2. Review of HHS and DKA
Various terminologies have been employed historically in the medical literature to describe hyperglycemic hyperosmolar syndrome (HHS), ranging from the broadly implicative "hyperosmolar hyperglycemic crisis" to more limiting and partially inclusive terms such as "hyperglycemic nonketotic syndrome or state," "hyperosmolar nonacidotic uncontrolled diabetes," and "hyperglycemic hyperosmolar coma" [
6]. These appellations represent aspects of the intricate hyperglycemic state, which may manifest in either new-onset or known diabetes mellitus and typically evolve acutely or indolently, potentially culminating in life-threatening emergencies [
4]. In comparison to diabetic ketoacidosis (DKA), HHS and hyperosmolar diabetic ketoacidosis are less prevalent, though they carry a higher mortality rate and exemplify the most severe hyperglycemic emergencies [
5]. While substantial differences and predominantly unique characteristics exist between DKA and HHS, some cases may exhibit overlapping pathogenesis, manifestations, and complications [
7]. Despite the increasing incidence of HHS, it is often misdiagnosed initially as DKA due to inadequate awareness about HHS and its exclusion from the differential diagnosis of complicated diabetes [
2]. This oversight precludes early recognition, timely monitoring, risk anticipation, and preventive therapy.
Classical HHS typically evolves gradually and follows a more protracted course than DKA. HHS induces profound dehydration and electrolyte losses, resulting in extreme hyperglycemia, hyperosmolality, and altered mental status in the absence of significant ketosis and corresponding acidosis [
2].
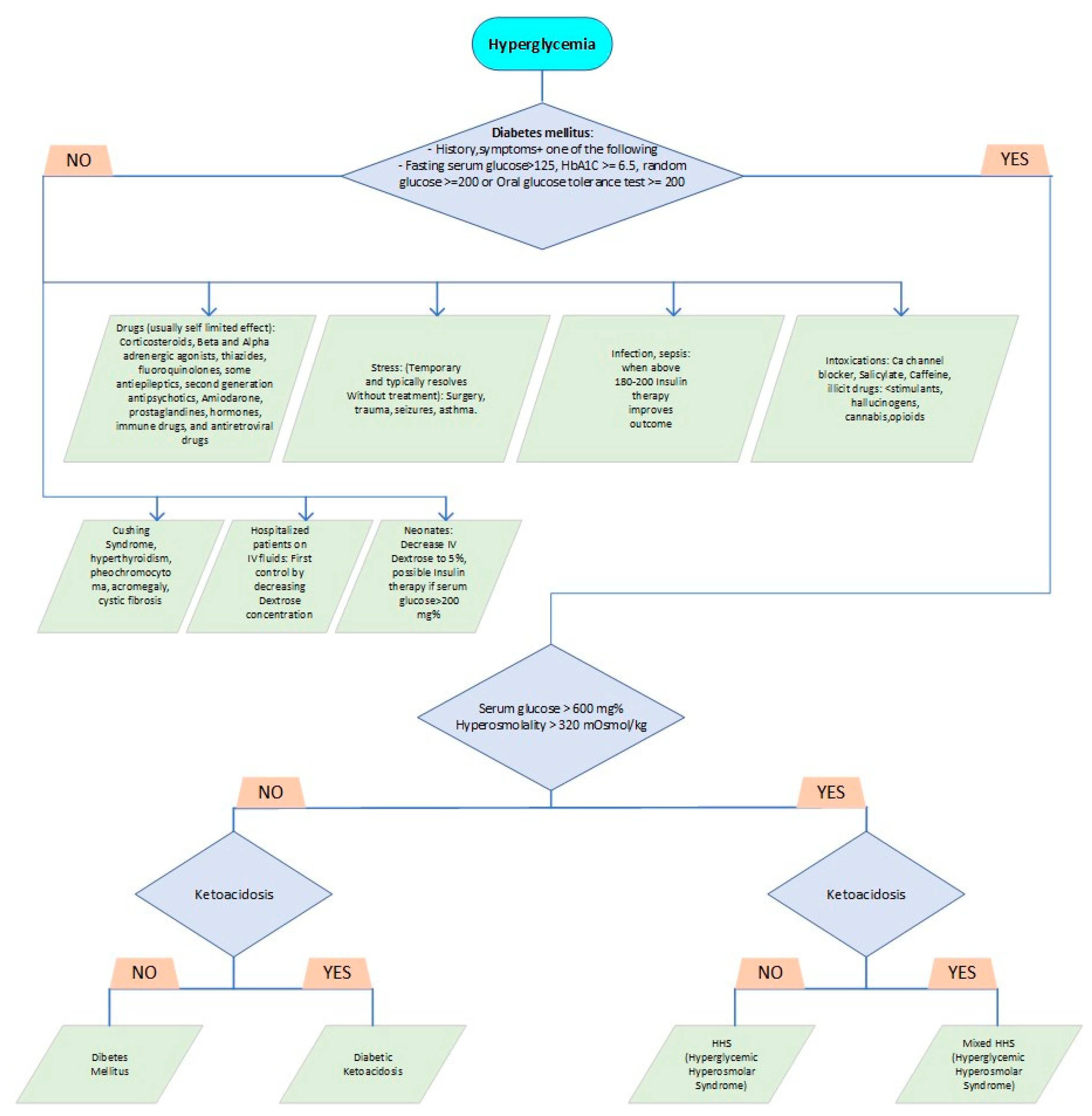
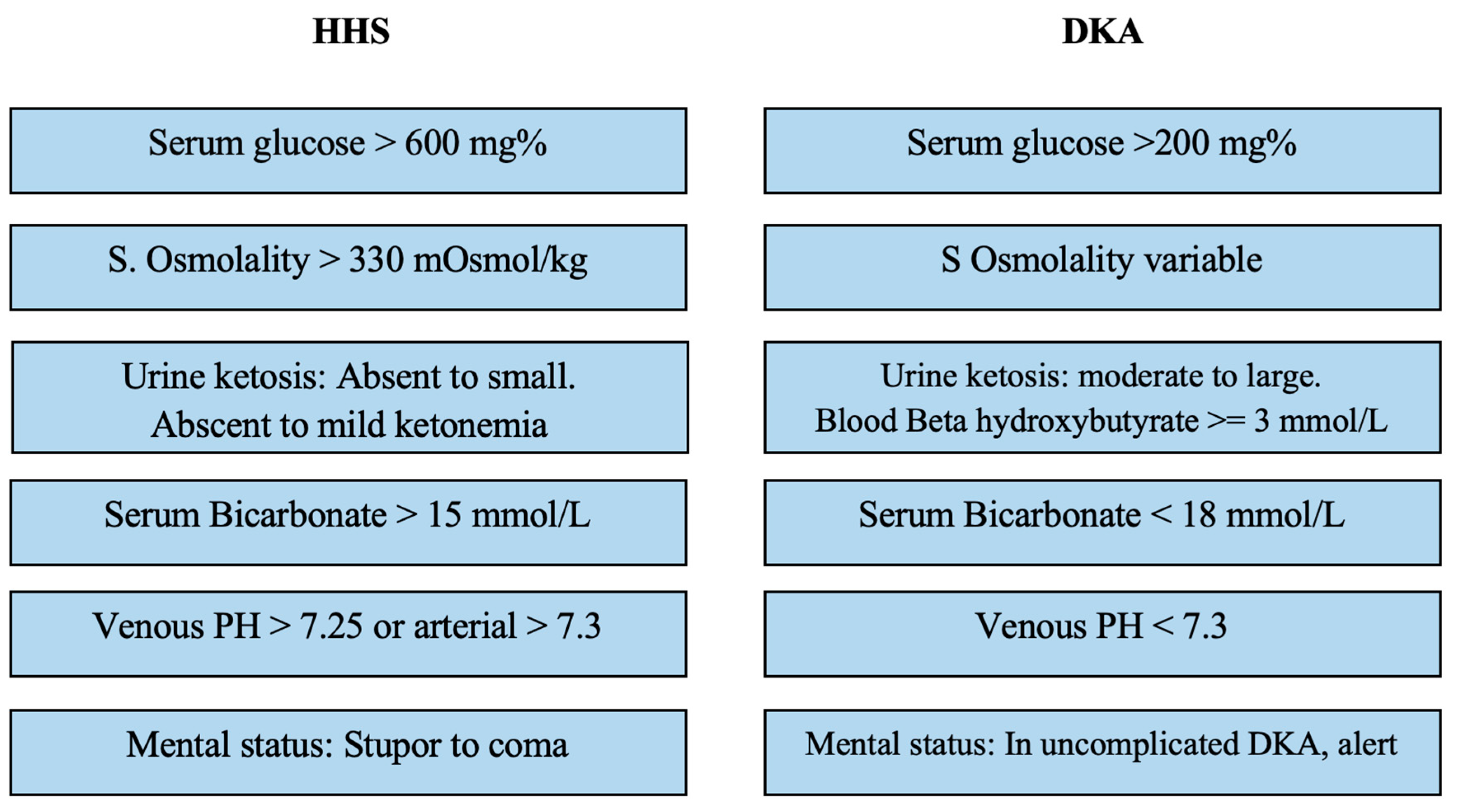
Table 1 delineates the Pediatric Endocrine Society's diagnostic criteria for distinguishing HHS from other hyperglycemia forms. While DKA is commonly associated with serum glucose levels exceeding 200 mg/dL, bicarbonate levels are usually below 15 mmol/L, and venous pH falls below 7.3. Additionally, DKA is characterized by the presence of ketonemia, ketonuria, and glucosuria [
8].
