1. Introduction
At the beginning of 2020, the emerging pandemic of COVID-19 (COrona VIrus Disease 2019) represented a difficult challenge for physicians and researchers. Initially the disease caused massive hospitalization due to severe acute respiratory syndrome, often leading to death or chronic respiratory failure (1). This scenario forced the Countries to apply strict lockdown (2), leading to the interruption of social life and severe job and financial loss. SARS-CoV-2 was implicated in 570 million infections and over 6.3 million deaths by July 27, 2022 (3). Among the several reasons for these high numbers in such a short time, both clinical and organizational reduced preparedness among healthcare workers have been recognized (4–8).
COVID-19 disease presents a wide spectrum of clinical manifestations, and it may involve any organ and system. Its symptoms range from mild cases including fever, fatigue, myalgia, rhinorrhea, coughing, and sore throat to more severe diseases that can cause acute respiratory distress syndrome (ARDS), septic shock, and multi-organ failure. The severe disease is more likely in presence of pre-existing risk factors such as: older age, chronic cardiac, pulmonary, metabolic and kidney disease, immunosuppression, obesity and neoplastic disease (9–11).
Therefore, the dramatic impact of the pandemic highlighted the need for development of preventive measures (vaccines) and specific therapies to avoid hospital overcrowding (12,13).
By 2021, several therapeutic options were developed. In February 2021 the scientific technical committee (CTS) of the Italian Drugs Agency (AIFA) approved, for emergency use, the first anti-SARS-CoV-2 monoclonal antibody (mAb) (14). mAbs effectively reduce hospitalization in patients at risk of disease progression but have some limitations. Primarily, the SARS-CoV-2 variants that spread in the last years carried mutations of those epitopes recognized by the neutralizing antibodies (15). This led to the emergence of viral resistance, with reduced efficacy and the need for new virion-binding mAbs. Moreover, mAbs are intravenous drugs that require dedicated staff and rooms, and a lot of time for preparation and administration. After a few months, the European Medicines Agency (EMA) authorized the antiviral drug Remdesivir for treatment of hospitalized patients undergoing oxygen therapy; clinical indication was then expanded as early therapy in non-hospitalized patients also (16). Like mAbs, Remdesivir is an intravenous drug; this does not facilitate quick access to therapy, especially in homecare settings (17). At the end of 2021, EMA authorized Paxlovid®, (Pfizer Europe MA EEIG), an association of two protease inhibitors (Nirmatrelvir-Ritonavir) (18,19) as the first oral antiviral treatment for COVID-19 patients at risk of disease progression. In a randomized, double-blind clinical trial, consisting of 3000 participants who tested positive for COVID-19, Paxlovid was reported to be 89% effective in avoiding hospitalization of vulnerable patients (20,21). Nevertheless, Paxlovid®, despite having very similar eligibility criteria, is not an easily prescribed medicine due to its interaction with many drugs and the necessity for dose adjustment on kidney function, requiring the patient to undergo frequent blood tests. Before the treatment, patients should undergo a careful clinic and drug history and have recent blood analysis. Patients with an estimated Glomerular Filter Rate (eGFR) > 60 ml/min take nirmatrelvir 150 mg 2 tablets and ritonavir 100 mg 1 tablets for 5 days. Nirmatrelvir dose needs to be halved for eGFR >30 < 60 ml/min.
In the same period, the Food and Drug Administration (FDA) authorized, for emergency use (22), the oral antiviral Molnupiravir (Merck Sharp & Dohme, MSD) for treatment of mild-to-moderate COVID-19 in adults infected with SARS-CoV-2 and who are at high risk for progression. In the phase 3 study Molnupiravir has shown to be 31% effective in reducing hospitalization and 89% in reducing death compared with placebo (22). The drug was approved for emergency use in those patients with contraindications to alternative COVID-19 treatment options (23).
Molnupiravir was the first oral antiviral to be available in Italy; it is a small-molecule ribonucleoside prodrug of N-hydroxycytidine (NHC), which, interfering with viral RNA polymerase, misdirects viral replication thus making the virus noninfectious and unable to replicate (24). This mechanism of action being independent of spike protein mutations, makes Molnupiravir effective against SARS-CoV-2 variants.
Molnupiravir is a short-term oral therapy (5 days) and it is easy to administer in outpatients and it has no serious drug interaction, does not require dose adjustment up to glomerular filtration rate (GFR) = 15 ml/min (24) and has been proven to be well tolerated and safe in phase 1, 2, and 3 clinical trials, at least in short-term therapy regimens.
Clinical trials and systematic reviews have showed that Molnupiravir seems to have both clinical efficacy in lowering the risk of hospitalization or death for non-hospitalized patients with COVID-19 and also good safety and tolerability, reporting few and mainly low-grade adverse events (25–27).
All these characteristics make the use of Molnupiravir especially feasible as treatment in mild-to-moderate COVID-19 non- hospitalized frail patients undergoing multidrug chronic therapies and at risk for clinical progression. Although it was withdrawn from the market by decision of the European Medicines Agency, Molnupiravir demonstrates efficacy and safety with mild-to-moderate COVID-19 patients, especially if already COVID-19 vaccinated (23).
