1. Introduction
The comprehensive care of patients diagnosed with acute promyelocytic leukemia (APL) involves four essential components - early-diagnos is and prompt ATRA administration; mitigation of early mortality; recognition and management of complications associated with arsenic trioxide (As
2O
3, ATO) and all-trans retinoic acid (ATRA) treatment; and monitoring of measurable residual disease (MRD). Taking into consideration outstanding outcomes of this treatment regimen and the probability of cure for more than 90% of patients, our main objectives nowadays are to reduce the risk of early death, to reduce treatment-related toxicities and to achieve a sustained molecular response [
1,
2,
3,
4,
5].
ATO has a long and intriguing course in medicine, spanning from its historical uses as a toxic substance during the MiddleAges, including the purported poisoning of Napoleon through arsenic-contaminated wine, to its contemporary utilization as a targeted therapeutic agent exhibiting exceptional efficacy in the treatment of APL [
1]. During the Middle Ages and Renaissance, ATO was administered as a treatment for diseases such as syphilis and trypanosomiasis [
2]. More recently, in the 1970s and 1980s, Chinese researchers pioneered the use of ATO (10mg/d, intravenous infusion) as a treatment for relapsed APL, with complete remission rates ranging from 65.5% to 84% and a survival rate of over 10 years in 9 patients [
6]. In 2000, the U.S. Food and Drug Administration (FDA) approved ATO as a treatment for relapsed or refractory APL. Later on, the FDA (2018) and EMA (2016) approved the use of the ATO-ATRA combination as a front-line therapy for non-high risk APL, based on the results of the APL0406 phase III randomized trial published by LoCoco et al. [
3].
Since its first use in 1980, it has been observed that the use of ATO demonstrates very reduced hematologic toxicity[
4].Moreover, this drug has a dose-dependent effect, specifically it induces apoptosis at higher concentrations (0.5 to 2 µM) and differentiation at lower concentrations (0.1 to 0.5 µM), both scenarios being associated with PML::RARA degradation [
5]. Clinical trials have highlighted the particular early-side effects associated with ATO therapy, such as hepatic toxic effects, leukocytosis, differentiation syndrome, prolongation of the QTc interval, skin toxicity, and neurotoxicity, most of which occur during induction therapy [
3,
6]. Regimens containing ATO and ATRA have completely changed the clinical course of patients with APL in terms of outcomes, leading to high rates of overall survival, extremely low rates of relapse, and successful cures for the majority of patients [3, 6, 7]. The synergistic mechanisms of the two molecules induce differentiation, apoptosis, degradation of PML::RARA,
ATRA targets the RARα moiety of the protein, while ATO targets PML [
8].
The APL0406 trial, and its extended follow-up demonstrated that the treatment regimen comprising ATRA and ATO is significantly superior to standard chemotherapy regarding both event-free survival (97% versus 86%, p=0.02) and overall survival (with a 2-year overall survival probability of 99% and 91% respectively) for patients diagnosed with non-high-risk APL [3, 7]. Regarding the toxicity profile, it was observed that patients who received the combination of ATRA and ATO exhibited a reduced occurrence of infections compared to those treated with ATRA-chemotherapy (26 episodes versus 59 episodes, p<0.001). Additionally, the ATRA plus ATO combination frequently resulted in a lower transfusion requirement. However, it is worth noting that the ATO-ATRA arm showed a higher incidence of high-grade hepatotoxicity compared to the ATRA-chemotherapy arm (63% versus 6%, p<0.001), as well as a higher frequency of QTc prolongation (16% versus 0%, p<0.001) [
3].
The AML17 trial enrolled patients diagnosed with both low-risk and high-risk APL. In this study, a different dosage regimen of ATO was administered alongside a standard dose of ATRA to all patients included in ATRA plus ATO arm. Additionally, those identified as having high-risk APL received an additional dose of gemtuzumab ozogamicin as part of their treatment protocol. It was shown that patients treated with ATRA and ATO experienced reduced hospitalization durations and required fewer blood products and antibiotics compared to the ATRA and chemotherapy group. However, the incidence of hyperbilirubinemia of any grade was higher in the ATRA and Idarubicin group, while higher levels of aspartatetransaminase (AST) wereobserved in the ATRA plus ATO group [
6]. The incidence of cardiac side-effects were higher in ATRA plus ATO arm compared with standard chemotherapy (21% and 10%, respectively). After two treatment cycles, 11% of patients in the ATO and ATRA group experienced cardiac complications, whereas no such complications were observed in the ATRA and Idarubicin group [
6]. Moreover, other non-hematological side-effects including elevated creatinine levels, proteinuria, alopecia, nausea, haematuria, and diarrhea, were more common in the ATRA and idarubicin regimen when compared to chemo-free regimen [
6]
.
The grade 3-4 treatment-related toxicities are managed with the temporary discontinuation of ATRA, ATO, or both and subsequent dose decreased, with patients being followed up closely. [
3,
6].
In this paper, we propose to show our „real-life” experience with the ATO and ATRA combination and to review literature data pertaining to early-adverse events related to ATO, with particular regard to their incidence, predisposing risk factors and management.
2. Materials and Methods
Patients diagnosed with APL, 18 years of age or older, who received ATRA and ATO in Fundeni Clinical Institute, between January 2019 to December 2022, were included in this monocentric real-world retrospective study.
Our objective was to analyze the incidence, severity and the time of onset of non-hematologic adverse events during induction and consolidation using the ATRA and ATO regimen. The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was utilized to assess the toxicity grade (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf). We discuss strategies for the management of non hematologic adverse events related to ATO/ATRA therapy.