Historically, hyperglycemic hyperosmolar syndrome (HHS) has been more commonly documented in elderly, obese, African American, and type 2 diabetic patients [
9]. However, recent literature suggests an increasing incidence of HHS in younger, non-obese, and insulin-dependent diabetic patients. Male predominance, a poor social health network, and a positive family history of type 2 diabetes have been reported as risk factors [
10]. Ingestion of large volumes of sugar-rich fluids, patients on diabetogenic medications including long-term systemic steroids, L-asparaginase, tacrolimus, growth hormone, and atypical antipsychotics, in addition to cystic fibrosis, have been identified as tangible triggers of HHS in some patients [
11,
12]. An increased risk with rare diseases, such as Kearns-Sayre syndrome, has also been published [
13]. In many cases, concurrent infection and lack of compliance are the commonly known precipitating triggers [
4].
Any physician managing pediatric patients can encounter HHS; though it is uncommon, it is not rare. In one report, 2-4% of pediatric patients with type 2 diabetes had HHS at diagnosis [
14]. Despite the sparse statistical data, substantially rising numbers of HHS in patients with type 1 diabetes continue to be published [
15]. HHS admissions in some reports are increasing around 4% annually [
16].
Both HHS and diabetic ketoacidosis (DKA) are due to insulin deficiency. The insulin level in HHS is low enough to induce extracellular hyperglycemia by decreasing tissue utilization and stimulating the release of glucagon and other counter-regulatory stress hormones to initiate glycogenolysis and gluconeogenesis (like DKA). However, in contrast to DKA, the insulin drop in HHS remains above the critical threshold of ketone formation through catabolic lipolysis and fatty acid oxidation. Hypothetically, the indolent HHS state is characterized by a prolonged, intermediately low insulin level that fails to meet glucose utilization demands, though the insulin nadir is not critically low and the subsequent cellular starvation from glucose deprivation is not sufficient to trigger compensatory adipose tissue oxidation and liver ketone production as an alternative energy source [
17].
In DKA, the catabolic mechanism leads to elevation in ketone bodies composed of acetone, acetoacetic acid, and beta-3-hydroxybutyrate, whereas HHS lacks significant ketoacidosis. The relatively longer period of symptomatic hyperglycemia in HHS produces an extracellular osmotic gradient, shifting fluids out of the cells, and precipitating osmotic diuresis; coupled with poor fluid intake, there is an exacerbation of the negative fluids balance, resulting in extreme dehydration and severe hyperosmolar hyperglycemia without significant ketoacidosis [
10]. This represents the main distinguishing difference between HHS and DKA. Longstanding and profound osmotic diuresis in HHS ultimately diminishes renal function, accelerating electrolyte waste to a greater extent than seen in DKA. This leads to significant sodium, chloride, potassium, phosphate, and magnesium tissue depletion that is not necessarily reflected in laboratory intravascular level measurements at the time of severe volume contraction [
17]. Some of these derangements, particularly hypophosphatemia, have been implicated in Na-K-ATPase imbalance, which contributes to rhabdomyolysis in some HHS patients. Hyperglycemia itself activates a systemic stress response characterized by rising levels of inflammatory markers, including interleukins and tumor necrosis factor, among others [
4,
18]. These inflammatory markers further exacerbate the clinical manifestations of HHS and contribute to its morbidity and mortality.