During the observation period (January-July 2022), the prevalence of Omicron B.1.1.529 variant was over 99% with a constant spread of BA.5 variant in the last 2 months of observation, according to the national reports of Italian Istituto Superiore di Sanità (National Institute of Health) (28,29).
Aims of this study are to: 1) confirm efficacy and safety of Molnupiravir use in the real world for non- hospitalized adults with mild-to-moderate, laboratory-confirmed COVID-19 patients who had at least one risk factor for severe progression of illness; 2) compare the results with those of clinical trials and to a subgroup of patients treated with the other available oral antiviral Paxlovid ; 3) perform an economic evaluation (30,31) comparing use of Molnupiravir versus Paxlovid, to assess potential saving associated with Molnupiravir use.
2. Materials and Methods
Study Design
This observational, prospective monocentric study was performed according to the Declaration of Helsinki statements and was approved by the Ethic Committee Lazio 2 with number 112.22, protocol 0178316/22. The study was reported according to the STROBE guidelines (32,33).
Setting and Population
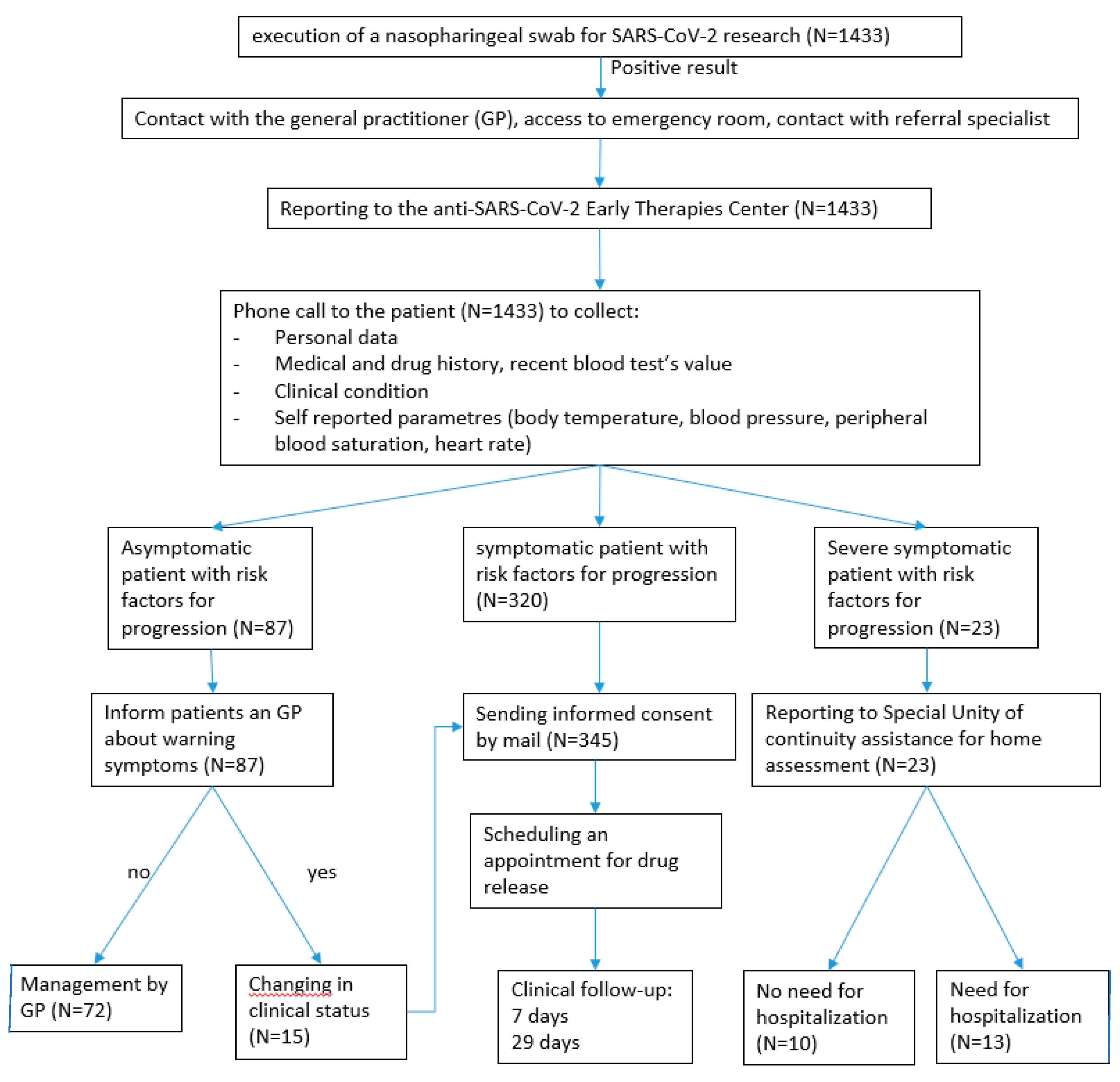
Since April 2021, the Ospedale dei Castelli hosts the COVID-19 Early Therapies Center of ASL Roma 6, specialized in the management of home COVID-19 patients with monoclonal antibodies and antivirals (34).
