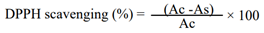1. Introduction
In developing countries, agricultural researches on crops were emphasized at increasing production to reduce hunger. However, limited research attention was given to nutritional quality improvement of crops and for this reason, malnutrition problems are becoming the main public health challenges. In Ethiopia, for example, micronutrient deficiency, among which iron and zinc are common, remains a substantial public health problem. All forms of malnutrition are estimated to contribute to 45% child death in developing countries [
1]. It was estimated that about 2 billion people in the world lack key micronutrients like iron and vitamin A and thus, they are experienced malnutrition because they mostly consume staples to avoid hunger [
2].
Plant breeding can be one of the mitigating strategies to reduce malnutrition [
3,
4]. However, before the initiation of breeding strategies, it is necessary to explore the grain quality and composition of the diverse crop genetic resources intended to be improved. Thus, it is critical to harness the genetic diversity of crops particularly landraces such as of sorghum landraces for improved nutritional quality traits, thereby reducing malnutrition. Landraces (also called “farmers’ varieties”) are dynamic populations of cultivated crops that have historical origin, distinct identity, lacking formal crop improvement program and associated with the traditional farming systems that are maintained and owned by local farmers to meet their socio-economic and cultural needs [
5,
6]. They can also be defined as plant populations maintained through conscious selection of farmers for their stable functional attributes and morphological characteristics within a defined biophysical and social environment [
7]. They own locally adapted and untapped reservoir of useful genes that have unique and potential source of traits for improved nutrition and biotic and abiotic tolerances [
8].
Sorghum, the third most important cereal crop after teff and maize in terms of production in Ethiopia [
9], is one of the stable foods in many parts of Ethiopia, including Tigray that is mainly consumed in the form of enjera (Ethiopian pancake-like flat bread) and Swa (local beverage) [
10,
11]. Sorghum is one of the healthiest and nutritious food crops in view of its richness in minerals, energy, protein, vitamins, fibre, phenolic compounds, and gluten-free properties for the people of semi-arid tropics [
12,
13,
14]. It is one of the important sources of mineral elements such as iron and zinc in the dishes of Ethiopians, particularly in the low-income communities. Although sorghum is nutritious crop, the nutritional content and composition varies among its genotypes and the environment it grows [
15,
16,
17,
18]. Genetic variation in nutritional composition were reported among Ethiopian [
15] and Malawian, Tanzanian, and Zambian [
19] sorghum landraces. The variability among South African [
17] and Ethiopian [
20] sorghum genotypes for protein content were reported. The starch content and composition of sorghum was also influenced by its genetic properties and environment [
15,
20,
21,
22]. In addition, the mineral content of sorghum was affected by the site of cultivation and countries of origin [
18].
Phenolic compounds, found in whole grains of some cereals including sorghum, are among the health promoting phytochemicals as they have unique bioactive properties. These phenolics act as antioxidants due to their ability to scavenge free radicals before they cause oxidative damage to the cellular structure [
23]. The availability of phenolic compounds such as flavonoids and tannin in sorghum, which are found in its pericarp layer, has been reported [
24,
25,
26]. It is well known that the presence of pigments in the pericarp layer is due to the presence of B1 and B2 genes and for the expression of these genes the existence of tannins is essential. The most unique beneficial aspect of sorghum as human food is due to the composition of polyphenol compounds [
27]. Sorghum has a diverse range of phenolic compounds that are not commonly found in other cereal grains [
28]. The content of phenolics varies widely among sorghums and their antioxidant activity level varies accordingly that is influenced by the genetics of the crop and environment [
14,
28].
Despite that sorghum is a diverse and stable food crop used for different end-uses in Tigray, northern Ethiopia, comprehensive information on nutritional and antinutritional profiling and variability among the sorghum landraces of Tigray is not documented yet. This makes critical to profile the level of genetic diversity for proximate, mineral, phenolic compounds, and antioxidant activities. Therefore, the main objective of this study was to determine the extent of genetic variation in sorghum landraces with respect to protein, starch, minerals, total flavonoid, total tannin, and antioxidant activities. It was also intended to select nutritionally superior landraces to be utilized in the future for complementary food development as well as to be used as breeding materials for improved grain quality traits of sorghum.
2. Materials And Methods
2.1. Plant Materials
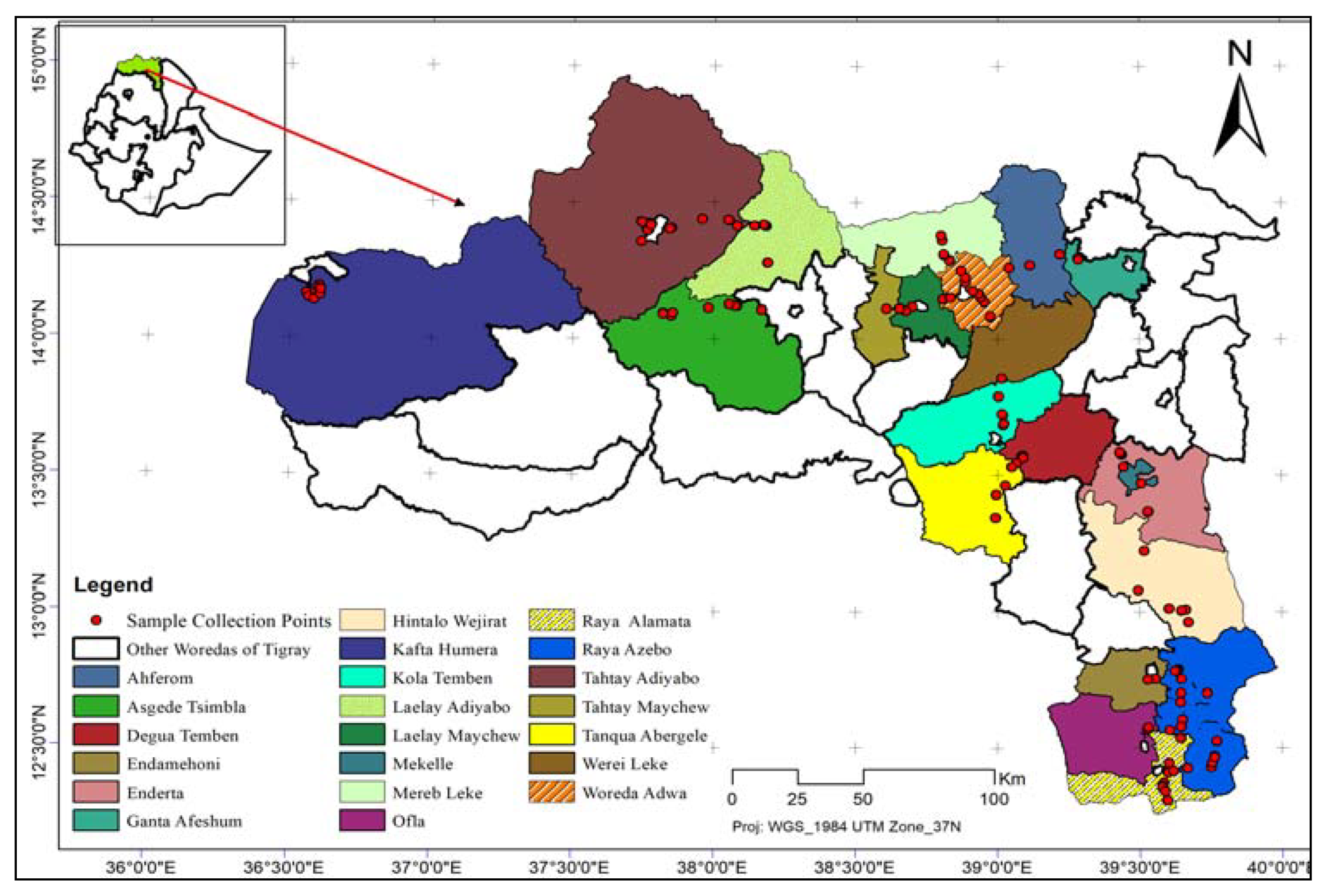
All the sorghum samples used in the current study are landraces collected in situ (on-farm). Panicles of these landraces were collected from 20 sorghum growing localities (‘weredas’) of the Tigray region, northern Ethiopia (
Figure 1). The unit of collection was vernacular names (local names) based as traced by farmers and farmers were also involved in describing their end use qualities. Three major races (bicolor, durra, and caudatum) and one intermediate race (durra-bicolor) were included in the collection. As part of this collection mission, sweet sorghum landraces (locally named as ‘Tinkish’) and the wild relative of sorghum were included. A total of 358 landraces were used for the analysis of protein and starch. However, for the determination of minerals, total flavonoid content (TFC), total tannin content (TTC), and antioxidant activity, 21 selected sorghum landraces were used. These 21 landraces were selected based on their merit of wide spectrum of utilization in the traditional food culture (different end-uses attributes) in the study area, Tigray (
Table 1).
2.2. Sample Preparation And Extraction
Panicles of each sample were dried and trashed separately. The sun-dried samples were subjected to cleaning and only pure seeds were kept for further analysis. The samples were grounded to fine powder using electric grinder. Extract was prepared by dissolving 10 g of fine powder in 100 ml of methanol and the contents were kept in orbital shaker for 8 hours at room temperature. Thereafter, each extract was filtered using Whatman no.1 filter paper and evaporated to dryness under vacuum at 40 oC using rotary evaporator. The resulting extracts were kept in a sealed glass bottle and stored at -20 oC until the analysis of TFC, TTC, and antioxidant activities.
2.3. Determination Of Protein And Starch
Protein and starch contents of the sorghum samples were analyzed using near infrared spectrophotometers (DA 720). NIR is most effective and non-destructive technique for the analysis of grain quality traits including protein and starch. A 100 g of pure sorghum seeds of each sample were placed in a sample cup for scanning of the whole seeds. The samples were scanned twice, and average values of protein and starch were recorded.
2.4. Estimation Of Mineral Concentration
The concentrations of mineral elements including iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), and chromium (Cr) of the sorghum landraces were estimated using atomic absorption spectrophotometer at Ezana mining Plc, Mekelle, Ethiopia. A dried seed sample of each landrace was ground to fine powder and 2 g powder was taken in to ashing vessel that was ashed at 550 oC. To complete the ashing process, the ash was dissolved in a volume of HCL-H2O (1:1). The samples were then subjected to mineral analysis with atomic absorption spectrophotometer using air-acetylene flame. The estimated concentrations of minerals were expressed as milligram per 100 grams (mg/100g) of the sample.
2.5. Determination Of Phenolic Compounds
2.5.1. Determination of Total Flavonoid Content (TFC)
TFC of the samples were estimated according to the method of [
29]. The methanol extract (1 mg/ml) was diluted with 1.25 ml of distilled water and then 75 μl NaNO2 was added to the mixture. AlCl3 (150 μl; 10%) and NaOH (1 ml; 1M) were added to the mixture separately each after 5 minutes interval. Absorbance of the mixture having pink color was then read at 415 nm against the blank (methanol extract without AlCl3). The measurement was performed in duplicate and the average absorbance was taken. TFC was determined using a standard curve of catechin (1-40 μg/ml) and values were calculated as milligram of catechin equivalent per gram of dried extract (mg CE/g).
