Submitted:
10 August 2023
Posted:
11 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Macrophages as immune system cells
2.1. Two macrophage phenotypes
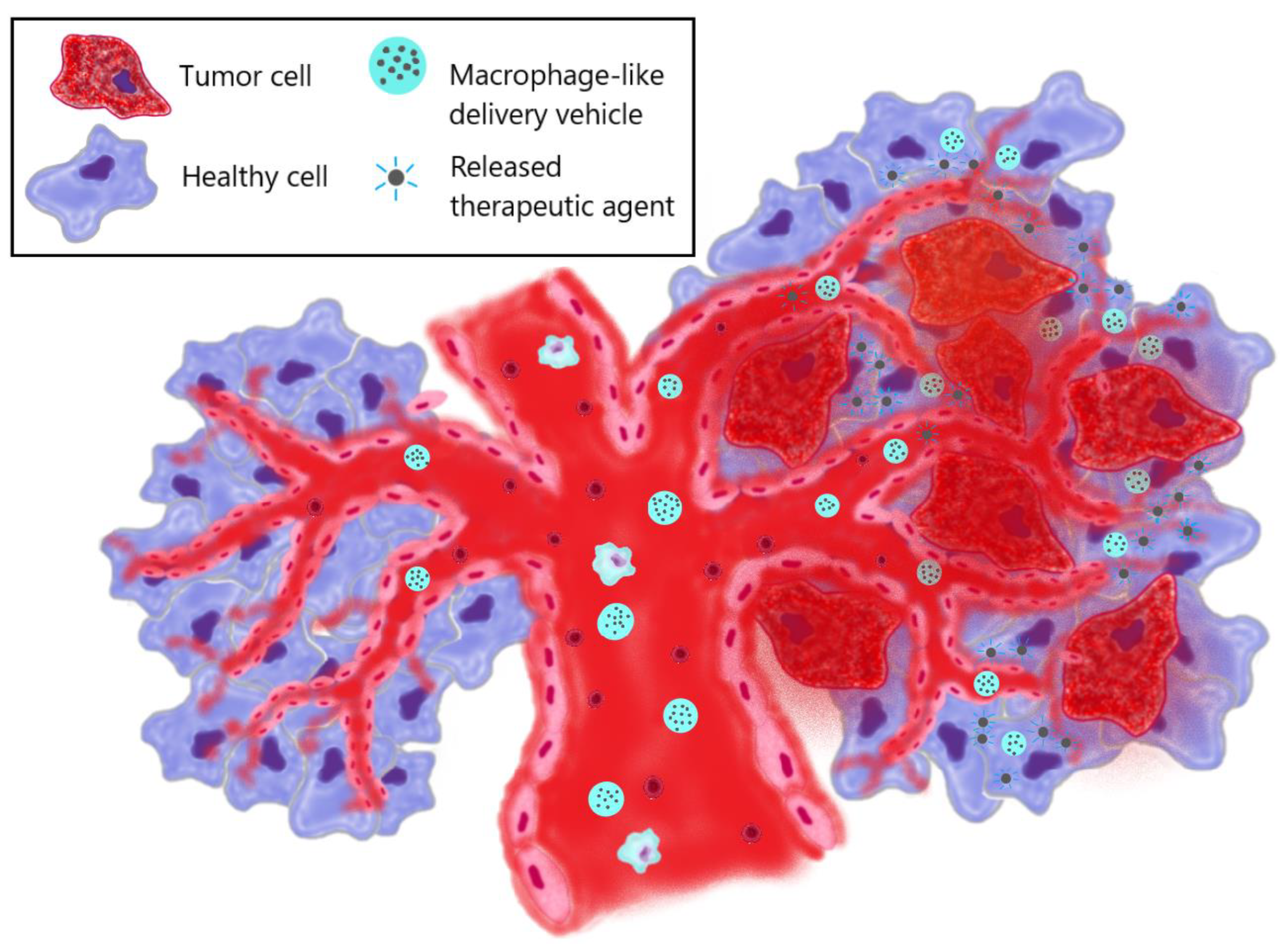
3. The use of macrophage-derived vesicles in therapy
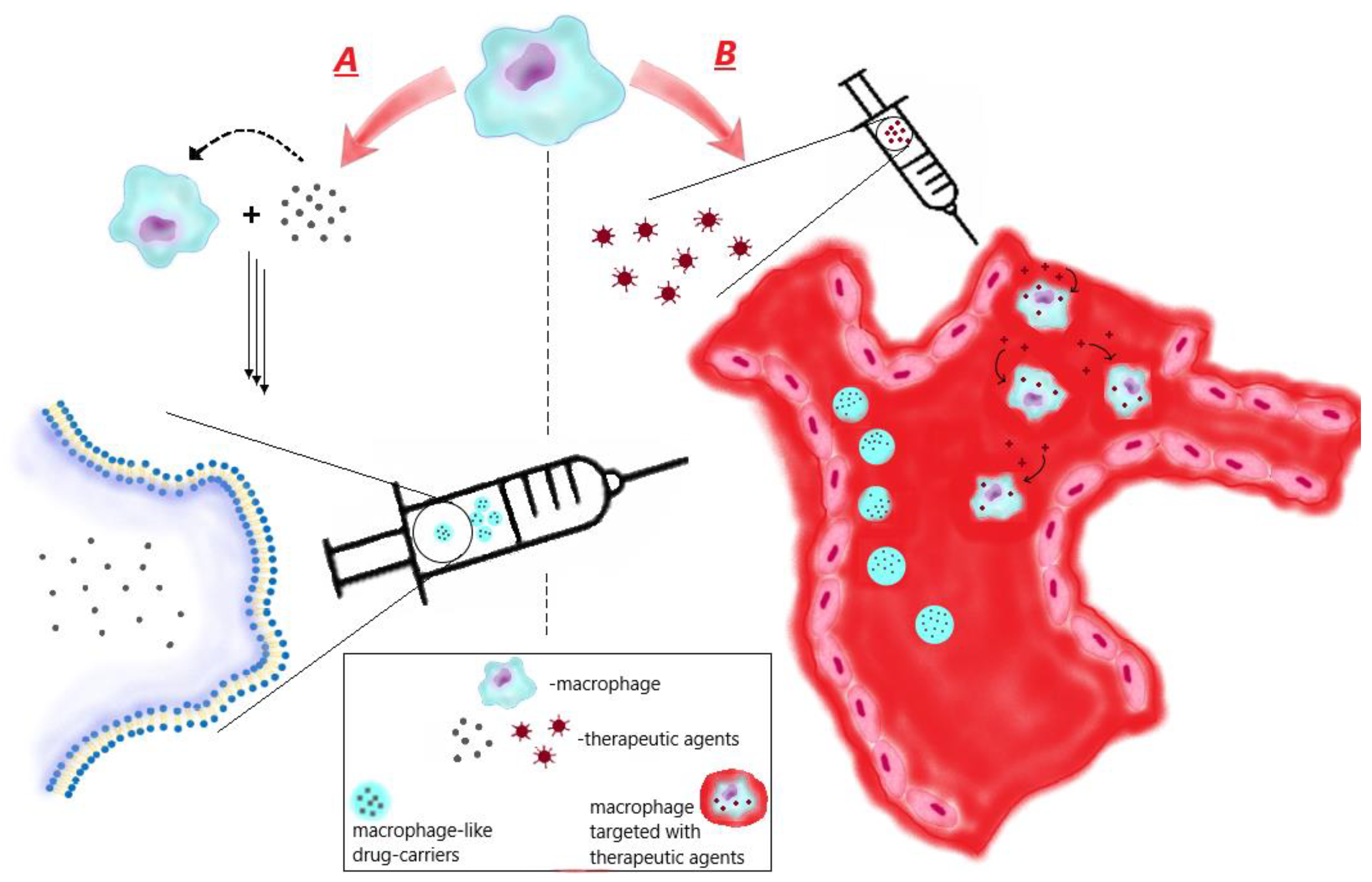
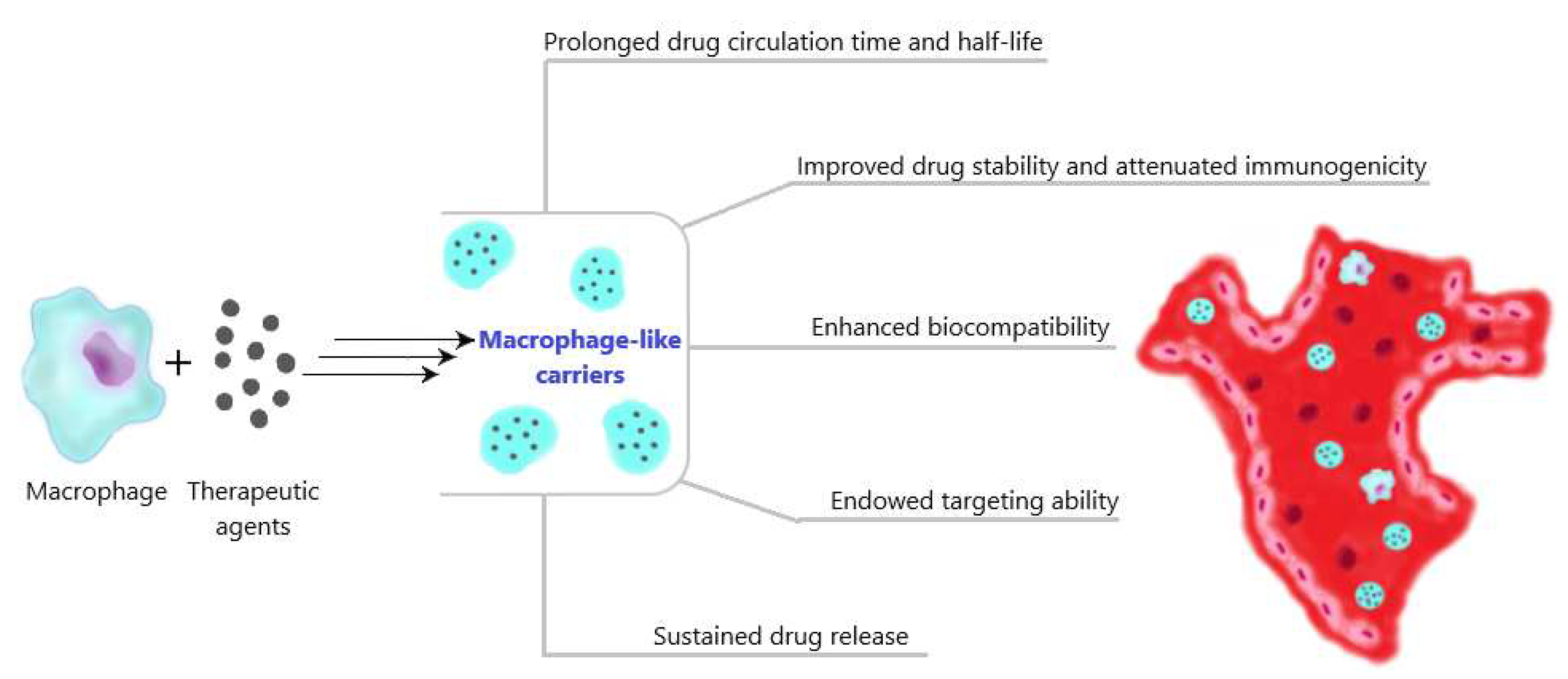
3.1. Ex vivo preparation of macrophage-like carriers of therapeutic agents
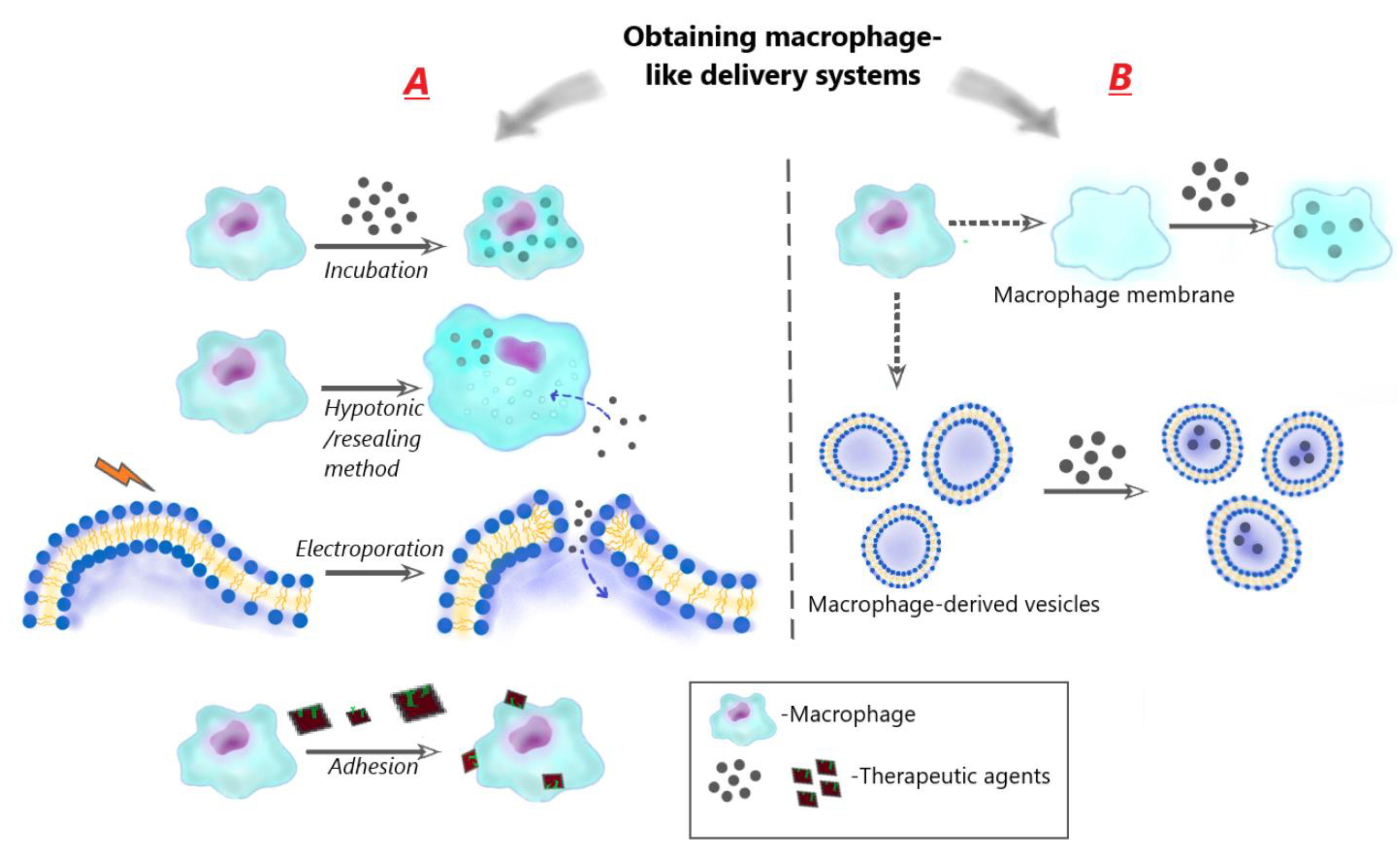
3.1.1. Sources of macrophages
3.1.2. Obtaining of macrophage-like carriers
3.1.2.1. Using of living cells
- Encapsulation of drugs in macrophages via incubation
- b.