The diagnosis was confirmed on bone marrow or peripheral blood samples by fluorescence in situ hybridization (FISH) and reverse transcriptase polymerase chain reaction (RT-PCR) analysis for the PML::RARA fusion oncogene. All patients were stratified according to the Sanz risk score [
9].
ATRA administration was divided into two daily doses totaling 45mg/m
2 while ATO was given intravenously at 0.15 mg/kg daily until complete remission (CR), or for a maximum of 60 days. Patients received hydroxyurea to control hyperleukocytosis when it occurred. Once in CR, patients received 4 cycles of ATO consolidation and 7 cycles of ATRA as per APL0406 trial [
3]. Four patients received Idarubicine and ATRA as induction according to PETHEMA protocol, followed by ATO and ATRA consolidation.
The following data from hospital-based patient records were collected: age, gender, transcript subtype of PML::RARA, treatment protocol, clinical and laboratory characteristics at diagnosis and during the course of induction and consolidation. The study received approval from the hospital's ethics committee. We determined the incidence of each complication by calculating it as a percentage of the entire study population.
3. Results and Discussion
A total of 26 patients treated with ATRA plus ATO from our center were included in this study. Among them, 50% were female and the median age at the time of treatment initiation was 42 years (range 18-69). 76.9% of patients had low/intermediate-risk APL, 7.69% had high-risk APL and four patients (15.38%) were diagnosed with relapsed disease (two patients with hematological relapse, one patient with combined molecular and central nervous system -CNS relapse, and another patient with combined morphological and CNS relapse). All patients received ATRA and ATO as induction therapy, except for 4 patients who were treated according to the PETHEMA regimen (two patients with high-risk APL and two patients with low-risk APL). Patient characteristics are shown in
Table 1.
The median follow-up was 21.5 months (range, 1-48 months). At the end of this period, 23 (88.4%) patients were in molecular remission and one patient had persistent CNS disease. The rate of early-death was 3.84% – one patient died from multiorgan failure during induction therapy. One patient died during the first consolidation therapy owing to multiple complications associated with COVID-19 infection. At the last follow-up,one patient had persistent CNS disease despite several lines of therapy (high-dose cytarabine and methotrexate, CNS radiotherapy, ATRA plus ATO, and multiple intrathecal chemotherapy administrations). It should be noted that all of our patients with relapsed APL treated with ATRA plus ATO refused to undergo auto-SCT or allogeneic SCT. The outcomes of our patients are shown in
Table 2.
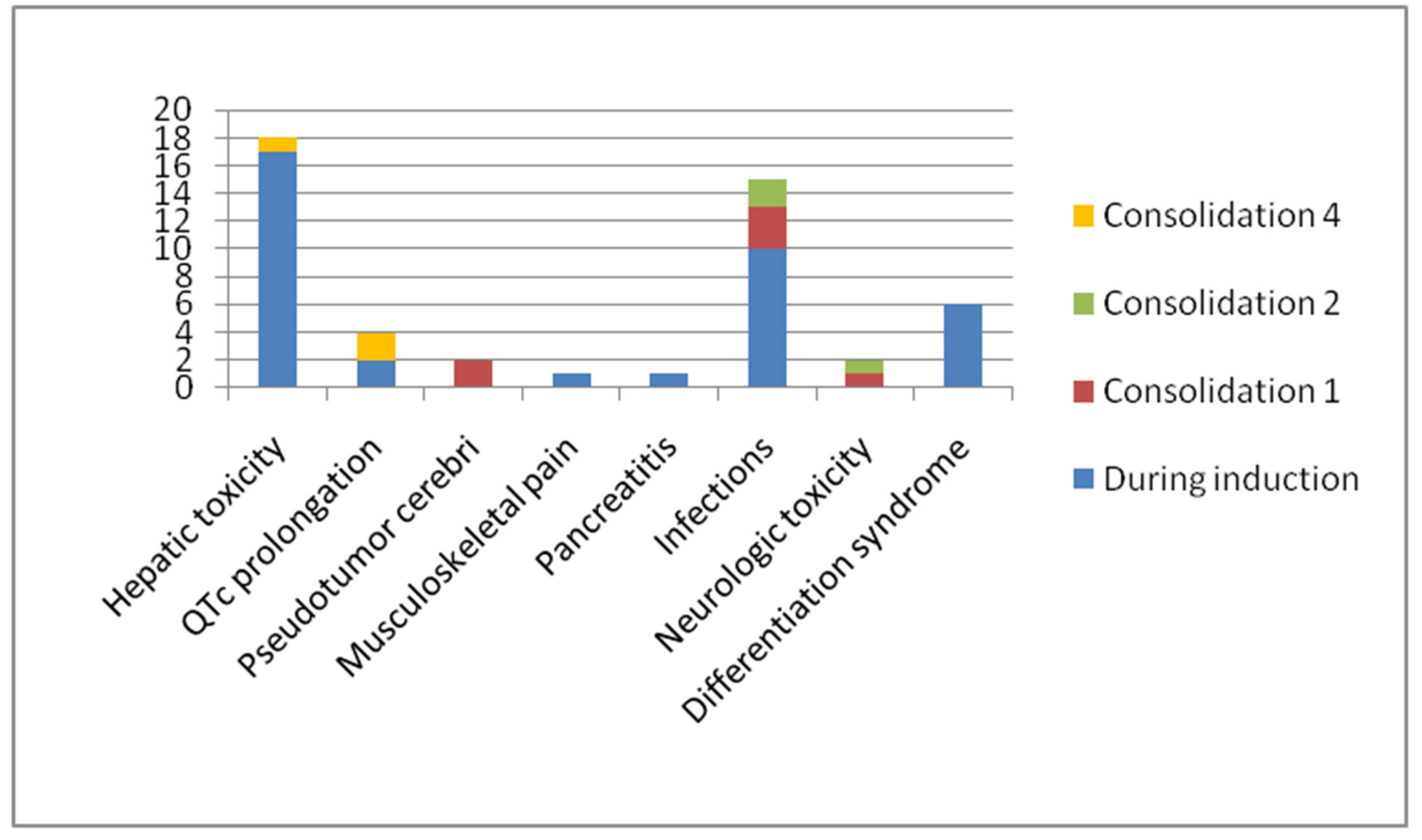
The most commonly reported adverse events were hepatotoxicity (69.2%), infections (46.1%), differentiation syndrome (23%) and QTc prolongation (11.5%). In total, 57.6% of patients experienced grade 3 or 4 complications. All these patients, except for one patient who developed differentiation syndrome, resumed treatment with no recurrence of a specific toxicity.