2.5.2. Determination of Total Tannin Content (TTC)
TTC was determined based on the method described by [
30]. A 0.5 ml of undiluted crude extract (1 mg/ml) was mixed with 3 ml of vanillin reagent (4% w/v, in absolute methanol), followed by an addition of 1.5 ml of concentrated HCl. The mixture was then stored for 15 min at room temperature in dark environment. Blank was prepared by replacing the 0.5 ml of undiluted crude extract with 0.5 ml of deionized water. The absorbance of the mixture was measured at 500 nm against blank using spectrophotometer. The measurement was performed in duplicate and the average absorbance was taken. Catechin (1-40 μg/ml) was used for the calibration of standard curve and the results were expressed as milligram of catechin equivalent per 100 g of dry weight sample (mg CE/100g).
2.6. Determination of Antioxidant Capacity
Since antioxidant activity is a complex procedure that cannot be fully estimated with a single method, three independent assays: namely, DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical scavenging activity, Ferric Reducing Antioxidant Power (FRAP), and phosphomolybdenum assays were used.
2.6.1. DPPH Radical Scavenging Assay
The DPPH radical scavenging activities of the sorghum extracts were determined according to the method of [
31]. Different concentrations (50-1000 µg/ml) of each extract were taken in different test tubes and a solution of DPPH in methanol (2 ml, 0.006% w/v) was added to each test tube. The reaction mixture was shaken vigorously and left in the dark for 30 minutes and then absorbance was measured at 520 nm using spectrophotometer. Methanol was used as a blank. The measurement was performed in duplicate and the average absorbance was taken. The antioxidant activity was expressed as percent of DPPH radical scavenging, which is calculated using the equation:
Where, Ac is absorbance of the control and As is absorbance of the sample. The effective concentration (EC50) at which 50% of DPPH radicals are scavenged was calculated from the graph percentage of DPPH scavenging activity against extract concentration. The EC50 was expressed in μg/ml.
2.6.2. Ferric Reducing Antioxidant Power
The ferric reducing antioxidant power (FRAP) assay was performed as described by [
32]. Sample extract solution (1 mg/ml) was mixed with sodium phosphate buffer (2.5 ml, 0.2 M, PH 6.6) and potassium ferricyanide (2.5 ml, 1% w/v). After the mixture was incubated at 50 oC for 20 minutes, trichloroacetic acid (2.5 ml, 10% w/v) was added followed by centrifugation at 3000 rpm for 5 min. Finally, 2.5 ml of the supernatant solution was mixed with 2.5 ml of distilled water and 0.5 ml of FeCl3 (0.1% w/v). After incubation at room temperature in the dark for 30 minutes, absorbance was measured at 700 nm using a spectrophotometer. The measurement was performed in duplicate and the average absorbance was taken. Antioxidant activity was expressed as milligram of ascorbic acid equivalents per gram of dried extract (mg AAE/g).
2.6.3. Total Antioxidant Capacity With Phosphomolybdenum Assay
The total antioxidant activities (TAC) of the samples were determined using phosphomolybdenum assay according to the method of [
33] with some modifications. Sample extract of 0.3 ml was mixed with 2.5 ml of phosphomolybdenum reagent (0.6M sulphuric acid, 28mM sodium sulphate and 4mM ammonium molybdate mixed in 250 ml distilled water). The absorbance of the reaction mixture was measured at 695 nm on spectrophotometer. Total antioxidant capacity was expressed as milligram equivalent of butylated hydroxytoluene per gram of dried extract (mg BHTE/g).
2.7. Statistical Data Analysis
The results obtained were reported as mean values ± standard error of mean. Significant tests were done using t-test in IBM-SPSS. Pearson’s correlation coefficients were used to assess associations among the parameters. Cluster analysis was conducted on the standardized data using the Ward Linkage method and Squared Euclidean Distance measure in MINITAB v19. Discriminant analysis (DA) was conducted to validate the groups created by the cluster analysis. Principal component analysis (PCA) was performed using a correlation matrix to define the existing pattern of variation among populations using Past4.0 software.
3. Results
3.1. Variabilities For Protein And Starch
Statistically significant (P<0.001) variation for protein and starch contents were found among the 358 sorghum landraces (Table 2). The protein content was ranged from 6.21 to 18% with a mean value of 11.40%. The sorghum landrace Arfa’agdm was superior in protein content followed by the landraces Tsaeda Jargte and Keyih Afincho with equal protein content of 17%. The lowest protein content (6.21%) was observed in the landrace Lequa. Protein content variability among the sorghum landraces that have similar local name was observed. For example, protein content of sorghum landraces named as Arfa’agdm (15.36-18%), Lequa (6.21-13.96%), Kodem (8.88-15.17%), and Shilkuit (7.31-13.31%) were variable. In this study, five sweet sorghum types that the farmers locally called these types as “Tinkish” were part of the analysis. These all were collected from the southern zone of Tigray, particularly from the districts of Raya-Alamata. Higher protein was found in Lewaso Tinkish with a value of 15.77% followed by Tinkish varieties Gorid and Gegebsa with protein contents of 14.67% and 14.52%, respectively, whereas lower protein content was found in Tinkish Afincho (11.54%) and Tinkish Hawaye (12.87%).
The starch content of the sorghum landraces was in the range of 33.42 to 78.30%. Next to Kodem, with 78.30% protein, the highest starch content was recorded from landrace Tsaeda Kubi (77.48%). The lowest starch was found from Zeri-Sheytan [the Devil’s sorghum], which is a wild type sorghum, followed by Lequa with a value of 43%. As it is shown in protein, variabilities for starch within the same locally named landraces were also observed, for example; the starch content of Kodem was varied from 51.65 to 78.3% and that of Lequa from 45.84 to 76.92%. The wild types of sorghum showed wide variability for protein (10.4-14.78%) and starch (33.42-63.9%) contents and some of the wild types had higher protein and starch contents than many of the cultivated sorghum landraces.
3.2. Variabilities For Mineral Elements
The result revealed the presence of statistically significant (P<0.001) variability for mineral concentrations among the landraces (Table 2). Fe concentration was ranged between 32 and 101 mg/100g. The highest concentration of Fe was obtained from landrace Jamuye followed by Dagnew with a concentration of 79 mg/100g. Next to Dengele, Aba’are and Tirbish had scored lowest Fe with a value of36.54 and 38.31 mg/100g, respectively. For zinc, maximum (42.98 mg/100g) and minimum (16.90 mg/100g) concentrations were found from the landraces of Dagnew and Zengada, respectively. Mn concentration in ten samples was ranged from 9.21 to 20.23 mg/100g. The highest Mn concentration was recorded from Aba’are followed by Wedi-Sbuh (18 mg/100g), while the lowest was obtained from Tirbish followed by Gumbil (10.46 mg/100g). Among the landraces, highest copper and chromium concentrations were recorded from Jamuye (5.25 mg/100g) and Dagnew (1.5 mg/100g), respectively. The lowest copper content (1.48 mg/100g) was obtained from Gumbil. Chromium was below the detection limit in the sorghum landrace Kodem.
3.3. Variabilities For Total Flavonoid And Tannin Contents
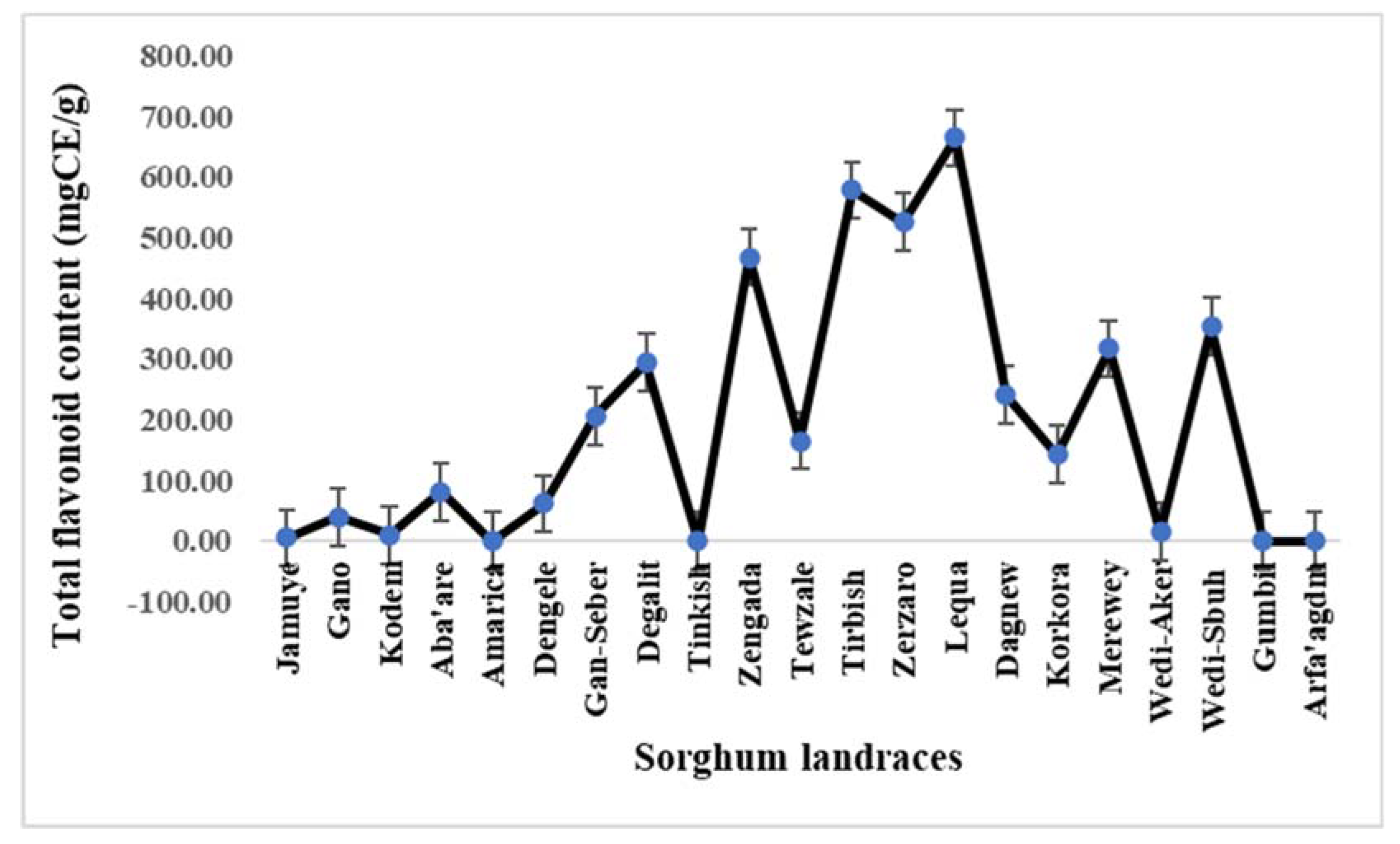
Extensive variability with significant difference (P<0.001) for total flavonoid content was observed among the evaluated sorghum landraces (
Table 2). TFC was ranged from 0 to 665 with a median value of 142.5 mg CE/g. The highest TFC was found in Lequa followed by Tirbish (578.50 mg CE/g), Zerzaro (526.25 mg CE/g), and Zengada (467.50 mg CE/g). Landraces Jamuye and Kodem scored lowest TFC with a value of 5.25 and 9.5 mg CE/g, respectively (
Figure 2). Out of the 21 evaluated sorghum landraces, flavonoid was below the detection limit in four landraces including Amarica, Tinkish, Gumbil, and Arfa’agdm.
