1. Introduction
Psoriasis is a chronic disease with a genetic predisposition that affects about 2% of the population. The etiopathogenesis of the disease is complex and not fully understood. Although multiple clinical types of psoriasis have been reported, most scientific research, including the present study, refers to the plaque variant, which affects up to 90% of all psoriatic patients. The hallmark of the disease is sustained inflammation that leads to uncontrolled keratinocyte proliferation. It is conjectured that disturbances in the innate and adaptive cutaneous immune responses are responsible for the development and maintenance of inflammation.
Recent research indicates that mitochondrial dysfunction may also be an important factor in the development of the disease [
1,
2].
A reliable diagnostic tool is therefore needed to assess mitochondrial dysfunction. One promising approach is to measure NADH autofluorescence [
3]. Wollina et al. have shown that the intensity of NADH fluorescence is much lower in psoriatic lesions compared to unaffected skin [
4]. This points to the presence of NADH/NAD
+ redox imbalance in psoriatic lesions, assuming that skin pigmentation is similar in both regions. However, is well known that melanin concentrations in the skin seriously affect the measured fluorescence intensity [
5,
6]. The measured level of skin NADH fluorescence should therefore be used with caution for characterization of mitochondrial dysfunction.
Flow Mediated Skin Fluorescence (FMSF) is a new non-invasive technique for assessing vascular circulation and metabolic regulation [
7,
8,
9]. It measures stimulation of the vascular circulation in response to post-occlusive reactive hyperemia, based on dynamical changes in NADH fluorescence from skin tissue. Very recently, the FMSF technique has been used to analyze mitochondrial dysfunction in patients with newly diagnosed primary hypertension, based on the ischemic phase of skin NADH dynamics [
10]. This approach is particularly promising because the dynamics of NADH ischemic growth are independent of skin pigmentation.
In this report, we provide unambiguous evidence of NADH/NAD+ redox imbalance in psoriatic lesions, based on analysis of recorded FMSF traces. This observation, which was independent of skin pigmentation, clearly indicates that the NADH–NAD+ equilibrium is shifted towards oxidation in psoriatic lesions. The therapeutic implications of this observation are discussed.
2. Materials and Methods
Study Population and Clinical Characteristics
The studied population consisted of eight psoriatic patients newly admitted to the university hospital. The FMSF measurements were performed on the first day, before any topical treatment had been introduced that might interfere with the fluorescence measurements. Two patients, from whom FMSF traces were successfully collected from forearm skin with psoriatic lesions and from a closely located unaffected area, were selected for detailed analysis. One of the patients (code 0004L) was a 50-year old male. The other patient (code 0005L) was a 24-year old female. Both patients had been suffering from psoriasis for 1–3 years. The diagnosis was confirmed by histopathological examination.
Brief Description of the FMSF Technique and the Measurement Protocol
Measurements were performed using AngioExpert, a device constructed by Angionica Ltd. The AngioExpert device uses the Flow Mediated Skin Fluorescence (FMSF) technique, which measures changes in the intensity of nicotinamide adenine dinucleotide (NADH) fluorescence from the skin on the forearm as a response to blocking and releasing blood flow. The skin is the largest organ of the human body, and is characterized by a specific metabolism. The epidermal layer of skin is not directly vascularized, and oxygen and nutrients are transported from the dermis by diffusion. Therefore, epidermal cell metabolism can be considered a unique and sensitive marker of early dysfunction in vascular circulation and metabolic regulation.
The measurement protocol has been described elsewhere.
9 For analysis of the results, four parameters were used. Two fluorescence parameters, which measure the fluorescence level at baseline and the half-time of ischemic growth (t
1/2), characterize the FMSF trace. The other two parameters, which measure the Reactive Hyperemia Response (RHR) and Hypoxia Sensitivity (HS) expressed as log(HS), have been demonstrated and discussed previously [
8,
9]. The RHR is a unique parameter, based on a combination of both the ischemic and hyperemic parts of the measured FMSF trace. It characterizes endothelial function related predominantly to the production of nitric oxide (NO) in the vasculature due to reactive hyperemia. The log(HS) parameter characterizes the intensity of myogenic microcirculatory oscillations (0.052–0.15 Hz) observed on the reperfusion line. It represents the response of microcirculation to transient ischemia.