Table 3 and
Figure 1 detail the incidence of side-effects during induction and consolidations.
3.1. Hepatic Toxicity – Results and Discussion
In our study, 69.2% (18 patients) had experienced hepatotoxicity during induction or consolidation. Among them, 50% developed grade 3-4 hepatotoxicity which resolved with temporary treatment discontinuation. All patients received N-acetylcysteine intravenous administration and hepatoprotective agents, which included soy essential phospholipids, silymarin, and ursodeoxycholic acid. Significantly, no recurrence of severe hepatic toxicity was observed when treatment was resumed. The vast majority of patients experienced hepatic side-effects on week 3-4 of induction treatment. ATO-induced hepatotoxicity is a diagnosis of exclusion; therefore, other etiologies must be considered such as viral reactivations including hepatitis B, hepatitis C or cytomegalovirus (CMV), imaging assessments to exclude local conditions such as cholecystitis or local thrombosis, and identifying other medications that could potentially contribute to liver toxicity. The liver toxicity primarily manifested through elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Studies have shown that the hepatotoxicity is more frequent in patients treated with ATO and ATRA regimen compared with ATRA and conventional chemotherapy [
7]. The incidence of liver-function abnormalities varies between 44% in the APL0406 trial to 25% in AML17 study [
6,
7]. The hepatic toxicity rates reported by Burnett et al. were lower, possibly because of the differend schedule of ATO administration. LoCoco et al. reported an incidence of 63% of grade 3-4 hepatic side-effects, which is similar to our findings [
3,
6].
The management strategy needs to be adapted to the severity of the manifestations. In patients with grade 1-2 liver-function abnormalities, closer monitoring of the liver function is required while continuing with the same dose of ATO. In the context of grade 3-4 hepatotoxicity, a necessary step is the temporary discontinuation of ATO. Subsequently, ATO can be resumed at 50% of the initial dose for a duration of 7 days, provided that liver enzyme levels decrease to less than 3 times the upper limit of normal (ULN) or total bilirubin reduces to less than 1.5 times ULN. In the absence of any aggravation in liver markers during this 7-day period, ATRA and/or ATO can be reintroduced at their full dosages. [3, 10].
Different trials have investigated the possible predictive factors for ATO-induced hepatotoxicity such as hemoglobin ≥ 80 g/L, the absence of prophylactic hepatoprotective agents, non-single-agent ATO, fibrinogen < 1 g/L [
11] and the homozygous mutation of MTHFR 1298 (C/C) [
12]. However, further studies are necessary to establish the role that each of these factors plays in liver toxicity. Evidence suggests that differentiation syndromemay serve as both a risk factor and an exacerbating factor for hepatotoxicity[
13].
Our study found that liver toxicity is transient in nature and does not lead to liver failure or to permanent treatment discontinuation. These findings are consistent with the results reported in various clinical trials and case-series[3,6,9, 13].
3.2. QT Prolongation – Results and Discussion
QTc (corrected QT) prolongation was defined as QTc greater than 450 milliseconds for men and greater than 460 milliseconds for women, with correction calculated using the Framingham formula [
3]. In our study, we performed a 12-lead ECG at least four times per week during the induction course and two times per week during consolidation; the Friedericia formula was used for rate-correction. QT-interval prolongation could be associated with torsades de Pointes (TdP) and sudden cardiac death. In general, risk factors associated with TdP include electrolyte abnormalities (such as hypokalemia and hypomagnesemia), female sex, existing cardiovascular conditions (e.g., heart failure, bradycardia), drug interactions, genetic predisposition, and QTc prolongation[
14].
In our study, four patients (11.5%) experienced QTc prolongation, two of them during induction and the other two during consolidation therapy. One patient had grade 2 QTc interval prolongation and three patients had grade 3, but none of them developed severe arrhythmias or clinically significant symptoms. The majority of patients with QT prolongation were female (75%), the median age was 48.5 and 50% of them had a previous history of cardiac failure and atrial fibrillation.