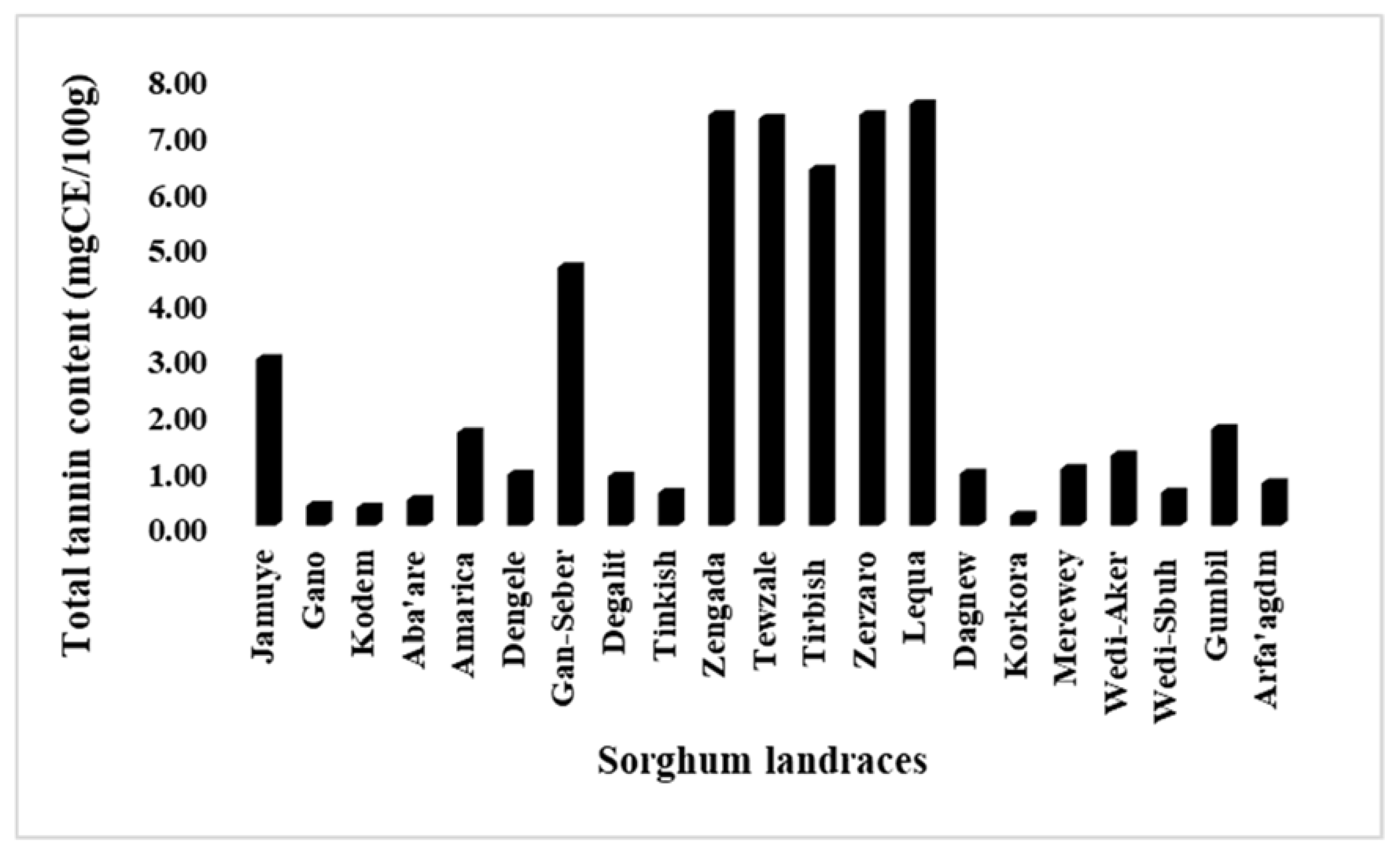
Significant variation (P<0.001) for total tannin content were observed among the landraces (
Table 2). The maximum (7.5 mg CE/100g) and minimum (0.18 mg CE/100g) TTCs were found from landraces Lequa and Korkora, respectively, with a median value of 1.01 mg CE/100g. Next to Lequa, the landraces Zerzaro, Zengada and Tewzale had highest TTC (with approximately 7.3 mg CE/100g) (
Figure 3). All the landraces with highest total tannin and flavonoid contents are from central zone of Tigray. Respectively, landraces Kodem, Gano and Aba’are scored lowest TTC next to Korkora with a value of 0.32, 0.36 and 0.46 mg CE/100g.
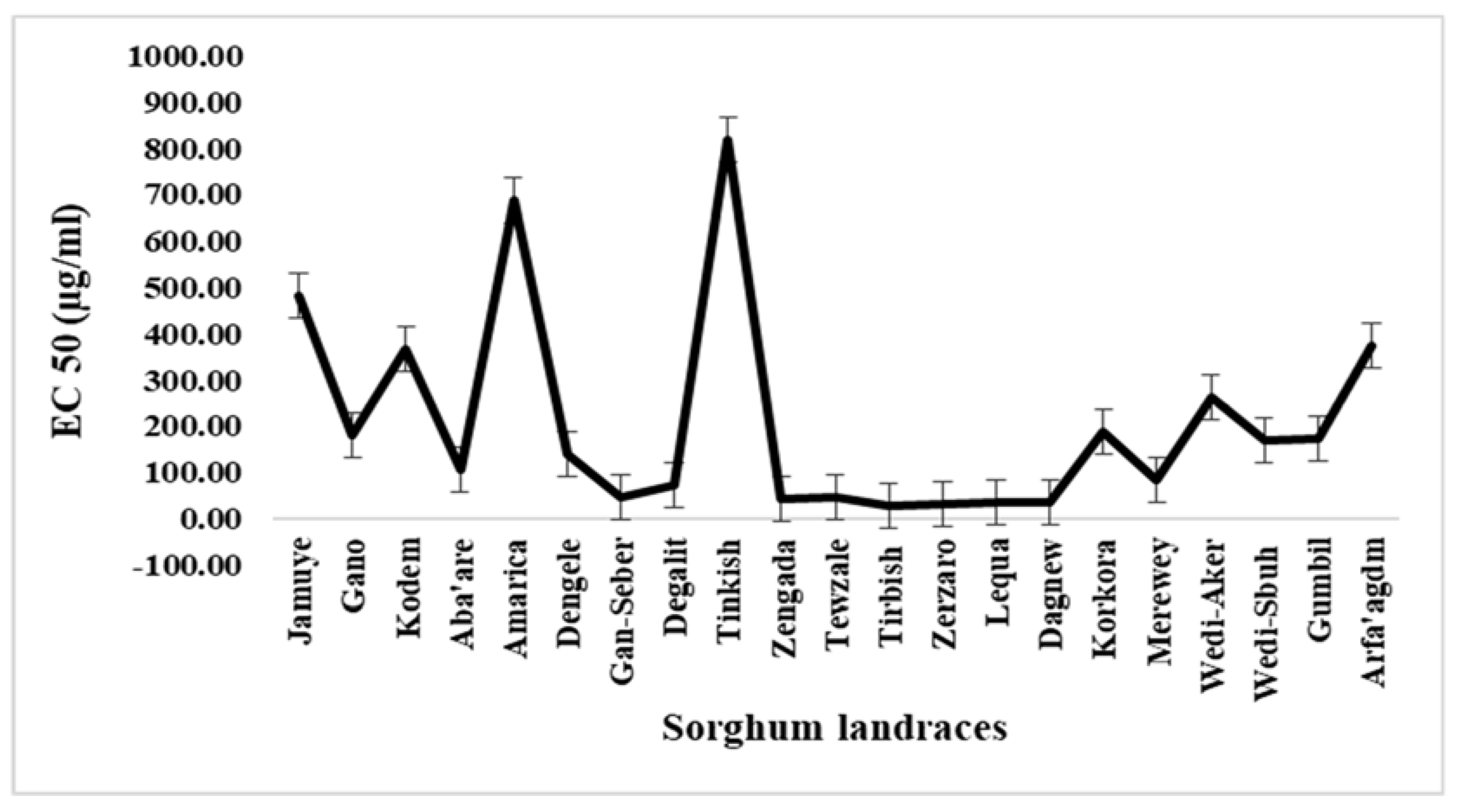
3.4. Variabilities For Antioxidant Activities
The antioxidant activities among the landraces showed significant difference (P<0.001) (
Table 2). DPPH radical scavenging for the sorghum sample extracts were evaluated at different concentrations ranging from 50 to 1000 µg/ml. At higher concentration (1000 µg/ml), the DPPH radical scavenging were ranged from 54.63% to 99.29%, whereas at lower concentration (50 µg/ml) it was in the range of 25.59 to 93.45%. Widespread ranges of variability in EC
50 (i.e., the sample extract concentration in µg/ml required to scavenge DPPH free radical by 50%) among the evaluated sorghum landraces were observed (
Figure 4). The EC
50 values were ranged between 29.09 and 818.37µg/ml. The sorghum landrace Tirbish had lower EC
50 value (29.09µg/ml) than the control (Ascorbic acid) with EC
50 value of 31.51µg/ml. Among other sorghum landraces, Zerzaro (33.38 µg/ml), Lequa (33.52 µg/ml), and Dagnew (33.6 µg/ml) scored lower EC
50 value, whereas higher EC
50 value were obtained from the landraces Tinkish (818.37 µg/ml), Amarica (687.6 µg/ml), and Jamuye (484.38 µg/ml). The landraces with higher phenolics (flavonoid and tannin) were also found to have higher DPPH scavenging activities.
There was high variation in the ferric reducing antioxidant power (FRAP) of the sorghum samples that was ranged between 17.85 and 334.81 mg AAE/g. The strongest ferric reducing antioxidant power was obtained from sorghum landrace Lequa (334.81 mg AAE/g) followed by Tirbish (333.75 mg AAE/g), Zerzaro (318.39 mg AAE/g), and Zengada (225.5 mg AAE/g). Respectively, sorghum landraces that had weakest ferric reducing antioxidant power were Tinkish (17.85 mg AAE/g), Amarica (18 mg AAE/g), Kodem (23 mg AAE/g), and Arfa’agdm (27.59 mg AAE/g).
Strongest and weakest total antioxidant capacity, as assayed using phosphomolybdenum, were also found in sorghum landraces Zengada (63.89 mg BHTE/g) and Tinkish (1.71 mg BHTE/g), respectively. Next to Zengada, the top three sorghum landraces with highest total antioxidant activity were Tirbish (62.18 mg BHTE/g), Zerzaro (61.28 mg BHTE/g), and Lequa (60.43 mg BHTE/g), whereas the sorghum landraces Amarica (6.01 mg BHTE/g), Wedi-Aker (6.55 mg BHTE/g), and Korkora (7.61 mg BHTE/g) were among the landraces that had weakest total antioxidant activity.
