Submitted:
10 August 2023
Posted:
11 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Synthesis of SiO2@Au nanocomposite
2.5. Nanozyme activity assay
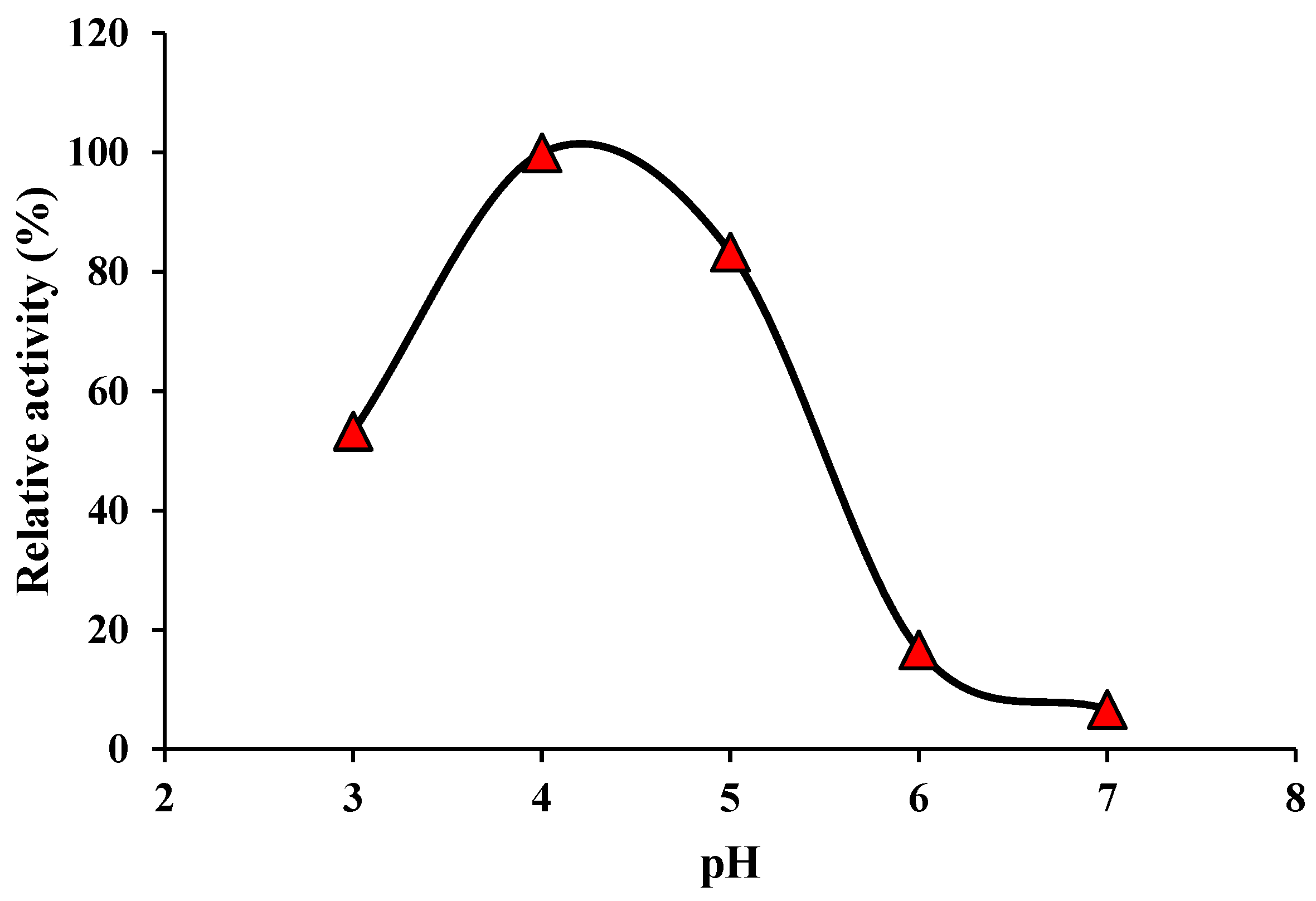
3. Results and discussion
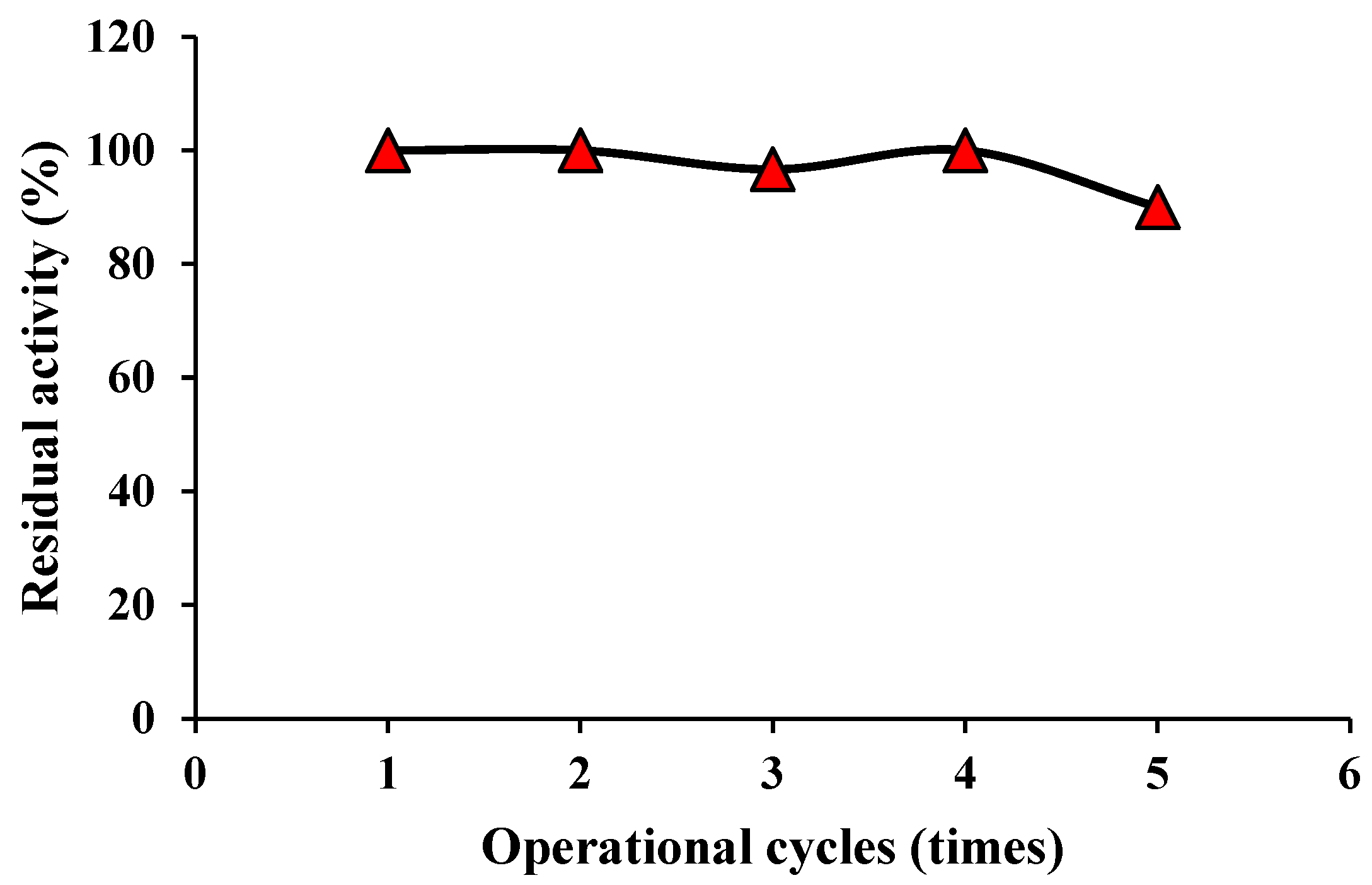
3.2. Cycling stability of SiO2@Au nanocomposite
3.3. Storage stability of SiO2@Au nanocomposite
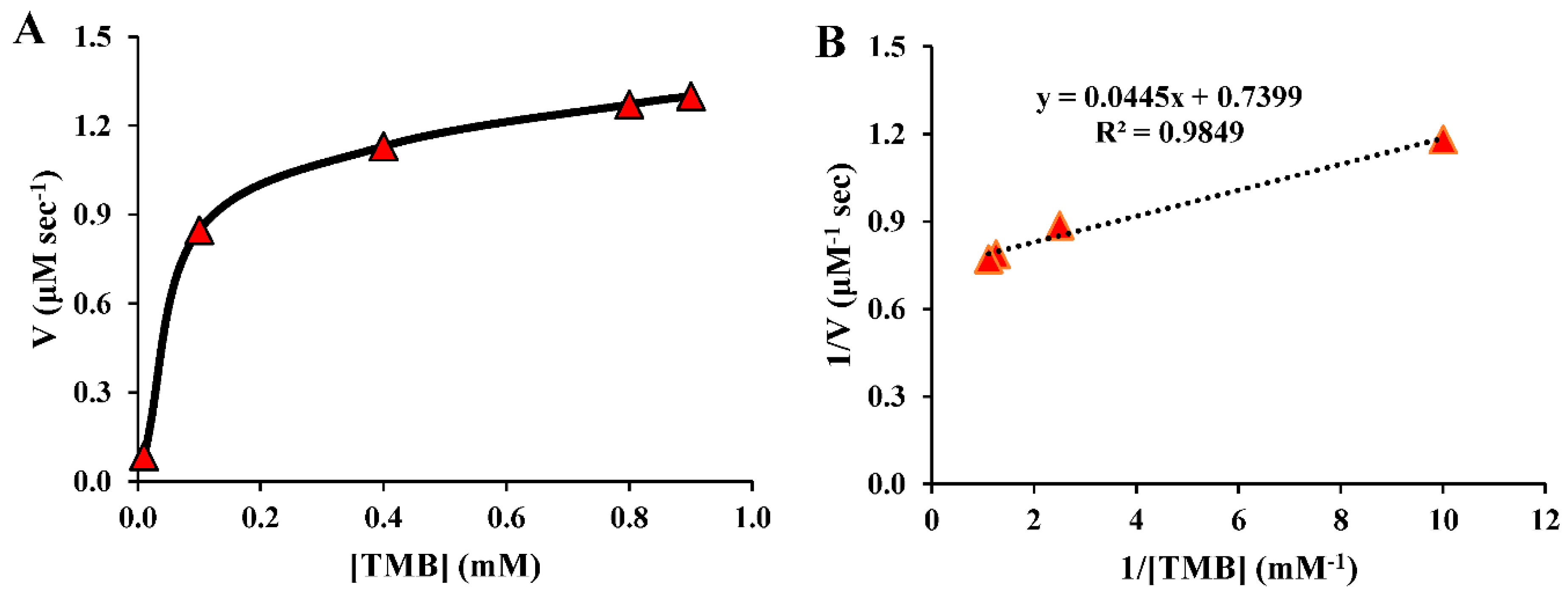
3.4. Kinetic performances of SiO2@AuNPs nanocomposite
4. Conclusions
Acknowledgments
Conflict of interest
References
- Kelly, K. L., Coronado, E., Zhao, L. L., & Schatz, G. C. (2003). The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. The Journal of Physical Chemistry B, 107(3), 668-677. [CrossRef]
