Submitted:
10 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and housing
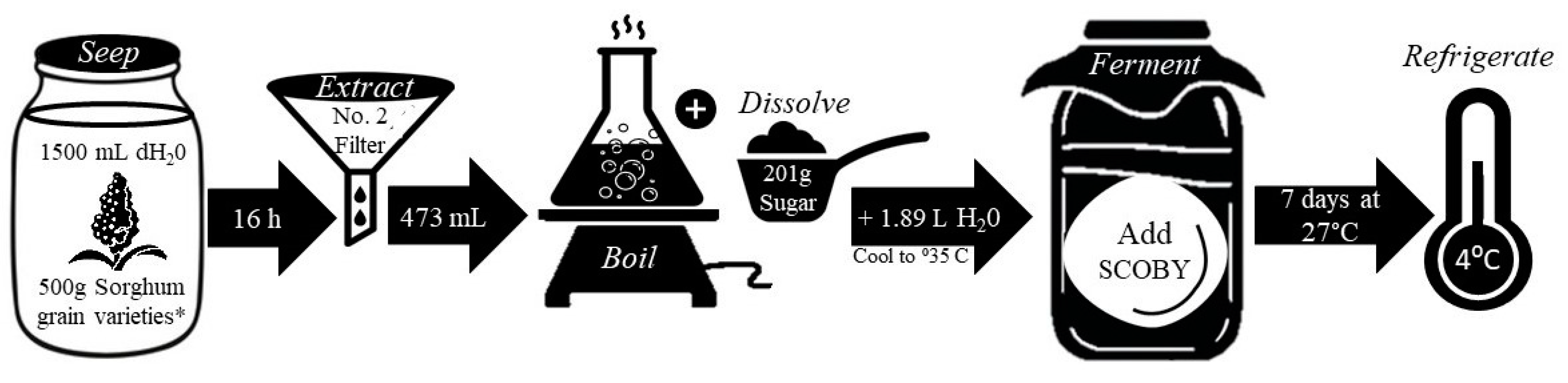
2.2. Formulation of solutions
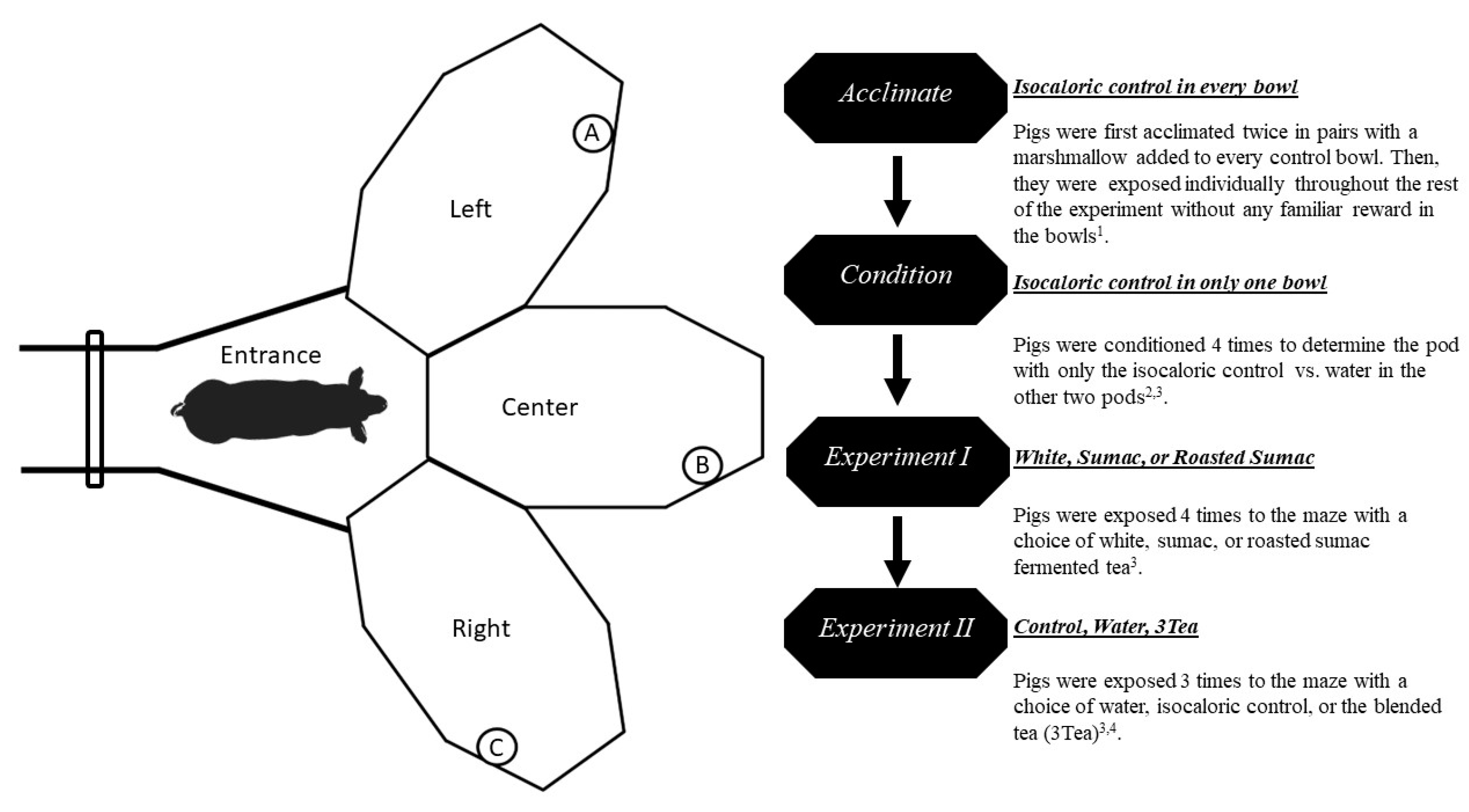
2.3. Preference Testing
2.4. Video Behavioral quantification
2.5. Statistical Analysis
3. Results
3.1. General Results
3.2. Experiment 1
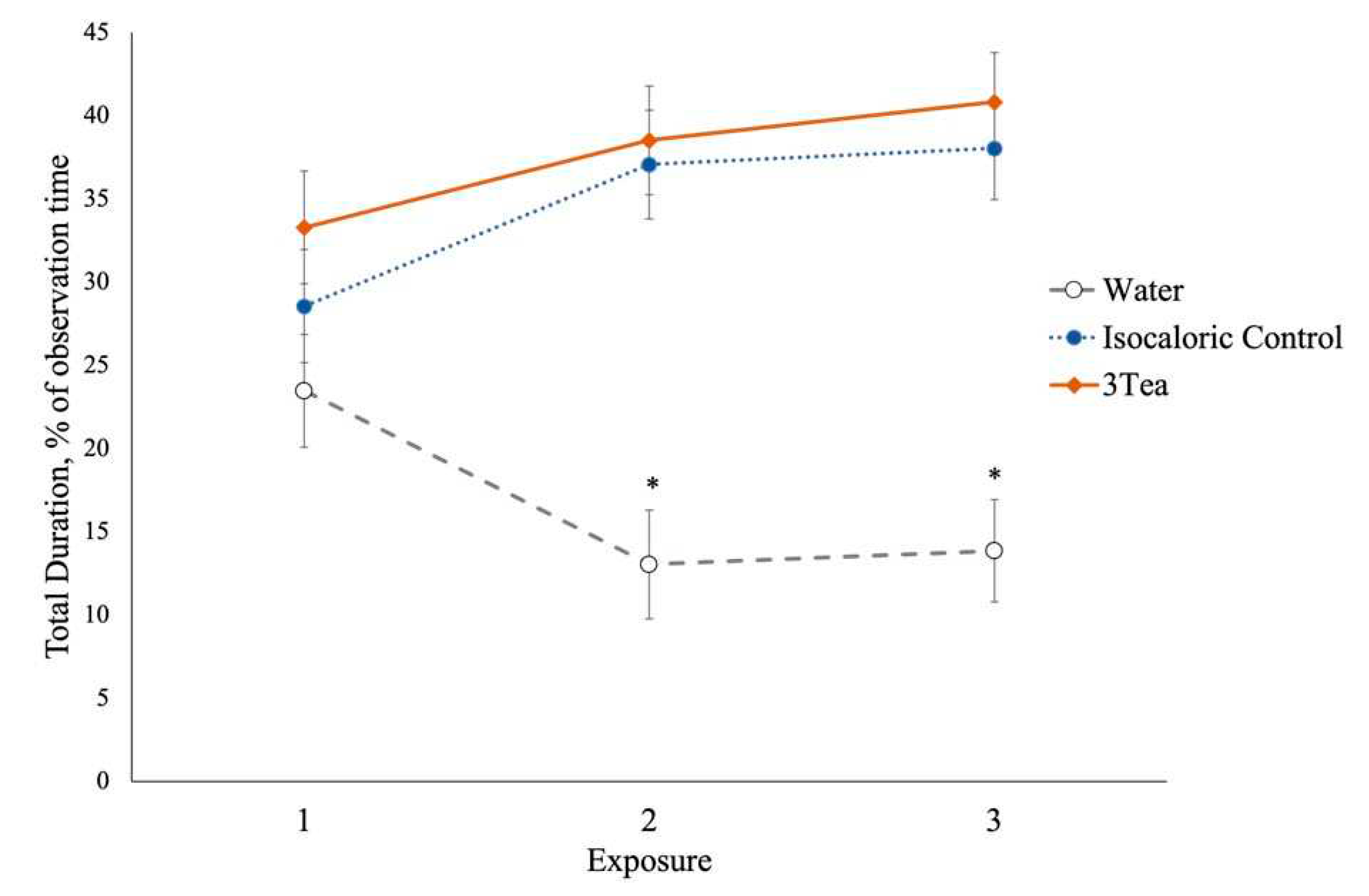
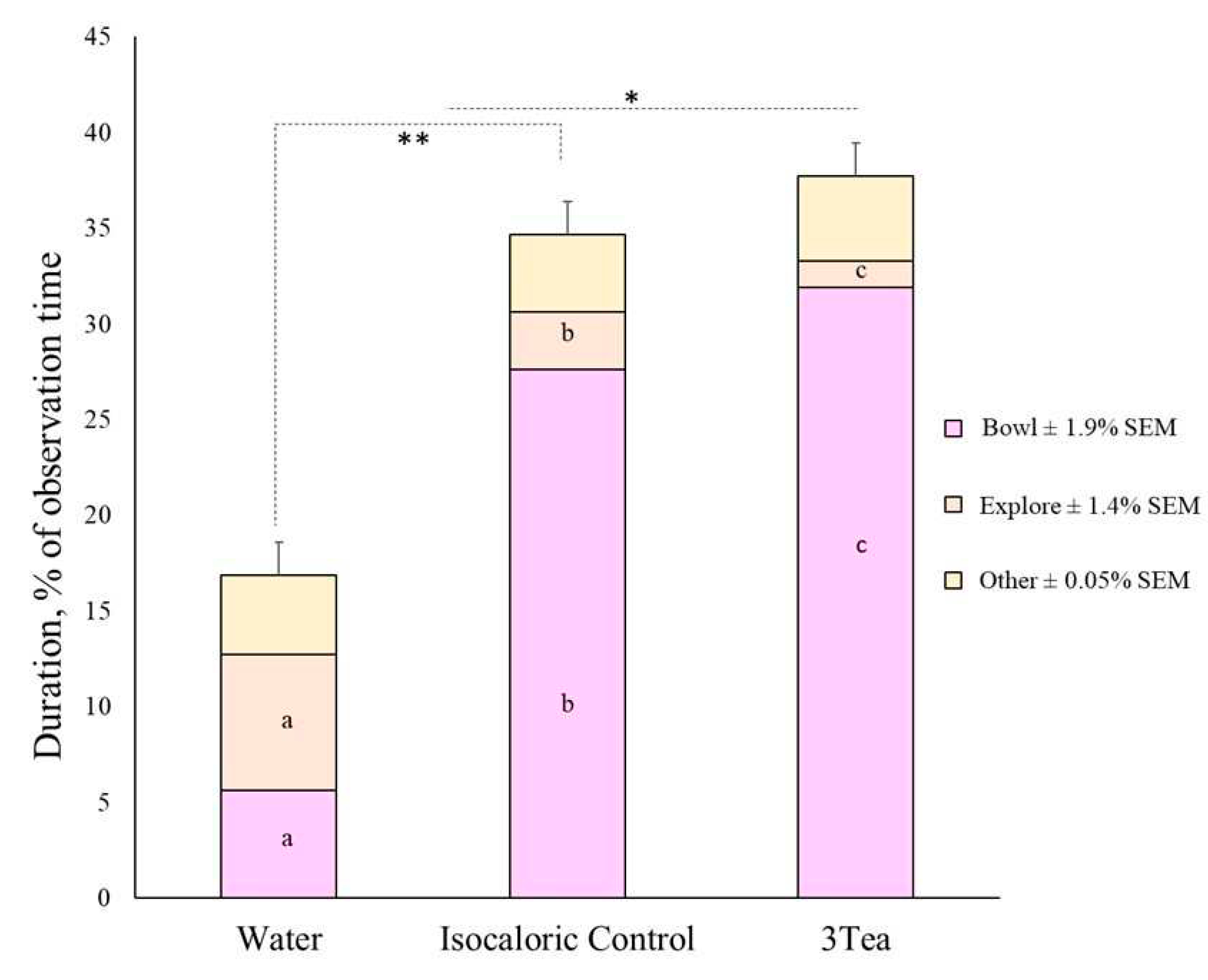
3.3. Experiment 2
4. Discussion
5. Conclusions
6. Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chipanshi, A.C.; Chanda, R.; Totolo, O. Vulnerability Assessment of the Maize and Sorghum Crops to Climate Change in Botswana. Clim. Change 2003, 61, 339–360. [Google Scholar] [CrossRef]
- Chadalavada, K.; Kumari, B.D.R.; Kumar, T.S. Sorghum mitigates climate variability and change on crop yield and quality. Planta 2021, 253, 113. [Google Scholar] [CrossRef]
- Warsame, A.A.; Sheik-Ali, I.A.; Jama, O.M.; Hassan, A.A.; Barre, G.M. Assessing the effects of climate change and political instability on sorghum production: Empirical evidence from Somalia. J. Clean. Prod. 2022, 360, 131893. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Zhao, Y. Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits. Foods 2021, 10, 10–8. [Google Scholar]
- Punia, H.; Tokas, J.; Malik, A.; Satpal; Sangwan, S. Characterization of phenolic compounds and antioxidant activity in sorghum [Sorghum bicolor (L.) Moench] grains. Cereal Res. Commun. 2021, 49, 343–353. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20.18, 4567. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; Wu, J. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity, J. Nutr. Biochem. 2020, 78, 108–324. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013, 61.7, 1525–1533. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 11, 2590. [Google Scholar] [CrossRef]
- Duressa, D.; Weerasoriya, D.; Bean, S.R.; Tilley, M.; Tesso, T. Genetic basis of protein digestibility in grain sorghum. Crop Sci. 2018, 58, 2183–2199. [Google Scholar] [CrossRef]
- Kazanas, N.; Fields, M.L. Nutritional improvement of sorghum by fermentation. J. Food Sci. 1981, 46.3, 819–821. [Google Scholar] [CrossRef]
- Schons, P.F.; Battestin, V.; Macedo, G.A. Fermentation and enzyme treatments for sorghum. Braz. J. Microbiol. 2012, 43.1, 89–97. [Google Scholar] [CrossRef]