3.5. Relationships Of Seed Color With Phenolics And Antioxidant Activities
The diversity of seed colors of the sorghum landraces used in this study (
Figure 5) resulted in excessive variability in the contents of the studied nutritional traits particularly phenolics and antioxidants. Those with red and brown seed colors had highest flavonoid, tannin and antioxidant capacity as compared to yellow and white seeded sorghum landraces. Those sorghum landraces with the highest phenolic compounds (flavonoid and tannin contents) had recorded highest antioxidant capacity.
3.6. Association Between Variables
Starch had very weak and non-significant correlation with all the minerals and protein (
Table 3). Weak and non-significant correlation was observed between protein and all minerals except Cu that was moderate. Protein had moderate and negative but non-significant correlation (r = -0.43, P>0.05) with tannin. Copper recorded a high positive and significant correlation with Fe (r = 0.73, P<0.01), Zn (r = 0.53, P<0.05) and Mn (r = 0.48, P<0.05). The correlation coefficients of the phenolic compounds and antioxidant activities were positive and highly significant. Flavonoid was significantly and strongly correlated with ferric reducing antioxidant power (r = 0.92, P<0.01) and total antioxidant activity (r = 0.87, P<0.01). Similarly, tannin was also strongly correlated with ferric reducing antioxidant power (r = 0.86, P<0.01) and total antioxidant activity (r = 0.90, P<0.01).
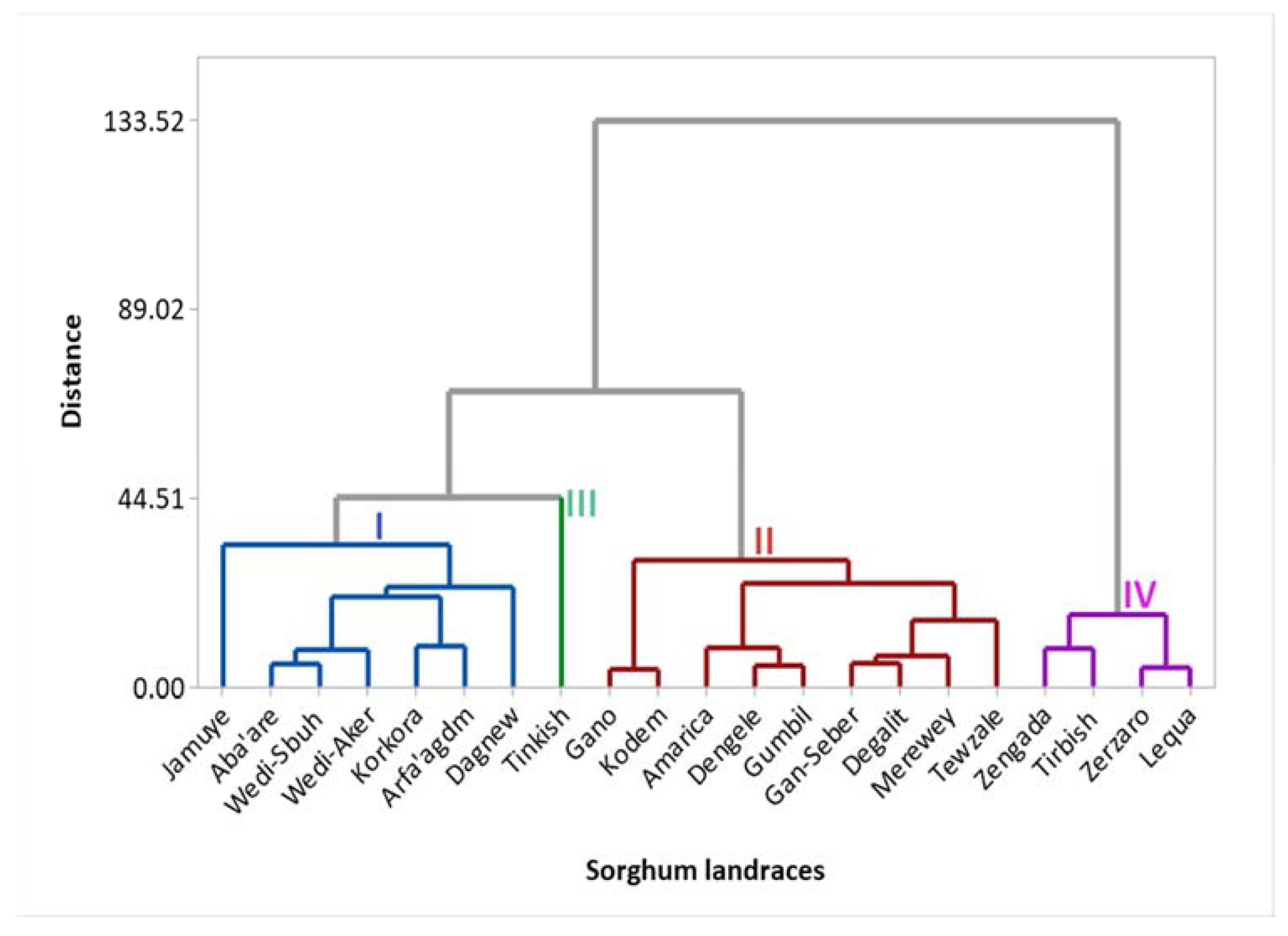
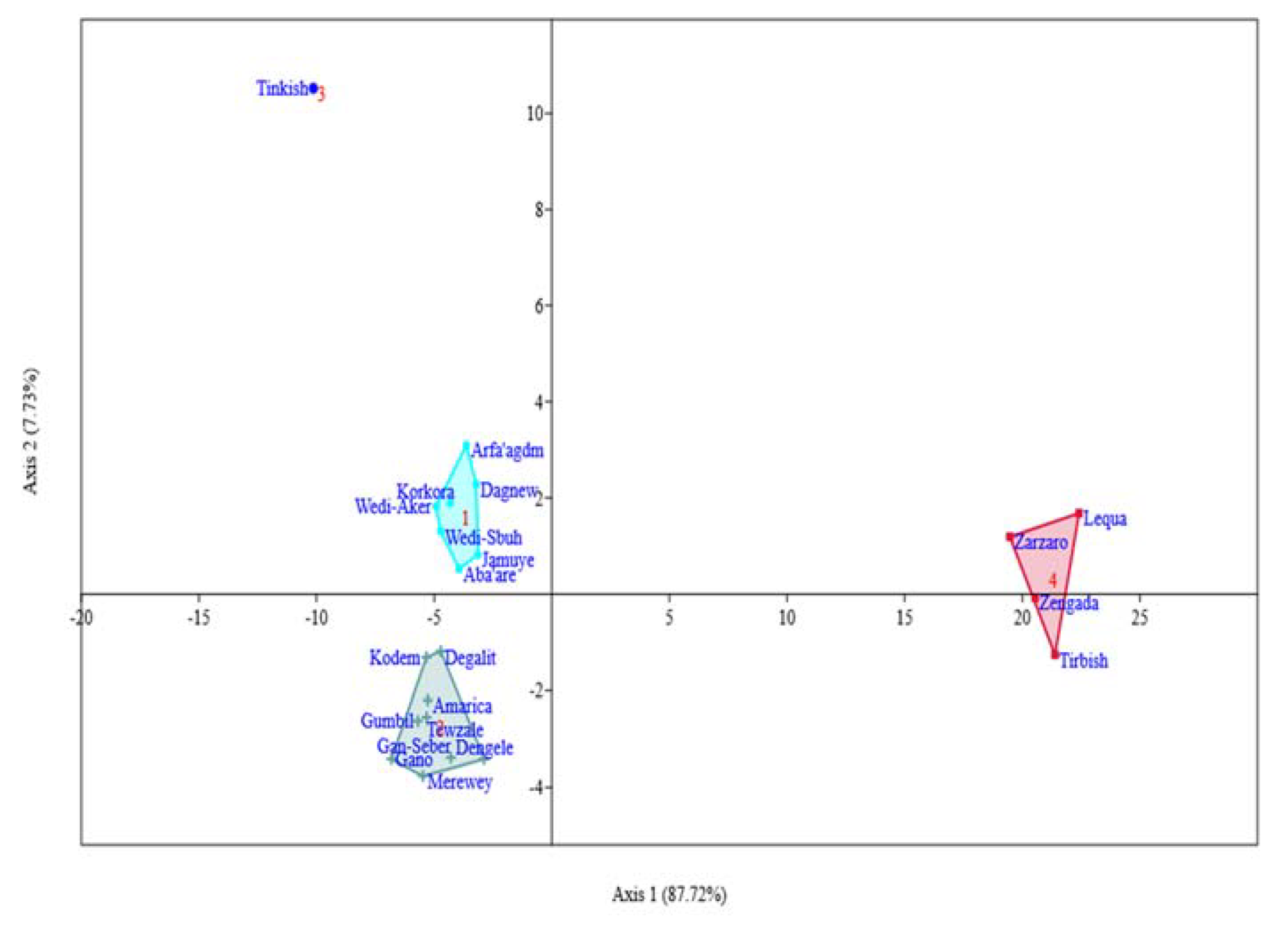
3.7. Grouping And Ordination Of Genotypes
3.7.1. Cluster Analysis
The result shown that the twenty-one sorghum landraces were clustered into four groups (
Figure 6). Respectively, clusters I, II, III, and IV consists of 7, 9, 1, and 4 landraces. Genotypes gathered under cluster I are characterized by their higher mineral elements, high starch, and lower flavonoid, tannin and antioxidant activities. Those in cluster II have highest starch, lower proteins, lower mineral concentrations, high tannin, and high antioxidant potentials. Interestingly, the landrace Tinkish (a sweet sorghum type) was uniquely grouped alone in cluster III. The highest protein and lowest starch, the presence of very low phenolic compounds, and weakest antioxidant capacity may be the reasons that made Tinkish to stand alone from the rest of the group. Besides, it is the only sweet sorghum included in the determination of minerals concentrations, flavonoid, and tannin as well as antioxidant activities. Members in cluster IV are characterized by highest phenolic compounds (flavonoid and tannin) and antioxidant potentials. Besides, all the landraces gathered under cluster IV have red/brown seed color. High variability for the cluster means of all traits were observed (
Table 4). Discriminant analysis was performed to further confirm the genotype grouping analyzed by cluster analysis (Figure 7). The first axis accounts for 87.72% of the total variability, while the second axis accounts for 7.73%. According to the squared distance between clusters (Mahalanobis distance, D
2), cluster IV was the most divergent than others (
Table 5). Clusters IV and III had the highest distance followed by clusters IV and II and clusters IV and I.