- Dehghani Z., Akhond M., Hormozi Jangi S.R., Absalan G. (2024) Highly sensitive enantioselective spectrofluorimetric determination of R-/S-mandelic acid using l-tryptophan-modified amino-functional silica-coated N-doped carbon dots as novel high-throughput chiral nanoprobes, Talanta, 266, 1, 124977. [CrossRef]
- Hormozi Jangi, S. R., & Gholamhosseinzadeh, E. (2023). Developing an ultra-reproducible and ultrasensitive label-free nanoassay for L-methionine quantification in biological samples toward application in homocystinuria diagnosis. Chemical Papers, 1-13. [CrossRef]
- Hormozi Jangi, S. R. (2023). Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: a new approach toward reuse instead of common mineralization. Chemical Papers, 1-15. [CrossRef]
- Hormozi Jangi S. R.; Akhond M. (2020). High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. Journal of Chemical Sciences, 132, 1-8. [CrossRef]
- Singh, R., & Nalwa, H. S. (2011). Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs. Journal of biomedical nanotechnology, 7(4), 489-503. [CrossRef]
- Shi, Y., Shan, S., Li, C., Song, X., Zhang, C., Chen, J., ... & Xiong, J. (2020). Application of the tumor site recognizable and dual-responsive nanoparticles for combinational treatment of the drug-resistant colorectal cancer. Pharmaceutical Research, 37, 1-14. [CrossRef]
- Amany, A., El-Rab, S. F. G., & Gad, F. (2012). Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Der Pharma Chemica, 4(1), 53-65.