For treatment with Molnupiravir, adult patients were selected according to the indications of AIFA’s CTS (24).
Eligibility criteria included the positivity for a nasopharyngeal swab, mild-moderate symptoms which started no later than 5 days, no indication for hospitalization, and the presence of at least one of the following risk factors: age > 65 years, severe cardiovascular disease with chronic complications, severe chronic lung disease, obesity (body mass index≥30), primary or secondary immunodeficiency, neoplastic or onco-hematological active disease, chronic kidney disease (eGFR >15 ml/min), diabetes mellitus with chronic damage, severe neurovascular disease, neurodegenerative diseases or neurodevelopmental pathologies.
Exclusion criteria were absence of symptoms, necessity to start or increase oxygen therapy, hospitalization, pregnancy, pre-dialysis renal failure.
Patients included in the study were treated with 800 mg of Molnupiravir every 12 hours for 5 days, were informed of the potential teratogenicity of the drug and received all information on contraception timing.
From 01st January 2022 to 31th December 2022, patients were reported to the Center team via e-mail by either General Practitioners, specialists, or Emergency Department physicians(2). The Center team, once assessed patient eligibility, contacted them and collected the demographic, clinical, and laboratory data in a standardized and electronically filled form.
According to literature (23,35–43), the primary efficacy end point was the incidence of hospitalization for any cause (defined as ≥24 hours of acute care in a hospital or any similar facility) or death, while the primary safety end point was the incidence of any adverse events during the 29 days period of follow-up.
Clinical data included past and present diseases, pharmacological therapies, and the self-reported vital signs (blood pressure, heart frequency, body temperature and peripheral oxygen saturation).
Laboratory data included kidney and hepatic function.
All enrolled patients received informed consent to sign, information about potential teratogenic issues, dosage and duration prescription, and their drug doses.
Patients were contacted again twice, after 7 and 29 days respectively, to record the disease course and to investigate for any adverse effects.
Furthermore, a comparison with Nirmatrelvir-Ritonavir treated-patients was performed (44,45). Ospedale dei Castelli dispensed Molnupiravir when Nirmatrelvir-Ritonavir was not allowed due to drugs interactions or to clinical controindications : eligible patients underwent to a careful clinic and drug history, due to ritonavir many interactions, and then were treated with nirmatrelvir 150 mg 2 tablets and ritonavir 100 mg 1 tablet twice a day for 5 days (eGFR ≥ 60 ml/min) or nirmatrelvir 150 mg 1 tablet and ritonavir 100 mg 1 tablet twice a day (eGFR >30 <60 ml/min).
Statistical Analysis
Patient demographic and clinical characteristics were evaluated using descriptive analysis: frequencies and percentages for qualitative data; median and range for quantitative data. Chi-square test was used to evaluate statistical significantly differences among groups. OpenEpi software, version 3.01 (Open-Source Epidemiologic Statistics for Public Health,
www.OpenEpi.com) was used.
Economic Evaluation
A cost-effectiveness analysis was performed to compare the scenario in which patients were treated with Molnupiravir in the absence of a standard of care. A cost effectiveness analysis (25–27) is a method of comparing the cost of a program with its expected outcomes that are qualitative in nature and it compares alternative ways to achieve a specific set of results. A “what-if” approach was followed so that the control scenario was modelled as if the patient cohort was administered a treatment without antiviral agents instead of Molnupiravir. According to literature (23,35–43), it was assumed a reduction of 31% in hospitalization and of 89% in mortality after administration of Molnupiravir. According to the baseline characteristics of the patients included in the study, it was assumed a probability of hospitalization of 10% and an overall mortality of 0.18%. Among those hospitalized, the probability of accessing the Intensive Care Unit (ICU) was set at 24%.
According to the estimation of the Italian Public Accounting Service (Ragioneria Generale dello Stato) (46), each day of hospitalization was associated to an average cost of € 674, whilst each day in ICU was assigned a tariff of €1,650. The average length of stay in hospital was 20 days for patients admitted to ward and 10 days for patients admitted to ICU. Deaths were assigned an average length of stay of 8 days. Molnupiravir was associated to a cost of € 610 per patient (47).
The economic evaluation allowed to calculate the value for money of Molnupiravir compared to the standard of care.
The value for money was expressed in terms of cost per avoided hospitalization.
A probabilistic sensitivity analysis within a Monte Carlo framework allowed to estimate a simulated 95% confidence interval as well as the percentile of the cumulated distribution resulting from the combination of costs and effectiveness parameters. In order to carry out the probabilistic analysis cost data were assigned Gamma-type random variable, whilst effectiveness parameters (i.e. hospitalization, treatment effectiveness, mortality rates, rate of admission in ICU) were assigned Beta-type random distributions (48).
Table 1 shows the clinical and the economics inputs as well as the scale and slope parameters (alpha and beta) used to run the probabilistic sensitivity analysis.