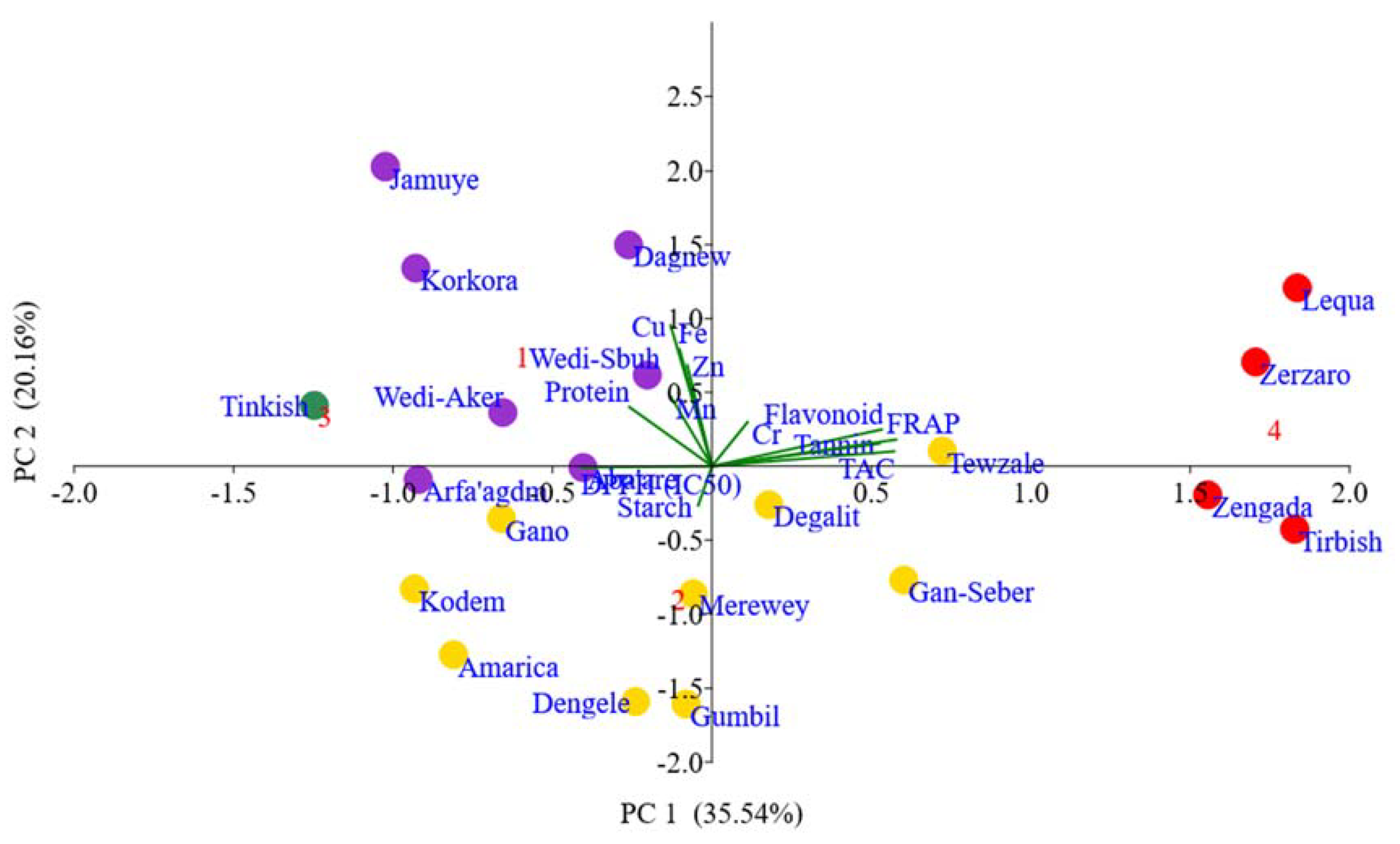
3.7.2. . Principal Component Analysis
Among the total of 12 principal components (PCs), the first four principal components (PCs) contributed much of the variability with eigenvalues greater than unity and cumulatively accounted for 78.48% of the total variation among the landraces. Thus, these four PCs were considered important for this study but the first two component scores were plotted to aid visualization of the overall variability among the populations (Figure 8). Respectively, the four PCs explained 35.54, 20.16, 13.08, and 9.71% of the total variability (
Table 6). The result of PCA revealed that the nutritional traits showed different patterns of contribution to the variability among the landraces. In PC 1, the variables ferric reducing antioxidant power, total antioxidant capacity, flavonoid, and tannin, respectively had significant contribution with positive loading, whereas protein and DPPH had high contribution in the negative direction. The mineral elements Cu, Fe, Zn, and Mn had greatly contributed for the variability in PC 2. The nutritional traits starch and Zn had significant influence in PC3 in the positive direction, while DPPH is in the negative direction. The traits Cr (positive loading) and Mn (negative loading) contributed more to the fourth component.
4. Discussion
4.1. Proximate Analysis
The high variability in protein content among the landraces of the current study may be due to the genetics of the landraces and environmental effects. The highest protein content found in the current study (18%) is higher than previous studies conducted in Ethiopian sorghum in which they found a maximum of 15.26% (15) and 16.48% [
34]. Variability in grain protein in Ethiopian sorghum that varied from 77 to 114 g/kg was also reported [
19]. According to [
35], the protein content in whole sorghum grain is in the range of 7 to 15%, relatively a narrower range than the current work. Grain protein content analysis of South African sorghum was also reported from 7.69 to 16.30% [
17,
19].
Farmers in the study area purposely sow Tinkish (sweet sorghum) in the middle of other sorghum types to hide them from herdsmen. This is because their stem is very sweet and the herdsmen mainly children like to chew them before they reach at maturity stage. In Ethiopia, unique sweet sorghums that, in terms of nutrition and tastiness, extremely exceeded other sorghum types are available. The local farmers called these landraces in Amharic as 'Wetet Begunche' (to mean "milk in my mouth") and Marchuke (to mean "honey squirts out of it"). It was reported that of the 9,000 varieties tested, these two were unique that contained 30% more protein, but more importantly, their protein is about twice the normal level of lysine, an amino acid critical to nutritional quality [
36]. High lysine mutant with the
hl gene has been identified from Ethiopian line [
37] where lysine content was enhanced by about 40-60%. Thus, the higher protein content of Tinkish landraces in this study may be due to the high lysine content of the sweet sorghum.
The great variability in starch also reflects the presence of genetic difference among the sorghum landraces and environmental effects. Such a wide variability is in line with previous studies of [
20,
21,
22]. The current result in starch content is much higher than the sorghum samples of western Ethiopia [
15] indicating that sorghum genetic resources of Tigray are important to consider in sorghum starch improvement programme. The discrepancy in protein and starch content within the locally similar named landraces shows the impact of environment on the nutritional status of the landraces. It is reported that the accumulation of quantitatively inherited traits, such as starch, in grains is affected by environment [
40]. The higher protein and starch content of some wild relatives than the cultivated landraces indicate the significance of including the wild gene pool in sorghum nutritional enrichment and improvement program as they might have valuable alleles for the nutritional trait. High variability for protein content (10.2-14.6%) among ten Sudanese wild genotypes of sorghum was also reported [
38].
Cereals and pulse grains are the main sources of protein for the poor people who rely in those grains as a staple food as in the cases of Ethiopia, which is known for its lowest per capita consumption of meat in the world [
39]. In Tigray and other parts of the country, sorghum is widely used for human consumption as enjera (mainly in the rural areas where about 85% of the population lives) and it is considered as one of the cheap sources of protein [
34]. The sorghum landraces identified to have highest protein content could be targeted for complementary food product development to enrich the nutritional profile of food products. In addition, these landraces could be considered by plant breeders as potential starting parent materials for grain quality improvements of sorghum.
The wide variability in mineral elements among the currently studied sorghum landraces could be due to the genetic differences among the landraces, environment, the variability in the concentration of minerals in the soil, and the differences in the ability of the landraces to absorb the nutrients from the soil. This agreed with [
15] who reported the concentrations of iron, zinc, and manganese ranging from 41.17 to 127.50, 13.5 to 34.67, and 9.5 to 23.83 mg kg
−1, respectively. Significant variation in Fe (12.10-83.40 mg kg
−1) and Zn (6.30-51.40 mg kg
−1) were also detected among cultivars, breeding lines and selected sorghum accessions [
41]. Respectively, iron, zinc, and copper content for sorghum flour was reported to be 2.24 mg/100g, 0.75 mg/100g and 0.61 mg/100g [
42].
An adequate amount of about 51 nutrients is required for nourished and healthy lives of human being [
43] among which mineral elements are essential trace elements in the human nutrition. Nowadays, micronutrient malnutrition situations affecting the human population, such as Fe and Zn deficiencies, are getting major concern because their deficiencies have serious health consequences including poor growth of children, reduced immunity, weakness and illness [
44]. This makes critical to determine the micronutrient contents in grains including sorghum for use in breeding strategies for the improvements of nutritional quality traits. Sorghum is reported to be a good source for more than 20 minerals [
36]. It exceeds other major cereals such as wheat, rice, and maize and compares well with pulses in terms of grain Fe and Zn [
45].
Biofortification, improving the density and availability of micronutrients, is considered as one of the most suitable solutions for the severe malnutrition problems mainly in the developing countries [
46]. Thus, the high concentration of the mineral elements in the studied sorghum landraces might offer a probable source for biofortification. The landraces such as Jamuye and Dagnew, with extremely high Fe and Zn, shall be taken in to consideration for those people suffering from these micronutrient deficiencies. Generally, those landraces with enhanced mineral elements may be directly consumed as whole grain to satisfy the daily micronutrient intake or used indirectly as candidate materials for breeding to generate new and nutritionally rich variety.
4.2. Phenolic Compounds And Antioxidant Activities
Flavonoids, the most abundant and diverse group of phenolics in plants, play a vital role in plant development as it controls auxin transport. The absence of flavonoid in some of the landraces suggests that these samples have insignificant antioxidant potential because the higher flavonoid corresponds to the higher antioxidant activity [
24]. Previous studies showed that TFC was in the range of 0-8138.22 μg CE g
-1 dm
-1 in sorghum landraces and breeding lines [
47], 0-22,606 μg CE g
-1 dm
-1 in sorghum panels [
48] and 15.33-42.84 mg RE/100g in five genotypes of sorghum [
49]. Genetic variability among the landraces for TFC was very clear (
Figure 2). In addition to the genotypic difference, the variation in flavonoid among the landraces may also be due to the differences in the sites of collection of the samples (i.e., environmental differences). Like the present result, significant variation of flavonoid concentration in sorghum grains was observed due to the variation in genotype and environment [
50].
In agreement to the total tannin content of this current study, [
34] found lower tannin content for the landrace Abuare, which is the same to the landrace Aba’are of our sample despite their spelling. Sorghum tannins have the ability of binding to minerals, mainly iron and zinc, and reduce their bioavailability, thereby negatively impacting the nutritional profile of sorghum [
51]. In line with this, we found that most of the landraces with high TTC had shown low mineral content. In addition, tannins can bind to proteins and significantly restricts their digestibility [
52,
53]. However, it was also stated that the reduction of protein digestibility in sorghum may not be entirely due to tannins [
24,
27]. In support of this; sorghums with equal levels of tannins had different digestibility signifying that tannins are only partially responsible for low protein digestibility [
54]. On the other hand, tannins are regarded as beneficial secondary metabolites to our body as they are associated with various health benefits due to their antioxidant activity. They also help in increasing plant resistant to predators such as birds, for example, black sorghum cannot be easily attacked by birds due to the existence of tannins in this type of sorghum [
26].
Since high tannins have the properties of undesirable texture, flavor, and taste, the sensory of food products made from food crops having high tannin may not be easily accepted by end users [
34]. Thus, the sorghum landraces with low tannin such as the landraces of Korkora, Kodem, Gano, and Aba’are are preferable for food product making for acceptable sensory attribute and nutritional value. However, processing such as decortication/dehulling improves the sensory appeal of sorghum grain by reducing the level of tannins by 80%-95% [
51] thereby increasing the bioavailability of minerals. On the other hand, the sorghum landraces with high tannin such as of Lequa are not preferable for enjera rather for local beverage (Swa), which is widely used in the study area. Sorghum grains with more than 18.13 mg/100ml tannin content are not suitable for malting and brewing [
55] because higher tannins have negative impact on the brewing process in that they can inhibit the activity of alpha amylase and this in turn lowers starch hydrolysis, which is vital for brewing [
56].
