1. Introduction
During the translation of the messenger RNA (mRNA), the ribosome needs to stop at the first natural stop codon (NTC) found on the mRNA sequence. Nonsense codons are three nucleotide triplets that represent a “STOP” signal for the translation of all mRNAs in the cell. Natural termination codons are identified as the first stop codon at the end of the coding mRNA sequence. The nonsense codons are represented by UAG, UGA, and UAA nucleotide triplets, also known as
amber,
opal, and
ochre codons, respectively [
1].
When the ribosome reaches an NTC, the translation is stopped, and the translation-termination complex induces the release of nascent polypeptide. The translation termination complex is composed of two protein factors named eukaryotic release factors 1 and 3 (eRF1 and eRF3). The eRF1 factor mimics a tRNA and it enters the A site of the ribosome to bind and recognize the stop codon (all three stop codons). The eRF3 protein is a GTPase that is stimulated by the interaction with polyA-binding protein (PABP) and it modifies the conformation of eRF1 promoting the release of nascent polypeptide and the termination of the translation [
2,
3].
Below the coding mRNA sequence and the NTC, there is an untranslated region named UTR-region containing regulatory elements of the translation such as poly-A tail and
multiple mRNA 3′-UTR binding sites for specific regulatory proteins [
4]. In addition, the probability to find several in-frame nonsense codons inside the UTR region is higher for all expressed genes [
4].
Nonsense mutations are a class of mutations in which an extra nonsense codon results in the mRNA sequence after transcription. This anomalous stop codon inside the coding mRNA sequence is named premature termination codon (PTC). The PTCs are recognized as canonical stop signals by the translation termination complex and that induces an early termination of the translation. The presence of the PTC is involved in the synthesis of truncated and dysfunctional proteins that are degraded by cellular pathways [
6].
Several genetic diseases are caused by nonsense mutations. Among the most diffuse nonsense-related diseases there are Cystic fibrosis, Duchenne muscular dystrophy, Choroideremia, β-thalassemia, Primary immune deficiencies,
Shwachman-Diamond syndrome, and some inherited cancer typologies [
7,
8,
9,
10,
11,
12,
13].
A strategy to restore the correct translation of the proteins deriving from the expression of nonsense mutated genes is nonsense suppression therapy using translational readthrough-inducing drugs (TRIDs). Different classes of compounds such as geneticin (also known as G418), ELX-02, gentamicin, paromomycin, neomycin, diaminopurine (DAP), and PTC124 (Ataluren) have the ability to induce ribosome translational readthrough [
14,
15,
16]. This mechanism consists of the misreading of the PTC during translation by TRIDs and in the insertion of a near-cognate tRNA instead of the eRF1 factor. It results in the synthesis of a full-length protein and in the rescue of its function [
5].
Only the readthrough mechanism of action of the aminoglycoside antibiotics, such as G418, is well clarified. These classes of compounds interact with the minor subunit of the eukaryotic ribosome inside the decoding center, increasing the miscoding errors at the PTCs and the recruitment of near-cognate tRNA [
17].
Recently, some evidence regarding the Ataluren (PTC124) mechanism of action was found. According to Huang S. and colleagues, Ataluren could bind two different sites during translation: the 18S rRNA near to the decoding centre and to interact with the PTC in the mRNA [
18,
19].