- Encapsulation of drugs in macrophages using hypotonic/resealing method
- c.
- Encapsulation of drugs in macrophage cell membranes using electroporation/resealing method
- d.
- Adhesion of therapeutic particles to the macrophage membrane (cellular backpacks)
3.1.2.2. Encapsulation of drugs in macrophage-derived membrane structures
- Encapsulation inside macrophage cellular membranes
- b.
- Encapsulation inside macrophage-derived vesicles
3.2. Macrophage- derived membranes (or particles) as anti-inflammatory agents
3.3. Macrophage- derived membranes (or particles) particles as anti-tumor agents
- Therapeutic effect obtained from macrophages
- b.
- Therapeutic effect due to drug loaded nanoparticles inside macrophages
- c.
- Therapeutic effect due to surface engineering of macrophages
- d.
- Therapeutic effect due to bioengineered species
- e.
- Photothermal therapy
3.4. Macrophage-derived particles for the treatment of infectious diseases
- Treatment of viral infections
- b.
- Wound healing and treatment of bacterial infections
4. Macrophage-mediated therapy via macrophage targeting
4.1. Design of therapeutic agents targeting macrophages
4.1.1. Passive macrophage-targeting therapeutic agents
- Size
- b.
- Shape
- c.
- Surface charge and hydrophilicity
4.1.2. Active macrophage-targeting therapeutic agents
| Receptor targeting | Carrier formulation | Ligand modification/coating | Cargo | Purpose | Result | Ref. |
| Mannose receptor | Liposomes | Mannose | DNA | Stimulation of immune response | Mannosylated cationic liposomes exhibited improved DNA delivery | [147] |
| Polymeric micelles | siRNA | TAMs repolarization | Modified micelles could selectively deliver efficacious amounts of functional siRNA into TAMs | [148] | ||
| Liposomes | 64Cu | PET imaging of TAMs | High selective accumulation of the liposomes in TAMs was observed | [149] | ||
| Selenium NPs | Isoniazid | Treatment of tuberculosis | The NPs preferentially entered macrophages and accumulated in lysosomes releasing Isoniazid | [150] | ||
| Galactose receptor | Dextran NPs | Galactose | CpG, anti-IL-10 and anti-IL-10 receptor oligonucleotides | TAMs repolarization | NPs accumulated in the tumor and was taken up predominantly by TAMs | [151] |
| Chitosan-cysteine NPs | siRNA | Treatment of ulcerative colitis | Galactose modification significantly facilitated the uptake by macrophages and targeting ability of the NPs | [152] | ||
| Poly(lactic-co-glycolic acid) NPs | Dexamethasone | Developing of the strategy to catch macrophages during intestinal inflammation | NPs were effectively captured by macrophages | [153] | ||
| Dectin-1 | Polymer-lipid hybrid NPs | Yeast cell wall microparticles, containing β-1,3-D-glucan | Cabazitaxel | Developing of oral targeted drug delivery | The microparticles were rapidly and efficiently taken up by macrophages | [154] |
| Mesoporous silica NPs | Doxorubicin | Developing of anti-tumor therapy | Drug delivery to macrophages was enhanced compared to uncoated silica NPs | [155] | ||
| Fc receptor | Alginate NPs | Tuftsin | DNA | Developing of anti-inflammatory agents | Tuftsin-modified NPs were rapidly internalized in murine macrophages | [156] |
| Folate receptor-β (FRβ) | - | Anti-mouse FRβ monoclonal antibody | Pseudomonas exotoxin A | TAMs depletion | Direct eliminating of TAMs was attained | [157] |
| Poly(amidoamine) dendrimers | Folic acid | Methotrexate | Alleviating of the inflammatory disease of arthritis | High degree of specific binding and internalization of the dendrimers into macrophages was observed | [158] | |
| Human serum albumin nanocapsules | - | Evaluating targeting ability of folic acid-modified agents | The internalization of nanocapsules was enhanced via FR specificity | [159] | ||
| CD44 | Hyaluronic acid-tocopherol succinate micelles | Hyaluronic acid | Rifampicin | Developing of tuberculosis treatment | Micelles exhibited a significant phygocytosis and CD44-dependent uptake in comparison to free drug | [160] |
| Liposomes | Prednisolone | Developing of rheumatoid arthritis therapy | Enhanced cellular uptake, mainly mediated by caveolae- and clathrin-dependent endocytosis, was acheived | [161] | ||
| Poly(lactic-co-glycolic acid) NPs | Curcumin | Alleviating of ulcerative colitis | Enhanced drug delivery to intestinal macrophages and selective accumulation in inflamed colitis tissue with minimal accumulation in healthy colon tissue was observed | [162] | ||
| Siglec-1 | Liposomes | Sialic acid | Epirubicin | Tumor therapy | The tumor targeting efficiency and the accumulation of epirubicin in monocytes was improved | [163] |
| Zoledronic acid | TAMs depleting and repolarization | Good targeting ability was observed | [164] |
- Toll-like receptors targeting
- b.
- Scavenger receptors targeting
- c.
- Fc-receptors targeting
- d.
- Other receptors targeting
- e.
- Tumor-associated macrophage targeting
4.2. Macrophage-targeting in anti-inflammation therapy
4.3. Macrophage-targeting in anti-tumor therapy
- Inhibition of macrophage recruitment
- b.
- Targeting Anti-Phagocytic Checkpoints
- c.
- TAMs depletion
- d.
- Reprogramming of TAMs
4.4. Macrophage-targeting in the therapy of infectious diseases
- Viral infectious diseases
- b.
- Tuberculosis
- c.
- Protozoan infectious diseases
4.5. Potency of macrophage targeting via CD206 receptor and future perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J Nanobiotechnology 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; Macparland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard-Nanomaterial Clearance by the Liver. Nat Mater 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J Cell Physiol 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef]
- Gautiar, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-Expression Profiles and Transcriptional Regulatory Pathways That Underlie the Identity and Diversity of Mouse Tissue Macrophages. Nat Immunol 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Hoeffel, G.; Ginhoux, F. Fetal Monocytes and the Origins of Tissue-Resident Macrophages. Cell Immunol 2018, 330, 5–15. [Google Scholar] [CrossRef]
- Wu, Y.; Hirschi, K.K. Tissue-Resident Macrophage Development and Function. Front Cell Dev Biol 2021, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Mosser, D.M. Macrophage Activation by Endogenous Danger Signals. Journal of Pathology 2008, 214, 161–178. [Google Scholar] [CrossRef]
- Tacke, F. Monozyten-Subpopulationen in Entzündungsprozessen: Prinzip Und Perspektive. Deutsche Medizinische Wochenschrift 2009, 134, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annu Rev Immunol 2009, 27, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J. Immunology: No Need to Coax Monocytes. Science (1979) 2011, 332, 1268–1269. [Google Scholar]
- Gordon, S.; Plüddemann, A. Tissue Macrophages: Heterogeneity and Functions. BMC Biol 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Cassol, E.; Poli, G. Macrophage Polarization in Health and Disease. ScientificWorldJournal 2011, 11, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. The Journal of Immunology 2005, 175, 342–349. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2 Mononuclear Phagocytes; 2002; Vol. 23.
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu Rev Immunol 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat Immunol 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Gordon, S. The Macrophage: Past, Present and Future. Eur J Immunol 2007, 37. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur J Pharmacol 2020, 877. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Artis, D. Innate Lymphoid Cells Control Signaling Circuits to Regulate Tissue-Specific Immunity. Cell Res 2020, 30, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mosser, D.M. Macrophage Activation by Endogenous Danger Signals. Journal of Pathology 2008, 214, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O. Regulators of Macrophage Activation. Eur J Immunol 2011, 41, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Nakane, A.; Minagawa, T.; Kasai, N.; Yamamoto, K.-I.; Sato, N.; Tsuruoka, N. Human Tumor Necrosis Factor Increases the Resistance against Listeria Infection in Mice; 1989; Vol. 178.
- Hutchings, M.I.; Palmer, T.; Harrington, D.J.; Sutcliffe, I.C. Lipoprotein Biogenesis in Gram-Positive Bacteria: Knowing When to Hold ’em, Knowing When to Fold ’Em. Trends Microbiol 2009, 17, 13–21. [Google Scholar] [CrossRef]
- Rumbo, M.; Nempont, C.; Kraehenbuhl, J.P.; Sirard, J.C. Mucosal Interplay among Commensal and Pathogenic Bacteria: Lessons from Flagellin and Toll-like Receptor 5. FEBS Lett 2006, 580, 2976–2984. [Google Scholar] [CrossRef]
- Xue, Q.; Lu, Y.; Eisele, M.R.; Sulistijo, E.S.; Khan, N.; Fan, R.; Miller-Jensen, K. Analysis of Single-Cell Cytokine Secretion Reveals a Role for Paracrine Signaling in Coordinating Macrophage Responses to TLR4 Stimulation.
- Fieren, M.W.J.A. The Local Inflammatory Responses to Infection of the Peritoneal Cavity in Humans: Their Regulation by Cytokines, Macrophages, and Other Leukocytes. Mediators Inflamm 2012, 2012. [Google Scholar] [CrossRef]
- Wang, J.; Nikrad, M.P.; Travanty, E.A.; Zhou, B.; Phang, T.; Gao, B.; Alford, T.; Ito, Y.; Nahreini, P.; Hartshorn, K.; et al. Innate Immune Response of Human Alveolar Macrophages during Influenza a Infection. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix Metalloproteinases as Regulators of Inflammatory Processes. Biochim Biophys Acta Mol Cell Res 2017, 1864, 2036–2042. [Google Scholar] [CrossRef]
- Lagente, V.; Le Quement, C.; Boichot, E. Macrophage Metalloelastase (MMP-12) as a Target for Inflammatory Respiratory Diseases. Expert Opin Ther Targets 2009, 13, 287–295. [Google Scholar] [CrossRef]
- Van Lint, P.; Libert, C. Chemokine and Cytokine Processing by Matrix Metalloproteinases and Its Effect on Leukocyte Migration and Inflammation. J Leukoc Biol 2007, 82, 1375–1381. [Google Scholar] [CrossRef]
- Nénan, S.; Boichot, E.; Lagente, V.; Bertrand, C.P. Macrophage Elastase (MMP-12): A pro-Inflammatory Mediator?; 2005; Vol. 100.