- Rodríguez-España, M.; Figueroa-Hernández, C.Y.; de Dios Figueroa-Cárdenas, J.; Rayas-Duarte, P.; Hernández-Estrada, Z.J. Effects of germination and lactic acid fermentation on nutritional and rheological properties of sorghum: A graphical review. Curr. Res. Food Sci. 2022, 5, 807–812. [Google Scholar] [CrossRef]
- Elkhier MK, S.; Abd-ALRaheem, A. A. Effect of fermentation period on the chemical composition, in-vitro protein digestibility and tannin content in two sorghum cultivars (Dabar and Tabat) in Sudan. J. Appl. Biosci 2011, 39, 2602–2606. [Google Scholar]
- Sorour, M.A.; Mehanni, A.E.; Taha, E.M.; Rashwan, A.K. Changes of total phenolics, tannins, phytate and antioxidant activity of two sorghum cultivars as affected by processing. J. Food & Dairy Sci. 2017, 8, 267–274. [Google Scholar]
- Bwamu, K.E.; Nkirote, K.C.; Kahiu, N.E.; Edgar, K.D. Optimization of fermentation and malting process of sorghum beverage and effects on nutritional quality. Afr. J. Food Sci. 2022, 16, 252–260. [Google Scholar]
- Kapp, J.M.; Sumner, W. Kombucha: a systematic review of the empirical evidence of human health benefit. Ann Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Zhang, P.; Ajlouni, S.; Fang, Z. Changes in phenolic content, antioxidant activity, and volatile compounds during processing of fermented sorghum grain tea. Cereal Chem. 97, 612–625. [CrossRef]
- Xu, W.; Tong, Y.; Tong, Q.; Liu, Y.; Wang, Z. Effects of different re-fermentation methods on the quality characteristics of kombucha beverages. J. Food Sci. Technol. 2023, 60.4, 1414–1424. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Sterneder, S.; Stoeger, V.; Dugulin, C.A.; Liszt, K.I.; Di Pizio, A.; Korntheuer, K. ,... & Somoza, V. (2021). Astringent gallic acid in red wine regulates mechanisms of gastric acid secretion via activation of bitter taste sensing receptor TAS2R4. J. Agric. Food Chem. 2021, 69, 10550–10561. [Google Scholar]
- Kennedy, J.M.; Baldwin, B.A. Taste preferences in pigs for nutritive and non-nutritive sweet solutions. Anim. Behav. 1972, 20.4, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Nelson Sandra, L.; Sanregret John, D. Response of Pigs to Bitter-tasting Compounds, Chem. Senses, 1997, 22, 129–132. [Google Scholar] [CrossRef]
- Kittawornrat, A.; Zimmerman, J. Toward a better understanding of pig behavior and pig welfare. Anim. Health Res. Rev. 2011, 12.1, 25–32. [Google Scholar] [CrossRef]