In the last years, three new TRIDs were identified by our group and tested to validate their translational readthrough ability and tolerability
in vivo [
20,
21,
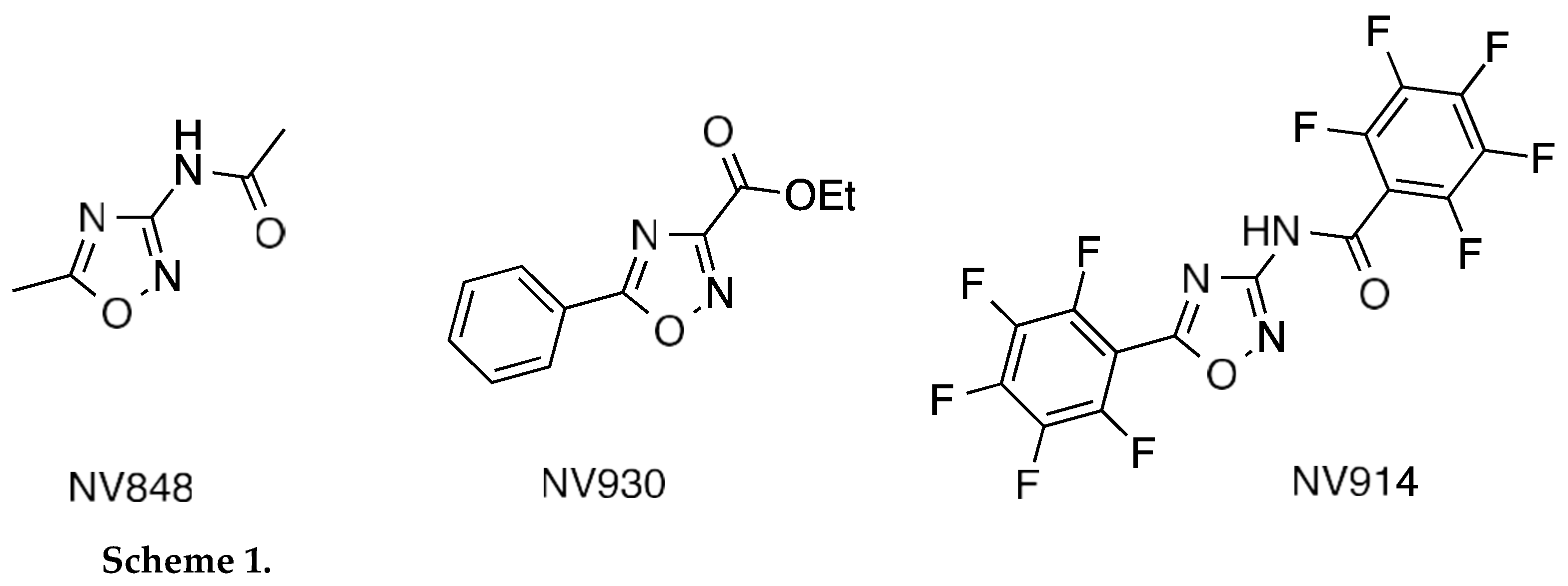
22]. These compounds named NV848, NV914, and NV930 (also known as NV molecules; PTC Int. Appl. WO 2019/101709 A1 20190531) present an oxadiazole-core chemical structure, like the Ataluren core, but with different functional groups (Scheme 1).
NV molecules showed a good translational readthrough activity in Cystic fibrosis
in vitro systems and a suitable tolerability
in vivo experiments [
20,
21]. Recently, we explored the possible MOA of these molecules to identify interaction with specific proteins involved in the translation process. Our results showed that NV914 and NV930 could interact with the FTSJ1 2’-O-methyltransferase [
23].
An important aspect of the functionality and efficiency of the TRIDs is their specific action against PTCs. The possible readthrough action and suppression of the NTCs by TRIDs is considered an off-target that hypothetically alters the translational process of the cell.
The purpose of this work is the investigation of the possible NV848, NV914, and NV930 readthrough effects against NTCs, to validate the specific action of these compounds on PTCs. Two different in vitro approaches were used to assess the translation alteration of different proteins after treatment with NV molecules.
The first one is a study of the molecular weight and functionality of the p53 protein, used as an inducible protein model, after its translational increase (stimulated by DNA damage response induction) and treatment with NV848, NV914, or NV930.
In addition, the second approach is the evaluation of two housekeeping proteins (Cys-C and β2M) molecular weights after treatment with NV molecules. The evaluation of translational errors using these housekeeping proteins was used previously in a study of the ELX-02 readthrough effect [
23].
