Submitted:
09 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Animals and samples
2.2. Determination of lipid oxidation
2.3. Determination of protein oxidation
2.4. Β-carotene and α-tocopherol content
2.5. Determination of fat content and fatty acid composition
2.6. Color measurement
2.7. Heme iron content
2.8. Statistical analysis
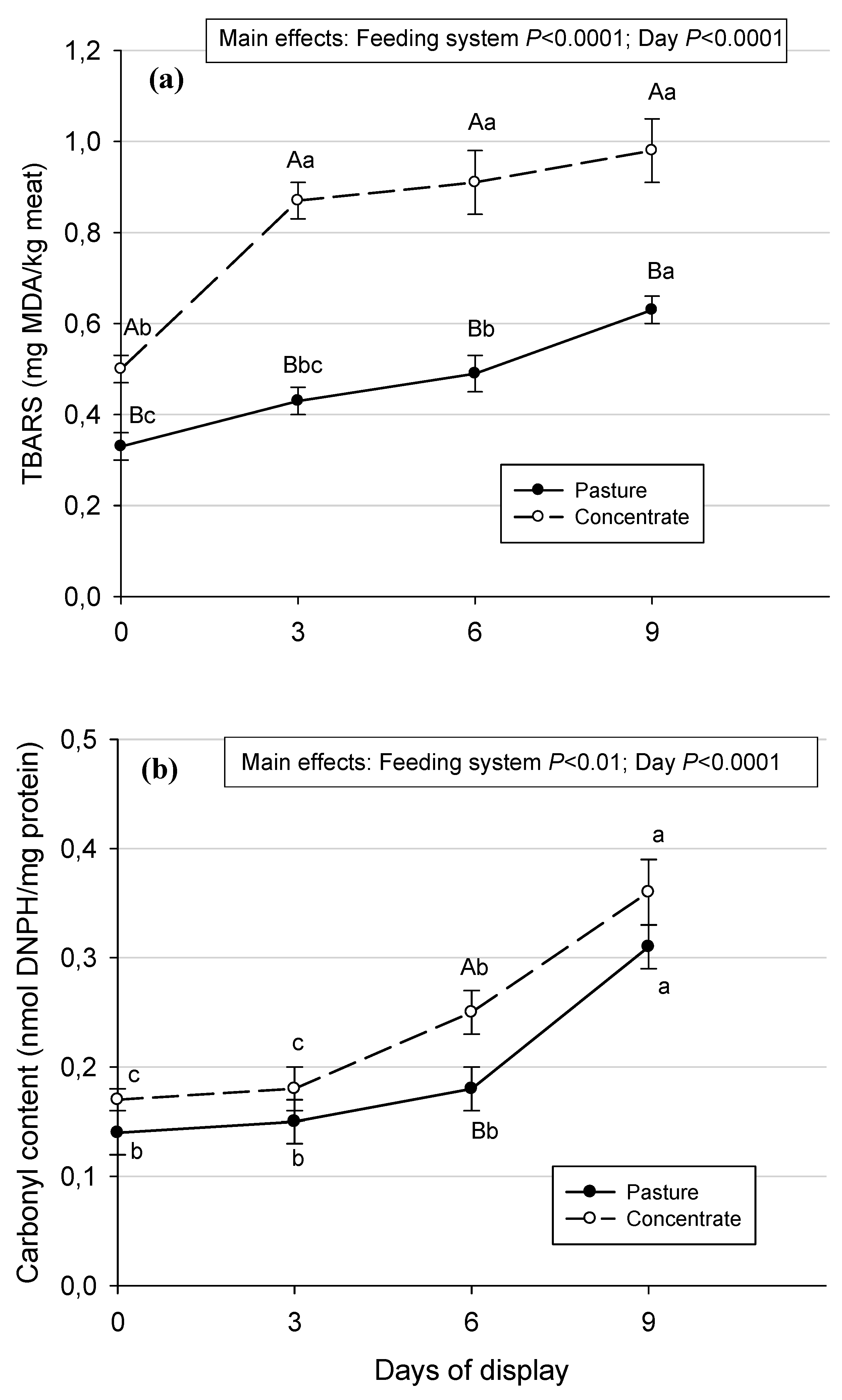
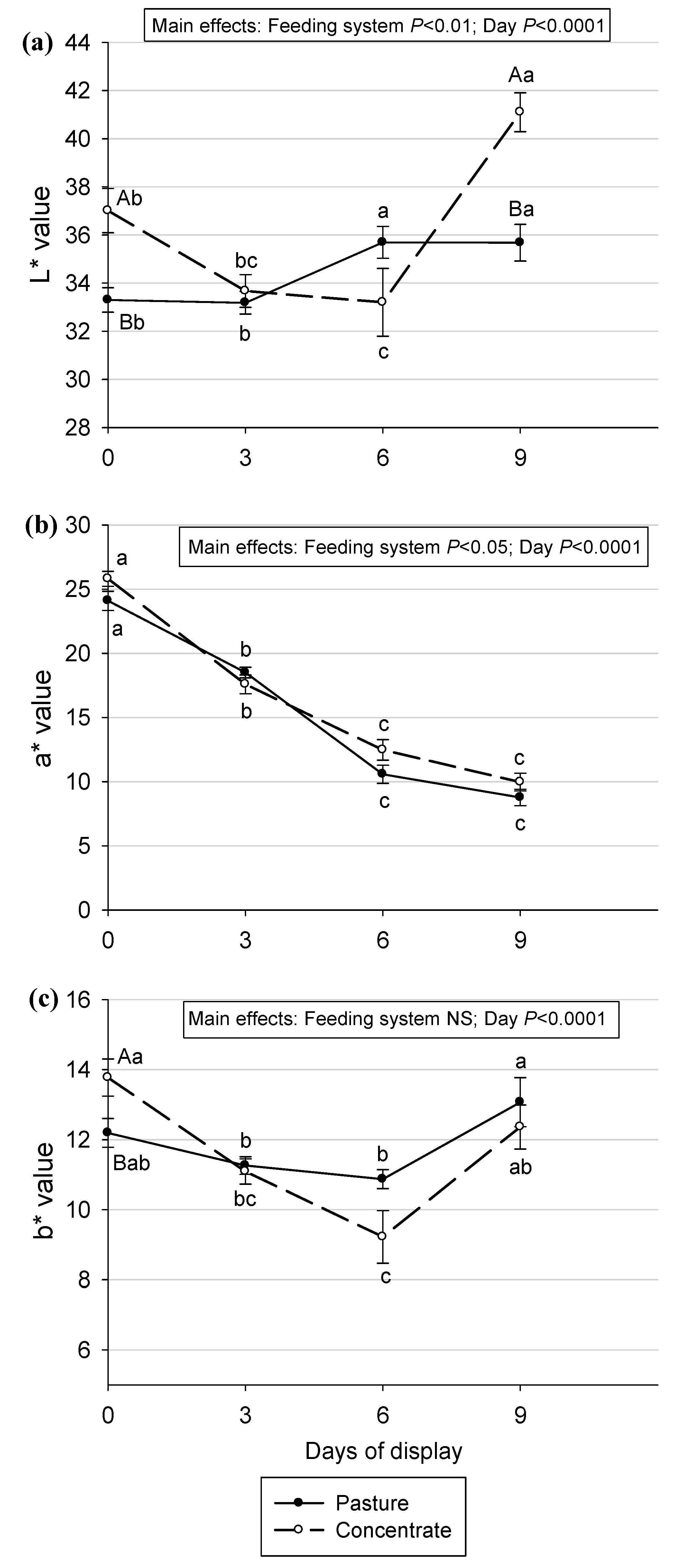
3. Results and discussion
3.1. Lipid and protein oxidation
3.2. β-carotene and α-tocopherol content
3.3. Lipid content and fatty acid composition
3.4. Color
3.5. Heme iron content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M.A. Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antiox. (Basel). 2019, 8(10), 429. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.I.; Tejeda, J.F.; Parra, V.; Andrés, A.I. Food Tech Evolution of the fatty acid composition and oxidative stability of Merino lamb meat stored under different modified atmospheres. Irish J. Agric. Food Res. 2013, 52, 81–92. [Google Scholar]
- Pereira, A.L.F.; Abreu, V.K.G. Lipid peroxidation in meat and meat products. In Lipid Peroxidation Research, IntechOpen Ed., 2018; pp. 1-15. https://www.intechopen.com/chapters/63958#:~:text=DOI%3A%2010.5772/intechopen. 8153. [Google Scholar]
- Insani, E.M.; Eyherabide, A.; Grigioni, G.; Sancho, A.M.; Pensel, N.A.; Descalzo, A.M. Oxidative stability and its relationship with natural antioxidants during refrigerated display of beef produced in Argentina. Meat Sci. 2008, 79, 444–452. [Google Scholar] [CrossRef]
- Ripoll, G.; Panea, B.; Albertí, P. Apreciación visual de la carne bovina y su relación con el espacio de color CIELab. Inf. Téc. Econ. Agr. 2012, 108, 222–232. [Google Scholar]