3. The challenge of Differentiating HHS and DKA at Clinical Presentation
Formulating a diagnosis and distinguishing between Hyperglycemic Hyperosmolar State (HHS) and Diabetic Ketoacidosis (DKA) purely based on clinical symptoms is an intricate undertaking, in spite of identifiable patterns in their progression, frequency, and associated risk factors. There's a frequent delay in the diagnosis of HHS, with patients often being initially treated as though they have DKA [
2]. DKA commonly manifests in a much quicker timeframe, evolving over mere hours or days, whereas HHS generally pursues a more gradual course, unfolding over several days to weeks, notably in instances of type 2 diabetes [
19]. Both conditions share common symptoms such as polyuria, polydipsia, weight loss, and fatigue. However, DKA is often associated with vomiting, widespread abdominal discomfort, and in severe cases, hyperventilation. Conversely, HHS may present itself through varying degrees of cognitive impairment and even seizures. Encephalopathy is generally seen when serum osmolality surpasses 330 mOs-mol/kg and sodium levels climb over 160 mEq/L [
4]. In some research, less than one-third of patients with HHS enter a comatose state [
20]. Indications of dehydration in HHS may be camouflaged by hyperosmolality, potentially appearing less evident clinically and being underappreciated in overweight patients, despite their suffering from intense fluid losses that exceed those observed in DKA dehydration [
21]. For patients demonstrating mild to severe symptoms of either condition, the associated signs could merge, creating a unique amalgamation of varying characteristics, similarities, and severity levels (
Figure 2). This presents a fascinating mosaic portrait of each patient's unique disease presentation.
Hyperglycemia can lead to dilutional hyponatremia, and the actual corrected serum sodium is calculated by adding 1.6 mEq/L for each 100 mg/dL glucose elevation above the normal serum glucose of 100 mg/dL [
22]. Head imaging for suspected cerebral edema is rarely positive despite severe hyperglycemia, and brain swelling is less common in HHS than in DKA, even during treatment. If it occurs, brain edema can have severe consequences, necessitating prompt recognition and immediate appropriate intervention [
23]. Muir et al. previously described abnormal verbal or motor response to pain, posturing, abnormal pupillary response, cranial nerve deficits (especially the III, IV, and VI nerves), abnormal respiratory patterns, and decreased oxygen saturation as signs of brain edema. They proposed a list of alarming signs and criteria for the clinical diagnosis of brain edema, with major criteria including (1) altered mentation; (2) sustained heart rate drop by more than 20 bpm unexplained by other factors; and (3) age-inappropriate incontinence14. Minor criteria encompass (1) ensuing vomiting or worsening severity if present before initial therapy despite treatment; (2) worsening headache; (3) lethargy; (4) increasing blood pressure with diastolic blood pressure (DBP) >90 mmHg; and (5) younger age (<5 years) [
24]. These criteria can be utilized to avoid missing imminent cerebral edema, based on the presence of two major criteria or one major and two minor criteria.
4. Complications in HHS and DKA
Cerebral edema represents a critical complication of diabetic ketoacidosis (DKA), occurring in approximately 1% of patients, with a greater number of subclinical cases; it is less frequent and even rare in hyperosmolar hyperglycemic state (HHS) [
25]. Cerebral edema typically develops within the first day of treatment, peaking between 3-12 hours of DKA therapy [
26]. Historically, it was believed to result from rapid rehydration or changes in osmolality, particularly through insulin therapy. However, recent studies have provided alternative explanations and additional perspectives. For instance, animal models of hyperglycemia treatment demonstrated intracerebral accumulation of cations and complex carbohydrates, potentially affecting neuron membrane sodium-proton ion exchange, thereby exacerbating brain edema during fluid infusion [
27,
28]. This explanation, however, may only partially account for the phenomenon, as it does not address the preexisting cerebral edema observed in some patients before therapeutic intervention. Some researchers propose that hyperosmolality and cerebral hypoperfusion, coupled with antidiuretic hormone dysregulation and hyponatremia, initiate brain edema [
29]. Other hypotheses suggest that the protective role of cerebral intracellular idiogenic osmoles becomes overwhelmed, failing to counteract and reduce the plasma osmotic gradient necessary for maintaining cerebral euvolemia [
30]. The disruption of euvolemia is partially induced by a rapid decrease in plasma osmolality that surpasses the reciprocal capacity of the autonomic physiological intracerebral osmotic drop; this results in an increasing osmotic gradient gap, subsequent intracerebral fluid shift, and brain edema [
31]. Some studies indicate that the degree of hypocapnia may predict the onset of brain edema [
32].