2. Results
2.1. Evaluation of p53 molecular weight alteration after DNA damage induction mediated by Doxorubicin combined with NV848, NV914, or NV930 treatment
To exclude the possible off-target effects on NTCs by NV848, NV914, or NV930, p53 was chosen as a model of an inducible protein to analyze its molecular weight after induction of DNA damage in association with NV molecules treatment. In fact, the eventual NTCs readthrough could cause the production of proteins with a greater molecular weight respect to the normal one of the wild-type p53.
p53 is one of the most characterized proteins in the research field, because it is involved in cell cycle arrest, DNA repair, senescence, and apoptosis, all processes involved in cancer [
24]. In order to exert its proper function, this protein needs a series of important events: aggregation in tetramer form, phosphorylation of specific amino acidic residues, nuclear localization, and interaction with transcription-promoting DNA sequences of specific genes [
25].
One of the earliest genes activated by the p53 transcriptional factor in response to DNA damage is
CDKN1A [
26]. This gene encodes for a cyclin-dependent kinase inhibitor also known as p21, that is fundamental to arresting the cell cycle during DNA repair response [
27].
Immediately, 24 hours after DNA damage, p53 mRNAs and proteins are stabilized and the translation of p53 is increased in order to accelerate DNA damage response [
28]. p53 alterations can interfere with the functionality and correct localization of this protein in several ways [
29].
Based on these observations, to evaluate a possible readthrough activity of the NV molecules (NV848, NV914, and NV30) on the NTCs during the translation of the p53 protein, DNA damage was induced by Doxorubicin, in HCT116 cells in presence of NV molecules. The purpose of these experiments was to visualize the possible expression of greater molecular weight forms of p53 after treatment with NV molecules for 24 hours. Moreover, the experiment aimed to assess the presence of not functional forms of the p53 protein due to an incorrect translational process.
HTC116 cells are tumor and immortalized cells with a high replication rate. These cells were used according to the higher translation capacity respect to normal cells.