- Gorelik, S.; Kanner, J. Oxymyoglobin oxidation and membranal lipid peroxidation initiated by iron redox cycle. J. Agric. Food Chem. 2001, 49(12), 5939–5944. [Google Scholar] [CrossRef]
- Realini, C.E.; Duckett, S.K.; Windham, W.R. Effect of vitamin C addition to ground beef from grass-fed or grain-fed sources on color and lipid stability, and prediction of fatty acid composition by near-infrared reflectance analysis. Meat Sci. 2004, 68, 35–43. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; De Mello, A.; Trombetta, F.; Stefanello, F.S.; Speroni, C.S.; De Vargas, D.P.; De Souza, A.N.M.; Rosado Júnior, A.G.; Tonetto, C.J.; Nörnberg, J.L. Oxidative stability of beef from steers finished exclusively with concentrate, supplemented, or on legume-grass pasture. Meat Sci. 2018, 145, 121–126. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Sancho, A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Livisay, S.A.; Zhou, S. Mechanisms of endogenous skeletal muscle antioxidants: chemical and physical aspects. In Antioxidants in Muscle Foods: Nutritional Strategies to Improve Quality; Decker, E.A., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley & Sons: New York, USA, 2000; pp. 25–60. [Google Scholar]
- Gregory, N.G. Animal Welfare and Meat Science; CABI Publishing: Oxon, UK, 1998. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br. J. Nutr. 1997, 78, S49–S60. [Google Scholar] [CrossRef] [PubMed]
- Luciano, G.; Moloney, A.P.; Priolo, A.; Rohrle, F.T.; Vasta, V.; Biondi, L.; López-Andrés, P.; Grasso, S.; Monahan, F. J. Vitamin E and polyunsaturated fatty acids in bovine muscle and the oxidative stability of beef from cattle receiving grass or concentrate-based rations. J. Anim Sci. 2011, 89(11), 3759–3768. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Butler, K.L.; McDonagh, M.B.; Jacobs, J.L.; Hopkins, D.L. Relationship between muscle antioxidant status, forms of iron, polyunsaturated fatty acids and functionality (retail colour) of meat in lambs. Meat Sci. 2012, 90, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Terevinto, A.; Cabrera, M.C.; Saadoun, A. Oxidative stability, fatty acid composition and health lipid indices of Longissimus dorsi muscle from Aberdeen Angus steers produced in different feeding systems. Ciênc. Rural. [CrossRef]
- Terevinto, A.; Saadoun, A.; Cabrera, M.C. From the fatty acid content perspective, is it healthier to eat a hindquarter or a forequarter cut? Angus steers in pasture or concentrate systems. CyTA – J. Food. [CrossRef]
- Jood, S.; Kapoor, A.C.; Singh, R. Polyphenol and phytic acid contents of cereal grains as affected by insect infestation. J. Agric. Food Chem. 1995, 43, 435–438. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Rossetti, L.; Sancho, A.M.; García, P.T.; Biolatto, A.; Carduza, F.; Grigioni, G.M. Antioxidant consumption and development of oxidation during ageing of buffalo meat produced in Argentina. Meat Sci. 2008, 79, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- Faustman, C.; Chan, W.K.M.; Lynch, M.P.; Joo, S.T. Strategies for increasing oxidative stability of (fresh) meat color. In Proceedings of the 49th Annual Reciprocal Meat Conference, Kansas City, USA, 9-11 June 1996; pp. 73–78. [Google Scholar]
- Nassu, R.T.; Dugan, M.E.R.; Juárez, M.; Basarab, J.A.; Baron, V.S.; Aalhus, J.L. Effect of α-tocopherol tissue levels on beef quality. Animal. 2011, 5(12), 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, K. Dynamics of lipid peroxidation and antioxidion of α-tocopherol in membranes. J. Nutr. Sci. Vitaminol. 2008, 54(4), 273–285. [Google Scholar] [CrossRef]
- 23. INAC; Instituto Nacional de Carnes. Manual de cortes bovinos para abasto, /: 2008. pp. 1-107. Available at: https, 2008.
