, G.; Araguas-Araguas, L.; Aggarwal, P.K. A simple polymer electrolyte membrane system for enrichment of low-level tritium (3H) in environmental water samples. Isot. Environ. Heal. Stud. 2018, 54, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Pajuste, E.; Reinholds, I.; Vaivars, G.; Antuzevičs, A.; Avotiņa, L.; Sprūģis, E.; Mikko, R.; Heikki, K.; Meri, R.; Kaparkalējs, R. Evaluation of radiation stability of electron beam irradiated Nafion® and sulfonated poly(ether ether ketone) membranes. Polym. Degrad. Stab. 2022, 200. [Google Scholar] [CrossRef]
- Yagizatli, Y.; Ulas, B.; Sahin, A.; Ar, I. Investigation of sulfonation reaction kinetics and effect of sulfonation degree on membrane characteristics for PEMFC performance. Ionics 2022, 28, 2323–2336. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Gashoul, F. Comprehensive investigation of physicochemical and electrochemical properties of sulfonated poly (ether ether ketone) membranes with different degrees of sulfonation for proton exchange membrane fuel cell applications. Energy 2017, 125, 614–628. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, X.; Hu, J.; Xu, W.; Cao, J.; Zhang, H. Degradation mechanism of sulfonated poly(ether ether ketone) (SPEEK) ion exchange membranes under vanadium flow battery medium. Phys. Chem. Chem. Phys. 2014, 16, 19841–19847. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Springer, T.; Davcy, J.; Valerie, J.; Gottesfeld, S. Water transport properties of fuel cell ionomer, in The Electrochemical Society Proceedings of the Symposium on Modeling of Batteries and Fuel Cells, Phoenix, AZ, October 13–18, 1991.
- Di Vona, M.L.; Licoccia, S.; Knauth, P. Organic–inorganic hybrid membranes based on sulfonated polyaryl–ether–ketones: Correlation between water uptake and electrical conductivity. Solid State Ionics 2008, 179, 1161. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Shao, P.; Burns, C.M.; Feng, X. Sulfonation of Poly(Ether Ether Ketone)(PEEK): Kinetic Study and Characterisation. Journal of Appl Polym. Sci 2001, 82, 2651–2660. [Google Scholar] [CrossRef]
- Suzuki, K.; Owen, R.; Mok, J.; Mochihara, H.; Hosokawa, T.; Kubota, H.; Sakamoto, H.; Matsuda, A.; Tashiro, Y.; Futamata, H. Comparison of electrochemical and microbiological characterization of microbial fuel cells equipped with SPEEK and Nafion membrane electrode assemblies. J. Biosci. Bioeng. 2016, 122, 322–328. [Google Scholar] [CrossRef]
- Do, K.N.T.; Kim, D. Synthesis and characterization of homogeneously sulfonated poly(ether ether ketone) membranes: Effect of casting solvent. J. Appl. Polym. Sci. 2008, 110, 1763–1770. [Google Scholar] [CrossRef]
- Yee, R.S.L.; Zhang, K.; Ladewig, B.P. The Effects of Sulfonated Poly(ether ether ketone) Ion Exchange Preparation Conditions on Membrane Properties. Membranes 2013, 3, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Wilski, H. Radiation stability of polymers. Int. J. Radiat. Appl. Instrumentation. Part C. Radiat. Phys. Chem. 1990, 35, 186–189. [Google Scholar] [CrossRef]
- Pajuste, E.; Vaivars, G.; Reinholds, I.; Lescinskis, A.; Avotina, L.; Teimane, A.S.; Kizilovs, A.; Zabolockis, R.J.; Kalnina, P. “Extraction And Separation Of Tritium, The Nuclear Fusion Fuel And The By-product Of Fission”, oral contribution at Technical Meeting on Synergies between Nuclear Fusion Technology Developments and Advanced Nuclear Fission Technologies, IAEA, June 6 – 10, 2022, IAEA HQ, Vienna, Austria https://conferences.iaea.org/event/285/contributions/21951/attachments/11825/19640/Pajuste_synergies.pdf.