3. Results and Discussion
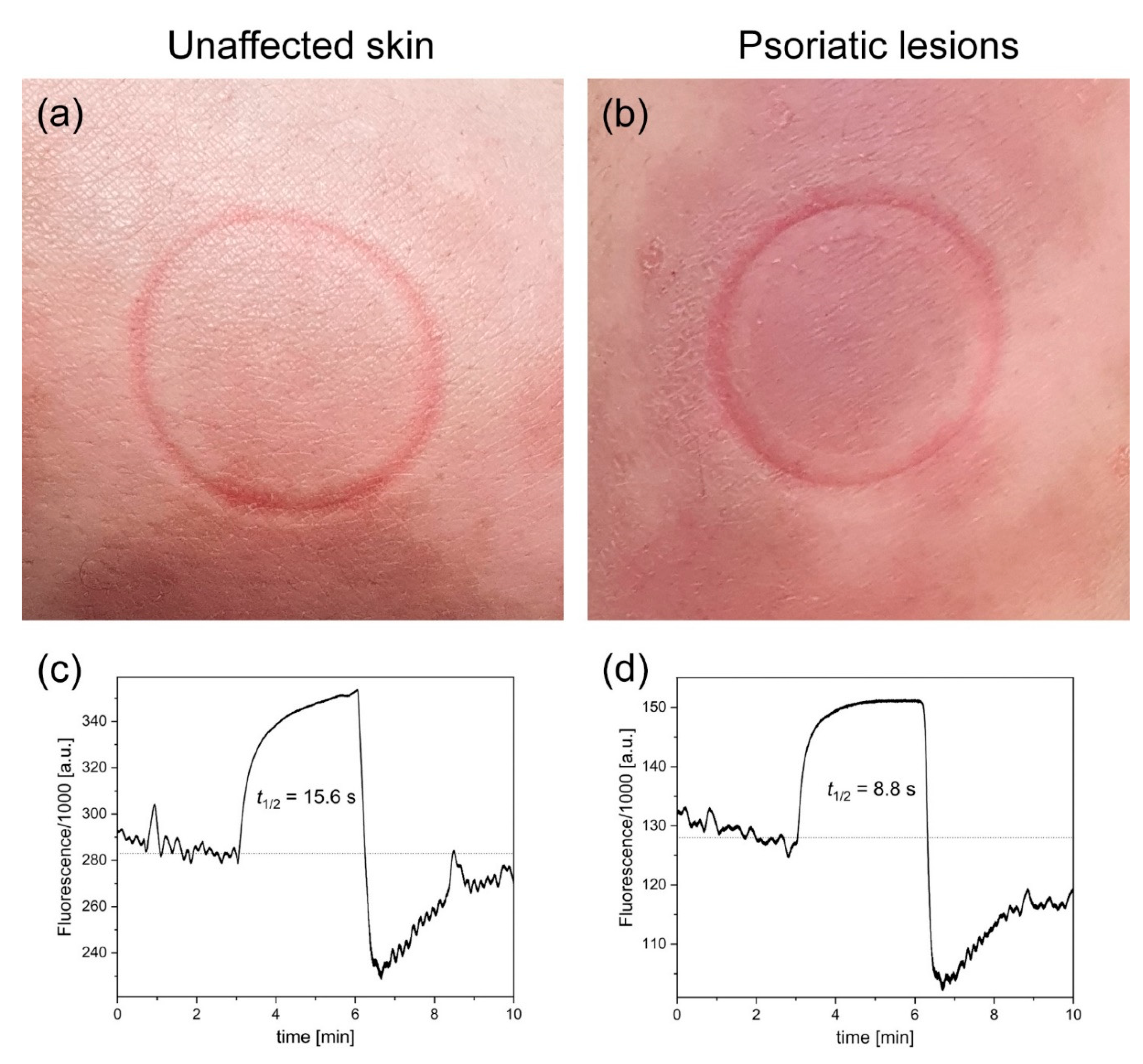
Figure 1 presents example FMSF traces collected for psoriatic lesions and unaffected skin from patient (code 004L), along with photographs of the studied skin areas including characteristic imprints from the optical window of the AngioExpert diagnostic device. The fluorescence level at baseline value collected from unaffected skin was 284,000 a.u. and the half-time (t
1/2) of ischemic growth was 15.6 s. The results for the psoriatic lesions display two characteristic features compared to the unaffected skin: a lower fluorescence level at baseline (128,000 a.u.) and a shorter half-time (t
1/2) of ischemic growth (8.8 s). Very similar results were obtained for the other patient (code 005L). The results for both patients are presented in
Table 1, along with the measured FMSF parameters. As can be seen, the FMSF parameters collected from psoriatic skin were lower than those collected from unaffected skin. A particularly pronounced difference is visible in the log(HS) parameter.