Rhabdomyolysis is a rare condition, encountered more frequently in HHS than in DKA. Statistically, it correlates with elevated blood urea nitrogen (BUN) and creatinine levels, as well as severe hyperglycemia and hypophosphatemia [
33]. In extreme cases, it can potentially lead to a high risk of compartment syndrome and renal failure [
34]. Disturbances in electrolytes and hypokalemic arrhythmias have been reported [
35]. Malignant hyperthermia is a rare phenomenon characterized by a temperature exceeding 40 °C due to muscle calcium flux disruption, causing a significant shift from the sarcoplasmic reticulum to the myoplasm16. Several pediatric HHS cases complicated by hyperthermia have been documented, with a high mortality rate [
36]. Various etiologies have been proposed, including rhabdomyolysis; however, the lack of muscle breakdown in some hyperthermic patients challenges this theory. In the past, insulin cresol preservative was implicated as a potential cause, supported by animal studies demonstrating hyperthermia induction [
37]. Nevertheless, in certain cases, hyperthermia occurred prior to insulin therapy initiation, suggesting cytokines inflammatory effect and the theoretical possibility of central thermoregulation impairment.
Cardiogenic shock and pancreatitis are more severe and frequent complications in new-onset type 2 diabetes with HHS, and occur rarely in pediatric type 1 DKA [
38]. The risk of thrombosis is heightened in both DKA and HHS due to the hyperosmolar dehydration state. In some cases, thrombosis has revealed previously unknown underlying thrombophilia, particularly factor V Leiden gene mutation; in these instances, central line placement may increase the risk of thrombosis [
39]. Respiratory distress syndrome and seizures, although rare, may present as complications in both DKA and HHS [
40]. It is important for clinicians to recognize and promptly address these complications to improve patient outcomes.
Additionally, it is crucial to consider various risk factors and comorbidities when managing patients with DKA and HHS. For instance, age, sex, ethnicity, and the presence of underlying medical conditions may influence the development and progression of these hyperglycemic emergencies [
41]. Prompt and accurate diagnosis, followed by appropriate therapeutic interventions, are essential to minimize the risk of complications and mortality.
5. Treatment of HHS
Early recognition and timely diagnosis of HHS are the cornerstones of optimal therapy to achieve good outcomes and reduce complications. Treatment of HHS has evolved from DKA protocols, and there are no established standards for HHS therapy [
40]. However, the 2022 ISPAD treatment recommendations for DKA, HHS, and mixed cases provide valuable guidance for managing these conditions [
42].
Typical fluid loss in HHS averages 1.5 to 2 times that of DKA, making vigorous fluid management and rapid deficit correction crucial therapeutic steps to restore euvolemic hydration and renal perfusion in HHS [
43]. Fluid losses usually range between 110-220 mL/kg, equivalent to 3-6 L in adults, and up to 4000 mL/m2/day. The goal is to achieve full deficit replacement within 24 hours, typically by administering normal saline (NS) at an initial rate of 20 mL/kg and repeating as needed, with a minimum of 40 mL/kg in the first 6 hours until peripheral perfusion is restored. Transitioning to hypotonic 0.45 NS is reserved for fully resuscitated patients with persistent hypernatremia and hyperosmolality to avoid overcorrection and promote a gradual fall, reducing potential risk of brain edema [
27]. Some authors recommend replacing urinary losses with an isotonic solution if the patient is hemodynamically unstable [
21,
38,
40].
In addition to fluid management, monitoring and managing electrolyte imbalances, such as potassium and phosphate, are essential for a comprehensive treatment approach. Insulin therapy should also be initiated cautiously, as HHS patients may be more sensitive to insulin than those with DKA [
40,
43]. Moreover, addressing the underlying cause of HHS, whether it be infection, medication noncompliance, or other factors, is crucial for the prevention of future occurrences and long-term management.
Hemodialysis has been advocated by some for rhabdomyolysis and renal failure in patients who do not achieve the desired rate of serum Na and osmolality drop. The gradual decline with dialysis can reduce complications [
44]. Furthermore, close monitoring of the patient's clinical and biochemical parameters is vital during the treatment process to ensure optimal outcomes and minimize potential adverse events.
Contrary to insulin therapy in DKA, exogenous insulin for HHS should be delayed and decreased, as there is usually no significant ketosis [
45]. Fluids resuscitation alone substantially decreases serum glucose in HHS by intravascular volume expansion, producing a hemodilutional effect, tissue circulation improvement, increased metabolic and glucose utilization, and finally renal perfusion enhancement; the latter increases glycosuria, therefore, any additional and simultaneous insulin hypoglycemic effect could be detrimental by accelerating the glucose fall curve precipitously and exceeding the optimal desired serum glucose decline rate of 75-100 mg/dl/hour (4.1-5.5 mmol/hour) [
46].