The increase in the concentration of extracts led to an increase in the percentage of DPPH radical scavenging activity. Similarly, this was supported in related studies [
32,
57,
58]. The lower EC
50 value indicates the higher DPPH radical scavenging potential and vice versa [
59,
60]. It was reported that sorghum had shown higher DPPH scavenging capacity as compared to rye [
60]. This shows that sorghum contains DPPH free radical scavenging capacity that could have a beneficial action against abnormalities caused by generation of free radicals. The presence of antioxidants in plant extracts causes reduction of the ferric ion (Fe
3+) to ferrous (Fe
2+) and molybdenum (VI) to molybdenum (V). Thus, higher reducing values, as shown in sorghum landraces of Lequa, Tirbish, Zengada, and Zerzaro implies that they have higher antioxidant capacity because the value is based on the reduction of ions in the presence of antioxidants [
61]. The widespread variability in total antioxidant capacity in the present result was also supported in previous studies in sorghum genotypes [
14,
27,
48]. According to [
25], sorghum had revealed the highest total antioxidant capacity as compared to wheat, oat, barley, and maize. Hence, sorghum is one of the richest cereals in antioxidant capacity and it could be used as natural antioxidant against free radicals or other physiological or pathological abnormalities.
4.3. Relationship Of Seed Color With Phenolic Compounds And Antioxidant Activities
Seed color is one of the important morphologic characters used by local farmers to identify their varieties. Sorghum landraces with diverse color traits are appropriate depending on the test and preferences of the farming community for different end uses such as for enjera, Swa, and as popping (Figure 9). In line to our result, high level of antioxidant in red seeded than white seeded sorghum was reported [
49,
62]. Besides, [
47] identified a low level of antioxidant compounds in yellow or white seeded sorghum genotypes. The highest antioxidant for the landraces with highest phenolic compounds confirms that the phenolic compounds, which are found in the pericarp layer of black and red seeded varieties of sorghum grain contributed to the antioxidant activities [
24]. Strong association between pericarp color and antioxidant activity was reported in previous studies [
48,
63]. Hence, sorghum grains with red and brown seed color may possess extensive range of prevention against cellular damage due to free radicals.
In the study region (Tigray), sorghum is mainly utilized for human consumption as enjera in which yellow and white seeded sorghum are ideal, whereas sorghum with red seed color is used for making ‘Swa’ [
64]. Farmers in the same study area, also cultivate finger millets of different seed colors for different end use purposes in that black seeded are used to prepare local drinks, while white seeded are utilized for making enjera [
65]. According to [
28], red seeded sorghum is preferred for brewing of traditional beer. In most African countries, white or light white sorghums are more generally preferred for porridge making [
66]. Furthermore, in some parts of Africa where the bird quelea problem is severe, the high tannin brown seeded sorghum varieties, which are least preferred by birds, are grown for food and drinks [
67]. The presence of connection between kernel color and human consumption in Ethiopian barley was also stated [
68]. Such end use attributes associated with different colored sorghum variants could have conservation and utilization as well as market implications.
4.4. Correlation Analysis
Correlation analysis determines the association among traits under study and helps in designing selection strategies for simultaneous improvements of combinations of traits [
69]. The weak correlation of starch with other minerals agrees with [
19] who reported that starch showed non-significant correlation with protein, Zn, and Fe, whereas unlike the authors we found moderate correlation between Fe and Zn. In contrast to the present result, [
15] reported that protein had significant positive correlation with Zn and Mn. Although the positive significant correlation between Cu and these mineral elements has implications for the possibility of combining selection, it must be validated whether breeding for high concentration of Cu in such a manner increases the concentration of the other minerals. The negative correlation between tannin and protein, starch, Zn, and Cr may be because tannin can bind these nutritional traits, thereby inhibiting bioavailability [
51,
53]. The high correlation between phenolic compounds and antioxidant activities is in line with other studies [
47,
49,
68]. The strong correlation between the total antioxidant capacity and phenolic compounds (flavonoid and tannin) indicates that the phenolic compounds basically contribute to the antioxidant activities of the sorghum landraces.
4.5. Multivariate Analysis
Cluster analysis helps to facilitate future identification and selection of best parents for nutritional improvement of the sorghum landraces. The high range for the cluster means suggests the significant contribution of all traits in the genotype clustering. This also supports the extensive genetic diversity among the planting materials for the nutritional traits. Mahalanobis distance is a powerful statistical tool that measures the genetic divergence of populations under evaluation. The lower the inter-cluster distance, the lower is the chance to produce important recombinants [
63] that shows the similarity in the nutritional make-up of the landraces. On the other hand, the higher distance between the clusters tells that there is a wide variability between the landraces in the different clusters, which is important to conduct crossing to get better nutritional trait of interest. In other words, extremely divergent genotypes would yield a broad range of variability in the following generation [
69]. Thus, desirable recombinants could be found by crossing parents selected from clusters IV and III and clusters IV and II.
Principal component analysis (PCA) was performed to reduce the number of variables into a few correlated components that can explain much of variability and to estimate the relative importance and contribution of each nutritional trait to the total variance. The sign of the loading indicates the direction of the relationship between the components and the variable [
70]. The biplot of PCA clearly shows the associations and differences among the landraces and targeted traits. The distinctiveness of characters that appear less than 90° angle are positively correlated, while those that formed more than 90° angles are associated with negative correlation, and those that have a 90° angle do not show correlation in the biplot [
71]. The landraces remained scattered across the four quadrants showing large genetic variability in their nutritional make-up that could be used in the sorghum improvement program. The landraces relatively close to each other have similar nutritional properties, whereas those that are positioned far from each other are different. The landraces, which are far from the origin are separated from the rest of the group due to some specific distinctiveness in their nutritional characteristics. Thus, selection of these landraces as potential parent materials would result in successful crossing to develop specific sorghum variety with enhanced nutritional attributes. Landraces opposite side of the trait vector arrows indicate small value for that trait, while those landraces on the direction of vector arrows display high values for the trait. The length of the vector is proportional to the magnitude of the trait in grouping the landraces. For example, the longer vector as shown in the traits of flavonoid, tannin, and antioxidant capacities highly contributed in grouping the landraces Lequa, Zerzaro, Zengada, and Tirbish.
5. Conclusion
The overall result revealed that the examined sorghum landraces exhibited extensive variability in the studied nutritional and antinutritional traits and thus, there is prospect for enrichment of the nutritional attributes in sorghum to combat malnutrition. The landraces Arfa’agdm, Tsaeda Jargte, Keyih Afincho, Kodem, and Taeda Kubi were superior in proximate analysis that should be considered as source of energy, whereas the landraces Jamuye, Dagnew, Aba’are, and Wedi-Sbuh had higher mineral concentrations. The sorghum landraces mainly Lequa, Zerzaro, Zengada, Tewzale, and Tirbish were superior in phenolics and antioxidants that could be used as potential natural antioxidants.
Depending on the breeding objective, the landraces with superior nutritional profile can serve as potential resources for future grain quality improvements of sorghum. They can also be considered for new food product development like biscuits, snacs, and syrup. However, for the delivery of high grain minerals, protein, starch and phenolic compounds, it is also critical to consider those landraces that satisfy farmers’ preferred traits including maturity and grain yield. The rich genetic diversity in the nutritional and antinutritional attributes may provide some novel genes for enhanced grain quality of sorghum and such a great diversity have implications on conservation and utilization of sorghum genetic resources. Finally, the present result will help as a benchmark in designing bio-fortification and/or breeding for quality traits of sorghum, thereby sensitizing nutrition in sorghum production and consumption in the region.
Acknowledgments
The authors wish to acknowledge the Norwegian Embassy in Addis Ababa for the research fund through the institutional collaboration between Mekelle University and the Norwegian University of Life Sciences (NMBU).
References
- Singh, R.; Govindan, V. Zinc-Biofortified Wheat: Harnessing Genetic Diversity for improved Nutritional Quality. Science Brief: Biofortification No. 1. CIMMYT, HarvestPlus, and the Global Crop Trust. Bonn, Germany, 2017.
- Development Initiatives. Global Nutrition Report 2017: Nourishing the SDGs; Development Initiatives: Bristol, UK, 2017. [Google Scholar]
- Maberly, G.F.; Trowbridge, F.L.; Yip, R.; Sullivan, K.M.; E West, C. Programs Against Micronutrient Malnutrition: Ending Hidden Hunger. Annu. Rev. Public Heal. 1994, 15, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.E.; Pfeiffer, W.H.; Beyer, P. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.; Baum, B.R.; Fahrig, L.; Torrance, J.K.; Arnason, T.J.; Lambert, J.D. Sorghum (Sorghum bicolor (L.) Moench) landrace variation and classification in North Shewa and South Welo. Ethiopia. Euphytica 1997, 97, 255–263. [Google Scholar] [CrossRef]
- Villa, T.C.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and Identifying Crop Landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef]
- Seboka, B.; Theo van Hintum, T. The dynamics of on-farm management of sorghum in Ethiopia: Implication for the conservation and improvement of plant genetic resources. Genet. Resour. Crop Evol. 2006, 53, 1385–1403. [Google Scholar] [CrossRef]
- Newton, A.C.; Akar, T.; Baresel, J.P.; Bebeli, P.J.; Bettencourt, E.; Bladenopoulos, K.V.; Czembor, J.H.; Fasoula, D.A.; Katsiotis, A.; Koutis, K.; et al. Cereal Landraces for Sustainable Agriculture. 2011, 147–186. [CrossRef]
- Central Statistical Agency (CSA). Agricultural Sample Survey2015/2016 volume 1, a report on area and production of major crops. Statistical Bulletin 2016, 584 Addis Ababa, Ethiopia.
- Teshome, A.; Patterson, D.; Asfew, Z.; Torrance, J.K.; Arnason, J.T. Changes of Sorghum bicolor landrace diversity and farmers’ selection criteria over space and time, Ethiopia. Genet. Resour. Crop. Evol. 2007, 54, 1219–1233. [Google Scholar] [CrossRef]
- Tsehaye, Y.; Abera, Z.; Kebede, A.; Ghebremichael, B. A dynamic sorghum (Sorghum bicolor (L.) Moench) diversity management in situ and livelihood resilience in South and Central Tigray Region, Ethiopia. Momona Ethiopian Journal of Science.
- Duodu, K.; Taylor, J.; Belton, P.; Hamaker, B. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef]
- Saleh, S.M.; Q. Zhang, Q.; Chen, J.; Shen, Q. Millet grains: nutritional quality, processing, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Wu, G.; Johnson, S.K.; Blanchard, C.L. Characterization of phenolic compounds and antioxidant activity in sorghum grains. J. Cereal Sci. 2018, 84, 103–111. [Google Scholar] [CrossRef]
- Shegro, A.; Shargie, N.G.; van Biljon, A.; Labuschagne, M.T. Diversity in starch, protein and mineral composition of sorghum landrace accessions from Ethiopia. J. Crop. Sci. Biotechnol. 2012, 15, 275–280. [Google Scholar] [CrossRef]
- Shegro, A.; Labuschagne, M.T.; Shargie, N.G.; van Biljon, A. Multivariate analysis of nutriotonal diversity in sorghum accesions from wetsern Ethiopia. Journal of Biological Sciences 2013, 13, 67–74. [Google Scholar] [CrossRef]
- Mofokeng, M.A. Diversity analysis of South African sorghum genotypes using agronomic traits, SSR markers and protein content and amino acid composition. PhD Thesis. University of KwaZulu-Natal Pietermaritzburg South Africa, 2015.