- Gordon, S. Alternative Activation of Macrophages. Nat Rev Immunol 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Munitz, A.; Brandt, E.B.; Mingler, M.; Finkelman, F.D.; Rothenberg, M.E.; Austen, K.F. Distinct Roles for IL-13 and IL-4 via IL-13 Receptor 1 and the Type II IL-4 Receptor in Asthma Pathogenesis; 2008.
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidbal, J.G. Shifts in Macrophage Phenotypes and Macrophage Competition for Arginine Metabolism Affect the Severity of Muscle Pathology in Muscular Dystrophy. Hum Mol Genet 2009, 18, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and Tissue Injury: Agents of Defense or Destruction? Annu Rev Pharmacol Toxicol 2011, 51, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, D.E.; Comerford, S.A.; Hammer, R.E. Hepatic Fibrosis, Glomerulosclerosis, and a Lipodystrophy-like Syndrome in PEPCK-TGF-Β1 Transgenic Mice. Journal of Clinical Investigation 1997, 100, 2697–2713. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2 Mononuclear Phagocytes; 2002; Vol. 23.
- Gerber, J.S.; Mosser, D.M. Reversing Lipopolysaccharide Toxicity by Ligating the Macrophage Fcγ Receptors. The Journal of Immunology 2001, 166, 6861–6868. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Rantakari, P.; Patten, D.A.; Valtonen, J.; Karikoski, M.; Gerke, H.; Dawes, H.; Laurila, J.; Ohlmeier, S.; Elima, K.; Hübscher, S.G.; et al. Stabilin-1 Expression Defines a Subset of Macrophages That Mediate Tissue Homeostasis and Prevent Fibrosis in Chronic Liver Injury. Proc Natl Acad Sci U S A 2016, 113, 9298–9303. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Anderson, C.F.; Mosser, D.M. Cutting Edge: Biasing Immune Responses by Directing Antigen to Macrophage Fcγ Receptors. The Journal of Immunology 2002, 168, 3697–3701. [Google Scholar] [CrossRef]
- Pierce, G.E.; Mustoe, T.A.; Lingelbach, J.; Masakowski, V.R.; Gall, L.; Griffin, L.; Senior, R.M.; Deuel, T.F. Plateletoderived Growth Factor and Transforming Growth Factor-/ Enhance Tissue Repair Activities by Unique Mechanisms.
- Song, E.; Ouyang, N.; Hörbelt, M.; Antus, B.; Wang, M.; Exton, M.S. Influence of Alternatively and Classically Activated Macrophages on Fibrogenic Activities of Human Fibroblasts. Cell Immunol 2000, 204, 19–28. [Google Scholar] [CrossRef]
- Koch, A.E.; Steven, ?; Kunkel$, L.; Chensue, S.W.; Kenneth Haine~, M.G.; Strieter, R.M. Expression of Interleukin-I and Interleukin-I Receptor Antagonist by Human Rheumatoid Synovial Tissue Macrophages’; 1992; Vol. 65.
- Liang, T.; Zhang, R.; Liu, X.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Recent Advances in Macrophage-Mediated Drug Delivery Systems. Int J Nanomedicine 2021, 16, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Dou, H.; Boska, M.; Destache, C.J.; Nelson, J.; Poluektova, L.; Rabinow, B.E.; Gendelman, H.E.; Mosley, R.L. Quantitative Magnetic Resonance and SPECT Imaging for Macrophage Tissue Migration and Nanoformulated Drug Delivery. J Leukoc Biol 2006, 80, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Destache, C.J.; Morehead, J.R.; Mosley, R.L.; Boska, M.D.; Kingsley, J.; Gorantla, S.; Poluektova, L.; Nelson, J.A.; Chaubal, M.; et al. Development of a Macrophage-Based Nanoparticle Platform for Antiretroviral Drug Delivery. Blood 2006, 108, 2827–2835. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Monocytes: A Novel Drug Delivery System Targeting Atherosclerosis. J Drug Target 2014, 22, 138–145. [Google Scholar] [CrossRef]
- Evangelopoulos, M.; Yazdi, I.K.; Acciardo, S.; Palomba, R.; Giordano, F.; Pasto, A.; Sushnitha, M.; Martinez, J.O.; Basu, N.; Torres, A.; et al. Biomimetic Cellular Vectors for Enhancing Drug Delivery to the Lungs. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Angsantikul, P.; Escajadillo, T.; Zhang, Q.; Olson, J.; Luk, B.T.; Zhang, S.; Fang, R.H.; Gao, W.; Nizet, V.; et al. Macrophage-like Nanoparticles Concurrently Absorbing Endotoxins and Proinflammatory Cytokines for Sepsis Management. Proc Natl Acad Sci U S A 2017, 114, 11488–11493. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A. V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. Journal of Controlled Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhu, Y.; Jiang, L.; Zhang, S.; Qin, J.; Wu, Q.; Dai, W.; Shen, S.; Pang, Z.; et al. Route to Rheumatoid Arthritis by Macrophage-Derived Microvesicle-Coated Nanoparticles. Nano Lett 2019, 19, 124–134. [Google Scholar] [CrossRef]
- Weischenfeldt, J.; Porse, B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. Cold Spring Harb Protoc 2008, 3. [Google Scholar] [CrossRef]
- Busch, C.; Favret, J.; Geirsdóttir, L.; Molawi, K.; Sieweke, M. Isolation and Long-Term Cultivation of Mouse Alveolar Macrophages. Bio Protoc 2019, 9. [Google Scholar] [CrossRef]
- Pineda-Torra, I.; Gage, M.; De Juan, A.; Pello, O.M. Isolation, Culture, and Polarization of Murine Bone Marrow-Derived and Peritoneal Macrophages. In Methods in Molecular Biology; Humana Press Inc., 2015; Vol. 1339, pp. 101–109.
- Kunjachan, S.; Gupta, S.; Dwivedi, A.K.; Dube, A.; Chourasia, M.K. Chitosan-Based Macrophage-Mediated Drug Targeting for the Treatment of Experimental Visceral Leishmaniasis. J Microencapsul 2011, 28, 301–310. [Google Scholar] [CrossRef]
- Nguyen, V. Du; Min, H.K.; Kim, D.H.; Kim, C.S.; Han, J.; Park, J.O.; Choi, E. Macrophage-Mediated Delivery of Multifunctional Nanotherapeutics for Synergistic Chemo-Photothermal Therapy of Solid Tumors. ACS Appl Mater Interfaces 2020, 12, 10130–10141. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tan, T.; Zhu, D.; Yu, H.; Liu, Y.; Zhou, H.; Jin, Y.; Xia, Q. Paclitaxel-Loaded Macrophage Membrane Camouflaged Albumin Nanoparticles for Targeted Cancer Therapy. Int J Nanomedicine 2020, 15, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, D.; Mei, D.; Zhang, H.; Wang, Z.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. Macrophage Mediated Biomimetic Delivery System for the Treatment of Lung Metastasis of Breast Cancer. Journal of Controlled Release 2015, 204, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gupta, S. PLGA-Based Macrophage-Mediated Drug Targeting for the Treatment of Visceral Leishmaniasis; 2017; Vol. 3.
- Zhou, X.; Luo, B.; Kang, K.; Zhang, Y.; Jiang, P.; Lan, F.; Yi, Q.; Wu, Y. Leukocyte-Repelling Biomimetic Immunomagnetic Nanoplatform for High-Performance Circulating Tumor Cells Isolation. Small 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, C.; O’Sullivan, M.P.; Sivadas, N.; O’Leary, S.; Gallagher, P.J.; Keane, J.; Cryan, S.A. The Application of High-Content Analysis in the Study of Targeted Particulate Delivery Systems for Intracellular Drug Delivery to Alveolar Macrophages. Mol Pharm 2011, 8, 1100–1112. [Google Scholar] [CrossRef]
- He, W.; Frueh, J.; Wu, Z.; He, Q. Leucocyte Membrane-Coated Janus Microcapsules for Enhanced Photothermal Cancer Treatment. Langmuir 2016, 32, 3637–3644. [Google Scholar] [CrossRef]
- Tushinski, R.J.; Oliver, I.T.; Larry, +; Guilbert, J.; Tynan, P.W.; Warner, J.R.; Stanley, E.R. Survival of Mononuclear Phagocytes Depends on a Lineage-Specific Growth Factor That the Differentiated Cells Selectively Destroy; 1982; Vol. 28.
- Zhao, Y.; J. Haney, M. Active Targeted Macrophage-Mediated Delivery of Catalase to Affected Brain Regions in Models of Parkinson?S Disease. J Nanomed Nanotechnol 2011, 01. [Google Scholar] [CrossRef]
- Evans, M.A.; Huang, P.J.; Iwamoto, Y.; Ibsen, K.N.; Chan, E.M.; Hitomi, Y.; Ford, P.C.; Mitragotri, S. Macrophage-Mediated Delivery of Light Activated Nitric Oxide Prodrugs with Spatial, Temporal and Concentration Control. Chem Sci 2018, 9, 3729–3741. [Google Scholar] [CrossRef]
- Ren, K.; Qiu, Y.; Yu, Q.; He, J.; Mei, L.; Liu, Y.; Li, J.; Wang, X.; Li, M.; Zhang, Z.; et al. Macrophage-Mediated Multi-Mode Drug Release System for Photothermal Combined with Anti-Inflammatory Therapy against Postoperative Recurrence of Triple Negative Breast Cancer. Int J Pharm 2021, 607. [Google Scholar] [CrossRef]
- Lv, Y.; Jun, Y.; Tang, Z.; Li, X.; Tao, M.; Zhang, Z.; Liu, L.; Sun, S.; Wang, Q.; Luo, C.; et al. Enhanced Antitumor Efficacy of Macrophage-Mediated Egg Yolk Lipid-Derived Delivery System against Breast Cancer. Int J Nanomedicine 2020, 15, 10075–10084. [Google Scholar] [CrossRef] [PubMed]
- Kandekar, S.G.; Del Río-Sancho, S.; Lapteva, M.; Kalia, Y.N. Selective Delivery of Adapalene to the Human Hair Follicle under Finite Dose Conditions Using Polymeric Micelle Nanocarriers. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Tripathi, R.P.; Chen, L.; Caminade, A.M.; Shi, X.; Majoral, J.P. New Ways to Treat Tuberculosis Using Dendrimers as Nanocarriers. Pharmaceutics 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lin, S.; Nune, K.C.; Misra, R.D.K. Chitosan-Gelatin-Based Microgel for Sustained Drug Delivery. J Biomater Sci Polym Ed 2016, 27, 441–453. [Google Scholar] [CrossRef]
- Salmaso, S.; Caliceti, P. Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers. J Drug Deliv 2013, 2013, 1–19. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Hildgen, P.; Banquy, X. Assessment of PEG on Polymeric Particles Surface, a Key Step in Drug Carrier Translation. Journal of Controlled Release 2014, 185, 71–87. [Google Scholar] [CrossRef]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Li, S.; Liu, L.; Xin, W.; Li, C.; Yin, X.; Xu, X.; Bao, F.; Hua, Z. Macrophage-Mediated Tumor-Targeted Delivery of Engineered Salmonella Typhimurium VNP20009 in Anti-PD1 Therapy against Melanoma. Acta Pharm Sin B 2022, 12, 3952–3971. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.Y.; Ju, E.J.; Jung, J.; Park, J.; Chung, H.K.; Lee, J.S.; Lee, J.S.; Park, H.J.; Song, S.Y.; et al. Use of Macrophages to Deliver Therapeutic and Imaging Contrast Agents to Tumors. Biomaterials 2012, 33, 4195–4203. [Google Scholar] [CrossRef]