The initial management of this specific complication consists of temporarily stopping ATO treatment if the QTc exceeds 500 milliseconds. Additionally, it is crucial to maintain sufficient electrolyte levels, specifically potassium (> 4 mmol/L) and magnesium (> 0.74 mmol/L). Furthermore, potential interactions with other drugs that may prolong the QTc interval should be identified. After normalization of the QTc interval, ATO treatment was reintroduced at a dosage of 0.075 mg/kg (50% of the full dose) for an initial period of 7 days. If no additional QTc prolongation was observed during this period, the ATO dosage was increased to 0.11 mg/kg for the following week. Subsequently, if no further prolongation was detected, ATO was resumed at the full prescribed dose [
3,
10,
15].
The study of LoCoco et al. reported an incidence of QTc prolongation of 16%, which is higher than the results published by Burnett et al. [
3,
6]. According to available literature data, the vast majority of patients had an asymptomatic QT prolongation [
6,
16,
17]. In the APL0406 trial one patient required permanent discontinuation of ATO [
3]. In the study of Soignet et al. one patient (1/40) had an episode of 7-beat run of torsade de pointes in the setting of hypopotassemia, hypomagnesemia and co-administration of other drugs that prolong QT interval [
16]. Outside of clinical trials, there are some case reports of transient nonsustained ventricular tachycardia without QT prolongation resolved after nadolol administration[
18], and in the paper published by Unnikrishnan et al. 3 cases of patients who developed torsades de pointes following ATO administration were described[
19]
. It has also been shown that patients with moderate or severe renal impairment have an increased incidence of QT prolongation and liver toxicity [
20]
The exact mechanism responsible for ATO-induced cardiotoxicity remains largely uncertain; it has been hypothesized that it may involve elevated oxidative stress and intracellular calcium overload. Several ongoing studies are investigating the potential of anti-oxidation and anti-inflammation drugs, such as Sacubitril/valsartan, resveratrol, L-ascorbic acid, and omega-3 fatty acids, as potential therapeutic options to mitigate arsenic-induced cardiotoxicity. Nevertheless, further research is necessary to comprehensively explore and validate the efficacy of these treatments [
13,
21,
22,
23].
3.3. Acute Musculoskeletal Pain (MSK) Syndrome
Acute musculoskeletal pain (MSK) syndrome is a less common toxicity of ATO therapy. Overall, data about the incidence and predisposing factors for MSK pain is still lacking and further studies are needed in order to define them.
In our study, one patient (3.84%) developed MSK syndrome during induction. This patient was a 27-year old male, diagnosed with non-high risk APL. On day 12 of the induction course, he experienced intense muscle and bone pain in both lower extremities. Pain levels reached 8 on a 0-to-10 numeric analog pain score. These symptoms showed poor responsiveness to conventional analgesics therapy. However, following the discontinuation of ATO and ATRA, and the initiation of dexamethasone administration, there was noticeable improvement within 4 days. On the first day of MSK syndrome the WBC count was 23 x 103 /uL, while the creatine kinase levels were within normal range.
There are several case reports which indicated that the ATRA therapy during induction could induce myositis [
24,
25], especially in the setting of Sweet’s syndrome. He H et al. describe the case of a 68-year-old patient who developed rhabdomyolysis secondary to ATO treatment [
26]. Kubiak et al. showed that 21.4% patients (9/42) developed acute MSK during induction, with a median duration of pain of 5 days and a median time of presentation of 11 days after treatment initiation. This side-effect seems to be related to rapid increase of the WBC in patients with low-risk APL [
27]. There is no standard recommendation for the management of this rare and specific side-effect. However, the most commonly adopted strategy involves temporarily discontinuing ATO and ATRA while administering corticosteroids [
27].
3.4. Treatment-Related Pancreatitis
The occurrence of acute pancreatitis during induction with ATRA and ATO is an uncommon complication. In the few cases in which it has been reported, acute pancreatitis developed in the setting of ATRA-induced hypertriglyceridemia or ATRA-induced differentiation syndrome [
28,
29].
In our study, one patient (3.84%) developed a grade 4 ATO-related pancreatitis during induction therapy, with no clinical manifestations. The patient was a 55-year-old woman with a medical history of dyslipidemia and type 2 diabetes mellitus, diagnosed with low-risk APL according to Sanz risk score. Starting on day 10 of induction therapy, the laboratory work-up showed a gradual increase of serum lipase and amylase levels (peak value 3738 IU/dl and 332 IU/dl, respectively), along with mild hypertriglyceridemia (305 mg/dl). The abdominal CT scan was compatible with an acute edematous pancreatitis with no local complications. Other possible causes of pancreatitis - such as gallstones, alcohol use, severe hypertriglyceridemia (> 1000 mg/dl) [
30] – have all been excluded. The management consisted of intravenous hydration, dexamethasone, and a 5-day pause of the induction therapy. The biological evolution was favorable and ATRA treatment was resumed at full dose. ATO was restarted at the dose of 0.075mg/kg/day for 7 days and then increased to the full dose. Our patient had no sign of differentiation syndrome.
A review of the literature showed 3 cases of patients diagnosed with APL who developed acute pancreatitis during ATO therapy are listed in
Table 4.
The mechanism of ATO-related pancreatitis is not well understood. Several cases of arsenic intoxication associated with signs of pancreatitis, gastroenteritis and neurologic manifestations have been reported [
34,
35]. Given the limited understanding of the possible implications of this rare complication, we advocate for vigilant monitoring of amylase and lipase levels during the induction therapy. This approach will facilitate dose adjustments or, if needed, the consideration of discontinuing ATO treatment.