3. Results
Molnupiravir
A total of 435 patients (225 males, 210 females; median age 72,9 years, 72,8 for men and 73 for women) affected by COVID-19 with mild-moderate symptoms and at least one risk factor for severe progression were enrolled in the study and treated with Molnupiravir. 276 patients (63,4%) had ≥2 risk factors. As shown in
Table 2, the main risk factor was old age (70%), followed by severe cardiovascular disease (65%), mostly chronic coronary heart disease and chronic heart failure. Considering the numerous comorbidities, almost 70% of the patients were on poly-pharmacological therapy.
A total of 124 patients (46 males and 78 females; median age 64 years), were treated with Nirmatrelvir-Ritonavir (Paxlovid) with mild-moderate symptoms. 72 (58,1%) patients had ≥2 risk factors. As shown in
Table 2, main risk factors were age >65 years (48%), obesity (38.8%), immunodeficiency (33.9%), severe chronic lung diseases (32%). Almost 40% of the patients had 1 (38,4%) and 2 (39,5%) comorbidities, while 18,4% had 3-4 comorbidities, only old age 5 pz (4%).
Overall, 11 patients needed hospitalization after a few days of therapy due to disease progression; 6 after completing therapy, 5 after partial therapy.
Out of 11, 10 hospitalized patients were dismissed after recovery; only one death was observed.
After 29 days of follow up, survival status was confirmed for all but a single patient who died due to COVID-19, after completing full therapy cycle. She was a 73-year-old immunocompromised patient whose clinical conditions and several comorbidities worsened 2 weeks after an apparent clinical remission. Since no autopsy was performed on the deceased patient, it is not possible to establish with certainty the cause of death.
The medium time for swab negativization was 12.4 days (DS 3.2) for patients treated with Molnupiravir and Median swab negativization 8 days (IQR 3, DS 5.6) for patients treated with Nirmatrel-Ritonavir.
For patients in treatment with Molnupiravir, residual symptoms were complained by 153 patients (37.1%) and 72 patients (15.9%) respectively after 7 (
Table 3) and 29 (
Table 4) days and the most frequently reported symptoms were fatigue and cough.
Among those in treatment with Nirmatrelvir-Ritonavir, after 7 days, 52 (41.6%) patients complained for residual symptoms. 60 people had ≥2 risk factors.
After 7 days, the most common residual symptoms were fatigue and cough, followed by myalgia, sore throat, headache and dyspnea and nasal congestion; after 29 days, the proportions were still very close to the same (
Table 3 and
Table 4).
After 29 days, 331 (76%) and 94 (75.8%) patients under Molnupiravir and Nirmatrelvir-Ritonavir, respectively, had no residual symptoms (
Table 4). Concerning safety, in patients treated with Molnupiravir, adverse events were reported by 62 patients (14,2%); they are summarized in
Table 5. The most frequently complained adverse events were gastrointestinal disorders, in agreement with data already reported in clinical trials (23). In 4 patients the symptoms were so invalidating to require therapy discontinuation. Moreover 3 patients did not finish therapy due to poor compliance, and 5 patients did not finish therapy due to hospitalization. These 5 patients had multiple comorbidities and required hospitalization within the first two days of therapy.
In patients treated with Nirmatrelvir-Ritonavir, adverse events were reported by 43 patients (34,68%); they are summarized in
Table 5. The most frequently complained adverse events were dysgeusia and diarrhea, followed by nausea and vomiting.
In
Table 6 is presented a comparison between the results of this real-world clinical practice study and the results of an important clinical trial (22). The observed number of hospitalizations and adverse effects (overall and severe) are far lower in this study and the differences are statistically significant.
Nirmatrelvir-Ritonavir
Economic Evaluation
Table 7 shows the results of the economic evaluation. Overall, the treatment scenario (involving the administration of Molnupiravir to the 435 patients included in the study) produced a total cost of € 694,000 including treatment costs (€210,450), hospitalizations (€320,891), ICUs (€158,870) and the cost of deaths (€ 3,847).
On the other side, under the assumption that the same cohort of 435 patients was treated withouth oral antiviral agents (not involving the administration of Molnupiravir), the total costs would have been € 757,012, including: €465,000 due to hospitalization in ward, € 248,234 due to ICU admissions and € 43,718 due to deaths (50).
This cost structure led to a dominant result of Molnupiravir versus treatment without oral antiviral agents, thus involving a potential total savings of about € 92,954 per patient (8% of treatment without oral antiviral agents cost). Economic Number-To-Treat was 12.01, meaning that for each group of 12 patients treated, Molnupiravir usage allows the availability of enough resources to treat an extra patient, compared to standard of care usage.
Figure 2 shows the results of the Monte Carlo simulation, from which a simulated cost/effectiveness plan and a 95% confidence interval for both incremental costs and avoided hospitalizations were extrapolated.
100% percent of simulations resulted in a saving consequent to the use of Molnupiravir in non-hospitalized patients. As for the savings magnitude, a 95% confidence interval ranging from € 144,000 to € 602,000 was estimated with a 50th percentile of € 327,000 savings (circa € 750 per patient).
Concerning avoided hospitalizations, the 95% confidence interval ranged from 25 to 36 with a 50th percentile of 32 avoided hospitalizations (7.36 %).