The lower fluorescence level at baseline values recorded for psoriatic lesions compared to unaffected skin support previous observations by Wollina et al. [
4] The important new finding made in the present study is based on the shorter half-time of ischemic growth recorded for psoriatic lesions. This observation, which was independent of skin pigmentation, clearly indicates that the NADH–NAD
+ equilibrium is shifted towards oxidation in psoriatic lesions, and that transient ischemia caused by blocking of the blood flow in brachial artery results in very rapid reduction of NAD
+ to NADH in skin keratinocytes. Shorter half-time of ischemic growth (t
1/2) in psoriatic lesions was seen in all studied patients, and in an extreme case (patient code 008L) was as low as 4.2 s. The half-time of ischemic growth calculated for the healthy group (n = 16) is 15.8 ± 2.9 s. It can generally be assumed that a t
1/2 of ischemic growth in the recorded FMSF traces below 10 s is indicative of mitochondrial dysfunction, represented as a shift of NADH/NAD
+ redox imbalance towards oxidation. The lower log(HS) parameters collected from psoriatic lesions compared to unaffected skin seem to confirm this assumption, as the highly oxidated skin keratinocytes seen in psoriatic lesions should have a lower response to transient hypoxia. Thus, the observed fluorescence and FMSF parameters presented in
Table 1 can be used for non-invasive assessment of mitochondrial dysfunction.
The evidence that psoriasis lesions show NADH/NAD+ redox imbalance towards oxidation may have important therapeutic implications. A deviation represented as a shift in the fluorescence and FMSF parameters collected from unaffected skin vs. psoriatic lesions could potentially be used as a measure of disease status and progress. This could in turn allow for better understanding of therapeutic options to treat psoriasis.
Identification of biomarkers for psoriasis based on metabolomics suggests the operation of the compensatory effect, involving activation of glycolytic activity resulting in elevated levels of lactic acid [
11,
12]. Indeed, the NADH/NAD
+ ratio may correlate with the pyruvate/lactate ratio. Surprisingly, potentiation of the NADH/NAD
+ redox imbalance by increasing the level of NAD
+ in psoriatic lesions could be considered as a therapeutic option. We have already shown that the topical composition of NAD
+ can effectively be used for treatment of psoriasis, with effects comparable to conventional therapy with anthralin [
13]. More recent research has confirmed that the augmentation of NAD
+ can result in amelioration of psoriasis-like dermatitis [
14]. This effect is linked to elevated Sirt1 activity, which is an NAD
+-dependent process. The therapeutic effect of hyperbaric oxygen in psoriasis can also be explained by activation of NADH/NAD
+ redox imbalance [
15]. On other hand, such effects may also be explained as mitohormesis (biological response where the induction of a reduced amount of mitochondrial stress leads to an increment in health and viability within a cell, tissue or organism) [
16].
Application of the presented methodology for diagnosis of psoriasis is greatly facilitated by the fact that results can be collected from both psoriatic lesions and unaffected skin of individual patients. However, research should be extended to evaluate the application of this methodology to other diseases and disorders.