3.5. Neurologic Toxicity
No data regarding neurotoxicity was included in the study published by LoCoco et al., while the final analysis of the APL0406 trial showed that peripheral neuropathy was the most common neurologic side-effect. The incidence of neurotoxicity was greater among patients treated with ATRA and ATO regimen, especially during consolidation (5% and 5.9% during the second consolidation and the third consolidation, respectively) [
3,
7]. Additionally, Zacholski et al. conducted a study investigating the dose-dependence of ATO-related neuropathy. Their findings revealed that implementing a dose capping at 10 mg resulted in reduced rates of neurotoxicity during the consolidation phase [
15].
In our study, four patients (15.3%) developed neurologic toxicity, two of which experienced peripheral neuropathy and another two pseudotumor cerebri (PTC). All patients completed the therapy and achieved molecular remission. The first patient was a 46-year-old man who had previously undergone surgery for a spinal dural arteriovenous fistula, resulting in grade 1 peripheral sensorimotor axonal polyneuropathy. He was diagnosed with low-risk APL and experienced grade 2 neurotoxicity during the consolidation phase of the ATO-ATRA regimen. In this particular instance, we did not reduce the dosage of ATO. Instead, the patient was prescribed pregabalin and a dietary supplement containing alpha-lipoic acid and gamma-linolenic acid. Over the three months following the end of treatment, the neurologic symptoms gradually improved. The second patient, a 64-year-old female diagnosed with low-risk APL, experienced grade 3 peripheral neuropathy in both lower extremities, accompanied by grade 2 constipation after the completion of the first consolidation course. In this specific case, during the second consolidation phase, the dose of ATO was reduced by 25% with improvement of neurotoxicity and resolution of constipation. ATO was later increased to full dose for the last two cycles of consolidation treatment.
Additionally, we identified two patients (7.69%) who were diagnosed with "probable" and "possible" PTC respectively. Both patients are young females under the age of 26, with a BMI exceeding 30 kg/m
2. The onset of this complication occurred during the first consolidation phase of ATO and ATRA treatment. The characteristics of the patients and their corresponding management are outlined in
Table 5. The Dandy criteria (papilledema, a normal neurological exam except for cranial nerve abnormalities, elevated lumbar puncture opening pressure (≥250 mmHg or ≥280 mmHg for non-obese children), and normal neuroimaging) were used to establish the diagnosis of PTC [
36]. Risk factors for the development of pseudotumor cerebri include female sex, age (childbearing years), obesity, endocrine disorders, and hypervitaminosis A [
37,
38]. This complication has a high incidence in the pediatric population and among young adults, especially within 2 -3 weeks of induction treatment for APL [
39]. Its various clinical manifestations may include persistent severe headache, diplopia, vomiting, nausea, and pulsatile tinnitus [
40].
Effective management of this condition is crucial in order to prevent blindness, which is the primary complication caused by progressive swelling and atrophy of the optic disc. The management strategies primarily aim to reduce intracranial pressure and may involve discontinuation of ATRA and ATO, therapeutic lumbar punctures to remove cerebrospinal fluid (CSF), administration of acetazolamide, corticotherapy, diuretics (mannitol or furosemide), and analgesics [
36,
40,
41]. Moreover, it is crucial to note the potential interaction between ATRA and other drugs which may be administered during leukemia treatment, particularly antifungal triazoles, which can increase the risk of developing pseudotumor cerebri (PTC)[
42,
43].
The neurotoxicity mechanism of ATRA shares similarities with vitamin A intoxication, leading to impaired CSF reabsorption. Conversely, the precise mechanism by which ATO induces these specific side effects has not been extensively elucidated in the available literature. [
39].
Limited data are available regarding the incidence of PTC in patients undergoing dual differentiation therapy. In a study by Smith et al, five cases of patients diagnosed with PTC were described. Among the patients, four experienced the onset of symptoms during the induction phase of treatment, while one patient developed PTC during consolidation. The management approach included temporarily discontinuing ATRA and ATO, along with the administration of acetazolamide and topiramate to reduce symptoms and control intracranial pressure [
44].
In a study conducted by Montesino et al., a total of 1034 patients from the LPA96, LPA99, and LPA2005 trials were analyzed. They found an incidence rate of PTC of 3% (32 patients), with the majority of cases occurring during the induction phase and only two cases during consolidation. The study also revealed a higher incidence of PTC in patients under 18 years old, those with fibrinogen levels below 170 mg/dl, and those with an ECOG (Eastern Cooperative Oncology Group) score above 1 [
41]. Importantly, this type of neurotoxicity was found to be reversible and did not impact treatment outcomes [
41].
PTC is an uncommon complication observed during consolidation therapy for APL. In one of our cases, its occurrence may be explained by the fact that the patient was initially treated according to the PETHEMA protocol for the induction phase and subsequently transitioned to ATRA and ATO consolidation therapy.
3.6. Infections
In our study population, 12 patients (46.15%) experienced documented infections and prolonged fever of unknown origin during both the induction and consolidation phases. Among these cases, urinary tract infections (UTIs) were observed in five patients (41.6%), while SARS-CoV-2 accounted for the second most common type of infection (4 patients, 28.5%). The majority of the infectious complications occurred during the induction phase (10 patients, 83.3%).
In our study, the prevalence of infections as one of the most common complications can be attributed to specific factors inherent to our hematology ward. Notably, we experienced two episodes of COVID-19 outbreaks during the second (March-May 2021) and fourth (January-April 2022) waves of the SARS-CoV-2 pandemic. Additionally, there was an outbreak of Klebsiella infection in our facility. These circumstances likely contributed to the increased infection incidence in our patient population.