4. Discussion
At present, information on the efficacy and safety of Molnupiravir for the prevention of COVID-19 progression, in daily clinical practice in vaccinated non-hospitalized patients with mild to moderate COVID-19 is scarce and controversy (17,39,44,51–53). In literature it is highly recommended to investigate this aspect since now a good proportion of the population worldwide is currently vaccinated (39,44,51–53)
The percentage of hospitalizations observed in this study was 2.5% which is statistically significantly lower than that observed in the benchmark clinical trial considered (6.8%) (20), but very close to that observed in the study of Vena and co-authors (2.7%) (44).This latter study is a real-world study in which patient characteristics (age and comorbidities) were similar to ours, but sample size was much smaller (only 145 versus 435 patients).
Considering vaccination, in the study of Vena et al. all patients were “fully vaccinated” but the minimum number of doses received is not specified. In our study, 87.8% of patients had 3 or more doses of vaccine, whereas the landmark clinical trial enrolled only unvaccinated patients. (44)
For what concern safety, in this study the percentages of adverse events (overall =14.3% and serious = 2.6%) were statistically lower than those observed in the benchmark clinical trial considered (30,4% and 6.9% respectively) (23). In the study of Vena et al., only 2 patients experienced mild adverse events and nobody experienced serious ones, maybe because of the relatively low number of enrolled patients (44).
It deserves attention to point out that the lower hospitalization and occurrence of adverse events observed in our study refers to frail patients. Our cohort consists mostly in elderly patients (median age 72.9 years), with several comorbidities such as cardiovascular diseases, chronic respiratory failure and malignancies, factors that are associated with COVID-19 progression and increased risk of hospitalization.
In our experience Molnupiravir seems to be an effective treatment, thanks to its manageability especially in fragile old patients suffering from multiple pathologies and taking several drugs. Indeed, the possibility of taking Molnupiravir at home and associate it with other drugs, allowed patients to continue their daily routine and avoid the discomfort of the hospital. Moreover, the reported data shown how Molnupiravir represents a feaseble and safe alternative in those patients with controindications to Nirmatrelvir-Ritonavir treatment.
The prevention of COVID-19 worsening, especially if performed in outpatient setting, allows to relieve the pressure on medical departments and health services, representing a substantial saving for the health system. In fact, COVID-19 hospitalizations require a substantial economic consumption in terms of clinical and nursing care and days of hospital stay. This, without considering the additional burden in terms of organizational impact on health structures, emergency medical service usage, and costs due to prolonged care and delayed discharge (54).
Molnupiravir seems to be also an economically effective treatment. Different studies (22, 31,46) reported the cost of single day in a COVID-19 department ranging from € 427.77 in a low intensity ward to € 1,278.50 in a high intensity setting. Considering that the average hospital stay in a sub-intensive care unit is 15.5 days, this means an amount of € 14,873.48. On the other hand, in Italy, the cost of Molnupiravir is about € 610 for the 5 days treatment, its usage resulting in a potential relief for public spending and for Hospital facilities burdening.
Patients groups with indications for Molnupiravir and for Nirmatrelvir/ritonavir are not usually comparable. In this study, a comparison between Molnupiravir and Nirmatrelvir/ritonavir was performed because both therapeutic regimens were proposed and used against the same Sars CoV 2 variants, and their effects on patients in terms of clinical and management impact were therefore directly comparable.
Strengths and Limitations
To our knowledge, this is the largest and most updated study on clinical effectiveness and cost-efficacy of Molnupiravir usage in early onset of COVID-19 in the routine clinical practice.
In analogy with a previous conducted study, its usage was favorable compared to standard supportive therapy. (37,39,54,55) Supportive therapy is not the standard of care, because currently scientists do not agree on any single therapy for COVID 19 to be considered as standard of care.
The major limitation of the study lies in the usage of Monte Carlo and what-if scenario for comparison evaluation. We believe that this limitation could only be overcome by a double-blind placebo-controlled trial, and doubts on its ethical feasibility would arise, since the usage of placebo against a proven working molecule would represent, at least, a harsh moral hazard. Henceforth, we chose to use simulation data for comparison, instead of actual patients not treated despite clinical indication for doing so. Another limitation is that the study is a single-center design.
5. Conclusions
The COVID-19 pandemic represented a hard challenge for physicians and researchers. During these last 2 years, the scientific world has faced a heterogeneous disease with different degrees of severity. This was partially due to viral mutations and to the development of new vaccines which allowed contain the disease in the vulnerable population. Moreover, the advent of specific, early treatment helped those patients who partially responded to vaccine, or with absolute contraindications to vaccination, to overcome COVID-19 without the need of hospitalization.
In our experience, Molnupiravir represented a clinical and economical effective treatment, avoiding disease progression especially in frailty, old patients suffering of multiple pathologies and taking several medications, thanks to its manageability. Our experience confirms its efficacy as reported in literature data.