- Rajasekaran, J., & Viswanathan, P. (2023). Anti-bacterial and antibiofilm properties of seaweed polysaccharide-based nanoparticles. Aquaculture International, 1-25. [CrossRef]
- Li, G., Liu, Z., Gao, W., & Tang, B. (2023). Recent advancement in graphene quantum dots based fluorescent sensor: Design, construction and bio-medical applications. Coordination Chemistry Reviews, 478, 214966. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2021). Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sensors and Actuators B: Chemical, 339, 129901. [CrossRef]
- Jangi, S. R. H. (2023). Determining kinetics parameters of bovine serum albumin-protected gold nanozymes toward different substrates. Qeios. [CrossRef]
- Hormozi Jangi, S. R. (2023). Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios. [CrossRef]
- Dongsar, T. T., Dongsar, T. S., Abourehab, M. A., Gupta, N., & Kesharwani, P. (2023). Emerging application of magnetic nanoparticles for breast cancer therapy. European Polymer Journal, 111898. [CrossRef]
- Carrapiço, A., Martins, M. R., Caldeira, A. T., Mirão, J., & Dias, L. (2023). Biosynthesis of metal and metal oxide nanoparticles using microbial cultures: Mechanisms, antimicrobial activity and applications to cultural heritage. Microorganisms, 11(2), 378. [CrossRef]
- Hormozi Jangi, S. R. (2023). Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chemical Research and Nanomaterials, 1(4), 1-9.
- Hormozi Jangi, S. R., & Akhond, M. (2021). High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochemistry, 105, 79-90. [CrossRef]
- Li, W., Chen, B., Zhang, H., Sun, Y., Wang, J., Zhang, J., & Fu, Y. (2015). BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury (II) ions. Biosensors and Bioelectronics, 66, 251-258. [CrossRef]
- Hormozi Jangi, A. R., Hormozi Jangi, M. R., & Hormozi Jangi, S. R. (2020). Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chinese Journal of Chemical Engineering, 28(6), 1492-1503. [CrossRef]
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chemical Research and Nanomaterials 2 (1), 15-23.