3.7. Differentiation Syndrome (DS)
In our study population, a total of six patients (23%) developed differentiation syndrome (DS). Among these cases, one patient experienced a severe form of DS associating respiratory, renal, and cardiac failure, which unfortunately resulted in his death. The five remaining patients experienced a milder form of DS, and their condition showed improvement upon discontinuation of ATRA and ATO, along with the administration of dexamethasone.
DS can present as a diverse range of manifestations, encompassing unexplained fever, dyspnea, hypotension, weight gain, radiographic opacities, acute renal failure, and pleural effusion. Depending on the severity of the clinical presentation, DS may be classified as indeterminate (1 or 2 signs or symptoms), moderate (3 symptoms), or severe (more than 4 clinicalfeatures)[
45]. This range of manifestations underscores the importance of a thorough clinical evaluation and early recognition for appropriate management.
To mitigate the risk of DS, all patients in our study received prophylactic treatment with prednisone at a dose of 1mg/kg/day for the initial 15 days of their induction course. Alternative schedules of prophylactic corticosteroids have been proposed, such as prednisone 0.5mg/kg per day starting from day 1 until the completion of the induction phase, or dexamethasone 2.5mg/m
2 every 12 hours during days 1-15 [
45,
46]. In managing DS, the established protocols involve promptly discontinuing the administration of ATRA and ATO. Instead, the patients were initiated on dexamethasone at a dose of 10 mg twice daily until the symptoms resolved [
15], [
3].
The reported incidence of DS ranges from 2% to 48%, and typically carries an average mortality rate of 1% [
45,
47].In the study conducted by LoCoco et al., DS occurred in 19% of the patients treated with ATRA-ATO, while 16% of the patients in the ATRA-chemotherapy group developed DS (p=0.62). The incidence of severe DS was comparable in both treatment arms, but notably, two patients who received ATRA plus chemotherapy experienced fatal outcomes. These results suggest that there were no significant differences in the severity or incidence of DS between the two treatment groups [
3]. Its onset can occur anywhere between 0 to 46 days after the introduction of ATRA, with a median manifestation time of 12 days [
46]. Given its life-threatening nature and status as one of the primary contributors to early mortality in APL, prompt identification of DS signs and symptoms holds paramount importance. The incidence of DS shows significant variations between clinical trials and real-world scenarios due to the challenges posed by its very nature as a diagnosis of exclusion. It requires considering and ruling out other potential conditions, such as sepsis, hemorrhage, and cardiac failure. Furthermore, the lack of a pathognomonic biomarker adds to the complexity of accurately diagnosing DS.
Our study is subject to certain limitations, including a small sample size within the study population and the retrospective nature of the analysis.
4. Conclusions
In conclusion, the short-term complications associated with the ATO plus ATRA regimen are manageable and reversible in adult patients diagnosed with APL outside of a clinical trial. The differentiation syndrome continues to be a significant contributing factor to early mortality in APL. Real-life data provides a more detailed understanding of specific toxicities, including some rare side effects such as MSK pain, PTC and ATO-induced pancreatitis. Overall, our real-life data aligns with the results observed in the published clinical trials regarding non-hematologic side effects, with the exception of infection-related complications which are higher in our study.
Author Contributions
AG reviewed the published literature, designed, and drafted the manuscript, CD review and editing, BI, MCS, RH, MC, AT were involved in clinical management; MD, OG, DCP, DV, CJ were in involved in diagnosis and/or monitoring of each case using morphology, cytogenetics, flow-cytometry, and molecular analysis; AT, BI and DC critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Antman, K.H. Introduction: The History of Arsenic Trioxide in Cancer Therapy. Oncologist 2001, 6, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Aronson, S.M. Arsenic and old myths. R I Med. 1994, 77, 233–234. [Google Scholar] [PubMed]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.X.; Chen, G.Q.; Ni, J.H.; Li, X.S.; Xiong, S.M.; Qiu, Q.Y.; Zhu, J.; Tang, W.; Sun, G.L.; Yang, K.Q.; et al. Use of arsenic trioxide (As2O3) in thetreatment of acute promyelocyticleukemia (APL): II. Clinicalefficacyandpharmacokinetics in relapsedpatients. Blood 1997, 89, 3354–3360. [Google Scholar] [CrossRef]
- Yilmaz, M.; Kantarjian, H.; Ravandi, F. Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Bowen, D.; Kell, J.; Knapper, S.; Morgan, Y.G.; Lok, J.; Grech, A.; Jones, G.; et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 1295–1305. [Google Scholar] [CrossRef]
- Platzbecker, U.; Avvisati, G.; Cicconi, L.; Thiede, C.; Paoloni, F.; Vignetti, M.; Ferrara, F.; Divona, M.; Albano, F.; Efficace, F.; et al. Improved Outcomes With Retinoic Acid and Arsenic Trioxide Compared With Retinoic Acid and Chemotherapy in Non–High-Risk Acute Promyelocytic Leukemia: Final Results of the Randomized Italian-German APL0406 Trial. J. Clin. Oncol. 2017, 35, 605–612. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.-F.; Wu, C.-F.; Xu, F.; Shen, Z.-X.; Zhu, Y.-M.; Li, J.-M.; Tang, W.; Zhao, W.-L.; Wu, W.; et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. 2009, 106, 3342–3347. [Google Scholar] [CrossRef]
- Sanz, M.A.; LoCoco, F.; Martín, G.; Avvisati, G.; Rayón, C.; Barbui, T.; Díaz-Mediavilla, J.; Fioritoni, G.; González, J.D.; Liso, V.; et al. Definition of relapse riskand role of nonanthracyclinedrugs for consolidation in patientswith acute promyelocyticleukemia: a jointstudy of the PETHEMA and GIMEMA cooperative groups. Blood 2000, 96, 1247–1253. [Google Scholar]
- https://www.trisenox.com/globalassets/trisenoxhcp/trisenox-prescribing-information.pdf.