HCT116 cells were plated and, after 24 hours, were treated with Doxorubicin to induce DNA damage and p53 translation increasing in combination with NV molecules as shown in the graphic representation in
Figure 1.
p53 protein expression, functionality, and localization were analyzed after DNA damage induction in presence of NV848, NV914, or NV930.
In particular, HCT116 cells were seeded in 6-well plates and treated with Doxorubicin 0,2 μg/ml in combination with G418 or NV molecules at the indicated concentrations (
G418: 430 μM, 645μM and 1075 μM;
NV848,
NV914, and
NV930: 3 μM, 12 μM, 48 μM;
Figure 2).
The G418 aminoglycoside was used as a positive control considering its capacity of inducing NTCs genome-wide readthrough [
5].
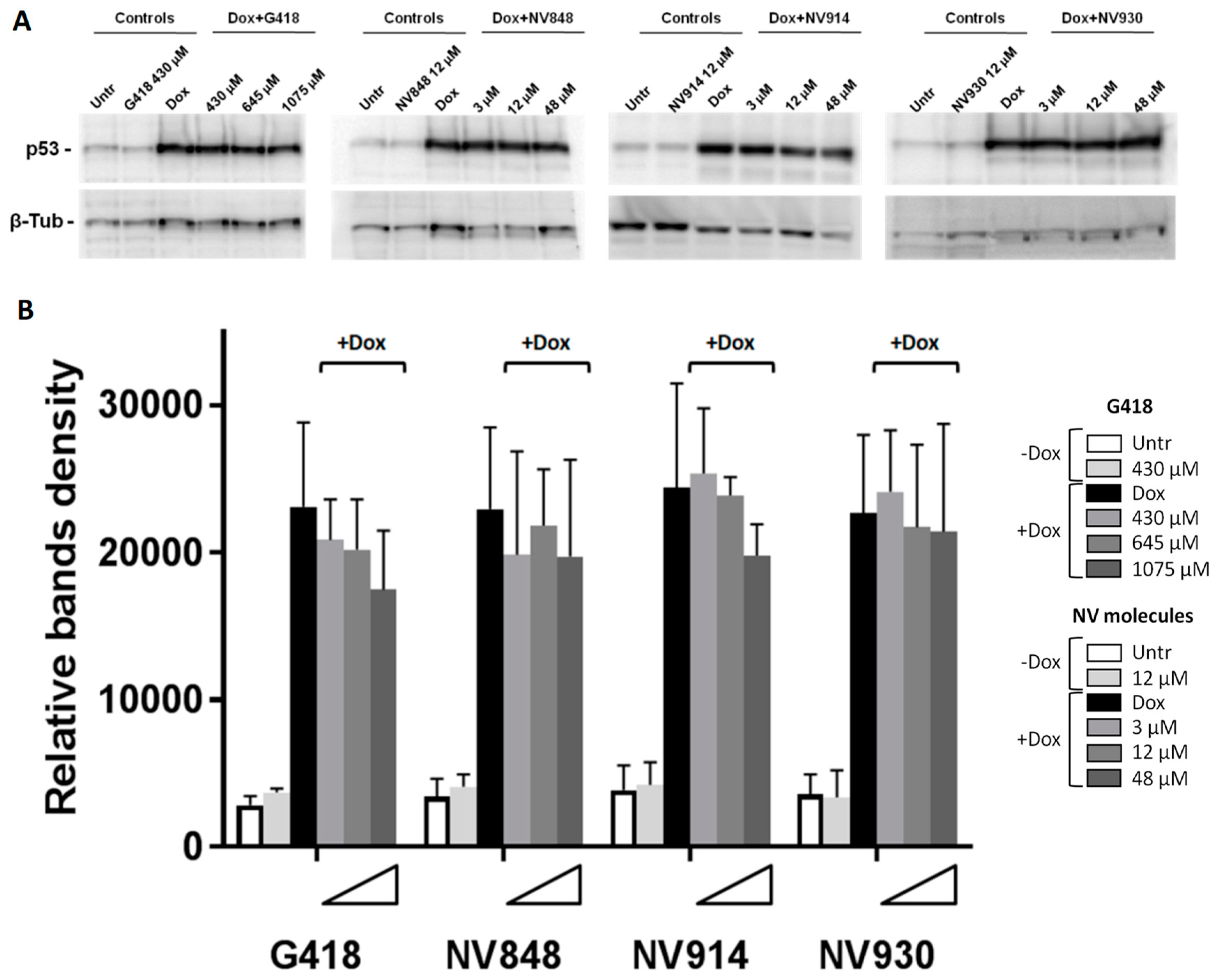
Western blot experiments and bands quantification analysis showed a little decrease of p53 protein levels in HCT116 treated with G418 and NV914 after DNA damage respect to Doxorubicin (Dox) samples (black bars in Figure 2-B). However, in all analyzed samples were no visible p53 bands with greater molecular weights.
2.2. Study of p53 protein localization and functionality after DNA damage response and NV molecules treatment
In response to DNA damage, the p53 protein is stabilized and its expression is increased. Post-translational modifications and other signals are involved in p53 tetramerization and its nuclear localization to activate specific gene transcription.
Nuclear translocation is a fundamental event to the correct p53 function and, in addition, this process is regulated by the presence of a protein nuclear localization sequence (NLS). Nuclear localization and export sequences together with the tetramerization domain are located in the C-terminal domain of p53. The suggestion is that: if any alteration in the p53 translation is induced by NV molecules treatment, protein functions would be impaired such as nuclear localization.
In order to visualize p53 protein localization after DNA damage induction and TRID treatments, the immunofluorescence assay was performed. HCT116 cells were treated with Doxorubicin and simultaneously with G418 or NV molecules at the indicated concentrations for 24 hours (
Figure 3 and
Figure 4).