- Guzmán-Pino, S.A.; Lazcano, C.; De Luca, V.; Figueroa, J.; Valenzuela, C.; Roura, E. Dietary Inclusion of Monosodium Glutamate in Gestating and Lactating Sows Modifies the Preference Thresholds and Sensory-Motivated Intake for Umami and Sweet Solutions in Post-Weaned Pigs. Animals 2019, 9.6, 336. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food chem. 2001, 73.3, 285–290. [Google Scholar] [CrossRef]
- Singh, R.P. , Chidambara Murthy, K.N. and Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Dhillon, B.; Awasthi, T.; Sodhi, N.S.; Sogi, D.S.; Jaiswal, S. A comparative study to investigate the effects of addition of milk and sugar on total polyphenol, flavonoid, catechin and tannin contents of green and black teas consumed in India. J. Food Meas. Charact. 2021, 15, 4652–4658. [Google Scholar] [CrossRef]
- Galef, B.G., Jr.; Whiskin, E.E. Preference for novel flavors in adult Norway Rats (Rattus norvegicus). J. Comp. Psychol. 2003, 117, 96–100. [Google Scholar] [CrossRef]
- Brown, G.R.; Nemes, C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav. Process. 2008, 78, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.J.; McClintock, M.K.; Cavigelli, S.A. Temperament moderates the influence of periadolescent social experience on behavior and adrenocortical activity in adult male rats. Horm. Behav. 2014, 66.3, 517–524. [Google Scholar] [CrossRef]
- Walters, E.M.; Prather, R.S. Advancing swine models for human health and diseases. Mo. Med. 2013, 110.3, 212. [Google Scholar]
- Fleming Stephen, A.; Dilger Ryan, N. Young pigs exhibit differential exploratory behavior during novelty preference tasks in response to age, sex, and delay, Behav. Brain Res. 2017, 321, 50–60. [Google Scholar]
- Roura, E.; Fu, M. Taste, nutrient sensing and feed intake in pigs (130 years of research: then, now and future), Anim. Feed Sci. Technol. 2017, 233, 3–12. [Google Scholar] [CrossRef]
- Cirera, S.; Clop, A.; Jacobsen, M.J.; Guerin, M.; Lesnik, P.; Jørgensen, C.B.; Karlskov-Mortensen, P. A targeted genotyping approach enhances identification of variants in taste receptor and appetite/reward genes of potential functional importance for obesity-related porcine traits. Anim. Genet. 2018, 49, 110–118. [Google Scholar] [CrossRef]