- Nowacek, A.S.; Balkundi, S.; McMillan, J.; Roy, U.; Martinez-Skinner, A.; Mosley, R.L.; Kanmogne, G.; Kabanov, A. V.; Bronich, T.; Gendelman, H.E. Analyses of Nanoformulated Antiretroviral Drug Charge, Size, Shape and Content for Uptake, Drug Release and Antiviral Activities in Human Monocyte-Derived Macrophages. Journal of Controlled Release 2011, 150, 204–211. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Polak, R.; Haney, M.J.; Zhao, Y.; Gomes Neto, R.J.; Hill, M.C.; Kabanov, A. V.; Cohen, R.E.; Rubner, M.F.; Batrakova, E. V. Macrophages with Cellular Backpacks for Targeted Drug Delivery to the Brain. Biomaterials 2017, 140, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-Based Drug Delivery Devices Using Phagocytosis-Resistant Backpacks. Advanced Materials 2011, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett 2018, 18, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, L.; Guo, H.; Zhang, W. Macrophage Membrane-Coated Nanovesicles for Dual-Targeted Drug Delivery to Inhibit Tumor and Induce Macrophage Polarization. Bioact Mater 2023, 23, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, L.; Zhang, J.; Zheng, C.; Ding, K.; Xiao, H.; Wang, L.; Zhang, Z. C-C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles to Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl Mater Interfaces 2018, 10, 31124–31135. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics 2019, 9, 1714–1727. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-Derived Exosome-Mimetic Hybrid Vesicles for Tumor Targeted Drug Delivery. Acta Biomater 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A. V. Macrophage Exosomes as Natural Nanocarriers for Protein Delivery to Inflamed Brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Pei, Y.; Yeo, Y. Drug Delivery to Macrophages: Challenges and Opportunities. Journal of Controlled Release 2016, 240, 202–211. [Google Scholar] [CrossRef]
- Choi, M.R.; Stanton-Maxey, K.J.; Stanley, J.K.; Levin, C.S.; Bardhan, R.; Akin, D.; Badve, S.; Sturgis, J.; Robinson, J.P.; Bashir, R.; et al. A Cellular Trojan Horse for Delivery of Therapeutic Nanoparticles into Tumors. Nano Lett 2007, 7, 3759–3765. [Google Scholar] [CrossRef]
- Batrakova, E. V.; Li, S.; Reynolds, A.D.; Mosley, R.L.; Bronich, T.K.; Kabanov, A. V.; Gendelman, H.E. A Macrophage-Nanozyme Delivery System for Parkinson’s Disease. Bioconjug Chem 2007, 18, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Ikada, Y. Effect of the Size and Surface Charge of Polymer Microspheres on Their Phagocytosis by Macrophage. Biomaterials 1988, 9, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated Nanoparticles for Biological and Pharmaceutical Applications. Adv Drug Deliv Rev 2003, 55, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of Engineered Nanomaterials: Focus on Biocompatibility, Biodistribution and Biodegradation. Biochim Biophys Acta Gen Subj 2011, 1810, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J.; Christie, C.; Hong, S.J.; Trinidad, A.; Peng, Q.; Uzal, F.A.; Hirschberg, H. Nanoparticle-Loaded Macrophage-Mediated Photothermal Therapy: Potential for Glioma Treatment. Lasers Med Sci 2015, 30, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Favretto, M.E.; Cluitmans, J.C.A.; Bosman, G.J.C.G.M.; Brock, R. Human Erythrocytes as Drug Carriers: Loading Efficiency and Side Effects of Hypotonic Dialysis, Chlorpromazine Treatment and Fusion with Liposomes. Journal of Controlled Release 2013, 170, 343–351. [Google Scholar] [CrossRef]
- Hamidi, M.; Rafiei, P.; Azadi, A.; Mohammadi-Samani, S. Encapsulation of Valproate-Loaded Hydrogel Nanoparticles in Intact Human Erythrocytes: A Novel Nano-Cell Composite for Drug Delivery. J Pharm Sci 2011, 100, 1702–1711. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of Cell Membranes. Biophys J 1991, 60, 297–306. [Google Scholar] [CrossRef]
- Rama Himaja Movva; Srinivasa Rao Yarraguntla; Venkata Kamala Kumari Paravastu Cellular Backpacks for Macrophage Immunotherapy- A Review. GSC Biological and Pharmaceutical Sciences 2022, 20, 126–133. [CrossRef]
- Swiston, A.J.; Cheng, C.; Um, S.H.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Surface Functionalization of Living Cells with Multilayer Patches. Nano Lett 2008, 8, 4446–4453. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll Receptors, CD14, and Macrophage Activation and Deactivation by LPS. Microbes Infect 2002, 4, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schönbein, G.W. Analysis of Inflammation. Annu Rev Biomed Eng 2006, 8, 93–151. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte Recruitment during Infection and Inflammation. Nat Rev Immunol 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol 2014, 5. [Google Scholar] [CrossRef]
- Tan, Q.; He, L.; Meng, X.; Wang, W.; Pan, H.; Yin, W.; Zhu, T.; Huang, X.; Shan, H. Macrophage Biomimetic Nanocarriers for Anti-Inflammation and Targeted Antiviral Treatment in COVID-19. J Nanobiotechnology 2021, 19. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of Atherosclerosis by Macrophage-Biomimetic Nanoparticles via Targeted Pharmacotherapy and Sequestration of Proinflammatory Cytokines. Nat Commun 2020, 11. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, S.S. Transmembrane TNFα-Expressed Macrophage Membrane-Coated Chitosan Nanoparticles as Cancer Therapeutics. ACS Omega 2020, 5, 1572–1580. [Google Scholar] [CrossRef]
- Tao, Y.; Ning, M.; Dou, H. A Novel Therapeutic System for Malignant Glioma: Nanoformulation, Pharmacokinetic, and Anticancer Properties of Cell-Nano-Drug Delivery. Nanomedicine 2013, 9, 222–232. [Google Scholar] [CrossRef]
- Evans, M.A.; Shields, C.W.; Krishnan, V.; Wang, L.L.; Zhao, Z.; Ukidve, A.; Lewandowski, M.; Gao, Y.; Mitragotri, S. Macrophage-Mediated Delivery of Hypoxia-Activated Prodrug Nanoparticles. Adv Ther (Weinh) 2020, 3, 1900162. [Google Scholar] [CrossRef]
- Wayne, E.C.; Long, C.; Haney, M.J.; Batrakova, E. V.; Leisner, T.M.; Parise, L. V.; Kabanov, A. V. Targeted Delivery of SiRNA Lipoplexes to Cancer Cells Using Macrophage Transient Horizontal Gene Transfer. Advanced Science 2019, 6, 1900582. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Iwasaki, Y. Surface Modification of Macrophages with Nucleic Acid Aptamers for Enhancing the Immune Response against Tumor Cells. Bioconjug Chem 2018, 29, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Muthana, M.; Giannoudis, A.; Scott, S.D.; Fang, H.Y.; Coffelt, S.B.; Morrow, F.J.; Murdoch, C.; Burton, J.; Cross, N.; Burke, B.; et al. Use of Macrophages to Target Therapeutic Adenovirus to Human Prostate Tumors. Cancer Res 2011, 71, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for in Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. ACS Appl Mater Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Qiu, Y.; Yu, Q.; He, J.; Mei, L.; Liu, Y.; Li, J.; Wang, X.; Li, M.; Zhang, Z.; et al. Macrophage-Mediated Multi-Mode Drug Release System for Photothermal Combined with Anti-Inflammatory Therapy against Postoperative Recurrence of Triple Negative Breast Cancer. Int J Pharm 2021, 607. [Google Scholar] [CrossRef]
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting Macrophages as Targeted Carrier to Guide Nanoparticles into Glioma. Oncotarget 2016, 7, 37081–37091. [Google Scholar] [CrossRef]
- Swiston, A.J.; Gilbert, J.B.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Freely Suspended Cellular “Backpacks” Lead to Cell Aggregate Self-Assembly. Biomacromolecules 2010, 11, 1826–1832. [Google Scholar] [CrossRef]
- Swiston, A.J.; Cheng, C.; Um, S.H.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Surface Functionalization of Living Cells with Multilayer Patches. Nano Lett 2008, 8, 4446–4453. [Google Scholar] [CrossRef]
- Rama Himaja Movva; Srinivasa Rao Yarraguntla; Venkata Kamala Kumari Paravastu Cellular Backpacks for Macrophage Immunotherapy- A Review. GSC Biological and Pharmaceutical Sciences 2022, 20, 126–133. [CrossRef]
- Holden, C.A.; Yuan, Q.; Yeudall, W.A.; Lebman, D.A.; Yang, H. Surface Engineering of Macrophages with Nanoparticles to Generate a Cell-Nanoparticle Hybrid Vehicle for Hypoxia-Targeted Drug Delivery; 2010.
- de Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv Healthc Mater 2017, 6, 1700073. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, F.; Ju, Y.; Hong, J.; Ding, Y. Gold Nanomaterial Engineering for Macrophage-Mediated Inflammation and Tumor Treatment. Adv Healthc Mater 2021, 10, 2000818. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Grotepas, C.B.; McMillan, J.M.; Destache, C.J.; Chaubal, M.; Werling, J.; Kipp, J.; Rabinow, B.; Gendelman, H.E. Macrophage Delivery of Nanoformulated Antiretroviral Drug to the Brain in a Murine Model of NeuroAIDS. J Immunol 2009, 183, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yang, J.; Liu, W. Bacteria Activated-Macrophage Membrane-Coated Tough Nanocomposite Hydrogel with Targeted Photothermal Antibacterial Ability for Infected Wound Healing. Chemical Engineering Journal 2021, 420. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated Macrophage-Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Advanced Materials 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Gu, J.; Jiang, Y.; Cui, W.; Chen, J.; Li, L.; Zheng, K.; Xu, Y. Pretreatment of Macrophage-Membrane-Coated Nanoparticles for Therapeutical Targeting of P. Gingivalis-Accelerated Atherosclerosis. Mater Des 2022, 223. [Google Scholar] [CrossRef]
- Meng, Z.; Pan, L.; Qian, S.; Yang, X.; Pan, L.; Chi, R.; Chen, J.; Pan, J.; Shi, C. Antimicrobial Peptide Nanoparticles Coated with Macrophage Cell Membrane for Targeted Antimicrobial Therapy of Sepsis. Mater Des 2023, 229, 111883. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Uptake Characteristics of Liposomes by Rat Alveolar Macrophages: Influence of Particle Size and Surface Mannose Modification. Journal of Pharmacy and Pharmacology 2010, 59, 75–80. [Google Scholar] [CrossRef]