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant'Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Nguni, D.; Geleta, M.; Hofvander, P.; Fatih, M.; Bryngelsson, T. Comparative genetic diversity and nutritional quality variation among some important southern African sorghum accessions [Sorghum bicolor (L.) Moench]. Aust. J. Crop Sci. 2012, 6, 56–64. [Google Scholar]
- Shewayrga, H.; A Sopade, P.; Jordan, D.R.; Godwin, I.D. Characterisation of grain quality in diverse sorghum germplasm using a Rapid Visco-Analyzer and near infrared reflectance spectroscopy. J. Sci. Food Agric. 2011, 92, 1402–1410. [Google Scholar] [CrossRef]
- Jambunathan, R.; Subramanian, V. Grain quality and utilization of sorghum and pearl millet. Biotechnology in tropical crop improvement 1988, 133–139. [Google Scholar]
- Njuguna, V.W.; Cheruiyot, E.K.; Mwonga, S.; Rono, J.K. Effect of genotype and environment on grain quality of sorghum (Sorghum bicolor L. Moench) lines evaluated in Kenya. Afr. J. Plant Sci. 2018, 12, 324–330. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. Journal of natural products 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Sorghum and millet phenols and antioxidants. J. Cereal Sci. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L. Phenolic compounds in cereal grains and their health benefits. Cereal foods world 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, W.L.; Rooney, L.W. Evaluation of phenolics and antioxidant activity of black sorghum hybrids. J. Cereal Sci. 2013, 58, 278–283. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W.; Waniska, R.D.; Rooney, W.L. Phenolic Compounds and Antioxidant Activity of Sorghum Grains of Varying Genotypes. J. Agric. Food Chem. 2005, 53, 6813–6818. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Aida, W.W.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427. [Google Scholar]
- Katerere, D.R.; Eloff, J. Antibacterial and antioxidant activity of Sutherlandia frutescens (Fabaceae), a reputed anti-HIV/AIDS. phytomedicine Phytother Res 2005, 19, 779–781. [Google Scholar] [CrossRef]
- Dessalegn, E. In vitro antioxidant and α-amylase inhibition activities of spiced red chili paste (datta) from South Ethiopia. Ethiop. Pharm. J. 2016, 31, 93. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Tasie, M.M.; Gebreyes, B.G. Characterization of Nutritional, Antinutritional, and Mineral Contents of Thirty-Five Sorghum Varieties Grown in Ethiopia. Int. J. Food Sci. 2020, 2020, 8243617–11. [Google Scholar] [CrossRef]
- FAO. Sorghum and Pearl Millets in Human Nutrition; Food and Agriculture Organization of the United Nations (FAO) Press: Rome, Italy, 1995. [Google Scholar]
- BSTID-NRC (Board on Science and Technology for International Development- National Research Council). Lost crops of Africa. Academic Press: Washington, DC, USA, 1996; pp. 127–213.
- Singh, R.; Axtell, J.D. High lysine mutant gene (hl) that improves protein quality and biochemical value of grain sorghum. Crop Science 1973, 13, 535–539. [Google Scholar] [CrossRef]
- Kumar, M.S.; Krishna, V.M. Experimental investigation on performance of hybrid PCM’s on addition of nano particles in thermal energy storage. Mater Today Proc. 2019, 17, 271–276. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nation (FAO). Current Worldwide Annual Meat Consumption per capita, Livestock and Fish Primary Equivalent. Food and Agriculture Organization of the United Nations, 2013.
- Yi, B.; Zhou, Y.-F.; Gao, M.-Y.; Zhang, Z.; Han, Y.; Yang, G.-D.; Xu, W.; Huang, R.-D. Effect of Drought Stress During Flowering Stage on Starch Accumulation and Starch Synthesis Enzymes in Sorghum Grains. J. Integr. Agric. 2014, 13, 2399–2406. [Google Scholar] [CrossRef]
- Hariprasanna, K.; Agte, V.; Elangovan, M.; Patil, J.V. Genetic variability for grain iron and zinc content in cultivars, breeding lines and selected germplasm accessions of sorghum [Sorghum bicolor (L.) Moench]. Indian Journal of Genetics 2014, 74, 42–49. [Google Scholar] [CrossRef]
- Mohammed, N.; Ahmed, I.; Babiker, E. Nutritional evaluation of sorghum flour (Sorghum bicolor L. Moench) during processing of injera. Int. J Bio Life Sci. 2010, 6, 35–39. [Google Scholar]
- Welch, R.M. Linkages Between Trace Elements in Food Crops and Human Health. 2008, 287–309. [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2010, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef]
- Chan, S.S.; Ferguson, E.L.; Bailey, K.; Fahmida, U.; Harper, T.B.; Gibson, R.S. The concentrations of iron, calcium, zinc and phytate in cereals and legumes habitually consumed by infants living in East Lombok, Indonesia. J. Food Compos. Anal. 2007, 20, 609–617. [Google Scholar] [CrossRef]
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014, 225, 52–57. [Google Scholar] [CrossRef]
- Alfieri, M.; Balconi, C.; Cabassi, G.; Habyarimana, E.; Redaelli, R. Antioxidant activity in a set of sorghum landraces and breeding lines. Maydica 2017, 62, 1–7. [Google Scholar]
- Williams, B.; Kabbage, M.; Kim, H.J.; Britt, R.; Dickman, M.B. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLOS Pathog. 2011, 7, e1002107. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Satpal; Sangwan, S. Characterization of phenolic compounds and antioxidant activity in sorghum [Sorghum bicolor (L.) Moench] grains. Cereal Res. Commun. 2021, 49, 343–353. [Google Scholar] [CrossRef]
- Taleon, V.; Dykes, L.; Rooney, W.; Rooney, L. Effect of genotype and environment on flavonoid concentration and profile of black sorghum grains. J. Cereal Sci. 2012, 56, 470–475. [Google Scholar] [CrossRef]
- Awika, J.M. Sorghum: Its Unique Nutritional and Health-Promoting Attributes: In Gluten-Free Ancient Grains: Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century. 2017.
- Kruger, J.; Taylor, J.R.; Du, X.; De Moura, F.F.; Lönnerdal, B.; Oelofse, A. Effect of phytate reduction of sorghum, through genetic modification, on iron and zinc availability as assessed by an in vitro dialysability bioaccessibility assay, Caco-2 cell uptake assay, and suckling rat pup absorption model. Food Chem. 2013, 141, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Karunakar, S.; Vivek, K. Millet Grain Processing, Utilization and its Role in Helath promotion. A review. Int. J. Nutr, Food Sci. 2016, 5, 318–329. [Google Scholar] [CrossRef]
- Elkin, R.G.; Freed, M.B.; Hamaker, B.R.; Zhang, Y.; Parsons, C.M. Condensed Tannins Are Only Partially Responsible for Variations in Nutrient Digestibilities of Sorghum Grain Cultivars. J. Agric. Food Chem. 1996, 44, 848–853. [Google Scholar] [CrossRef]
- Kiprotich, F.K.; Cheruiyot, E.K.; Mwendia, C.M.; Wachira, F.N.; Owuoche, J.O. Biochemical quality indices of sorghum genotypes from East Africa for malting and brewing. African Journal of Biotechnology 2014, 13, 313–321. [Google Scholar]
- Alonso, A.; Aguirre, A.; Marzo, F. Effect of extrusion and traditional processing methods on anti-nutrients and in vitro digestibility of protein and starch in faba bean and kidney beans. Food Chemistry 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Sharma, I.; Mathur, M.; Singh, G.P.; Rishi, A. Quantittaive estimation of phenolic and flavonoid content ns antioxidant activity of various extracts of different parts of Plumbago zeylanica Linn. Int. J. Drug Dev. & Res. 2014.
- Aregay, N.; Belew, D.; Zenebe, A.; Haile, M.; Gebresamuel, G.; Girma, A. Tree Age and Harvesting Season Affected Physico-chemical and Bioactive Compounds of Elite Type of Gunda Gundo Orange (Citrus Spp) in the Northern Ethiopia. Int. J. Fruit Sci. 2021, 21, 26–39. [Google Scholar] [CrossRef]
- Ljiljana, S.; Mihajlo, S.; Vensa, N.; Ljubisa, N.; Dusica, R.; Jasna, C.B.; Vensa, T. Antioxidant Activity and Total Phenolic and Flavonoid Contents of Hieracium pilosella L. Extracts. Sensors 2009, 9, 5702–5714; [Google Scholar]
- Alsayadi, M. Antioxidant activity and nutrient composition of Sorghum bicolor L. and Secale cereale L. in Algeria. Acad. J. Food Res. 2013, 1, 059–065. [Google Scholar]
- Rohman, A.; Riyanto, S.; Yuniarti, N.; Saputra, W.R.; Utami, R.; Mulatsih, W. Antioxidant activity, total phenolic, and total flavaonoid of extracts and fractions of red fruit (Pandanus conoideus Lam). Int. Food Res. J. 2010, 17, 97–106. [Google Scholar]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic Compositions and Antioxidant Activities Differ Significantly among Sorghum Grains with Different Applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef]
- Awika, J.M. Sorghum phenols as antioxidants. M.Sc. Thesis, Texas A&M University, usa, College Station, TX, 2000. [Google Scholar]
- Semere, T.; Fjellheim, S.; Tsehaye, Y.; Westengen, O.T. Inventory of sorghum landraces diversity on-farm and adaptability responses to changing climate: implications for sorghum breeding and conservation. Genetic Resources and Crop Evolution 2023, 1–18. [Google Scholar]
- Tsehaye, Y.; Berg, T.; Tsegaye, B. & Tanto, T. Farmers’ management of finger millet (Eleusine coracana L.) diversity in Tigray, Ethiopia and implications for on-farm conservation. Biodivers. Conserv. 2006, 15, 4289–4308. [Google Scholar]
- Hikeezi. The Importance of Sorghum Grain Colour and Hardness, and Their Causes and Measurement. International Sorghum and Millet Collaborative Research Support Program (INTSORMIL CRSP) Presentations. 2010. Available online: https://digitalcommons.unl.edu/intsormilpresent/18.
- Harris, H.B.; Burns, R.E. Influence of tannin content on preharvest seed germination in sorghum seed molding. Agronomy Journal 1970, 65, 957–959. [Google Scholar] [CrossRef]
- Asfaw, Z. Variation in the Morphology of the Spike within Ethiopian Barley,Hordeum vulgareL. (Poaceae). Acta Agric. Scand. 1988, 38, 277–288. [Google Scholar] [CrossRef]
- Deepika, K.; Lavuri, K.; Rathod, S.; Yeshala, C.M.; Jukanti, A.K.; Reddy, S.N.; Lv, S.R.; Badri, J. Multivariate analysis of geographically diverse rice germplasm for genetic improvement of yield, dormancy and shattering-related traits. Plant Genet. Resour. Charact. Util. 2021, 19, 144–152. [Google Scholar] [CrossRef]
- Seiler, G.J.; Stafford, R.E. Factor Analysis of Components of Yield in Guar 1. Crop. Sci. 1985, 25, 905–908. [Google Scholar] [CrossRef]
- Yan, W.; Fregeau-Reid, J.A. Breeding line selection based on multiple traits. Crop Sci. 2008, 48, 417–423. [Google Scholar] [CrossRef]
Figure 1.