- Clouard, C.; Val-Laillet, D. Impact of sensory feed additives on feed intake, feed preferences, and growth of female piglets during the early postweaning period. J. Anim. Sci. 2014, 92.5, 2133–2140. [Google Scholar] [CrossRef]
- Guzmán-Pino, S. A. , Solà-Oriol, D. , Figueroa, J., & Pérez, J. F. Influence of the protein status of piglets on their ability to select and prefer protein sources, Physiol. Behav. 2014, 129, 43–49. [Google Scholar] [PubMed]
- Wang, J.; Yang, M.; Xu, S.; Lin, Y.; Che, L.; Fang, Z.; Wu, D. Comparative effects of sodium butyrate and flavors on feed intake of lactating sows and growth performance of piglets. Anim. Sci. J. 2014, 85.6, 683–689. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, G.; Dong, B.; Peng, C.C.; Tian, Y.Y.; Gong, L.M. Effects of sweetener neotame on diet preference, performance and hematological and biochemical parameters of weaned piglets, Anim. Feed Sci. Technol. 2016, 214, 86–94. [Google Scholar] [CrossRef]
- Reyes-Camacho, D.; Pérez, J.F.; Vinyeta, E.; Aumiller, T.; Van der Klis, J.D.; Solà-Oriol, D. Prenatal Exposure to Innately Preferred D-Limonene and Trans-Anethole Does Not Overcome Innate Aversion to Eucalyptol. Affecting Growth Performance of Weanling Piglets. Animals 2021, 11, 2062. [Google Scholar]
- Ronda, V.; Aruna, C.; Visarada KB, R.S.; Bhat, B.V. Sorghum for animal feed. In Breeding Sorghum for diverse end uses, 1st ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 229–238. [Google Scholar]
- Pan, L.; An, D.; Zhu, W.Y. Sorghum as a dietary substitute for corn reduces the activities of digestive enzymes and antioxidant enzymes in pigs. Anim. Feed Sci. Technol. 2021, 273, 114831. [Google Scholar] [CrossRef]
- Corassa, A.; Stuani, J.L.; Ton, A.P.S.; Kiefer, C.; Sbardella, M.; Brito, C.O.; Amorim, A.B.; Gonçalves, D.B.C. Nutritional value of distillers dried grains with solubles from corn and sorghum and xylanase in diets for pigs. Rev. Bras. Zootec. 2019, 48, 20190012. [Google Scholar] [CrossRef]
- Brambillasca, S.; Fernández–García, M.; Aguerre, M.; Repetto, J.L.; Cajarville, C. Characterization of the in vitro digestion of starch and fermentation kinetics of dry sorghum grains soaked or rehydrated and ensiled to be used in pig nutrition. J. Cereal Sci. 2019, 89, 102817. [Google Scholar] [CrossRef]
- McCuistion, K.C.; Selle, P.H.; Liu, S.Y.; Goodband, R.D. Sorghum as a feed grain for animal production. In Sorghum and Millets, 2nd ed.; Duodu K.G.; Elsevier: Amsterdam, NL, 2019; pp. 355–391. [Google Scholar]