Author Contributions
Conceptualization, E.C. and M.L.; methodology, A.V., M.R.; software, A.V., M.M.; validation, R.I., F.R.; formal analysis, M.L.F, N.P., F.P.; investigation, M.D.C., F.V., A.C.; resources, A.C.; data curation, M.R., L.F., G.G.; writing—original draft preparation, L.M.C.C, E.C. and M.L.; writing—review and editing, F.P., F.R., M.S.C.; supervision, Ma.M., R.C., M.S.C.; project administration, D.A., A.S., M. S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee Lazio 2, number 112.22, protocol 0178316/22.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgement
Authors said many thanks to Associazione Fratello Cuore for its inconditionated support
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Internal medicine Working group: Filomena Pietrantonio, Fabio Vinci, Michela Delli Castelli, Angela Ciamei, Margherita Londi, Enrica Cipriano
Hygiene working group: Francesco Rosiello, Marise Sabato, Aurelio Spartà, Davide Acco, Maria Sofia Cattaruzza
Lawyer: Rosa Iodice, Luca Fortunato
References
- Rosiello, D.F.; Anwar, S.; Yufika, A.; Adam, R.Y.; Ismaeil, M.I.; Ismail, A.Y.; Dahman, N.B.; Hafsi, M.; Ferjani, M.; Sami, F.S.; et al. Acceptance of COVID-19 vaccination at different hypothetical efficacy and safety levels in ten countries in Asia, Africa, and South America. Narra J 2021, 1. [Google Scholar] [CrossRef]
- Vinci, A.; Pasquarella, A.; Corradi, M.P.; Chatzichristou, P.; D’agostino, G.; Iannazzo, S.; Trani, N.; Parafati, M.A.; Palombi, L.; Ientile, D.A. Emergency Medical Services Calls Analysis for Trend Prediction during Epidemic Outbreaks: Interrupted Time Series Analysis on 2020–2021 COVID-19 Epidemic in Lazio, Italy. Int. J. Environ. Res. Public Health 2022, 19, 5951. [Google Scholar] [CrossRef] [PubMed]
- Alfonsi, V.; Scarpelli, S.; Gorgoni, M.; Couyoumdjian, A.; Rosiello, F.; Sandroni, C.; Corsi, R.; Pietrantonio, F.; De Gennaro, L. Healthcare Workers after Two Years of COVID-19: The Consequences of the Pandemic on Psychological Health and Sleep among Nurses and Physicians. Int. J. Environ. Res. Public Health 2023, 20, 1410. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Rosiello, F.; Alessi, E.; Pascucci, M.; Rainone, M.; Cipriano, E.; Di Berardino, A.; Vinci, A.; Ruggeri, M.; Ricci, S. Burden of COVID-19 on Italian Internal Medicine Wards: Delphi, SWOT, and Performance Analysis after Two Pandemic Waves in the Local Health Authority “Roma 6” Hospital Structures. Int. J. Environ. Res. Public Health 2021, 18, 5999. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Weekly Epidemiological Update on COVID-19—21 September 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19 (accessed on 21 September 2021).
- Huy, N.T.; Chico, R.M.; Huan, V.T.; Shaikhkhalil, H.W.; Uyen, V.N.T.; Qarawi, A.T.A.; Alhady, S.T.M.; Vuong, N.L.; Van Truong, L.; Luu, M.N.; et al. Awareness and preparedness of healthcare workers against the first wave of the COVID-19 pandemic: A cross-sectional survey across 57 countries. PLoS ONE 2021, 16, e0258348. [Google Scholar] [CrossRef]
- Harapan, H.; Yufika, A.; Anwar, S.; Ophinni, Y.; Yamada, C.; Sharun, K.; Gachabayov, M.; Fahriani, M.; Husnah, M.; Raad, R.; et al. Beliefs on social distancing and face mask practices during the COVID-19 pandemic in low- and middle-income countries: A cross-sectional study. F1000Research 2022, 11, 206. [Google Scholar] [CrossRef]
- Salzberger B, Buder F, Lampl B, Ehrenstein B, Hitzenbichler F, Holzmann, T. ; et al. Epidemiology of SARS-CoV-2. Infection 2021, 49, 233–239. [Google Scholar] [CrossRef]
- Fahriani, M.; Ilmawan, M.; Fajar, J.K.; Maliga, H.A.; Frediansyah, A.; Masyeni, S.; Yusuf, H.; Nainu, F.; Rosiello, F.; Sirinam, S.; et al. Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis - A systematic review and meta-analysis. Narra J 2021, 1. [Google Scholar] [CrossRef]
- Fajar, J.K.; Ilmawan, M.; Mamada, S.; Mutiawati, E.; Husnah, M.; Yusuf, H.; Nainu, F.; Sirinam, S.; Keam, S.; Ophinni, Y.; et al. Global prevalence of persistent neuromuscular symptoms and the possible pathomechanisms in COVID-19 recovered individuals: A systematic review and meta-analysis. Narra J 2021, 1. [Google Scholar] [CrossRef]
- Harapan, H.; Anwar, S.; Yufika, A.; Sharun, K.; Gachabayov, M.; Fahriani, M.; Husnah, M.; Raad, R.; Abdalla, R.Y.; Adam, R.Y.; et al. Vaccine hesitancy among communities in ten countries in Asia, Africa, and South America during the COVID-19 pandemic. Ann. Trop. Med. Parasitol. 2021, 116, 236–243. [Google Scholar] [CrossRef]
- Rosiello, F.; , D’Oca, E. Vaccinations and the movement of antivaccers. Eur J Public Health [Internet]. 2020. Available online: https://academic.oup.com/eurpub/article/doi/10.1093/eurpub/ckaa166.700/5916042 (accessed on 3January 2021).