The overlaps and defining factors of HHS and DKA differences approach can be emphasized through comprehensive and distinguished management steps included in a summarizing DI-FF-ER-EN-CE-S mnemonic, which serves as an invaluable guide for healthcare professionals to remember the specific management strategies unique to HHS. This mnemonic also highlights the necessity to focus treatment on the underlying cause of HHS, such as infection or medication non-compliance, for long-term management and prevention of recurrence. Furthermore, it underscores the importance of education and training for healthcare professionals in recognizing and managing HHS, including mixed cases with DKA, as a key factor in improving patient care. The mnemonic breaks down as follows:
- 'DI' - Delay and Decrease insulin administration: HHS patients require a later initiation and a lower dose of insulin compared to those with DKA. This stage emphasizes the need to manage the decline of serum glucose primarily through fluid resuscitation before insulin therapy is initiated.
- 'FF' - Fluid and Electrolyte Replacement: Fluid management is pivotal in HHS due to severe dehydration, often double that observed in DKA.
- 'ER'- Electrolyte imbalances, especially potassium, phosphate, and magnesium, are more pronounced in HHS and necessitate frequent monitoring and replacement, initially as often as every 2 hours.
- 'EN' - Encephalopathy: HHS can potentially lead to encephalopathy, underlining the need for controlled serum osmolality reduction. Regular monitoring of clinical and biochemical parameters, including close hourly glucose monitoring, is essential during treatment.
- 'CE' - Cerebral Edema: While rare in HHS, cerebral edema requires aggressive therapy when it occurs. Early recognition and management of this and other complications, such as rhabdomyolysis and malignant hyperthermia, are crucial for improving patient outcomes.
- 'S' - Systemic Multiorgan Failure: HHS can potentially lead to systemic multiorgan failure. For ease of awareness and screening, we categorize the associated risks into three mnemonic groups: the 3Rs (Renal failure, Respiratory distress, Rhabdomyolysis), the 3Hs (Heart failure, Hypercoagulation, Hyperthermia), and AP (Arrhythmias, Pancreatitis).
Premature insulin use in under-resuscitated HHS patients with vasospasm may cause hypotension due to a prominent extravascular fluid shift. A rapid decline in serum glucose of >100 mg/dL may occur in the first hours of fluid expansion alone [
17]. Therefore, insulin infusion should be held temporarily if it was already initiated. Some authors advise adding low glucose (2.5-5%) if a significant drop continues [
47]. Once serum glucose begins to fall at a rate below 50 mg/dL/hour (2.7 mmol/liter/hour), an insulin drip is added to fluid therapy at a lower dose of 0.05-0.025 unit/kg/h. This approach contrasts with that used for patients with severe ketoacidosis typical of DKA, where insulin infusion is initiated earlier and at higher dose (around 0.1 unit/kg/hour) [
48]. The initial goal of hyperglycemia level control for HHS is between 200-300 mg/dL with an IV insulin drip titrated at a lower rate than in DKA. By comparison, the blood glucose goal for DKA on IV Insulin is 150-200 mg/dL. A hypernatremic sodium drop of 0.5 meq/L/hour is advocated in some studies [
49]. Generally, electrolytes losses in HHS, including losses in potassium, phosphate, and magnesium, are higher than in DKA. Potassium losses are extreme despite the common initial hyperkalemia secondary to acidosis ion shift [
50]. If serum potassium is <5 mEq/L with adequate renal function, it is recommended that patients are provided 40 mEq/L of potassium phosphate/KCl. Magnesium may be needed for hypomagnesemia or hypocalcemia [
40,
43,
45,
47,
48].
Dantrolene is the drug of choice for malignant hyperthermia in HHS, to stabilize myoplasm calcium [
51]. Heparin prophylaxis to prevent deep vein thrombosis (DVT) is recommended in HHS patients who require a central line and are immobilized for 1-2 days [
52].
Brain edema treatment should be initiated with a clinical diagnosis and should not require head CT confirmation since false negative results have been reported. Standard treatment includes head elevation, respiratory support, and specific therapy of Mannitol as the first line treatment [
2,
24,
26]. Hypertonic saline is reserved for unresponsive cases after infusing 1 gm/kg of Mannitol. Bicarb should be avoided unless pH is less than 6.9, which is not characteristic for HHS but can occur in severe DKA [
53].