- Zhang, Z.; Zhang, S.; Zhang, F.; Zhang, Q.; Wei, H.; Xiu, R.; Zhao, Y.; Sui, M. Clinical Indicators of Hepatotoxicity in Newly Diagnosed Acute Promyelocytic Leukemia Patients Undergoing Arsenic Trioxide Treatment. Biol. Trace Element Res. 2023, 1–11. [Google Scholar] [CrossRef]
- Mathews, V.; Desire, S.; George, B.; Lakshmi, K.M.; Rao, J.G.; Viswabandya, A.; Bajel, A.; Srivastava, V.M.; Srivastava, A.; Chandy, M. Hepatotoxicity profile of single agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia 2006, 20, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhao, J.; Wang, X.; Wang, H.; Wang, H.; Xu, G. Hepatotoxicity From Arsenic Trioxide for Pediatric Acute Promyelocytic Leukemia. J. Pediatr. Hematol. 2013, e67–e70. [Google Scholar] [CrossRef] [PubMed]
- Trinkley, K.E.; Page, R.L.; Lien, H.; Yamanouye, K.; Tisdale, J.E. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr. Med Res. Opin. 2013, 29, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.-J.; et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Soignet, S.L.; Frankel, S.R.; Douer, D.; Tallman, M.S.; Kantarjian, H.; Calleja, E.; Stone, R.M.; Kalaycio, M.; Scheinberg, D.A.; Steinherz, P.; et al. United States Multicenter Study of Arsenic Trioxide in Relapsed Acute Promyelocytic Leukemia. J. Clin. Oncol. 2001, 19, 3852–3860. [Google Scholar] [CrossRef]
- Roboz, G.J.; Ritchie, E.K.; Carlin, R.F.; Samuel, M.; Gale, L.; Provenzano-Gober, J.L.; Curcio, T.J.; Feldman, E.J.; Kligfield, P.D. Prevalence, Management, and Clinical Consequences of QT Interval Prolongation During Treatment With Arsenic Trioxide. J. Clin. Oncol. 2014, 32, 3723–3728. [Google Scholar] [CrossRef]
- Zeitjian, V.; Moazez, C.; Arslan, W.; Saririan, M. QT Independent Ventricular Tachycardia Induced by Arsenic Trioxide. Case Rep. Cardiol. 2019, 2019, 9870283–3. [Google Scholar] [CrossRef]
- Unnikrishnan, D.; Dutcher, J.P.; Varshneya, N.; Lucariello, R.; Api, M.; Garl, S.; Wiernik, P.H.; Chiaramida, S. Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood 2001, 97, 1514–1516. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, M.; Hostetter, T.H.; Chen, H.; Lin, L.; Hai, X. Effect of renal impairment on arsenic accumulation, methylation capacity, and safety in acute promyelocytic leukemia (APL) patients treated with arsenic trioxide. Expert Rev. Clin. Pharmacol. 2021, 14, 1173–1182. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Sun, G.B.; Wang, M.; Liao, P.; Du, Y.Y.; Yang, K.; Sun, X.B. Arsenic trioxidetriggeredcalciumhomeostasisimbalanceandinducedendoplasmicreticulumstress-mediatedapoptosis in adult rat ventricular myocytes. Toxicol. Res. 2016, 5, 682–688. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H.; Lin, L.; Lu, J.; Zhao, Q.; Dong, Z.; Hai, X. Sacubitril/valsartan protects against arsenic trioxide induced cardiotoxicity in vivo and in vitro. Toxicol. Res. 2022, 11, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Jodat, H.; Saki, N. Strategies to inhibit arsenic trioxide-induced cardiotoxicity in acute promyelocytic leukemia. J. Cell. Physiol. 2019, 234, 14500–14506. [Google Scholar] [CrossRef] [PubMed]
- van DerVliet, H.J.; Roberson, A.E.; Hogan, M.C.; Morales, C.E.; Crader, S.C.; Letendre, L.; Pruthi, R.K. All-trans-retinoic acid-inducedmyositis: a description of twopatients. Am J Hematol. 2000, 63, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Fabbiano, F.; Magrin, S.; Cangialosi, C.; Felice, R.; Mirto, S.; Pitrolo, F. All-trans retinoic acid induced cardiac and skeletal myositis in induction therapy of acute promyelocytic leukaemia. Br. J. Haematol. 2005, 129, 444–445. [Google Scholar] [CrossRef]
- He, H.; An, R.; Hou, J.; Fu, W. Arsenic trioxide induced rhabdomyolysis, a rare but severe side effect, in an APL patient: a case report. Front. Med. 2017, 11, 284–286. [Google Scholar] [CrossRef]
- JakubKubiak, M.; Jillella, A.P.; Bradshaw, D.; Savage, N.M.; Bryan, L.J.; Kota, V.K. MusculoskeletalPainSyndrome in a Subset of APL PatientsduringInductionTherapy. Blood 2022, 140 (Suppl. 1), 8948–8949. [Google Scholar]
- Abou, C.L.; Ghosn, M.; Ghayad, E.; Honein, K. A case of pancreatitisassociatedwithall-trans-retinoic acid therapy in acute promyelocyticleukemia. Hematol. J. 2001, 2, 406–407. [Google Scholar] [CrossRef]