Map of the Tigray region (study site) and Ethiopia (upper left). The colour highlighted maps are Woredas/districts from where sorghum landraces were collected.
Figure 1.
Map of the Tigray region (study site) and Ethiopia (upper left). The colour highlighted maps are Woredas/districts from where sorghum landraces were collected.
Figure 2.
Variability for total flavonoid contents among 21 sorghum landraces.
Figure 2.
Variability for total flavonoid contents among 21 sorghum landraces.
Figure 3.
Variability for total tannin contents among 21 sorghum landraces.
Figure 3.
Variability for total tannin contents among 21 sorghum landraces.
Figure 4.
Comparisons of effective concentration (EC50) of sorghum landraces.
Figure 4.
Comparisons of effective concentration (EC50) of sorghum landraces.
Figure 5.
Sorghum landraces of the study area, Tigray with different seed colours.
Figure 5.
Sorghum landraces of the study area, Tigray with different seed colours.
Figure 6.
Dendrogram showing sorghum landraces clustered into different distinct groups.
Figure 6.
Dendrogram showing sorghum landraces clustered into different distinct groups.
Figure 7.
Discriminant analysis for 21 sorghum landrace collections. The axes represent the first two Linear Discriminants (LD). Each convex hull represents a cluster and each dot represents an individual landrace.
Figure 7.
Discriminant analysis for 21 sorghum landrace collections. The axes represent the first two Linear Discriminants (LD). Each convex hull represents a cluster and each dot represents an individual landrace.
Figure 8.
Biplot of the 21 sorghum landraces and nutritional traits for the first and second principal components.
Figure 8.
Biplot of the 21 sorghum landraces and nutritional traits for the first and second principal components.
Figure 9.
Some end uses of sorghum including as Enjera (pancake-like Ethiopian flat bread); popping, and Swa’ (local beverage), respectively from left to right.
Figure 9.
Some end uses of sorghum including as Enjera (pancake-like Ethiopian flat bread); popping, and Swa’ (local beverage), respectively from left to right.
Table 1.
The 21 selected sorghum landraces used for the analysis of minerals, flavonoid, tannin, and antioxidant activities with some of their description.
Table 1.
The 21 selected sorghum landraces used for the analysis of minerals, flavonoid, tannin, and antioxidant activities with some of their description.
| No. |
Landrace name |
Zone of origin |
Race |
Seed color |
Preferred end-use |
| 1 |
Jamuye |
Southern Tigray |
Durra |
Yellow |
Swa/Enjera |
| 2 |
Gano |
Southern Tigray |
Durra |
Yellow |
Enjera |
| 3 |
Kodem |
Southern Tigray |
Durra |
Yellow |
Enjera/Swa |
| 4 |
Aba'are |
Southern Tigray |
Durra |
Yellow |
Enjera |
| 5 |
Amarica |
Southern Tigray |
Durra |
White |
Enjera |
| 6 |
Dengele |
Southern Tigray |
Durra |
Yellow |
Enjera/Swa |
| 7 |
Gan-Seber |
Southern Tigray |
Durra |
White |
Swa/Enjera |
| 8 |
Degalit |
Southern Tigray |
Durra |
Yellow |
Swa/Enjera |
| 9 |
Tinkish |
Southern Tigray |
Durra |
Yellow |
Enjera/popping |
| 10 |
Zengada |
Southern Tigray |
Bicolor |
Red |
Enjera/Swa |
| 11 |
Tewzale |
Central Tigray |
Caudatum |
Red |
Enjera/Swa |
| 12 |
Tirbish |
Central Tigray |
Durra |
Yellow |
Swa/Enjera |
| 13 |
Zerzaro |
Central Tigray |
Durra |
Brown |
Swa/Enjera |
| 14 |
Lequa |
Central Tigray |
Bicolor |
Brown |
Swa |
| 15 |
Dagnew |
Western Tigray |
Bicolor |
Yellow |
Swa/Enjera |
| 16 |
Korkora |
Western Tigray |
Caudatum |
White |
Enjera |
| 17 |
Merewey |
Western Tigray |
Durra |
Yellow |
Enjera |
| 18 |
Wedi-Aker |
Western Tigray |
Caudatum |
White |
Swa |
| 19 |
Wedi-Sbuh |
Western Tigray |
Durra |
White |
Enjera |
| 20 |
Gumbil |
Central Tigray |
Durra |
Yellow |
Enjera |
| 21 |
Arfa’agdm |
Western Tigray |
Caudatum |
White |
Enjera |
Table 2.
Descriptive statistics for all the nutritional traits of sorghum landraces.
Table 2.
Descriptive statistics for all the nutritional traits of sorghum landraces.
| Variable |
Protein |
Starch |
Fe |
Zn |
Cu |
Mn |
Cr |
TFC |
TTC |
EC50 |
FRAP |
TAC |
| Max |
18 |
78.3 |
101 |
43 |
5.3 |
20.2 |
1.5 |
665 |
7.5 |
818.4 |
334.8 |
63.9 |
| Min |
6.21 |
33.4 |
32 |
16.9 |
1.5 |
9.21 |
0 |
0 |
0.18 |
29.1 |
17.9 |
1.71 |
| Mean |
11.4 |
69.3 |
51.6 |
27 |
2.9 |
14.7 |
1.01 |
198.4 |
2.61 |
208 |
115 |
23.5 |
| SE |
0.12 |
0.32 |
3.29 |
1.42 |
0.2 |
0.55 |
0.1 |
46.7 |
0.61 |
48.6 |
23.1 |
4.45 |
| SD |
2.2 |
6.12 |
15.1 |
6.5 |
0.9 |
2.51 |
0.35 |
214.2 |
2.78 |
222.6 |
105.9 |
20.4 |
| CV |
19.34 |
8.83 |
29.2 |
24.1 |
32 |
17.1 |
34.3 |
108 |
106.5 |
106.9 |
92.35 |
86.8 |
| T-test |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
Table 3.
Pearson’s correlation coefficients showing pair-wise association among the nutritional traits of sorghum.
Table 3.
Pearson’s correlation coefficients showing pair-wise association among the nutritional traits of sorghum.
| |
Protein |
Starch |
Fe |
Zn |
Cu |
Mn |
Cr |
TFC |
TTC |
EC50 |
FRAP |
| Starch |
-0.26 |
|
|
|
|
|
|
|
|
|
|
| Fe |
0.21 |
-0.11 |
|
|
|
|
|
|
|
|
|
| Zn |
0.17 |
0.21 |
0.34 |
|
|
|
|
|
|
|
|
| Cu |
0.40 |
-0.17 |
0.73** |
0.53* |
|
|
|
|
|
|
|
| Mn |
0.03 |
-0.04 |
0.16 |
0.26 |
0.48* |
|
|
|
|
|
|
| Cr |
0.03 |
-0.15 |
0.12 |
0.26 |
0.02 |
-0.10 |
|
|
|
|
|
| TFC |
-0.14 |
-0.10 |
-0.06 |
0.15 |
-0.04 |
-0.14 |
0.22 |
|
|
|
|
| TTC |
-0.43 |
-0.26 |
0.05 |
-0.23 |
0.00 |
-0.07 |
0.16 |
0.70** |
|
|
|
| EC50 |
0.28 |
-0.41 |
0.15 |
-0.12 |
0.11 |
0.07 |
-0.07 |
-0.63** |
-0.43 |
|
|
| FRAP |
-0.31 |
-0.15 |
-0.08 |
0.01 |
-0.05 |
-0.15 |
0.19 |
0.92** |
0.86** |
-0.63** |
|
| TAC |
-0.31 |
-0.18 |
-0.06 |
-0.18 |
-0.10 |
-0.15 |
0.10 |
0.87** |
0.90** |
-0.57** |
0.95** |
Table 4.
Mean of sorghum nutritional traits of the four clusters.
Table 4.
Mean of sorghum nutritional traits of the four clusters.
| Mean of nutritional traits |
|---|
| Cluster |
Protein |
Starch |
Fe |
Zn |
Cu |
Mn |
Cr |
TFC |
TTC |
EC50
|
FRAP |
TAC |
| I |
13.17 |
72.38 |
59.99 |
32.48 |
3.7 |
16.24 |
1.12 |
119.6 |
1.02 |
231.6 |
62 |
12.39 |
| II |
10.27 |
72.8 |
45.01 |
23.13 |
2.32 |
14.02 |
0.85 |
121.3 |
2.08 |
199.6 |
82 |
17.47 |
| III |
14.67 |
53 |
55.49 |
25.19 |
2.91 |
14.24 |
1.39 |
0 |
0.59 |
818.37 |
18 |
1.71 |
| IV |
10.34 |
67.88 |
50.74 |
26.28 |
2.83 |
13.7 |
1.07 |
559.3 |
7.12 |
34.8 |
303 |
61.94 |
Table 1.
Squared distances between clusters (Mahalanobis Distance, D2).
Table 1.
Squared distances between clusters (Mahalanobis Distance, D2).
| Clusters |
I |
II |
III |
IV |
| I |
- |
|
|
|
| II |
40.18** |
- |
|
|
| III |
212.12** |
238.15** |
- |
|
| IV |
673.98** |
729.15** |
1173.79** |
- |
Table 6.
Principal component matrix showing eigenvalues, total variance, cumulative variance, and eigen vector (loadings) for the 12 nutritional traits of sorghum landraces.
Table 6.
Principal component matrix showing eigenvalues, total variance, cumulative variance, and eigen vector (loadings) for the 12 nutritional traits of sorghum landraces.
| Trait |
PC 1 |
PC 2 |
PC 3 |
PC 4 |
| Protein |
-0.43 |
0.38 |
-0.27 |
0.32 |
| Starch |
-0.07 |
-0.25 |
0.89 |
-0.02 |
| Fe |
-0.17 |
0.75 |
-0.06 |
-0.07 |
| Zn |
-0.13 |
0.65 |
0.53 |
0.27 |
| Cu |
-0.22 |
0.91 |
0.01 |
-0.21 |
| Mn |
-0.22 |
0.45 |
0.16 |
-0.59 |
| Cr |
0.19 |
0.28 |
-0.05 |
0.72 |
| TFC |
0.89 |
0.24 |
0.05 |
0.15 |
| TTC |
0.88 |
0.15 |
-0.24 |
-0.21 |
| EC50 |
-0.69 |
-0.01 |
-0.56 |
-0.02 |
| FRAP |
0.97 |
0.17 |
-0.03 |
0.01 |
| TAC |
0.96 |
0.10 |
-0.14 |
-0.11 |
| Eigenvalue |
4.26 |
2.42 |
1.57 |
1.17 |
| % Total variance |
35.54 |
20.16 |
13.08 |
9.71 |
| % Cumulative variance |
35.54 |
55.70 |
68.78 |
78.48 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).