Resolution of HHS is achieved by rehydration, serum osmolality normalization, restoration of normal mental status in uncomplicated cases, and stabilization of electrolytes derangements. If present, any underlying hyperglycemic precipitating illness, including infection, should be targeted early.
7. Mixed HHS and DKA
The diagnostic landscape of diabetes-related conditions, particularly Hyperglycemic Hyperosmolar Syndrome (HHS) and Diabetic Ketoacidosis (DKA), is intricate and complex. Traditionally, these two conditions have been discerned based on their distinctive classical differential characteristics, providing clear diagnostic pathways. However, in practical clinical scenarios, particularly in pediatric cases, this differentiation isn't always so clear-cut. Increasingly, unique and challenging cases have emerged that present with a blend of symptoms or biochemical criteria traditionally associated with either HHS or DKA. This creates a unique spectrum of conditions that are not wholly HHS or DKA, but rather, a coalescence of both. This has led to the identification of hybrid conditions such as hyperosmolar DKA and ketotic HHS. These cases embody the cumulative risks of both HHS and DKA, highlighting the clinical complexity and potential severity of these overlapping conditions [
5,
59]. In certain manifestations of HHS, we may observe a phenomenon of mild to moderate acidosis, even in the absence of significant ketosis. Such occurrences can be perplexing, given the conventional understanding of HHS. The explanation for this atypical presentation can often be traced back to physiological processes like hypoperfusion and anaerobic metabolism. In such scenarios, due to insufficient oxygen supply or impaired blood flow, the body switches to anaerobic metabolism, resulting in the production and accumulation of lactic acid, and thereby, lactic acidemia [
58,
59]. Furthermore, a noteworthy clinical observation is the exacerbation of hyperglycemia in patients with DKA who attempt to alleviate their extreme thirst. Thirst is a common symptom of DKA, driving patients to consume beverages, often those high in carbohydrates. Paradoxically, this well-intentioned attempt to mitigate their symptoms can lead to severe hyperglycemia, further complicating their condition [
60].
Some HHS cases present along a continuum between two hyperglycemic poles, dependent at least on the degree of insulin deficiency or its tissue sensitivity, dehydration severity and fluid intake, symptoms duration, and patient’s status [
61,
62]. These factors affect the extent of ketosis and acidosis or range of serum glucose elevations, dehydration, hyperosmolality, and DKA. Patients presenting with some combination of HHS and DKA are particularly challenging, and diagnosis and treatment requires a higher degree of vigilance with attention to risks and treatment priorities of the HHS and DKA components.
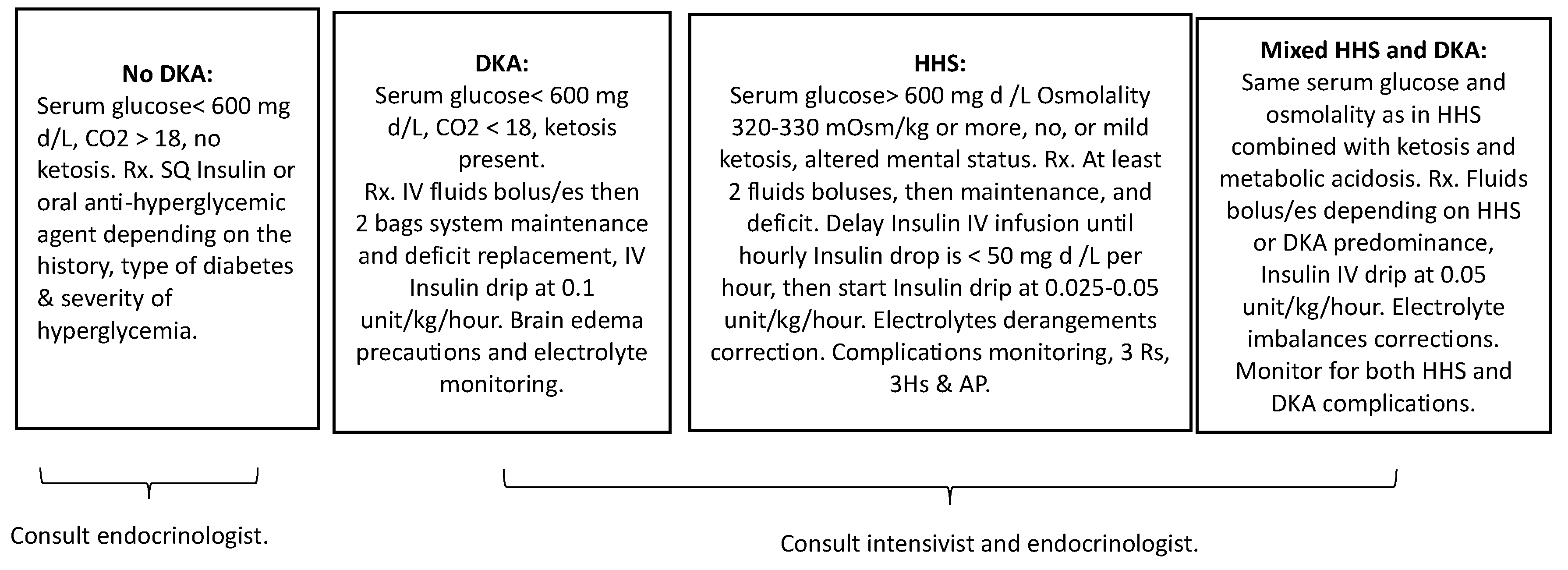
Management of these patients should be guided by biochemical profile and clinical judgment, evidence from the management of isolated HHS and DKA, and a strong focus on individualized patient care. Based on the predominant findings, in addition to recognized deficits and risks, treatment of patients with mild DKA and significant hyperosmolar dehydration should begin with saline boluses to restore fluid losses, with simultaneous monitoring of rehydration volumes response, electrolytes derangements precautions, and supplementation. Insulin therapy should be delayed until there is no significant drop in hyperglycemia as in classical HHS, starting at half the typical 0.1 unit/kg/hour insulin infusion dose used in DKA, with glucose and saline fluids adjustments based on the hyperglycemia and electrolytes status.