- Thomas, L.L.; Espinosa, C.D.; Goodband, R.D.; Stein, H.H.; Tokach, M.D.; Dritz, S.S.; Woodworth, J.C.; DeRouchey, J.M. Nutritional evaluation of different varieties of sorghum and the effects on nursery pig growth performance. J. Anim. Sci. 2020, 98, skaa120. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Sun, X.; Cui, G.; Li, Q.; Xu, Y.; Wang, L.; Li, X.; Hu, B.; Chi, Z. A strategy of co-fermentation of distillers dried grains with solubles (DDGS) and lignocellulosic feedstocks as swine feed. Crit. Rev. Biotechnol. 2023, 43.2, 212–226. [Google Scholar] [CrossRef] [PubMed]
- De Souza TC, R.; Árres IE, Á.; Gómez-Soto, J.G.; García, K.E.; Rodríguez, E.R.; Mariscal-Landín, G. Effect of tannins and kafirins on the nitrogen and energy balance and performance of pigs. Anim. Prod. Sci. 2023. [Google Scholar]
- Tran, T.; Billet, K.; Torres-Cobos, B.; Vichi, S.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Use of a minimal microbial consortium to determine the origin of kombucha flavor. Front. Microbiol. 2022, 13, 836617. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K. A. Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Redondo, N. , Gómez-Martínez, S., & Marcos, A. Sensory attributes of soft drinks and their influence on consumers' preferences. Food Funct. 2014, 5, 1686–1694. [Google Scholar]
- Barker, S.; Moss, R.; McSweeney, M.B. Carbonated emotions: Consumers’ sensory perception and emotional response to carbonated and still fruit juices. Food Res. Int. 2021, 147, 110534. [Google Scholar] [CrossRef]
- Wendrick, N.A.; Sims, C.A.; MacIntosh, A.J. The Effect of Carbonation Level on the Acceptability and Purchase Intent of Muscadine and Fruit Wines. Beverages 2021, 7, 6. [Google Scholar] [CrossRef]
- Jang, S.S. , McIntyre L., Chan M., Brown P.N., Finley J., Chen S.X. Ethanol Concentration of Kombucha Teas in British Columbia, Canada. J. Food Prot. 2021, 84, 1878–1883. [Google Scholar] [CrossRef]
- Tran TM, K.; Kirkman, T.; Nguyen, M.; Van Vuong, Q. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora). Heliyon 2020, 6. [Google Scholar]