- Lynch, S.M.; Frei, B. Mechanisms of copper- and iron-dependent oxidative modification of human low-density lipoprotein. J. Lipid Res. 1993, 34, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Gatellier, P.; Mercier, Y.; Renerre, M. Effect of diet finishing mode (pasture or mixed diet) on antioxidant status of Charolais bovine meat. Meat Sci. 2004, 67, 385–394. [Google Scholar] [CrossRef]
- Mercier, Y.; Gatellier, P.; Renerre, M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004, 66, 467–473. [Google Scholar] [CrossRef]
- Stoscheck, C.M. Quantitation of Protein. Meth. Enzymol. 1990, 182, 50–68. [Google Scholar] [CrossRef]
- Zaccari, F.; Cabrera, M.C.; Ramos, A.; Saadoun, A. In vitro bioaccessibility of β-carotene, Ca, Mg and Zn in landrace carrots (Daucus carota, L.). Food Chem. 2015, 166, 365–371. [Google Scholar] [CrossRef]
- Koprivnjak, J.F.; Lum, K.R.; Sisak, M.M.; Saborowski, R. Determination of α-, γ (+β)-, and δ-tocopherols in a variety of liver tissues by reverse-phase high pressure liquid chromatography. Comp. Biochem. Physiol. 1996, 113, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipid from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ichihara, K.; Shibahara, A.; Yamamoto, K.; Nakayama, T. An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids. 1996, 31(5), 535–539. [Google Scholar] [CrossRef] [PubMed]
- 32. CIE. Commisssion Internationale de L`Eleclerige, 2: Colorimetry, 15, 2004.
- Hornsey, H.C. The colour of cooked cured pork. I.-Estimation of the nitric oxide-haem pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- Ramos, A.; Cabrera, M.C.; del Puerto, M.; Saadoun, A. Minerals, haem iron and non-haem iron contents of rhea meat. Meat Sci. 2009, 81, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Cabrera, M.C.; Saadoun, A. Bioaccessibility of Se, Cu, Zn, Mn and Fe, and haem iron content in unaged and aged meat of Hereford and Braford steers fed pasture. Meat Sci. 2012, 91, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Duarte, T.L.; Bolkenov, B.; Klopatek, S.C.; Oltjen, J.W.; King, D.A.; Shackelford, S.D.; Wheeler, T.L.; Yang, X. Evaluating the shelf life and sensory properties of beef steaks from cattle raised on different grass feeding systems in the Western United States. Foods. 2022, 11(14), 2141. [Google Scholar] [CrossRef]
- Humada, M.J.; Sañudo, C.; Serrano, E. Chemical composition, vitamin E content, lipid oxidation, color and cooking losses in meat from Tudanca bulls finished on semi-extensive or intensive systems and slaughtered at 12 or 14 months. Meat Sci. 2014, 96, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.N.; Arp, S.C.; Scheller, K.K.; Williams, S.N.; Schaefer, D.M. Tissue equilibration and subcellular distribution of vitamin E relative to myoglobin and lipid oxidation in displayed beef. J. Anim. Sci. 1993, 71, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Renerre, M. Oxidative processes and myoglobin. In Antioxidants in muscle foods; Decker, E., Faustman, C., Lopez-Bote, C., Eds.; Wiley & Sons: New York, USA, 2000; pp. 113–135. [Google Scholar]
- Srivastava, R. Physicochemical, antioxidant properties of carotenoids and its optoelectronic and interaction studies with chlorophyll pigments. Sci. Rep. 2021, 11(1), 1–14. [Google Scholar] [CrossRef]
- Arend, F.A.; Murdoch, G.K.; Doumit, M.E.; Chibisa, G.E. Inclusion of grape pomace in finishing cattle diets: carcass traits, meat quality and fatty acid composition. Animals. 2022, 12(19), 2597. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kang, G.; Seong, P.; Park, B.; Kang, S.M. Effect of slaughter age on the antioxidant enzyme activity, color, and oxidative stability of Korean Hanwoo (Bos Taurus coreanae) cow beef. Meat Sci. 2015, 108, 44–49. [Google Scholar] [CrossRef]