- Yue, H.; Wei, W.; Yue, Z.; Lv, P.; Wang, L.; Ma, G.; Su, Z. Particle Size Affects the Cellular Response in Macrophages. European Journal of Pharmaceutical Sciences 2010, 41, 650–657. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc Natl Acad Sci U S A 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging Macrophages with Nanoparticles. Nat Mater 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J Pharm Sci 2021, 16, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat Rev Clin Oncol 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- GAO, W.J.; LIU, J.X.; LIU, M.N.; YAO, Y. Da; LIU, Z.Q.; LIU, L.; HE, H.H.; ZHOU, H. Macrophage 3D Migration: A Potential Therapeutic Target for Inflammation and Deleterious Progression in Diseases. Pharmacol Res 2021, 167, 105563. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, L.; Bai, L.; Li, M.; Guo, T.; Tian, B.; He, Z.; Fu, Q. Inflammatory Microenvironment-Targeted Nanotherapies. Journal of Controlled Release 2021, 334, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer Particle Shape Independently Influences Binding and Internalization by Macrophages. Journal of Controlled Release 2010, 147, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Herd, H.; Daum, N.; Jones, A.T.; Huwer, H.; Ghandehari, H.; Lehr, C.M. Nanoparticle Geometry and Surface Orientation Influence Mode of Cellular Uptake. ACS Nano 2013, 7, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Zahr, A.S.; Davis, C.A.; Pishko, M. V. Macrophage Uptake of Core-Shell Nanoparticles Surface Modified with Poly(Ethylene Glycol). Langmuir 2006, 22, 8178–8185. [Google Scholar] [CrossRef]
- Epstein-Barash, H.; Gutman, D.; Markovsky, E.; Mishan-Eisenberg, G.; Koroukhov, N.; Szebeni, J.; Golomb, G. Physicochemical Parameters Affecting Liposomal Bisphosphonates Bioactivity for Restenosis Therapy: Internalization, Cell Inhibition, Activation of Cytokines and Complement, and Mechanism of Cell Death. J Control Release 2010, 146, 182–195. [Google Scholar] [CrossRef]
- Liu, X.; Xie, X.; Jiang, J.; Lin, M.; Zheng, E.; Qiu, W.; Yeung, I.; Zhu, M.; Li, Q.; Xia, T.; et al. Use of Nanoformulation to Target Macrophages for Disease Treatment. Adv Funct Mater 2021, 31. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between Complement Activation, Cellular Uptake and Surface Physicochemical Aspects of Novel PEG-Modified Nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Motskin, M.; Müller, K.H.; Genoud, C.; Monteith, A.G.; Skepper, J.N. The Sequestration of Hydroxyapatite Nanoparticles by Human Monocyte-Macrophages in a Compartment That Allows Free Diffusion with the Extracellular Environment. Biomaterials 2011, 32, 9470–9482. [Google Scholar] [CrossRef] [PubMed]
- Sarparanta, M.; Bimbo, L.M.; Rytkoänen, J.; Mäkilä, E.; Laaksonen, T.J.; Laaksonen, P.; Nyman, M.; Salonen, J.; Linder, M.B.; Hirvonen, J.; et al. Intravenous Delivery of Hydrophobin-Functionalized Porous Silicon Nanoparticles: Stability, Plasma Protein Adsorption and Biodistribution. Mol Pharm 2012, 9, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or Not to PEGylate: Immunological Properties of Nanomedicine’s Most Popular Component, Polyethylene Glycol and Its Alternatives. Adv Drug Deliv Rev 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kawakami, S.; Suzuki, S.; Yamashita, F.; Hashida, M. Enhancement of Immune Responses by DNA Vaccination through Targeted Gene Delivery Using Mannosylated Cationic Liposome Formulations Following Intravenous Administration in Mice. Biochem Biophys Res Commun 2004, 317, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.A.; Barham, W.; Sharman, K.; Tikhomirov, O.; Giorgio, T.D.; Yull, F.E. Manipulating the NF-ΚB Pathway in Macrophages Using Mannosylated, SiRNA-Delivering Nanoparticles Can Induce Immunostimulatory and Tumor Cytotoxic Functions. Int J Nanomedicine 2016, 11, 2163–2177. [Google Scholar] [CrossRef]
- Locke, L.W.; Mayo, M.W.; Yoo, A.D.; Williams, M.B.; Berr, S.S. PET Imaging of Tumor Associated Macrophages Using Mannose Coated 64Cu Liposomes. Biomaterials 2012, 33, 7785–7793. [Google Scholar] [CrossRef]
- Pi, J.; Shen, L.; Yang, E.; Shen, H.; Huang, D.; Wang, R.; Hu, C.; Jin, H.; Cai, H.; Cai, J.; et al. Macrophage-Targeted Isoniazid–Selenium Nanoparticles Promote Antimicrobial Immunity and Synergize Bactericidal Destruction of Tuberculosis Bacilli. Angewandte Chemie - International Edition 2020, 59, 3226–3234. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Jiang, Y.; Zhang, D.; Chen, J.; Dong, L.; Zhang, J. Targeted Delivery of Oligonucleotides into Tumor-Associated Macrophages for Cancer Immunotherapy. Journal of Controlled Release 2012, 158, 286–292. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated Trimethyl Chitosan-Cysteine Nanoparticles Loaded with Map4k4 SiRNA for Targeting Activated Macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ali, H.; Ain, Q.U.; Mukhtar, M.; Gul, R.; Sarwar, A.; Khan, S. A Holistic QBD Approach to Design Galactose Conjugated PLGA Polymer and Nanoparticles to Catch Macrophages during Intestinal Inflammation. Materials Science and Engineering C 2021, 126. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Gou, J.; Sun, W.; Tao, X.; Tan, X.; Wang, P.; Zhang, Y.; He, H.; Yin, T.; Tang, X. Entrapping of Nanoparticles in Yeast Cell Wall Microparticles for Macrophage-Targeted Oral Delivery of Cabazitaxel. Mol Pharm 2018, 15, 2870–2882. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J Drug Deliv 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Amiji, M. Tuftsin-Modified Alginate Nanoparticles as a Noncondensing Macrophage-Targeted DNA Delivery System. Biomacromolecules 2012, 13, 1074–1085. [Google Scholar] [CrossRef]
- Nagai, T.; Tanaka, M.; Tsuneyoshi, Y.; Xu, B.; Michie, S.A.; Hasui, K.; Hirano, H.; Arita, K.; Matsuyama, T. Targeting Tumor-Associated Macrophages in an Experimental Glioma Model with a Recombinant Immunotoxin to Folate Receptor β. Cancer Immunology, Immunotherapy 2009, 58, 1577–1586. [Google Scholar] [CrossRef]
- Thomas, T.P.; Goonewardena, S.N.; Majoros, I.J.; Kotlyar, A.; Cao, Z.; Leroueil, P.R.; Baker, J.R. Folate-Targeted Nanoparticles Show Efficacy in the Treatment of Inflammatory Arthritis. Arthritis Rheum 2011, 63, 2671–2680. [Google Scholar] [CrossRef]
- Rollett, A.; Reiter, T.; Nogueira, P.; Cardinale, M.; Loureiro, A.; Gomes, A.; Cavaco-Paulo, A.; Moreira, A.; Carmo, A.M.; Guebitz, G.M. Folic Acid-Functionalized Human Serum Albumin Nanocapsules for Targeted Drug Delivery to Chronically Activated Macrophages. Int J Pharm 2012, 427, 460–466. [Google Scholar] [CrossRef]
- Gao, Y.; Sarfraz, M.K.; Clas, S.D.; Roa, W.; Löbenberg, R. Hyaluronic Acid-Tocopherol Succinate-Based Self-Assembling Micelles for Targeted Delivery of Rifampicin to Alveolar Macrophages. J Biomed Nanotechnol 2014, 11, 1312–1329. [Google Scholar] [CrossRef]
- Gouveia, V.M.; Lopes-De-Araújo, J.; Costa Lima, S.A.; Nunes, C.; Reis, S. Hyaluronic Acid-Conjugated PH-Sensitive Liposomes for Targeted Delivery of Prednisolone on Rheumatoid Arthritis Therapy. Nanomedicine 2018, 13, 1037–1049. [Google Scholar] [CrossRef]
- Hlaing, S.P.; Cao, J.; Lee, J.; Kim, J.; Saparbayeva, A.; Kwak, D.; Kim, H.; Hwang, S.; Yun, H.; Moon, H.R.; et al. Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Ding, J.; Sui, D.; Liu, M.; Su, Y.; Wang, Y.; Liu, M.; Luo, X.; Liu, X.; Deng, Y.; Song, Y. Sialic Acid Conjugate-Modified Liposomes Enable Tumor Homing of Epirubicin via Neutrophil/Monocyte Infiltration for Tumor Therapy. Acta Biomater 2021, 134, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sui, D.; Liu, M.; Zhang, H.; Liu, M.; Wang, S.; Zhao, D.; Sun, W.; Liu, M.; Luo, X.; et al. Targeted Delivery of Zoledronic Acid through the Sialic Acid - Siglec Axis for Killing and Reversal of M2 Phenotypic Tumor-Associated Macrophages – A Promising Cancer Immunotherapy. Int J Pharm 2020, 590. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Akira, S. Toll-like Receptor Function and Signaling. Journal of Allergy and Clinical Immunology 2006, 117, 979–987. [Google Scholar] [CrossRef]
- Federico, S.; Pozzetti, L.; Papa, A.; Carullo, G.; Gemma, S.; Butini, S.; Campiani, G.; Relitti, N. Modulation of the Innate Immune Response by Targeting Toll-like Receptors: A Perspective on Their Agonists and Antagonists. J Med Chem 2020, 63, 13466–13513. [Google Scholar] [CrossRef]
- Zeng, Q.; Jewell, C.M. Directing Toll-like Receptor Signaling in Macrophages to Enhance Tumor Immunotherapy. Curr Opin Biotechnol 2019, 60, 138–145. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger Receptors in Homeostasis and Immunity. Nat Rev Immunol 2013, 13, 621–634. [Google Scholar] [CrossRef]
- Areschoug, T.; Gordon, S. Scavenger Receptors: Role in Innate Immunity and Microbial Pathogenesis. Cell Microbiol 2009, 11, 1160–1169. [Google Scholar] [CrossRef]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-Type Lectin Receptors with Multivalent Carbohydrate Ligands. Adv Drug Deliv Rev 2013, 65, 1271–1281. [Google Scholar] [CrossRef]
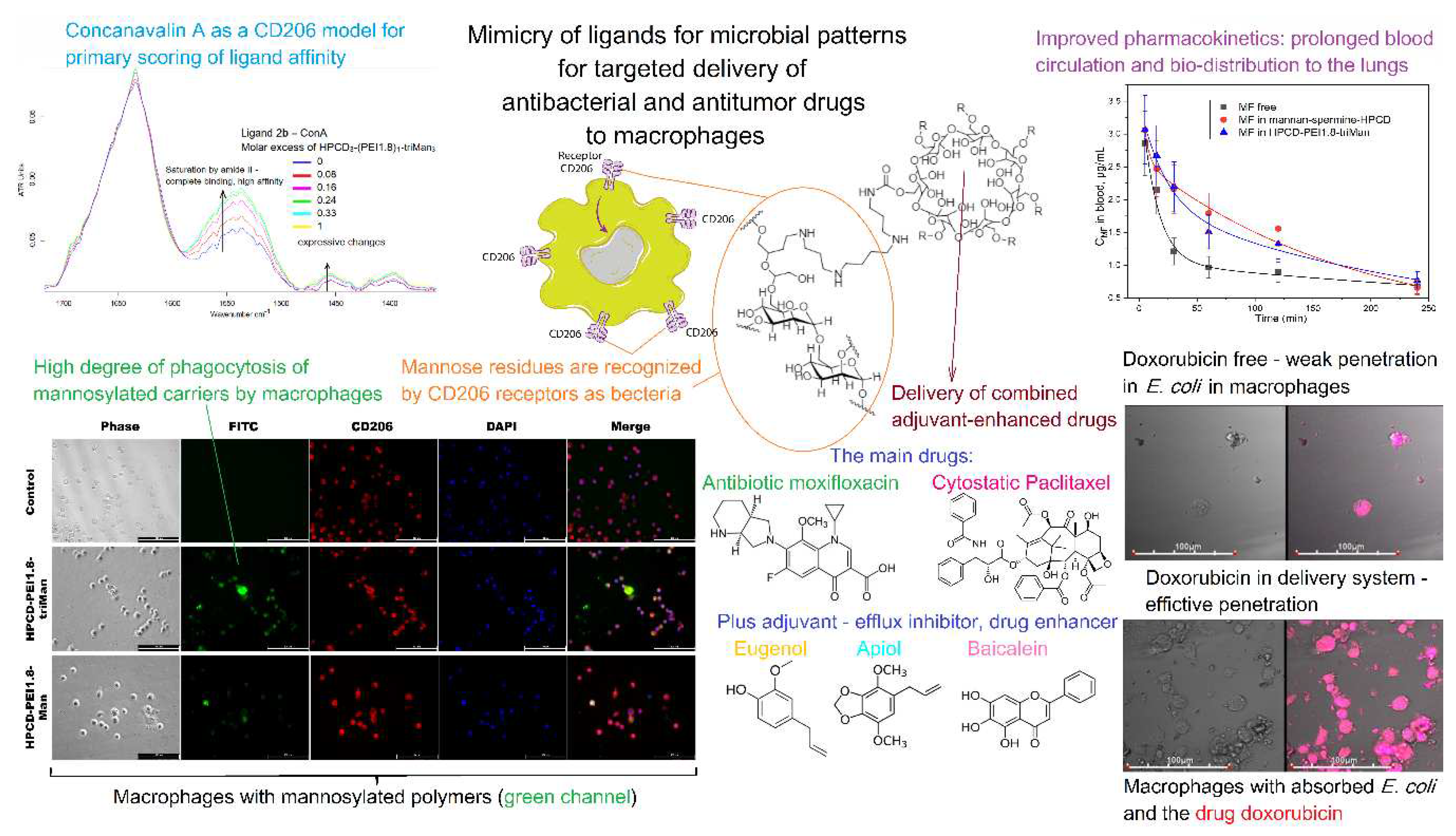
- Zlotnikov, I.D.; Ezhov, A.A.; Petrov, R.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Belogurova, N.G.; Kudryashova, E. V. Mannosylated Polymeric Ligands for Targeted Delivery of Antibacterials and Their Adjuvants to Macrophages for the Enhancement of the Drug Efficiency. Pharmaceuticals 2022, 15. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Vigovskiy, M.A.; Davydova, M.P.; Danilov, M.R.; Dyachkova, U.D.; Grigorieva, O.A.; Kudryashova, E. V. Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, M.; Ye, W.; Huang, Z.; Ma, X.; Wang, W.; Wang, W.; Huang, Y.; Pan, X.; Wu, C. Inhalable Solid Lipid Nanoparticles for Intracellular Tuberculosis Infection Therapy: Macrophage-Targeting and PH-Sensitive Properties. Drug Deliv Transl Res 2021, 11, 1218–1235. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yao, W.; Wang, B.; Zong, L. Mannosylated Chitosan Nanoparticles Based Macrophage-Targeting Gene Delivery System Enhanced Cellular Uptake and Improved Transfection Efficiency. J Nanosci Nanotechnol 2015, 15, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, X.; Jia, L.; Prud’Homme, R.K.; Szekely, Z.; Sinko, P.J. Optimal Structural Design of Mannosylated Nanocarriers for Macrophage Targeting. Journal of Controlled Release 2014, 194, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Ali, H.; Ain, Q.U.; Mukhtar, M.; Gul, R.; Sarwar, A.; Khan, S. A Holistic QBD Approach to Design Galactose Conjugated PLGA Polymer and Nanoparticles to Catch Macrophages during Intestinal Inflammation. Materials Science and Engineering: C 2021, 126, 112183. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Li, R.; Li, C.; Xu, L.; Qiao, W.; Dong, N. Galactose-Modified Nanoparticles for Delivery of MicroRNA to Mitigate the Progress of Abdominal Aortic Aneurysms via Regulating Macrophage Polarization. Nanomedicine 2022, 44, 102564. [Google Scholar] [CrossRef]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM Dendrimers Significantly Improves Tumor Macrophage Targeting and Specificity in Glioblastoma. Journal of Controlled Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Foerster, F.; Bamberger, D.; Schupp, J.; Weilbächer, M.; Kaps, L.; Strobl, S.; Radi, L.; Diken, M.; Strand, D.; Tuettenberg, A.; et al. Dextran-Based Therapeutic Nanoparticles for Hepatic Drug Delivery. https://doi.org/10.2217/nnm-2016-0156 2016, 11, 2663–2677. [CrossRef]