- Nofre, C. , Glaser, D., Tinti, J.M. and Wanner, M. Gustatory responses of pigs to sixty compounds tasting sweet to humans. J. Anim. Physiol. Anim. Nutr. 2002, 86, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Docking, C.M.; Van de Weerd, H.A.; Day JE, L.; Edwards, S. A. The influence of age on the use of potential enrichment objects and synchronisation of behaviour of pigs, Appl. Anim. Behav. Sci. 2008, 110, 244–257. [Google Scholar] [CrossRef]
- Jensen, P. (2017). The ethology of domestic animals: An Introductory Text, 3rd ed.; CABI: Boston, USA 2017; pp. 40–44. [Google Scholar]
- Urrutia, M.A.D.; Ramos, A.G.; Menegusso, R.B.; Lenz, R.D.; Ramos, M.G.; Tarone, A.G.; Cazarin, C.B.B.; Cottica, S.M.; da Silva, S.A.V.; Bernardi, D.M. Effects of supplementation with kombucha and green banana flour on Wistar rats fed with a cafeteria diet. Heliyon, 2021, 7. [Google Scholar] [CrossRef]
- Chen, X.; Chen, K.; Cheng, H.; Liang, L. Soluble aggregates of myofibrillar proteins engineered by gallic acid: Colloidal structure and resistance to in vitro gastric digestion. J. Agric. Food Chem. 2022, 70.13, 4066–4075. [Google Scholar] [CrossRef]
- Hithamani, G.; Srinivasan, K. Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef] [PubMed]
- de Morais Cardoso, L.; Pinheiro, S.S.; de Carvalho, C.W.P.; Queiroz, V.A.V.; de Menezes, C.B.; Moreira, A.V.B.; de Barros, F.A.R.; Awika, J.M.; Martino, H.S.D.; Pinheiro-Sant'Ana, H.M. Phenolic compounds profile in sorghum processed by extrusion cooking and dry heat in a conventional oven. J. Cereal Sci. 2015, 65, 220–226. [Google Scholar] [CrossRef]
- Lipsitch, M.; Tchetgen Tchetgen, E.; Cohen, T. Negative controls: a tool for detecting confounding and bias in observational studies. J. Epidemiol. 2010, 21.3, 383. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, L.E.; Bortoluzzi, E.M.; Luo, Y.; Mumm, J.M.; Coffin, M.J.; Becker, G.Y.; Vandevord, P.J.; McNeil, E.M.; Walilko, T.; Khaing, Z.Z.; Zai, L. Noninvasive, In-pen Approach Test for Laboratory-housed Pigs. JoVE, 2019, 148, 58597. [Google Scholar]
- Studnitz, M.; Jensen, M.B.; Pedersen, L.J. Why do pigs root and in what will they root?: A review on the exploratory behaviour of pigs in relation to environmental enrichment. Appl. Anim. Behav. Sci. 2007, 107, 183–197. [Google Scholar] [CrossRef]
- Tatemoto, P.; Broom, D.M.; Zanella, A.J. Changes in Stereotypies: Effects over Time and over Generations. Animals 2022, 12, 2504. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.X.; Mutukumira, A.N. Isolation and characterisation of dominant acetic acid bacteria and yeast isolated from Kombucha samples at point of sale in New Zealand. Curr. Res. Nutr. Food Sci. 2022, 5, 835–844. [Google Scholar] [CrossRef]