- Mitsumoto, M.; Ozawa, S.; Mitsuhashi, T.; Koide, K. Effect of dietary vitamin E supplementation for one week before slaughter on drip, colour and lipid stability during display in Japanese black steer beef. Meat Sci. 1998, 49, 165–174. [Google Scholar] [CrossRef]
- Zainudin, M.A.M.; Poojary, M.M.; Jongberg, S.; Lund, M.N. Light exposure accelerates oxidative protein polymerization in beef stored in high oxygen atmosphere. Food Chem. 2019, 299, 125132. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.; Nute, G.; Hughes, S.; Enser, M.; Wood, J.; Richardson, R. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.M.; Insani, E.M.; Biolatto, A.; Sancho, A.M.; García, P.T.; Pensel, N.A.; Josifovich, J.A. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 2005, 70, 35–44. [Google Scholar] [CrossRef]
- Simmone, A.H.; Green, N.R.; Bransby, D.I. Consumer acceptability and β-carotene content of beef as related to cattle finishing diets. J. Food Sci. 1996, 61(6), 1254–1257. [Google Scholar] [CrossRef]
- Yang, A.; Brewster, M.J.; Lanari, M.C.; Tunne, R.K. Effect of vitamin E supplementation on α-tocopherol and β-carotene concentrations in tissues from pasture- and grain-fed cattle. Meat Sci. 2002, 60, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Putnam, M.E.; Comben, N. Vitamin E—review article. Vet. Rec. 1987, 121(23), 541–545. [Google Scholar] [PubMed]
- De la Fuente, J.; Díaz, M.T.; Álvarez, I.; Oliver, M.A.; Font i Furnols, M.; Sañudo, C.; Campo, M.M.; Montossi, F.; Nute, G.R.; Cañeque, V. Fatty acid and vitamin E composition of intramuscular fat in cattle reared in different production systems. Meat Sci. 2009, 82(3), 331–337. [Google Scholar] [CrossRef] [PubMed]
- Realini, C.E.; Duckett, S.K.; Brito, G.W.; Dalla Rizza, M.; De Mattos, D. Effect of pasture vs concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci. 2004, 66, 567–577. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Norng, S.; Burnett, V.F.; Dunshea, F.R.; Jacobs, J.L.; Hopkins, D.L. The synergism of biochemical components controlling lipid oxidation in lamb muscle. Lipids. 2014, 49, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Manner, W.N.; Maxwell, R.J.; Williams, J.E. Effects of dietary regimen and tissue site on bovine fatty acid profiles. J. Anim. Sci. 1984, 59(1), 109–121. [Google Scholar] [CrossRef]
- Terevinto, A.; Cabrera, M.C.; Saadoun, A. Catalase, SOD and GPx activities in Triceps brachii muscle from Aberdeen Angus steers finished on pasture, pasture and concentrate, or concentrate. Am. J. Food Nutr. [CrossRef]
- Terevinto, A.; Cabrera, M.C.; Saadoun, A. Influence of feeding system on lipids and proteins oxidation, and antioxidant enzymes activities of meat from Aberdeen Angus steers. J. Food Nutr. Res. /: 581 – 586. http.
- Deckelbaum, R.J. n-6 and n-3 fatty acids and atherosclerosis. Ratios or amounts? Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2325–2326. [Google Scholar] [CrossRef] [PubMed]
- Pordomingo, A.J.; García, T.P.; Volpi Lagreca, G. Effect of feeding treatment during the backgrounding phase of beef production from pasture on: II. Longissimus muscle proximate composition, cholesterol and fatty acids. Meat Sci. [CrossRef]
- García, P.T.; Pensel, N.A.; Sancho, A.M.; Latimori, N.J.; Kloster, A.M.; Amigone, M.A.; Casal, J.J. Beef lipids in relation to animal breed and nutrition in Argentina. Meat Sci. 2008, 79, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Venkata Reddy, B.; Sivakumar, A.D.; Jeong, D.W.; Woo, Y-B. ; Park, S-J.; Lee, S-Y.; Byun, J-Y.; Kim, C-H.; Cho, S-H.; Hwang, I. Beef quality traits of heifer in comparison with steer, bull and cow at various feeding environments. Anim. Sci. J. 2015, 86, 1–16. [Google Scholar] [CrossRef]
- FAO-WHO. Fats and fatty acids in human nutrition. Rome: FAO Food and nutrition paper # 91. Report of an expert consultation, Geneva, 2010, 10-. https://www.who. 14 November.