Author Contributions
Conceptualization: JG, AW; Patient selection: AW; Data collection: TF; Data processing: TF, AM; Result discussion: JG, TF, AM, AW; Draft writing: JG; Supervision: JG, AW
Funding
This work was supported by the European Union from the resources of the European Regional Development Fund under the Smart Growth Operational Program, Grant No. POIR. 01.01.01-00-0540/15-00.
Informed Consent Statement
The study was conducted at the Medical University of Lodz (Poland). It conformed to the principles outlined in the Declaration of Helsinki and the study protocol was approved by the Bioethics Committee at the Medical University of Lodz. All the subjects were Polish nationality/Caucasian type and gave written informed consent prior to participation.
Data Availability Statement
Data presented in this paper are available on request.
Conflict of Interest
TF is employed by Angionica Ltd. JG and AM are inventors of the patents protecting the use of FMSF technology. AW declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Therianou, A.; Vasiadi, M.; Delivanis, D.A.; et al. Mitochondrial dysfunction in affected skin and increased mitochondrial DNA in serum from patients with psoriasis. Exp. Dermatol. 2019, 28, 72–75. [Google Scholar] [CrossRef]
- Alwehaidah, M.S.; AlFadhli, S.; Al-Kafaji, G. Leukocyte mitochondrial DNA copy number is a potential non-invasive biomarker for psoriasis. PLoS ONE 2022, 17, e0270714. [Google Scholar] [CrossRef]
- Schaefer, P.M.; Kalinina, S.; Rueck, A.; von Arnim, C.A.F.; von Einem, B. NADH autofluorescence-a marker on its way to boost bioenergetic research. Cytom. Part. A J. Int. Soc. Anal. Cytol. 2019, 95, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Schmidt, W.-D.; Koch, A.; Scheibe, A.; Erfurth, F.; Fassler, D. Fluorescence remission spectroscopy of psoriatic lesions and the effect of topical anthralin therapy. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Dremin, V.V.; Dunaev, A.V. How the melanin concentration in the skin affects the fluorescence-spectroscopy signal formation. J. Opt. Technol. 2016, 83, 43. [Google Scholar] [CrossRef]
- Heikal, A.A. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark. Med. 2010, 4, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Katarzynska, J.; Lipinski, Z.; Cholewinski, T.; et al. Non-invasive evaluation of microcirculation and metabolic regulation using flow mediated skin fluorescence (FMSF): Technical aspects and methodology. Rev. Sci. Instrum. 2019, 90, 104104. [Google Scholar] [CrossRef]
- Katarzyńska, J.; Zieliński, J.; Marcinek, A.; Gebicki, J. New approach to non-invasive assessment of vascular circulation based on the response to transient ischemia. Vasc. Health Risk Manag. 2022, 18, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Marcinek, A.; Katarzynska, J.; Sieron, L.; Skokowski, R.; Zielinski, J.; Gebicki, J. Non-invasive assessment of vascular circulation based on Flow Mediated Skin Fluorescence (FMSF). Biology 2023, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Chomicka, R.; Chomicki, W.; Krauze, T.; et al. Investigating the ischaemic phase of skin NADH fluorescence dynamics in recently diagnosed primary hypertension: A time series analysis. J. Clin. Med. 2023, 12, 1247. [Google Scholar] [CrossRef]
- Kang, H.; Li, X.; Zhou, Q.; et al. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br. J. Dermatol. 2017, 176, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Yan, J. Identifying biomarkers in human psoriasis: Revealed by a systems metabolomics approach. Br. J. Dermatol. 2017, 176, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Wozniacka, A.; Szajerski, P.; Adamus, J.; Gebicki, J.; Sysa-Jedrzejowska, A. In search for new antipsoriatic agents: NAD topical composition. Skin. Pharmacol. Physiol. 2007, 20, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.-J.; Oh, G.-S.; et al. Augmentation of NAD(+) by dunnione ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J. Inflamm. Res. 2022, 15, 4623–4636. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.; Michaels, J.C.; Al-Waili, N.; et al. Therapeutic effect of hyperbaric oxygen in psoriasis vulgaris: Two case reports and a review of the literature. J. Med. Case Rep. 2009, 3, 7023. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of Reactive Oxygen Species (ROS). Dose Response. 2014, 12, 288–341. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).