- Shah, N.K.; Gupta, S.K.; Wang, Z.; Meenach, S.A. Enhancement of Macrophage Uptake via Phosphatidylserine-Coated Acetalated Dextran Nanoparticles. J Drug Deliv Sci Technol 2019, 50, 57–65. [Google Scholar] [CrossRef]
- Han, J.; Na, R.; Zhao, N.; Yuan, X.; Fu, L.; Jing, J.; Qian, A.; Ye, W. Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Li, H.; Tatematsu, K.; Somiya, M.; Iijima, M.; Kuroda, S. Development of a Macrophage-Targeting and Phagocytosis-Inducing Bio-Nanocapsule-Based Nanocarrier for Drug Delivery. Acta Biomater 2018, 73, 412–423. [Google Scholar] [CrossRef]
- Tsutsui, Y.; Tomizawa, K.; Nagita, M.; Michiue, H.; Nishiki, T. ichi; Ohmori, I.; Seno, M.; Matsui, H. Development of Bionanocapsules Targeting Brain Tumors. Journal of Controlled Release 2007, 122, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, S.; Zhang, Z.; Chen, M.; Ran, P.; Li, X. Bacterial Ghosts for Targeting Delivery and Subsequent Responsive Release of Ciprofloxacin to Destruct Intracellular Bacteria. Chemical Engineering Journal 2020, 399. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J. V. Fc-Receptors as Regulators of Immunity. Adv Immunol 2007, 96, 179–204. [Google Scholar]
- Mkaddem, S. Ben; Benhamou, M.; Monteiro, R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front Immunol 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, Z.; Stabinsky, Y.; Fridkin, M.; Goldman, R. Tuftsin-Macrophage Interaction: Specific Binding and Augmentation of Phagocytosis. J Cell Physiol 1979, 100, 55–62. [Google Scholar] [CrossRef]
- Khan, M.A. Targeted Drug Delivery Using Tuftsin-Bearing Liposomes: Implications in the Treatment of Infectious Diseases and Tumors. Curr Drug Targets 2020, 22, 770–778. [Google Scholar] [CrossRef]
- Liang, D.S.; Wen, Z.J.; Wang, J.H.; Zhu, F.F.; Guo, F.; Zhou, J.L.; Xu, J.J.; Zhong, H.J. Legumain Protease-Sheddable PEGylated, Tuftsin-Modified Nanoparticles for Selective Targeting to Tumour-Associated Macrophages. J Drug Target 2022, 30, 82–93. [Google Scholar] [CrossRef]
- Horváti, K.; Bacsa, B.; Szabó, N.; Dávid, S.; Mezo, G.; Grolmusz, V.; Vértessy, B.; Hudecz, F.; Bo’sze, S. Enhanced Cellular Uptake of a New, in Silico Identified Antitubercular Candidate by Peptide Conjugation. Bioconjug Chem 2012, 23, 900–907. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the Folate Receptor α in Oncology. Nature Reviews Clinical Oncology 2020 17:6 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Rios de la Rosa, J.M.; Tirella, A.; Gennari, A.; Stratford, I.J.; Tirelli, N. The CD44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv Healthc Mater 2017, 6. [Google Scholar] [CrossRef]
- Paulos, C.M.; Turk, M.J.; Breur, G.J.; Low, P.S. Folate Receptor-Mediated Targeting of Therapeutic and Imaging Agents to Activated Macrophages in Rheumatoid Arthritis. Adv Drug Deliv Rev 2004, 56, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, S.; Kjems, J. Folic Acid Conjugated Chitosan for Targeted Delivery of SiRNA to Activated Macrophages in Vitro and in Vivo. J Mater Chem B 2014, 2, 8608–8615. [Google Scholar] [CrossRef] [PubMed]
- Poh, S.; Chelvam, V.; Ayala-López, W.; Putt, K.S.; Low, P.S. Selective Liposome Targeting of Folate Receptor Positive Immune Cells in Inflammatory Diseases. Nanomedicine 2018, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Y.; Xia, R.; Wei, Y.; Wei, X. Role of the CCL2-CCR2 Signalling Axis in Cancer: Mechanisms and Therapeutic Targeting. Cell Prolif 2021, 54. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Q.; Dong, M.; Guo, D.; Wu, X.; Wang, B. Glioblastoma Immunotherapy Targeting the Innate Immune Checkpoint CD47-SIRPα Axis. Front Immunol 2020, 11. [Google Scholar] [CrossRef]
- PF, L.; N, S.; B, J.; A, K.-B. Monocyte-Induced Prostate Cancer Cell Invasion Is Mediated by Chemokine Ligand 2 and Nuclear Factor-ΚB Activity. J Clin Cell Immunol 2015, 6. [Google Scholar] [CrossRef]
- Grossman, J.G.; Nywening, T.M.; Belt, B.A.; Panni, R.Z.; Krasnick, B.A.; DeNardo, D.G.; Hawkins, W.G.; Goedegebuure, S.P.; Linehan, D.C.; Fields, R.C. Recruitment of CCR2+ Tumor Associated Macrophage to Sites of Liver Metastasis Confers a Poor Prognosis in Human Colorectal Cancer. Oncoimmunology 2018, 7. [Google Scholar] [CrossRef]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 Inhibition Reduces Tumor Myeloid Cells and Unmasks a Checkpoint Inhibitor Effect to Slow Progression of Resistant Murine Gliomas. Proc Natl Acad Sci U S A 2020, 117, 1129–1138. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Scheeren, F.A.; Schumacher, T.N. The CD47-SIRPα Immune Checkpoint. Immunity 2020, 52, 742–752. [Google Scholar] [CrossRef]
- Russ, A.; Hua, A.B.; Montfort, W.R.; Rahman, B.; Riaz, I. Bin; Khalid, M.U.; Carew, J.S.; Nawrocki, S.T.; Persky, D.; Anwer, F. Blocking “Don’t Eat Me” Signal of CD47-SIRPα in Hematological Malignancies, an in-Depth Review. Blood Rev 2018, 32, 480–489. [Google Scholar] [CrossRef]
- Ho, C.C.M.; Guo, N.; Sockolosky, J.T.; Ring, A.M.; Weiskopf, K.; Özkan, E.; Mori, Y.; Weissman, I.L.; Garcia, K.C. “Velcro” Engineering of High Affinity CD47 Ectodomain as Signal Regulatory Protein α (SIRPα) Antagonists That Enhance Antibody-Dependent Cellular Phagocytosis. Journal of Biological Chemistry 2015, 290, 12650–12663. [Google Scholar] [CrossRef]
- Murata, Y.; Tanaka, D.; Hazama, D.; Yanagita, T.; Saito, Y.; Kotani, T.; Oldenborg, P.A.; Matozaki, T. Anti-Human SIRPα Antibody Is a New Tool for Cancer Immunotherapy. Cancer Sci 2018, 109, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPα Antibody Immunotherapy Enhances Neutrophil and Macrophage Antitumor Activity. Proc Natl Acad Sci U S A 2017, 114, E10578–E10585. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, T.; Peng, B.; Luo, X.; Liu, X.; Hu, L.; Liu, Y.; Di, D.; Song, Y.; Deng, Y. Targeted Delivery of Epirubicin to Tumor-Associated Macrophages by Sialic Acid-Cholesterol Conjugate Modified Liposomes with Improved Antitumor Activity. Int J Pharm 2017, 523, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.B.; Dutta, P.; Dahlman, J.E.; Hulsmans, M.; Courties, G.; Sun, Y.; Heidt, T.; Vinegoni, C.; Borodovsky, A.; Fitzgerald, K.; et al. RNAi Targeting Multiple Cell Adhesion Molecules Reduces Immune Cell Recruitment and Vascular Inflammation after Myocardial Infarction. Sci Transl Med 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.-S.; Chan Kwon, I.; Tae, G. Targeted Delivery of Anti-Inflammatory Cytokine by Nanocarrier Reduces Atherosclerosis in Apo E−/- Mice. Biomaterials 2020, 226, 119550. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.H.; Huh, B.K.; Yoo, Y.C.; Heo, C.Y.; Choy, Y. Bin; Park, J.H. Surgical Suture Releasing Macrophage-Targeted Drug-Loaded Nanoparticles for an Enhanced Anti-Inflammatory Effect. Biomater Sci 2017, 5, 1670–1677. [Google Scholar] [CrossRef]
- Ospelt, C.; Gay, S. TLRs and Chronic Inflammation. Int J Biochem Cell Biol 2010, 42, 495–505. [Google Scholar] [CrossRef]
- Murgueitio, M.S.; Henneke, P.; Glossmann, H.; Santos-Sierra, S.; Wolber, G. Prospective Virtual Screening in a Sparse Data Scenario: Design of Small-Molecule TLR2 Antagonists. ChemMedChem 2014, 9, 813–822. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Song, X.Y.; Li, Y.; Ye, L.L.; Zhou, Q.; Yang, W.B. Tumor-Associated Macrophages: A Promising Target for a Cancer Immunotherapeutic Strategy. Pharmacol Res 2020, 161. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Chen, K.G.; Luo, Y.L.; Wang, J. Cationic Polymeric Nanoparticle Delivering CCR2 SiRNA to Inflammatory Monocytes for Tumor Microenvironment Modification and Cancer Therapy. Mol Pharm 2018, 15, 3642–3653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Huang, L. Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention. Adv Funct Mater 2021, 31. [Google Scholar] [CrossRef]
- Veillette, A.; Tang, Z. Signaling Regulatory Protein (SIRP)a-CD47 Blockade Joins the Ranks of Immune Checkpoint Inhibition. Journal of Clinical Oncology 2019, 37, 1012–1014. [Google Scholar] [CrossRef]
- Yanagita, T.; Murata, Y.; Tanaka, D.; Motegi, S. ichiro; Arai, E.; Daniwijaya, E.W.; Hazama, D.; Washio, K.; Saito, Y.; Kotani, T.; et al. Anti-SIRPα Antibodies as a Potential New Tool for Cancer Immunotherapy. JCI Insight 2017, 2, 89140. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res 2011, 71, 1374–1384. [Google Scholar] [CrossRef]
- Koh, E.; Lee, E.J.; Nam, G.H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.S. Exosome-SIRPα, a CD47 Blockade Increases Cancer Cell Phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef]
- Paul, B.; Liedtke, M.; Khouri, J.; Rifkin, R.; Gandhi, M.D.; Kin, A.; Levy, M.Y.; Silbermann, R.; Cottini, F.; Sborov, D.W.; et al. A Phase II Multi-Arm Study of Magrolimab Combinations in Patients with Relapsed/Refractory Multiple Myeloma. Future Oncology 2023, 19, 7–17. [Google Scholar] [CrossRef]
- Voets, E.; Paradé, M.; Lutje Hulsik, D.; Spijkers, S.; Janssen, W.; Rens, J.; Reinieren-Beeren, I.; Van Den Tillaart, G.; Van Duijnhoven, S.; Driessen, L.; et al. Functional Characterization of the Selective Pan-Allele Anti-SIRPα Antibody ADU-1805 That Blocks the SIRPα-CD47 Innate Immune Checkpoint. J Immunother Cancer 2019, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Velez-Delgado, A.; Mathew, E.; Li, D.; Mendez, F.M.; Flannagan, K.; Rhim, A.D.; Simeone, D.M.; Beatty, G.L.; Di Magliano, M.P. Myeloid Cells Are Required for PD-1/PD-L1 Checkpoint Activation and the Establishment of an Immunosuppressive Environment in Pancreatic Cancer. Gut 2017, 66, 124–136. [Google Scholar] [CrossRef]