- Stajic, S.; Zivkovic, D.; Perunovic, M.; Sobajic, S.; Vranic, D. Cholesterol content and atherogenicity of fermented sausages made of pork meat from various breeds. In 11th International Congress on Engineering and Food. Procedia Food Sci. 2011, 1, 568–575. [Google Scholar] [CrossRef]
- Alfaia, C.P.M.; Alves, S.P.; Martins, S.I.V.; Costa, A.S.H.; Fontes, C.M.G.A.; Lemos, J.P.C.; Bessa, R.J.B.; Prates, J.A.M. Effect of feeding system on intramuscular fatty acids and conjugated linoleic acid isomers of beef cattle, with emphasis on their nutritional value and discriminatory ability. Food Chem. 2009, 114, 939–946. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Mann, N.J.; Sinclair, A.J. Effect of feeding systems on omega-3 fatty acids, conjugated linoleic acid and trans fatty acids in Australian beef cuts, potential impact on human health. Asia Pac. J. Clin. Nutr. 2006, 15(1), 21–29. [Google Scholar] [PubMed]
- Song, Z.; Xia, H.; Yang, L.; Wang, S.; Sun, G. Lowering the n-6/n-3 PUFAs ratio inhibits the formation of THP-1 macrophage-derived foam cell. Lipids Health Dis. 2018, 17(125), 1–8. [Google Scholar] [CrossRef] [PubMed]
- García, P.T.; Pensel, N.A.; Margaria, C.A.; Olga Rossco, C.M. Intramuscular fat, cholesterol and 18:2 n-6/18:3 n-3 ratio in total lipids in two frame steers under different dietary regimen. In Proceedings of the 45th International Congress of Meat Science and Technology, Yokohama, Japan, 1-7 August 1999; pp. 76–77. [Google Scholar]
- Yang, A.; Lanari, M.C.; Brewster, M.; Tume, R.K. Lipid stability and meat colour of beef from pasture- and grain-fed cattle with or without vitamin E supplement. Meat Sci. 2002, 60, 41–50. [Google Scholar] [CrossRef]
- Bennet, L.L.; Hammon, A.C.; Williams, M.J.; Kunkle, W.E.; Johnson, D.D.; Preston, R.L. Performance, carcass yield, and carcass quality characteristics of steers finished on rhizoma peanut-tropical grass pasture or concentrate. J. Anim. Sci. 1995, 73, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Bidner, T.D.; Schupp, A.R.; Mohamad, A.B.; Rumore, N.C.; Montgomery, R.E.; Bagley, C.P. Acceptability of beef from Angus-Hereford or Angus-Hereford-Brahman steers finished on all-forage or a high energy diet. J. Anim. Sci. 1986, 63, 381–387. [Google Scholar] [CrossRef]
- Schroeder, J.W.; Cramer, D.A.; Bowling, R.A.; Cook, C.W. Palatability, shelf-life and chemical differences between forage- and grain-finished beef. J. Anim. Sci. 1980, 50, 852–859. [Google Scholar] [CrossRef]
- Vestergaard, M.; Oksbjerg, N.; Henckel, P. Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of Semitendinosus, Longissimus dorsi and Supraspinatus muscles of young Bulls. Meat Sci. 2000, 54(2), 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.G.; Monahan, F.J.; Moloney, A.P. Current perspectives on the darker beef often reported from extensively-managed cattle: Does physical activity play a significant role? Livest. Sci. 2011, 142, 1–22. [Google Scholar] [CrossRef]
- Dunne, P.G.; Monahan, F.J.; O`Mara, F.P.; Moloney, A.P. Color stability, under simulated retail display conditions, of M. longissimus dorsi and M. semimembranosus from steers given long-term daily exercise and supplemented with vitamin E. Meat Sci. [CrossRef]