- Jangi, S. R. H. (2023). Introducing a High Throughput Nanozymatic Method for Eco-Friendly Nanozyme-Mediated Degradation of Methylene Blue in Real Water Media. Sustainable Chemical Engineering, 90-99. [CrossRef]
- Huang, Y., Ren, J., & Qu, X. (2019). Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chemical reviews, 119(6), 4357-4412. [CrossRef]
- Lu, L., Huang, M., Huang, Y., Corvini, P. F. X., Ji, R., & Zhao, L. (2020). Mn 3 O 4 nanozymes boost endogenous antioxidant metabolites in cucumber (Cucumis sativus) plant and enhance resistance to salinity stress. Environmental Science: Nano, 7(6), 1692-1703. [CrossRef]
- Xia, F., Shi, Q., & Nan, Z. (2020). Facile synthesis of Cu-CuFe 2 O 4 nanozymes for sensitive assay of H 2 O 2 and GSH. Dalton Transactions, 49(36), 12780-12792. [CrossRef]
- Dega, N. K., Ganganboina, A. B., Tran, H. L., Kuncoro, E. P., & Doong, R. A. (2022). BSA-stabilized manganese phosphate nanoflower with enhanced nanozyme activity for highly sensitive and rapid detection of glutathione. Talanta, 237, 122957. [CrossRef]
- Liu, J., Gao, J., Zhang, A., Guo, Y., Fan, S., He, Y., ... & Cheng, Y. (2020). Carbon nanocage-based nanozyme as an endogenous H 2 O 2-activated oxygenerator for real-time bimodal imaging and enhanced phototherapy of esophageal cancer. Nanoscale, 12(42), 21674-21686. [CrossRef]
- Hormozi Jangi, S. R., Davoudli, H. K., Delshad, Y., Hormozi Jangi, M. R., & Hormozi Jangi, A. R. H. (2020). A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surfaces and Interfaces, 21, 100771. [CrossRef]
- Sun, H., Zhou, Y., Ren, J., & Qu, X. (2018). Carbon nanozymes: enzymatic properties, catalytic mechanism, and applications. Angewandte Chemie International Edition, 57(30), 9224-9237. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3′-diaminobenzidine. Microchimica Acta, 187, 431. [CrossRef]
- Meng, X., Li, D., Chen, L., He, H., Wang, Q., Hong, C., ... & Fan, K. (2021). High-performance self-cascade pyrite nanozymes for apoptosis–ferroptosis synergistic tumor therapy. ACS nano, 15(3), 5735-5751. [CrossRef]
- Dong, H., Du, W., Dong, J., Che, R., Kong, F., Cheng, W., ... & Zhang, Y. (2022). Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions. Nature Communications, 13(1), 5365. [CrossRef]
- Hormozi Jangi, S. R., & Akhond, M. (2020). Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchemical Journal, 158, 105328. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Absalan, G. (2020). A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Analytica Chimica Acta, 1127, 1-8. [CrossRef]
- Chen, Y., Jiao, L., Yan, H., Xu, W., Wu, Y., Wang, H., ... & Zhu, C. (2020). Hierarchically porous S/N codoped carbon nanozymes with enhanced peroxidase-like activity for total antioxidant capacity biosensing. Analytical Chemistry, 92(19), 13518-13524. [CrossRef]
- Hormozi Jangi, S. R., & Dehghani, Z. (2023). Kinetics and biochemical characterization of silver nanozymes and investigating impact of storage conditions on their activity and shelf-life. Chemical Research and Nanomaterials, 1(4), 25-33.
- Zhou, X., Wang, M., Chen, J., & Su, X. (2022). Cascade reaction biosensor based on Cu/N co-doped two-dimensional carbon-based nanozyme for the detection of lactose and β-galactosidase. Talanta, 245, 123451. [CrossRef]
- Akhond, M., Hormozi Jangi, S. R., Barzegar, S., & Absalan, G. (2020). Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chemical Papers, 74, 1321-1330.blood. [CrossRef]
- Zeng, X., Ruan, Y., Chen, Q., Yan, S., & Huang, W. (2023). Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chemical Engineering Journal, 452, 138422. [CrossRef]
- Ahmadi-Leilakouhi, B., Hormozi Jangi, S. R., & Khorshidi, A. (2023). Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chemical Papers, 77(2), 1033-1046. [CrossRef]
- Hormozi Jangi, S. R. (2023). Evaluation of Biochemical Behavior and Stability of Gold Nanoparticles with High Intrinsic Peroxidase-Like Activity. Petro Chem Indus Intern, 6(4), 234-239. [CrossRef]
- Hormozi Jangi, S. R., Akhond, M., & Dehghani, Z. (2020). High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochemistry, 90, 102-112. [CrossRef]
- Jangi, S. R. H., & Akhond, M. (2022). Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochemistry, 120, 138-155. [CrossRef]
- Seong, B., Kim, J., Kim, W., Lee, S. H., Pham, X. H., & Jun, B. H. (2021). Synthesis of Finely Controllable Sizes of Au Nanoparticles on a Silica Template and Their Nanozyme Properties. International Journal of Molecular Sciences, 22(19), 10382. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