- Li, C.; Lai, C.; Qiu, Q.; Luo, X.; Hu, L.; Zheng, H.; Lu, Y.; Liu, M.; Zhang, H.; Liu, X.; et al. Dual-Ligand Modification of PEGylated Liposomes Used for Targeted Doxorubicin Delivery to Enhance Anticancer Efficacy. AAPS PharmSciTech 2019, 20. [Google Scholar] [CrossRef]
- Datta, M.; Coussens, L.M.; Nishikawa, H.; Hodi, F.S.; Jain, R.K. Reprogramming the Tumor Microenvironment to Improve Immunotherapy: Emerging Strategies and Combination Therapies. [CrossRef]
- Sousa, S.; Auriola, S.; Mönkkönen, J.; Määttä, J. Liposome Encapsulated Zoledronate Favours M1-like Behaviour in Murine Macrophages Cultured with Soluble Factors from Breast Cancer Cells. BMC Cancer 2015, 15. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.X.; Qiao, S.L.; An, H.W.; Ma, Y.; Qiao, Z.Y.; Rajapaksha, R.P.Y.J.; Wang, H. Polymeric Nanoparticles Enable Reversing Macrophage in Tumor Microenvironment for Immunotherapy. Biomaterials 2017, 112, 153–163. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, B.Y.; Ai, S.L.; Xu, L.; Zhuo, R.X.; Cheng, S.X. Functional Polymer/Inorganic Hybrid Nanoparticles for Macrophage Targeting Delivery of Oligodeoxynucleotides in Cancer Immunotherapy. Mater Today Chem 2017, 4, 106–116. [Google Scholar] [CrossRef]
- Sun, Y.; Cronin, M.F.; Mendonça, M.C.P.; Guo, J.; O’Driscoll, C.M. Sialic Acid-Targeted Cyclodextrin-Based Nanoparticles Deliver CSF-1R SiRNA and Reprogram Tumour-Associated Macrophages for Immunotherapy of Prostate Cancer. European Journal of Pharmaceutical Sciences 2023, 185. [Google Scholar] [CrossRef]
- Nascimento, C.; Castro, F.; Domingues, M.; Lage, A.; Alves, É.; de Oliveira, R.; de Melo, C.; Eduardo Calzavara-Silva, C.; Sarmento, B. Reprogramming of Tumor-Associated Macrophages by Polyaniline-Coated Iron Oxide Nanoparticles Applied to Treatment of Breast Cancer. Int J Pharm 2023, 636, 122866. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, B.; Wu, L.; Xiao, H.; Ding, K.; Zheng, C.; Song, Q.; Sun, L.; Wang, L.; Zhang, Z. Amplified Cancer Immunotherapy of a Surface-Engineered Antigenic Microparticle Vaccine by Synergistically Modulating Tumor Microenvironment. ACS Nano 2019, 13, 12553–12566. [Google Scholar] [CrossRef]
- Yoon, J.; Le, X.T.; Kim, J.; Lee, H.; Nguyen, N.T.; Lee, W.T.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Macrophage-Reprogramming Upconverting Nanoparticles for Enhanced TAM-Mediated Antitumor Therapy of Hypoxic Breast Cancer. Journal of Controlled Release 2023, 360, 482–495. [Google Scholar] [CrossRef]
- Djaldetti, M.; Salman, H.; Bergman, M.; Djaldetti, R.; Bessler, H. Phagocytosis - The Mighty Weapon of the Silent Warriors. Microsc Res Tech 2002, 57, 421–431. [Google Scholar] [CrossRef]
- KIRSH, R.; BUGELSKI, P.J.; POSTE, G. Drug Delivery to Macrophages for the Therapy of Cancer and Infectious Diseases. Ann N Y Acad Sci 1987, 507, 141–154. [Google Scholar] [CrossRef]
- Mosaiab, T.; Farr, D.C.; Kiefel, M.J.; Houston, T.A. Carbohydrate-Based Nanocarriers and Their Application to Target Macrophages and Deliver Antimicrobial Agents. Adv Drug Deliv Rev 2019, 151–152, 94–129.
- Kruize, Z.; Kootstra, N.A. The Role of Macrophages in HIV-1 Persistence and Pathogenesis. Front Microbiol 2019, 10. [Google Scholar] [CrossRef]
- Kumar, A.; Herbein, G. The Macrophage: A Therapeutic Target in HIV-1 Infection. Mol Cell Ther 2014, 2, 10. [Google Scholar] [CrossRef]
- Dutta, T.; Garg, M.; Jain, N.K. Targeting of Efavirenz Loaded Tuftsin Conjugated Poly(Propyleneimine) Dendrimers to HIV Infected Macrophages in Vitro. European Journal of Pharmaceutical Sciences 2008, 34, 181–189. [Google Scholar] [CrossRef]
- Garg, M.; Asthana, A.; Agashe, H.B.; Agrawal, G.P.; Jain, N.K. Stavudine-Loaded Mannosylated Liposomes: In-Vitro Anti-HIV-I Activity, Tissue Distribution and Pharmacokinetics. Journal of Pharmacy and Pharmacology 2010, 58, 605–616. [Google Scholar] [CrossRef]
- Adlin Jino Nesalin, J.; Anton Smith, A. Preparation and Evaluation of Stavudine Loaded Chitosan Nanoparticles. J Pharm Res 2013, 6, 268–274. [Google Scholar] [CrossRef]
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S. V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of Poly(Lactic Acid)/Chitosan Nanoparticles for Anti-HIV Drug Delivery Applications. Carbohydr Polym 2010, 80, 833–838. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Jafari, E.; Jahanian-Najafabadi, A.; Taheri, A. Formulation and Characterization of Cellulose Acetate Butyrate Nanoparticles Loaded with Nevirapine for HIV Treatment. J Drug Deliv Sci Technol 2018, 48, 9–20. [Google Scholar] [CrossRef]
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Evaluation of Chitosan Nanoformulations as Potent Anti-HIV Therapeutic Systems. Biochim Biophys Acta Gen Subj 2014, 1840, 476–484. [Google Scholar] [CrossRef]
- Pieters, J. Mycobacterium Tuberculosis and the Macrophage: Maintaining a Balance. Cell Host Microbe 2008, 3, 399–407. [Google Scholar] [CrossRef]
- Gairola, A.; Benjamin, A.; Weatherston, J.D.; Cirillo, J.D.; Wu, H. Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages. Adv Ther (Weinh) 2022, 5, 2100193. [Google Scholar] [CrossRef]
- Mukhtar, M.; Csaba, N.; Robla, S.; Varela-Calviño, R.; Nagy, A.; Burian, K.; Kókai, D.; Ambrus, R. Dry Powder Comprised of Isoniazid-Loaded Nanoparticles of Hyaluronic Acid in Conjugation with Mannose-Anchored Chitosan for Macrophage-Targeted Pulmonary Administration in Tuberculosis. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the Macrophage: A Multifaceted Interaction. Future Microbiol 2015, 10, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Jose, S.; Thomas, C.A.; Joseph, E.; Kiessling, F.; Lammers, T. Physicochemical and Biological Aspects of Macrophage-Mediated Drug Targeting in Anti-Microbial Therapy. Fundam Clin Pharmacol 2012, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.J.; Din, F.U.; Khan, G.M. Sodium Stibogluconate Loaded Nano-Deformable Liposomes for Topical Treatment of Leishmaniasis: Macrophage as a Target Cell. Drug Deliv 2018, 25, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, F.; Motazedian, M.H.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Erratum: Paromomycin-Loaded Mannosylated Chitosan Nanoparticles: Synthesis, Characterization and Targeted Drug Delivery against Leishmaniasis (Acta Tropica (2019) 197 (105045) PII: S0001-706X(19)30864-2). Acta Trop 2019, 197, 105072. [Google Scholar] [CrossRef]
- Dowari, P.; Roy, S.; Das, S.; Chowdhuri, S.; Kushwaha, R.; Das, B.K.; Ukil, A.; Das, D. Mannose-Decorated Composite Peptide Hydrogel with Thixotropic and Syneresis Properties and Its Application in Treatment of Leishmaniasis. Chem Asian J 2022, 17, e202200550. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Kudryashova, E. V. Computer Simulation of the Receptor–Ligand Interactions of Mannose Receptor CD206 in Comparison with the Lectin Concanavalin A Model. Biochemistry (Moscow) 2022, 87, 54–69. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Dyachkova, U.D.; Belogurova, N.G.; Kudryashova, E. V Application Prospects of FTIR Spectroscopy and CLSM to Monitor the Drugs Interaction with Bacteria Cells Localized in Macrophages for Diagnosis and Treatment Control of Respiratory Diseases. 2023, 1–23.
- Zlotnikov, I.D.; Kudryashova, E. V Spectroscopy Approach for Highly - Efficient Screening of Lectin - Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs. 2022.
- Zlotnikov, I.D.; Vanichkin, D.A.; Kudryashova, E.V. Methods for Determining the Parameters of Receptor-Ligand Interactions on the Model of Concanavalin A and Mannosylated Chitosans Promising Carriers for Drug Delivery to Alveolar Macrophages. Biotekhnologiya 2021, 37, 28–40. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Davydova, M.P.; Danilov, M.R.; Krylov, S.S.; Belogurova, N.G. Covalent Conjugates of Allylbenzenes and Terpenoids as Antibiotics Enhancers with the Function of Prolonged Action. 2023, 1–34.
- Zlotnikov, I.D.; Streltsov, D.A.; Belogurova, N.G.; Kudryashova, E. V. Chitosan or Cyclodextrin Grafted with Oleic Acid Self-Assemble into Stabilized Polymeric Micelles with Potential of Drug Carriers. Life 2023, 13. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V. V; Kudryashova, E. V Polymeric Micelles Formulation of Combretastatin Derivatives with Enhanced Solubility , Cytostatic Activity and Selectivity against Cancer Cells. 2023.
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. 2023.
- Zlotnikov, I.D.; Kudryashova, E. V. Mannose Receptors of Alveolar Macrophages as a Target for the Addressed Delivery of Medicines to the Lungs. Russ J Bioorg Chem 2022, 48, 46–75. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Vigovskiy, M.A.; Davydova, M.P.; Danilov, M.R.; Dyachkova, U.D.; Grigorieva, O.A.; Kudryashova, E. V Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. 2022, 1–29.
- Zlotnikov, I.D.; Belogurova, N.G.; Krylov, S.S.; Semenova, M.N.; Semenov, V. V; Kudryashova, E. V Plant Alkylbenzenes and Terpenoids in the Form of Cyclodextrin Inclusion Complexes as Antibacterial Agents and Levofloxacin Synergists. 2022.
- Zlotnikov, I.D.; Malashkeevich, S.M.; Belogurova, N.G.; Kudryashova, E. V. Thermoreversible Gels Based on Chitosan Copolymers as “Intelligent” Drug Delivery System with Prolonged Action for Intramuscular Injection. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Dobryakova, N. V; Ezhov, A.A.; Kudryashova, E. V Achievement of the Selectivity of Cytotoxic Agents against Can- Cer Cells by Creation of Combined Formulation with Terpenoid Adjuvants as Prospects to Overcome Multidrug Resistance. 2022, 1–34.
- Zlotnikov, I.D.; Savchenko, I. V; Kudryashova, E. V Fluorescent Probes with Förster Resonance Energy Transfer Function for Monitoring the Gelation and Formation of Nanoparticles Based on Chitosan Copolymers. 2023.