In the context of these complex overlapping cases, early and frequent interdisciplinary collaboration involving endocrinology, nephrology, and critical care specialists can optimize patient management and outcomes. In challenging overlapping cases of HHS and severe DKA, we propose dynamic adjustable combined therapy that addresses both conditions based on which state is predominant, with particular attention to HHS complications, coupled with close clinical and laboratory monitoring, starting with fluids boluses, at volumes and repeats determined by the severity of HHS and DKA and their manifestations. The insulin dose should be lower than that used for DKA and dropped to (0.05-0.1 U/kg/h). The risk of cerebral edema is higher in mixed cases than in isolated HHS, warranting greater awareness and clinical monitoring. Moreover, ongoing patient and family education about self-management strategies, including proper monitoring of blood glucose levels and timely administration of insulin, can play a significant role in preventing future episodes of HHS or DKA. To aid understanding and guide the clinical management of these convoluted diabetic states,
Figure 3 offers a comprehensive summary. This figure outlines the fundamental defining criteria of the diabetic states discussed and aligns them with the corresponding initial therapeutic interventions. This synthesis serves as an invaluable tool in navigating these multifaceted conditions, allowing for an effective and targeted therapeutic approach.
8. Conclusion.
In conclusion, the recognition and management of HHS as a distinct hyperglycemic emergency, separate from DKA, is crucial in reducing complications and improving patient outcomes. HHS presents a wide spectrum of cases, including mixed cases with DKA, necessitating prompt diagnosis, recognition of complications, and appropriate treatment that takes into account the unique aspects of HHS.
Hyperglycemic Hyperosmolar State (HHS) is a serious, though rarely encountered, hyperglycemic emergency that is often misperceived as Diabetic Ketoacidosis (DKA). Traditionally, HHS was primarily considered a possibility in adults with type 2 diabetes. However, recent reports suggest an increasing prevalence in the pediatric population, including those with type 1 diabetes, leading to a broad spectrum of presentations that may also include mixed cases with DKA. The delayed recognition and treatment of HHS can exacerbate risks of complications and negatively influence prognosis, underscoring the importance of expanding awareness about this condition and its inclusion in differential diagnoses for severe hyperglycemias. Effective management of HHS necessitates unique monitoring and treatment measures, which diverge from typical DKA protocols despite their commonalities. Prompt diagnosis and recognition of complications are paramount, as is the anticipation of the risks associated with both conditions. Implementing early treatment measures, utilizing DI-FF-ER-EN-CE-S mnemonic with the 3Rs, 3Hs, and AP complications acronyms monitoring, understanding predictive prognostic values, standardizing treatment regimens, and establishing practice guidelines are crucial steps to improve patient outcomes.