- Figueroa, J.; Frías, D.; Solà-Oriol, D.; Tadich, T.; Franco-Rosselló, R; Nuñez, V.; Dwyer, D.M. Palatability in pigs, the pleasure of consumption. J. Anim. Sci. 2019, 97, 2165–2174. [Google Scholar] [CrossRef]
- Charles, D.J.; Charles, D.J. 2013. Anise star. In Antioxidant Properties of Spices, Herbs and Other Sources, 1st ed; Springer: New York, USA, 2012; Volume 3, pp. 165–168. [Google Scholar]

| Choice | Exposure | p-Values | ||||||||||
| White | Sumac | Roasted | SEM1 | 1 | 2 | 3 | 4 | SEM1 | Choice | Exp | Choice x Exp | |
| Consumption2, mL | 95.2 | 97.9 | 95.6 | ±2.34 | 87.9a | 99.9b | 99.2b | 97.9b | ±2.64 | 0.628 | 0.005 | 0.653 |
| Gallic acid, mg | 3.3a | 13.7b | 12.7c | ±0.22 | 9.1a | 10.3b | 10.2b | 10.1b | ±0.25 | <0.001 | 0.008 | 0.201 |
| Duration, % | ||||||||||||
| Total | 28.2 | 31.8 | 30.0 | ±1.25 | 30.1 | 29.8 | 30.5 | 29.7 | ±1.48 | 0.208 | 0.988 | 0.922 |
| Bowl3 | 21.9 | 24.2 | 23.9 | ±1.69 | 23.7 | 21.0 | 24.6 | 24.0 | ±1.80 | 0.264 | 0.213 | 0.923 |
| Explore4,5 | 3.1 | 3.3 | 2.3 | ±0.92 | 3.3a | 4.7a | 1.7b | 1.8b | ±1.01 | 0.617 | 0.029 | 0.958 |
| Non-oral behaviors6 | 3.3a | 4.3b,c | 3.8c | ±0.45 | 3.1d | 4.0e | 4.1e | 3.9e | ±0.48 | 0.050 | 0.097 | 0.949 |
| Latency, % | ||||||||||||
| Bowl3 | 24.4 | 24.7 | 26.8 | ±3.60 | 30.9 | 19.3 | 24.3 | 26.7 | ±4.22 | 0.890 | 0.327 | 0.672 |
| Explore4 | 62.7 | 67.5 | 74.1 | ±6.10 | 56.6d | 68.7e | 75.8e | 71.1e | ±6.58 | 0.192 | 0.060 | 0.970 |
| Other6 | 23.5 | 23.8 | 27.5 | ±7.85 | 30.8 | 19.5 | 23.4 | 26.1 | ±8.10 | 0.696 | 0.338 | 0.626 |
| Rate, no./min | ||||||||||||
| Bowl3 | 0.68 | 0.70 | 0.68 | ±0.068 | 0.57d,e | 0.70e,f | 0.72e,f | 0.76f | ±0.073 | 0.904 | 0.069 | 0.982 |
| Explore4 | 0.40 | 0.43 | 0.32 | ±0.079 | 0.47 | 0.36 | 0.36 | 0.35 | ±0.084 | 0.302 | 0.360 | 0.907 |
| Other6 | 0.97 | 1.15 | 1.08 | ±0.120 | 0.81a | 1.1b | 1.2b | 1.2b | ±0.126 | 0.173 | 0.003 | 0.962 |