- Luo, H.; Ji, S.; Vaivars, G.; Bladergroen, B.; Linkov, V. Preparation and characterization of sulfonated poly (ether ether ketone)/phosphated zirconia nanoparticles composite proton-conducting membranes. South African Journal of Chemistry 2007, 60, 85–90. [Google Scholar]
- Yee, R.S.L.; Zhang, K.; Ladewig, B.P. The Effects of Sulfonated Poly(ether ether ketone) Ion Exchange Preparation Conditions on Membrane Properties. Membranes 2013, 3, 182–195. [Google Scholar] [CrossRef]
- Vaivars, G.; Krūkle-Bērziņa, K.; Markus, M. Modelling IR Spectra of Sulfonated Polyether Ether Ketone (SPEEK) Membranes for Fuel Cells. Key Eng. Mater. 2020, 850, 138–143. [Google Scholar] [CrossRef]
- Fedorenko, D.; Vaivars, G. Different approaches in sulfonated poly (ether ether ketone) conductivity measurements. IOP Conf. Series: Mater. Sci. Eng. 2019, 503, 012030. [Google Scholar] [CrossRef]
- Fedorenko, D.; Vaivars, G. Composite Membranes of Sulfonated Poly(ether ether ketone) with Active Carbon: Composite Preparation and Investigation of their Properties for Potential Application for CO2 Electrochemical Reduction. Mater. Sci. 2020, 26, 444–450. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Sarikhani, K.; Majedi, F.S.; Khanbabaei, G. Electrochemical investigation of sulfonated poly(ether ether ketone)/clay nanocomposite membranes for moderate temperature fuel cell applications. Journal of Power Sources 2010, 195, 2450–2456. [Google Scholar] [CrossRef]
- Schalenbach, M.; Keller, L.; Janotta, B.; Bauer, A.; Tempel, H.; Kungl, H.; Bonnet, M.; Eichel, R.-A. The Effect of Ion Exchange Poisoning on the Ion Transport and Conduction in Polymer Electrolyte Membranes (PEMs) for Water Electrolysis. J. Electrochem. Soc. 2022, 169, 094510. [Google Scholar] [CrossRef]
- Shabani, B.; Hafttananian, M.; Khamani, S.; Ramiar, A.; Ranjbar, A.A. Poisoning of proton exchange membrane fuel cells by contaminants and impurities: Review of mechanisms, effects, and mitigation strategies. J. Power Sources 2019, 427, 21–48. [Google Scholar] [CrossRef]
- Kanwal, F.; Imran, M.; Mitu, L.; Rashid, Z.; Razzaq, H.; Ain, Q.U. Removal of Chromium(III) Using Synthetic Polymers, Copolymers and their Sulfonated Derivatives as Adsorbents. E-Journal Chem. 2012, 9, 621–630. [Google Scholar] [CrossRef]
- Boef, G.D.; De Jong, W.; Krijn, G.; Poppe, H. Spectrophotometric determination of chromium(III) with EDTA. Anal. Chim. Acta 1960, 23, 557–564. [Google Scholar] [CrossRef]
- Venkatesan, P.N.; Dharmalingam, S. Characterization and performance study of phase inversed Sulfonated Poly Ether Ether Ketone – Silico tungstic composite membrane as an electrolyte for microbial fuel cell applications. Renew. Energy 2017, 102, 77–86. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Liu, X.; Chang, F.; Zhang, Y.; Xue, W.; Yang, S. Immobilizing Cr3+ with SO3H-functionalized solid polymeric ionic liquids as efficient and reusable catalysts for selective transformation of carbohydrates into 5-hydroxymethylfurfural. Bioresour. Technol. 2013, 144, 21–27. [Google Scholar] [CrossRef]
- Knauth, P.; Hou, H.; Bloch, E.; Sgreccia, E.; Di Vona, M. Thermogravimetric analysis of SPEEK membranes: Thermal stability, degree of sulfonation and cross-linking reaction. J. Anal. Appl. Pyrolysis 2011, 92, 361–365. [Google Scholar] [CrossRef]
- Yılmazoğlu, M.; Kanmaz, N.; Hızal, J. Highly efficient sulfonated poly (ether ether ketone) (sPEEK) adsorbent for removal of uranium (VI) from aqueous solution. Process. Saf. Environ. Prot. 2023, 174, 848–855. [Google Scholar] [CrossRef]
- Feng, S.; Shen, K.; Wang, Y.; Pang, J.; Jiang, Z. Concentrated sulfonated poly (ether sulfone)s as proton exchange membranes. J. Power Sources 2013, 224, 42–49. [Google Scholar] [CrossRef]
- Kanmaz, N.; Acar, M.; Yılmazoğlu, M.; Hızal, J. Rhodamine B and murexide retention onto sulfonated poly (ether ether ketone) (sPEEK). Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 605, 125341. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).