- Yutsudo, Y.; Imoto, S.; Ozuru, R.; et al. Acute pancreatitisafterall-trans retinoic acid therapy. Ann. Hematol. 1997, 74, 295–296. [Google Scholar] [CrossRef]
- Wang, G.-J.; Gao, C.-F.; Wei, D.; Wang, C.; Ding, S.-Q. Acute pancreatitis: Etiology and common pathogenesis. World J. Gastroenterol. 2009, 15, 1427–1430. [Google Scholar] [CrossRef]
- Yamano, T.; Yokote, T.; Akioka, T.; Hara, S.; Oka, T.; Tsuji, M.; Hanafusa, T. [Acute pancreatitisduringthetreatment of relapsed acute promyelocyticleukemiawith As2O3]. RinshoKetsueki 2006, 47, 23–25. [Google Scholar]
- De, D.; Nath, U.; Chakrabarti, P. Pancreatitis in acute promyelocytic leukemia: Drug-induced or differentiation syndrome? Indian J. Med. Paediatr. Oncol. 2017, 38, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Connelly, S.; Zancosky, K.; Farah, K. Arsenic-Induced Pancreatitis. Case Rep. Gastrointest. Med. 2011, 2011, 1–3. [Google Scholar] [CrossRef]
- Zaloga, G.P.; Deal, J.; Spurling, T.; Richter, J.; Chernow, B. Unusual Manifestations of Arsenic Intoxication. Am. J. Med Sci. 1985, 289, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Hantson, P.; Haufroid, V.; Buchet, J.-P.; Mahieu, P. Acute Arsenic Poisoning Treated by Intravenous Dimercaptosuccinic Acid (DMSA) and Combined Extrarenal Epuration Techniques. J. Toxicol. Clin. Toxicol. 2003, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.I.; Liu, G.T.; Digre, K.B. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013, 81, 1159–1165. [Google Scholar] [CrossRef]
- Chen, J.; Wall, M. Epidemiology and Risk Factors for Idiopathic Intracranial Hypertension. Int. Ophthalmol. Clin. 2014, 54, 1–11. [Google Scholar] [CrossRef]
- GlennBurkett, J.; Ailani, J. An Upto Date Review of Pseudotumor Cerebri Syndrome. Curr. Neurol. Neurosci. Rep. 2018, 18, 33. [Google Scholar]
- Anoop, T.; Jain, N.; Nair, S.; Narayanan, G. All-trans-retinoic acid-induced pseudotumor cerebri in acute promyelocytic leukemia. J. Neurosci. Rural. Pr. 2014, 5, 273–275. [Google Scholar] [CrossRef]
- Zacholski, K.; Hambley, B.; Hickey, E.; Kashanian, S.; Li, A.; Baer, M.R.; Duong, V.H.; Newman, M.J.; DeZern, A.; Gojo, I.; et al. Arsenic trioxide dose capping to decrease toxicity in the treatment of acute promyelocytic leukemia. J. Oncol. Pharm. Pr. 2021, 28, 1340–1349. [Google Scholar] [CrossRef]
- Montesinos, P.; Vellenga, E.; Holowiecka, A.; Rayon, C.; Milone, G.; de la Serna, J.; Leon, A.; Bergua, J.; Rivas, C.; Gonzalez, J.; et al. Incidence, OutcomeandRiskFactors of PseudotumorCerebriafterAll- Trans Retinoic Acid and Anthracycline-Based Chemotherapy in Patientswith Acute PromyelocyticLeukemia. Blood 2008, 112, 2992. [Google Scholar] [CrossRef]
- Atas, U.; Ersoy, M.A.; Iltar, U.; Yucel, O.K.; Turkoglu, E.B.; Salim, O. Papilledema and idiopathic intracranial hypertension due to the possible potentiation of ATRA by posaconazole in a case of acute promyelocytic leukemia. J. Oncol. Pharm. Pr. 2022, 28, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.; Vishnu, P.; Dorer, R.K.; Aboulafia, D.M. All-Trans Retinoic Acid-Induced Pseudotumor Cerebri during Induction Therapy for Acute Promyelocytic Leukemia: A Case Report and Literature Review. Case Rep. Oncol. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B.; Griffiths, E.A.; Thompson, J.E.; Wang, E.S.; Wetzler, M.; Freyer, C.W. High pseudotumor cerebri incidence in tretinoin and arsenic treated acute promyelocytic leukemia and the role of topiramate after acetazolamide failure. Leuk. Res. Rep. 2014, 3, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Tallman, M.S. Acute promyelocytic leukemia (APL): remaining challenges towards a cure for all. Leuk. Lymphoma 2019, 60, 3107–3115. [Google Scholar] [CrossRef]
- Iyer, S.G.; Elias, L.; Stanchina, M.; Watts, J. The treatment of acute promyelocytic leukemia in 2023: Paradigm, advances, and future directions. Front. Oncol. 2023, 12, 1062524. [Google Scholar] [CrossRef]
- De Botton, S.; Dombret, H.; Sanz, M.; Miguel, J.S.; Caillot, D.; Zittoun, R.; Gardembas, M.; Stamatoulas, A.; Condé, E.; Guerci, A.; et al. Incidence, clinicalfeatures, andoutcome of all trans-retinoic acid syndrome in 413 cases of newlydiagnosed acute promyelocyticleukemia. The European APL Group. Blood 1998, 92, 2712–2718. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).