- Sapp, P.H.; Williams, S.E.; McCann, M.A. Sensory attributes and retail display characteristics of pasture- and/or grain-fed beef aged 7, 14 or 21 days. J. Food Qual. 1998, 22, 257–274. [Google Scholar] [CrossRef]
- Gatellier, P.; Mercier, Y.; Juin, H.; Renerre, M. Effect of finishing mode (pasture- or mixed diet) on lipid composition, color stability and lipid oxidation in meat from Charolais cattle. Meat Sci. 2005, 69, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Razminowicz, R.H.; Kreuzer, M.; Scheeder, M.R.L. Quality of retail beef from two grass-based production systems in comparison with conventional beef. Meat Sci. 2006, 73, 351–361. [Google Scholar] [CrossRef]
- Purohit, A.; Singh, R.; Kerr, W.; Mohan, A. Effects of heme and nonheme iron on meat quality characteristics during retail display and storage. J. Food Meas. Charact. 2015, 9, 175–185. [Google Scholar] [CrossRef]
| Pasture | Concentrate CConcentratccConcentrat | p-value | |
|---|---|---|---|
| β-carotene | 1.73 ± 0.18 a | 0.27 ± 0.04 b | <0.0001 |
| α-tocopherol | 3.7 ± 0.5 a | 1.9 ± 0.3 b | <0.05 |
| Pasture | Concentrate | p-value | |
|---|---|---|---|
| % Fat | 1.78 ± 0.15b | 4.52 ± 0.46a | <0.0001 |
| C12:0 | 0.13 ± 0.04 | 0.08 ± 0.03 | NS |
| C14:0 | 3.46 ± 0.71 | 3.10 ± 0.85 | NS |
| C15:0i | 0.25 ± 0.04a | 0.12 ± 0.03b | 0.01 |
| C15:0ai | 0.26 ± 0.03 | 0.17 ± 0.04 | NS |
| C14:1 | 0.60 ± 0.12 | 0.59 ± 0.17 | NS |
| C15:0 | 0.72 ± 0.10 | 0.51 ± 0.11 | NS |
| C16:0i | 0.20 ± 0.03a | 0.10 ± 0.01b | <0.05 |
| C16:0 | 31.85 ± 2.71 | 28.75 ± 2.45 | NS |
| C16:1 | 4.16 ± 0.38 | 4.22 ± 0.35 | NS |
| C17:0 | 1.10 ± 0.04b | 1.27 ± 0.05a | 0.01 |
| C17:1 | 0.74 ± 0.24 | 0.94 ± 0.07 | NS |
| C18:0 | 12.12 ± 1.25 | 12.53 ± 1.08 | NS |
| C18:1 | 34.46 ± 2.91 | 41.53 ± 2.57 | NS |
| C18:2 n-6 | 2.84 ± 0.36 | 3.07 ± 0.22 | NS |
| C20:1 | 0.13 ± 0.04 | 0.21 ± 0.08 | NS |
| C18:3 n-3 | 0.61 ± 0.04a | 0.18 ± 0.03b | 0.001 |
| CLA | 0.31 ± 0.03 | 0.31 ± 0.09 | NS |
| C20:3 n-3 | 0.14 ± 0.04a | 0.03 ± 0.01b | 0.01 |
| C20:3 n-6 | 0.34 ± 0.10a | 0.11 ± 0.02b | 0.05 |
| C20:4 n-6 | 0.30 ± 0.12 | 0.21 ± 0.06 | NS |
| EPA n-3 | 0.04 ± 0.05 | 0.01 ± 0.01 | NS |
| DPA n-3 | 0.13 ± 0.08 | 0.04 ± 0.02 | NS |
| DHA n-3 | 0.41 ± 0.06a | 0.08 ± 0.07b | <0.01 |
| Others | 4.73 ± 1.37 | 1.92 ± 0.41 | …… |
| SFA | 50.09 ± 2.54 | 46.64 ± 2.39 | NS |
| MUFA | 40.08 ± 2.68b | 47.49 ± 2.19a | <0.05 |
| PUFA | 5.13 ± 0.62 | 4.02 ± 0.49 | NS |
| Σn-6 | 3.79 ± 0.41 | 3.69 ± 0.37 | NS |
| Σn-3 | 1.33 ± 0.24a | 0.33 ± 0.13b | <0.001 |
| n-6/n-3 | 2.85 ± 0.35b | 11.18 ± 3.96a | <0.05 |
| PUFA/SFA | 0.10 ± 0.02 | 0.09 ± 0.02 | NS |
| Atherogenic index | 1.02 ± 0.19 | 0.81 ± 0.15 | NS |
| Thrombogenic index | 1.82 ± 0.24 | 1.67 ± 0.19 | NS |
| Day 0 | Day 3 | Day 6 | Day 9 | p-value | |
|---|---|---|---|---|---|
| Pasture | 19.3 ± 1.0 | 20.5 ± 0.9 | 19.5 ± 0.7 | 19.2 ± 1.5 | NS |
| Concentrate | 20.2 ± 1.4 | 20.5 ± 2.5 | 21.0 ± 2.8 | 19.3 ± 2.8 | NS |
| p-value | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