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N. Engl. J. Med. 2021, 386, 305–315. [Google Scholar] [CrossRef]
- Drożdżal S, Rosik J, Lechowicz K, Machaj F, Szostak B, Przybyciński, J. ; et al. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist Updat. 2021, 59, 100794. [Google Scholar]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 [Internet]. 2021. Available online: https://www.fda.gov/newsevents/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oralantiviral-treatment-covid-19.
- Hashemian, S.M.R.; Sheida, A.; Taghizadieh, M.; Memar, M.Y.; Hamblin, M.R.; Bannazadeh Baghi, H.; et al. Paxlovid (Nirmatrelvir/Ritonavir): A new approach to Covid-19 therapy? Biomed. Pharmacother. 2023, 162, 114367. [Google Scholar]
- Cox, R.M.; Wolf, J.D.; Plemper, R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021, 6, 11–18. [Google Scholar]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Ciamei, A.; Vinci, A.; Ciarambino, T.; Alessi, E.; Pascucci, M.; Castelli, M.D.; Zito, S.; Sanguedolce, S.; Rainone, M.; et al. Polypharmacy Management in a Gender Perspective: At the Heart of the Problem: Analysis of Major Cardiac Diseases, SARS-CoV-2 Affection and Gender Distribution in a Cohort of Patients in Internal Medicine Ward. Int. J. Environ. Res. Public Health 2023, 20, 5711. [Google Scholar] [CrossRef]
- Harries, A.; Takarinda, K.C. ; A. , J.B.; Mm, G.d.S.; Db, M.; E., K.; A., G.; V., D.R.; A., M.-Q.; Y., C.; et al. Faculty Opinions recommendation of Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients.. 2022, 386. [Google Scholar] [CrossRef]
- AIFA. Modifiche al registro degli antivirali orali contro il COVID 19 [Internet]. Available online: https://www.aifa.gov.it/-/modifica-registro-antivirali-orali-covid-19.
- Ruggeri, M.; Drago, C.; Rosiello, F.; Orlando, V.; Santori, C. Economic Evaluation of Treatments for Migraine: An Assessment of the Generalizability Following a Systematic Review. PharmacoEconomics 2020, 38, 473–484. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Cost effectiveness. Available online: https://www.ncbi.nlm.nih.gov/mesh/?term=cost+effectiveness (accessed on 2 March 2023).
- Ruggeri, M.; Signorini, A.; Caravaggio, S.; Santori, C.; Rosiello, F.; Coluzzi, F. Cost-Effectiveness Analysis of Tapentadol Versus Oxycodone/Naloxone in both Branded and Generic Formulations in Patients with Musculoskeletal Pain. Clin. Drug Investig. 2021, 41, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Agenzia Nazionale per i Servizi Sanitari Regionali. Rapporto COVID-19. 2020.
- Istituto Superiore di Sanità. Varianti COVID [Internet]. Available online: https://www.iss.it/cov19-cosa-fa-iss-varianti.
- Ruggeri, M.; Signorini, A.; Drago, C.; Rosiello, F.; Marchetti, M. Model for estimating the healthcare costs and capacity of intensive care units in Italy in the treatment of patients with COVID-19: Remdesivir impact assessment. AboutOpen 2020, 7, 95–102. [Google Scholar] [CrossRef]
- Ruggeri, M.; Signorini, A.; Caravaggio, S.; Rua, J.; Luís, N.; Braz, S.; Aragão, F. Estimation Model for Healthcare Costs and Intensive Care Units Access for Covid-19 Patients and Evaluation of the Effects of Remdesivir in the Portuguese Context: Hypothetical Study. Clin. Drug Investig. 2022, 42, 345–354. [Google Scholar] [CrossRef] [PubMed]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar]
- Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Vinci, A.; Rosiello, F.; Alessi, E.; Pascucci, M.; Rainone, M.; Castelli, M.D.; Ciamei, A.; Montagnese, F.; D’amico, R.; et al. Green Line Hospital-Territory Study: A Single-Blind Randomized Clinical Trial for Evaluation of Technological Challenges of Continuous Wireless Monitoring in Internal Medicine, Preliminary Results. Int. J. Environ. Res. Public Health. 2021, 18, 10328. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr Clin Res Rev. 2021, 15, 102329. [Google Scholar] [CrossRef]
- Sendi, P.; Razonable, R.R.; Nelson, S.B.; Soriano, A.; Gandhi, R.T. First-generation oral antivirals against SARS-CoV-2. Clin Microbiol Infect. 2022, 28, 1230–1235. [Google Scholar]
- Atal, S.; Kaore, S. Molnupiravir in COVID-19: A scoping review. Curr. Drug Res. Rev. 2022. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19. Diabetes Metab Syndr Clin Res Rev. 2022, 16, 102396. [Google Scholar] [CrossRef] [PubMed]