- Pflumm, M.N.; Wang, J.L.; Edelman, G.M. Conformational Changes in Concanavalin A. Journal of Biological Chemistry 1971, 246, 4369–4370. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Belogurova, N.G.; Kudryashova, E. V. The Formation of Quasi-Regular Polymeric Network of Cross-Linked Sulfobutyl Ether Derivative of β-Cyclodextrin Synthesized with Moxifloxacin as a Template. React Funct Polym 2021, 159, 104811. [Google Scholar] [CrossRef]
- Rodriguez-perez, A.N.A.I.; Rodriguez-tenreiro, C.; Alvarez-lorenzo, C.; Taboada, P.; Concheiro, A.; Torres-labandeira, J.J. Sertaconazole / Hydroxypropyl- b -Cyclodextrin Complexation : Isothermal Titration Calorimetry and Solubility Approaches. 2006, 95, 1751–1762. [CrossRef]
- Castro, S.; Duff, M.; Snyder, N.L.; Morton, M.; Kumar, C. V.; Peczuh, M.W. Recognition of Septanose Carbohydrates by Concanavalin A. Org Biomol Chem 2005, 3, 3869–3872. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.; Auerbach, D.; Jung, G.; Wenz, G. Inclusion of Chemotherapeutic Agents in Substituted β-Cyclodextrin Derivatives. J Incl Phenom Macrocycl Chem 2011, 69, 303–307. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int J Mol Sci 2016, 17, 1–34. [Google Scholar] [CrossRef]
- Białas, N.; Sokolova, V.; van der Meer, S.B.; Knuschke, T.; Ruks, T.; Klein, K.; Westendorf, A.M.; Epple, M. Bacteria ( E. Coli ) Take up Ultrasmall Gold Nanoparticles (2 Nm) as Shown by Different Optical Microscopic Techniques (CLSM, SIM, STORM). Nano Select 2022, 3, 1407–1420. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Uporov, I. V.; Kudryashova, E. V. Thermodynamics and Molecular Insight in Guest–Host Complexes of Fluoroquinolones with β-Cyclodextrin Derivatives, as Revealed by ATR-FTIR Spectroscopy and Molecular Modeling Experiments. Anal Bioanal Chem 2017, 409, 6451–6462. [Google Scholar] [CrossRef]

| Utilization of living cells | ||||||
| Method of binding to macrophages | Source | Carrier formulation | Cargo | Loading efficiency | Cell viability | Ref. |
| Incubation (engulfment) | RAW264.7 | - | Doxorubicin (400 µg/ml) | ≈14% (after 10 s of incubation) | 79% at 72 h after incubation | [63] |
| - Liposomes |
AuNRs (150 µg/ml) + Doxorubicin (25 µg/ml) |
13.34% (after 6 h of incubation) 35.2% |
85% after 6 h of incubation | [61] | ||
| - | Bioengineered Salmonella typhimurium | 220 ± 13 CFU /100 cells (after 60 min of incubation) | >90% after 60 min of incubation | [79] | ||
| Mouse peritoneal macrophages | - | Doxorubicin (1-200 µg/ml) | No data | about 30-60% after 12 h of incubation | [80] | |
| Liposomes | Doxorubicin (1-200 µg/ml) | No data | about 80-90% at 12 h after incubation | |||
| BMM | Polymeric NPs (100 µg/mL) | Nitric oxide | ≈77% (after 2 h of incubation) | ≈100% for incubation period of 24 h and 48 h | [70] | |
| Human monocyte-derived macrophages | Liposomes (100 μM) | Indinavir | 85% (after 4 h of incubation) | No effect of drug encapsulation on macrophage viability was observed | [81] | |
| Hypotonic dialysis | THP-1 | - | Catalase (osmolality of 75.67 mOsm/L during 15 min of dialysis) |
53% | 89% after encapsulation | [52] |
| Electroporation | J774 | - | Doxorubicin (20mg/mL) | 5% (after <20 s of electroporation) | Drug-loading significantly decreased cell viability | [53] |
| Adhesion | Raw 264.7 | Multilayer microfilm (“backpack”) | Catalase (2.3 µU/cell backpack) | 80% (after a brief incubation with the “backpacks”) | Attachment of cell backpacks to macrophages did not alter their major functions | [82] |
| J774 | Multilayer microfilm (“backpack”) | Bovine serum albumin | ≈95% (after incubation with the “backpacks” for 4 h) | “Cellular backpacks” didn’t affect macrophage biological functions | [83] | |
| Utilization of macrophage-derived membrane structures | ||||||
| Source | Carrier formulation | Cargo | Method of encapsulation | Detected proteins | Ref. | |
| Cellular membranes | J774 | Polymeric NPs | - | Sonication | CD126, CD130, CD120, CD119, CD14 and TLR4 | [54] |
| Mouse peritoneal macrophages | Polymeric NPs | Paclitaxel | Sonication | No data | [84] | |
| RAW264.7 | - | Methyltransferase like 14 + RS09 | Coextrusion | No data | [85] | |
| RAW 264.7 | Bi2Se3 hollow mesoporous NPs | Quercetin | Coextrusion | α4 integrin, CCR2 | [86] | |
| Vesicles | RAW264.7 | - | Paclitaxel | Sonication | Alix, TSG101, CD9, iNOS, Arg-1 | [87] |
| J774A.1 | Liposomes | Doxorubicin | Vortexing, sonication and coextrusion | CD81, CD63 and CD9 | [88] | |
| RAW 264.7 | Polymeric NPs | - | Sonication | CD45, CD14, CD44, CD18, Mac-1 etc. | [56] | |
| RAW264.7 | - | Brain derived neurotrophic factor | Simple mixing | Alix, Tsg 101, LAMP 2 and cytosolic protein β-actin | [89] | |
| Vehicle | Carrier formulation | Cargo | Target | Highlighted features of macrophage-like particles | Therapeutic effect | Ref. |
| Macrophage membrane | Chitosan NPs | - | Tumor cells: HeLa, MCF7 and MDA-MB-231 (in vitro) | Stability Biocompatibility and hemocompatibility Triggering apoptosis due to the presence of TNFα in macrophage membrane |
Dose-dependent anti-tumor proliferative properties and triggering of apoptosis | [109] |
| Macrophage | - | Doxorubicin | 4T1 mouse breast cancer cells (in vivo) | Meaningful content of the drug High targeting ability |
Significant inhibition of tumor growth and increasing the survival rate among tumor-bearing mice | [63] |
| Macrophage | Poly(D,L-lactide-co-glycolide) micelles and Pluronic block copolymer micelles | Paclitaxel | Human glioma cell line U87 (in vitro) | Main biological functions of macrophages were preserved Anti-tumor effect was enhanced compared to nano-Paclitaxel |
Significant tumor cell growth inhibition | [110] |
| Macrophage | Poly(D,L-lactide-co-glycolide) NPs | Tirapazamine | 4T1 mouse breast cancer cells (in vivo) | Targeting ability Enhanced accumulation in hypoxic areas of tumor |
Inhibition of tumor growth and extension in the median survival time, especially in the synergetic chemotherapy | [111] |
| Macrophage | - | siRNA lipoplexes | MDA-MB-468 breast cancer model (in vivo) | Ability for horizontal gene transfer of siRNA in tumor site Anti-tumor effect was enhanced compared to pure siRNA Results indicated that exosomal secretion via M2 activation is involved with gene transfer |
A significant reduction in the tumor spheres growth | [112] |
| Macrophage | N-methacryloyl mannosamine (conjugated to macrophage surface) | Nucleic acid aptamers | CCRF-CEM tumor cells (in vitro) | Surface modification didn’t affect macrophage phenotype and viability The capture of tumor cells was improved |
Enhanced anticancer immune response via macrophages | [113] |
| Macrophage | - | Oncolytic adenovirus | Human prostate tumor model (in vivo) | Targeting ability Accumulation in hypoxic/perinecrotic areas of the tumor |
A lasting antitumor effect with negligible metastatic frequency | [114] |
| Macrophage membrane | Gold nanoshells (AuNSs) | Cy7 | 4T1 cancer cells (in vivo) | Active targeting ability High tumoritropic accumulation Good biocompatibility Prolonged circulation time Membrane coating didn’t affect NIR optical properties of AuNSs |
Effective inhibition of tumor growth and its complete eradication after 25 days of photothermal therapy | [115] |
| Macrophage | - Liposomes |
AuNRs + Doxorubicin |
4T1 mouse breast cancer cells (in vivo) | High targeting ability Effective infiltration into the tumor tissue High thermal sensitivity Controlled drug release via photothermal perfomance |
Synergetic chemo- and phototherapy allowed enhanced tumor growth inhibition | [61] |
| Macrophage | Liposomes | ICG (photothermal agent) + Resveratrol (anti-inflammatory drug) |
4T1 post-operative model (in vivo) | tumor-targeting ability good inflammatory tropism release of the liposomes was enhanced due to membrane destruction via phototherapy excellent photothermal performance |
Ablation of residual tumor tissues, inhibiting tumor postoperative relapse and reduction of the postoperative inflammation | [116] |
| Method | Applications | Brief description | References |
| FTIR spectroscopy | Macrophage CD206 receptor – ligand interaction studies on the example of ConA model and mannosylated polymers | The use of a model receptor protein allows for rapid primary screening of ligands and selection of the most affine ones, and it is not necessary to isolate hard-to-reach CD206 | [253,260] |
| Drug – delivery system (to macrophages) interactions | Registration of FTIR spectra of drug complexes with different polymer ratios and calculation of dissociation constants, entrapment efficiency. Study of molecular details of binding ( functional groups) | [258,260,261] | |
| Cells – drug formulation interactions. The effect of the drug on the cells. Selection of the optimal composition of the drug formulation | Provide information about the main components of the cell interacting with the drug. Using this technique, efflux and its inhibition on bacterial and cancer cells were demonstrated | [252,258] | |
| Quantification of living cells | Centrifugation of cell suspension and registration of the FTIR spectra of sediment. Low analysis time: does not require seeding of bacteria on a Petri dish | [255] | |
| Characterization of polymeric drug delivery systems | The presence of all components, and the success of crosslinking. Molecular architecture | [172,253,255,256,257,258,260,261,262,263] | |
| NMR spectroscopy | Drug interaction with the delivery system | The NMR spectrum provides valuable information about the functional groups involved | [261] |
| Characterization of polymer drug delivery systems | The presence of all components, and the success of crosslinking | [172,255,260] | |
| Fluorescence spectroscopy | Macrophage CD206 receptor – ligand interaction studies on the example of ConA model and mannosylated polymers | Quenching of tryptophan fluorescence in the receptor protein and an increase in fluorescence anisotropy during ligand binding. An alternative is using a FITC-labeled ligand | [254] |
| Inclusion of fluorophores-drugs in polymer particles | Change of fluorescent properties: the position and intensity of the maximum, as well as FRET | [264] | |
| Interaction of ligands with cells, adsorption and permeability over time, and the effect of efflux inhibitors on drug permeability and retention | |||
| UV spectroscopy | Macrophage CD206 receptor – ligand interaction studies on the example of ConA model and mannosylated polymers | Change in protein uptake during ligand binding and change in secondary structure | [254] |
| Loading and release of drugs from polymer carriers | Absorption characterizes the amount of drug loaded or released from nanoparticles | [253,256] | |
| Antibacteril activity | A600 correlates with the number of colony-forming units | [255,256,261] | |
| Circular dichroism spectroscopy | Secondary structure of macrophage CD206 receptor (or its model protein on the example of ConA model) during ligand binding | Changing the circular dichroism sometimes with a cardinal reversal of the spectrum | [265] |
| Loading of chiral drugs into polymeric particles | [266] | ||
| Isothermal titration calorimetry | Study of macrophage CD206 receptor – ligand interaction studies on the example of ConA model and mannosylated polymers | Thermodynamic parameters (enthalpy, entropy and Gibbs energy) of ligand–receptor complex formation | [267,268,269,270] |
| Atomic force microscopy, SEM and TEM | Study of the morphology of nanoparticles, simulating epitops of pathogenic microoragnisms recognized by macrophages. Study of the morphology of bacterial and macrophage cells with adsorbed polymers | High-quality images providing information about the structure of nanoparticles and their effect on bacteria | [257,264,266] |
| Nanoparticle tracking analysis (NTA) | Characterization of macrophage terget drug delivery system (nanoparticles) | The rate of particle movement is related to a sphere equivalent hydrodynamic radius as calculated through the Stokes–Einstein equation | [256] |
| Dynamic light scattering | Detection of polymeric nanoparticles interaction with cells surface by changing of zeta potential of bacteria and macrofages cells during polymer adsorption | The zeta potential characterizes the stability of nanoparticles. For cells, there is a recharge during the adsorption of polymers | [172,257,264] |
| Confocal laser scanning microscopy | Interaction of drug formulations with bacterial and eukaryotic (macrophage and cancerous) cells | Images from multiple cells at the micro and nanoscale. Inhibition of efflux (reverse release of drugs from cells) has been demonstrated | [172,252,271] |
| Microbiological studies | Study of the antibacterial effect of drugs including effect on bacteria inside macrophage | The strengthening and prolonged (in vitro) of antibacterial drugs due to the addition of adjuvants to them has been demonstrated | [172,253,255,256,260,261] |
| Pharmacokinetics studies | Testing the macrophage terget drug delivery system in terms od the drug circulation time in the bloodstream and bio-distribution | A multiple increase in the half-life of the drug is shown, especially for covalent pro-drugs, and accumulation in the lungs | [255,256,260] |
| Flow cytometry | The existence of fluorescent nanoparticles with the drug (not debris) | [257] | |
| Nanoparticles adsorption on E. coli cells, and quantification of living cells by DAPI staining | |||
| Computer modeling | Molecular dynamics and neural network analysis of macrophage CD206-ligand and drug-polymer interaction | The study of ligands does not require synthesis in the laboratory and complex experiments - as the primary stage of selecting candidates for drug delivery systems to macrophages. Molecular architecture of complexes, binding sites and prediction of binding energy. | [251,272] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