| 1Largest Standard Error Mean; 2Total volume consumed (100 mL maximum) data were non-parametric when Choice was considered. The Kruskal-Wallis Test for Consumption Choice was not significant (χ2 = 0.39; p = 0.82; df = 2) but was significant for gallic acid (χ2 = 97.5; p < 0.001; df = 2), 3Head over bowl, head moving, indicating drinking; 4p-values were derived from square root transformed data; 5All non-nutritive oral behaviors (NNOB); 6Walking, standing without any oral-related behavior (i.e. drinking, sniffing, licking, rooting, or rubbing using the mouth, snout, or face); a,b,c LS Means p ≤ 0.05; d,e,f LS Means p ≥ 0.05 ≤ 0.10. | ||||||||||||
| Choice | Exposure | p-values | |||||||||
| -control | +control | 3Tea | SEM1 | 1 | 2 | 3 | SEM1 | Choice | Exp | Choice x Exp | |
| Consumption2, mL | 18.0a | 96.6b | 99.0b | ±2.21 | 74.1 | 68.9 | 70.6 | ±2.97 | <0.001 | 0.136 | 0.087 |
| Gallic Acid, mg | 0.02a | 0.20b | 11.7c | ±0.03 | 3.8a | 3.8a | 4.3b | ±0.03 | <0.001 | <0.001 | <0.001 |
| Duration, % | |||||||||||
| Total | 16.8a | 34.5b | 37.7c | ±1.71 | 28.4 | 29.5 | 31.1 | ±1.71 | <0.001 | 0.597 | 0.033 |
| Bowl3 | 5.6a | 27.6b | 31.9c | ±1.87 | 20.3 | 21.8 | 23.0 | ±1.92 | <0.001 | 0.365 | 0.091 |
| Explore4,5 | 7.1a | 3.0b | 1.4b | ±1.45 | 4.4 | 3.5 | 3.5 | ±1.50 | 0.002 | 0.866 | 0.261 |
| Non-oral behaviors6 | 4.2 | 4.1 | 4.4 | ±0.45 | 4.5 | 4.2 | 4.0 | ±0.46 | 0.583 | 0.321 | 0.506 |
| Latency, % | |||||||||||
| Bowl3 | 20.8 | 16.2 | 14.8 | ±3.45 | 13.93 | 19.59 | 18.19 | ±3.61 | 0.420 | 0.481 | 0.681 |
| Explore4 | 60.1d | 71.2e | 76.0e | ±7.55 | 69.0 | 65.7 | 72.6 | ±7.74 | 0.086 | 0.608 | 0.714 |
| Other6 | 16.2 | 15.2 | 13.8 | ±3.11 | 12.2 | 15.6 | 17.4 | ±3.26 | 0.849 | 0.480 | 0.958 |
| Rate, no./min | |||||||||||
| Bowl3 | 20.75 | 16.18 | 14.77 | ±3.459 | 0.42 | 0.48 | 0.68 | ±3.615 | 0.420 | 0.481 | 0.681 |
| Explore4 | 0.55d | 0.38e | 0.35e | ±0.108 | 0.39 | 0.53 | 0.37 | ±0.111 | 0.102 | 0.196 | 0.646 |
| Other6 | 1.41 | 1.44 | 1.52 | ±0.150 | 1.49 | 1.46 | 1.41 | ±0.152 | 0.526 | 0.730 | 0.413 |
| 1Largest Standard Error Mean; 2Total volume consumed (100 mL maximum) data were non-parametric when Choice was considered. The Kruskal-Wallis Test for Choice was significant for consumption volume (χ2 = 0.71.6; P < 0.001; df = 2), and for gallic acid (χ2 = 88.9; p < 0.001; df = 2),4P-Values were derived from square root transformed data; 5All non-nutritive oral behaviors (NNOB); 6All non-oral behaviors walking or standing without any sniffing, drinking, chomping, licking, rooting, rubbing using the mouth, snout, or face); a,b,c LS Means ≤ 0.05; d,e LS Means ≥ 0.05 ≤ 0.10. | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