- Mali, K.R.; Eerike, M.; Raj, G.M.; Bisoi, D.; Priyadarshini, R.; Ravi, G.; Chaliserry, L.F.; Janti, S.S. Efficacy and safety of Molnupiravir in COVID-19 patients: A systematic review. Ir. J. Med Sci. ( 2022, 192, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Kamal, L.; Ramadan, A.; Farraj, S.; Bahig, L.; Ezzat, S. The pill of recovery; Molnupiravir for treatment of COVID-19 patients; a systematic review. Saudi Pharm. J. 2022, 30, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Wang, Y.-H.; Chen, K.-H.; Chen, C.-H.; Wang, C.-Y. The Clinical Efficacy and Safety of Anti-Viral Agents for Non-Hospitalized Patients with COVID-19: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Viruses 2022, 14, 1706. [Google Scholar] [CrossRef] [PubMed]
- Yip, T.C.-F.; Lui, G.C.-Y.; Lai, M.S.-M.; Wong, V.W.-S.; Tse, Y.-K.; Ma, B.H.-M.; Hui, E.; Leung, M.K.W.; Chan, H.L.-Y.; Hui, D.S.-C.; et al. Impact of the Use of Oral Antiviral Agents on the Risk of Hospitalization in Community Coronavirus Disease 2019 Patients (COVID-19). Clin. Infect. Dis. 2022, 76, e26–e33. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Traman, L.; Bavastro, M.; Limongelli, A.; Dentone, C.; Magnè, F.; Giacobbe, D.R.; Mikulska, M.; Taramasso, L.; Di Biagio, A.; et al. Early Clinical Experience with Molnupiravir for Mild to Moderate Breakthrough COVID-19 among Fully Vaccinated Patients at Risk for Disease Progression. Vaccines 2022, 10, 1141. [Google Scholar] [CrossRef]
- Gentile, I.; Scotto, R.; Shiano Moriello, N.; Pinchera, B.; Villari, R.; Trucillo, E.; Ametrano, L.; Fusco, L.; Castaldo, G.; Buonomo, A.R.; et al. Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study. Vaccines 2022, 10, 1731. [Google Scholar] [CrossRef]
- Ruggeri, M.; Signorini, A.; Caravaggio, S.; Alraddadi, B.; Alali, A.; Jarrett, J.; Kozma, S.; Harfouche, C.; Al Musawi, T. Modeling the Potential Impact of Remdesivir Treatment for Hospitalized Patients with COVID-19 in Saudi Arabia on Healthcare Resource Use and Direct Hospital Costs: A Hypothetical Study. Clin. Drug Investig. 2022, 42, 1–10. [Google Scholar] [CrossRef]
- Goswami H, Alsumali A, Jiang Y, Schindler M, Duke ER, Cohen J; et al. Cost-Effectiveness Analysis of Molnupiravir Versus Best Supportive Care for the Treatment of Outpatient COVID-19 in Adults in the US. PharmacoEconomics 2022, 40, 699–714. [Google Scholar] [CrossRef]
- Onen-Dumlu, Z.; Harper, A.L.; Forte, P.G.; Powell, A.L.; Pitt, M.; Vasilakis, C.; Wood, R.M. Optimising the balance of acute and intermediate care capacity for the complex discharge pathway: Computer modelling study during COVID-19 recovery in England. PLoS ONE 2022, 17, e0268837. [Google Scholar] [CrossRef]
- Liu, J.; Pan, X.; Zhang, S.; Li, M.; Ma, K.; Fan, C.; Lv, Y.; Guan, X.; Yang, Y.; Ye, X.; et al. Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: A multicenter randomized controlled study. Lancet Reg. Heal. - West. Pac. 2023, 33, 100694. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. J. Manag. Care Spéc. Pharm. 2022, 1–10. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: Molnupiravir does not cut hospital admissions or deaths in vaccinated people at high risk. trial finds 2022, 379, o3055. [Google Scholar] [CrossRef]
- Scioscia, G.; De Pace, C.C.; Giganti, G.; Tondo, P.; Barbaro, M.P.F.; Lacedonia, D. Real life experience of molnupiravir as a treatment of SARS-CoV-2 infection in vaccinated and unvaccinated patients: A letter on its effectiveness at preventing hospitalization. Ir. J. Med Sci. 2022, 1–3. [Google Scholar] [CrossRef]
- Khoo, S.H.; FitzGerald, R.; Saunders, G.; Middleton, C.; Ahmad, S.; Edwards, C.J.; et al. Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): A randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect Dis. 2023, 23, 183–195. [Google Scholar] [CrossRef]
- Parums, D.V.; Editorial: Long COVID, or Post-COVID Syndrome, and the Global Impact on Health Care. Med Sci Monit [Internet]. Available online: https://www.medscimonit.com/abstract/index/idArt/933446 (accessed on 30 November 2022).
- Fischer, W.A.; Eron, J.J.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022, 14, eabl7430. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).