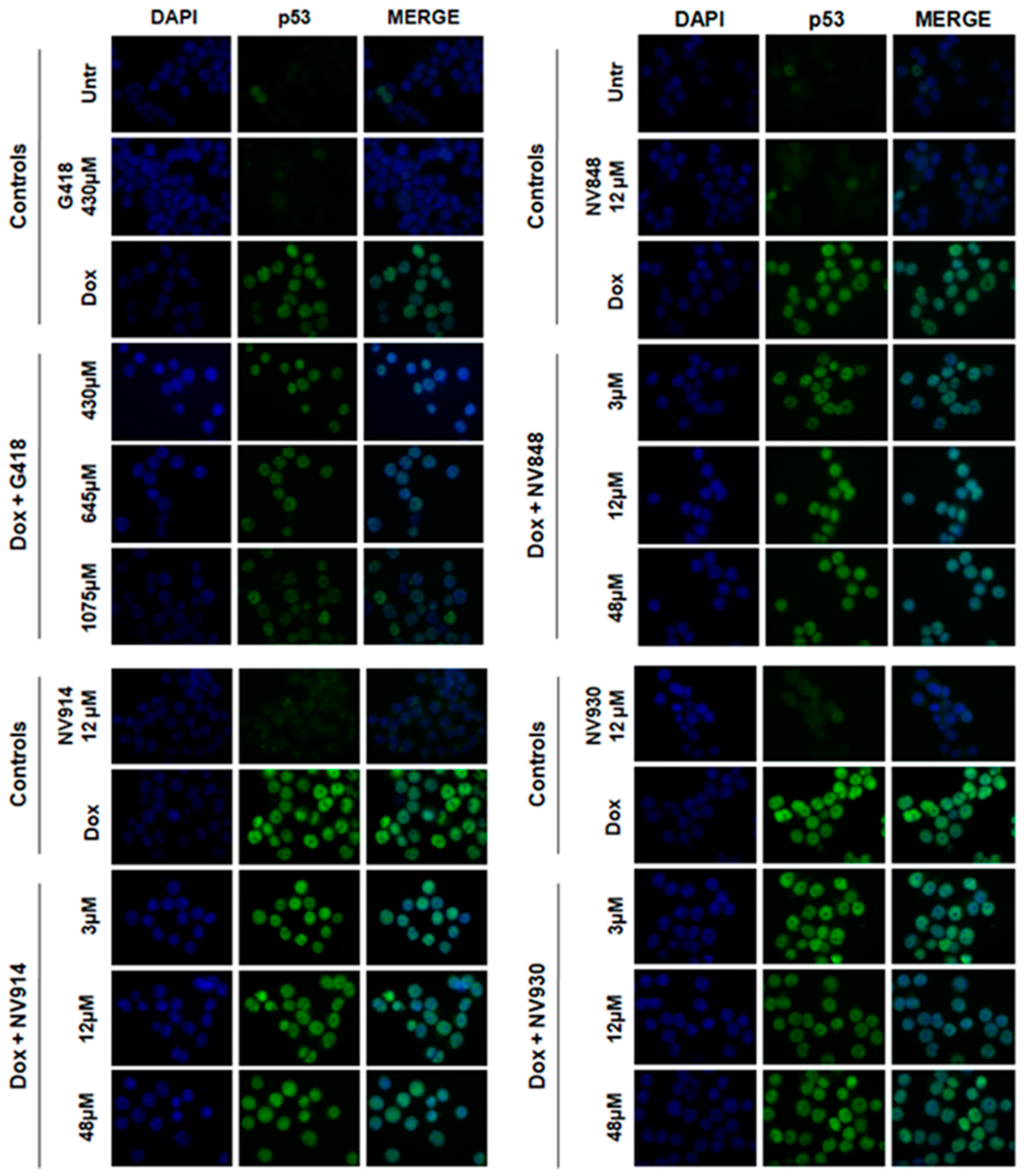
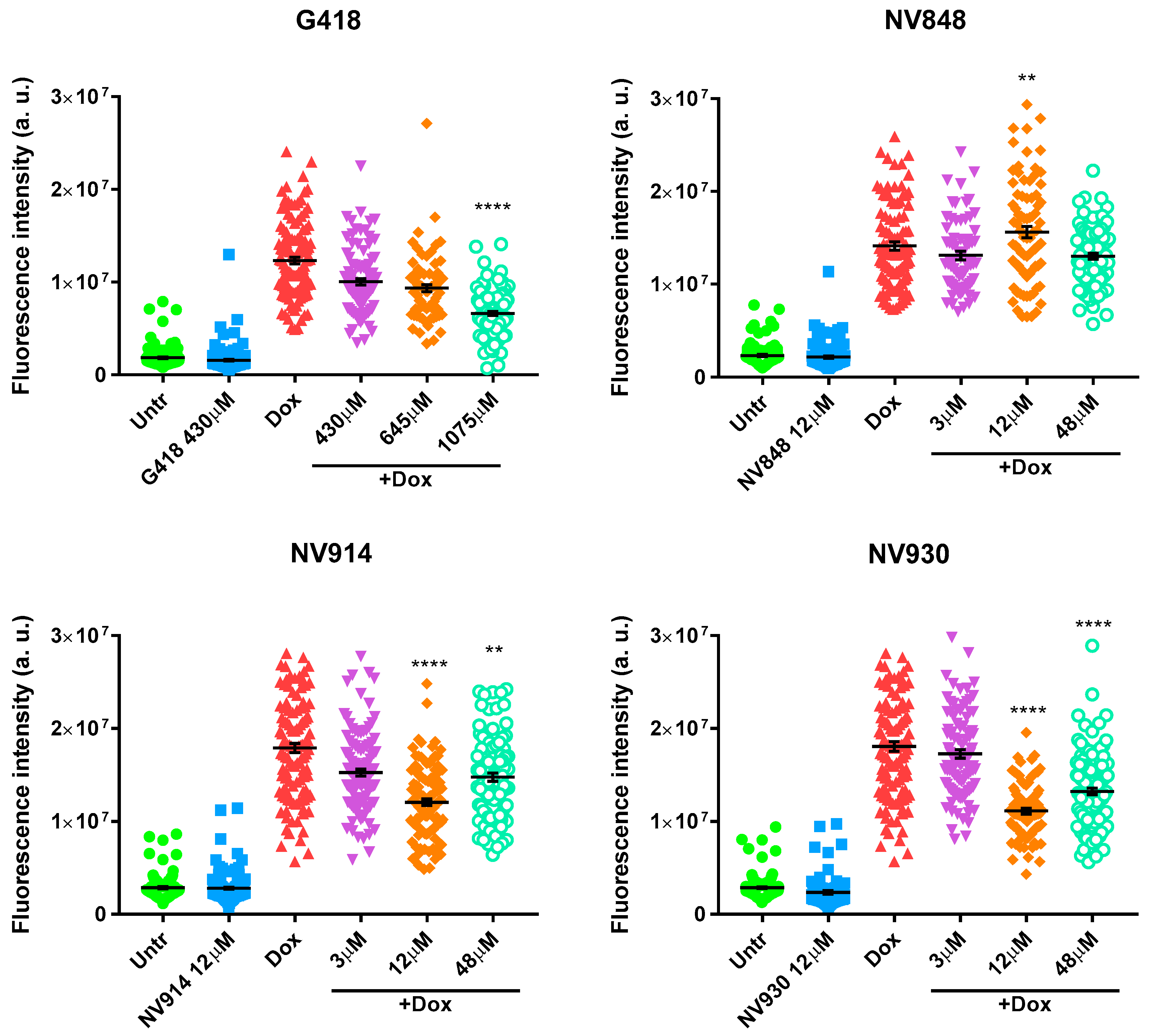
Interestingly, the samples treated with 1075 μM of G418 showed a decreased fluorescence intensity similar respect to the controls without induced DNA damage (Untr and G418 430 μM; Figure 4). This result could be indicative of the reduced ability of the p53 protein to respond against DNA damage as a consequence of aberrant protein production. Cells treated with NV848 and Doxorubicin showed a similar fluorescence intensity compared to the DNA damage positive control samples (Dox).
On the other hand, samples treated with NV914 and NV930 showed a decreased fluorescence signal at 12 μM. However, the p53 fluorescent signal was localized every time in the cell nuclei in all samples treated with Doxorubicin and in combination with TRIDs (G418 and NV molecules).
Since no significant change in p53 nuclear localization was revealed after treatment with Doxorubicin and NV molecules, the transcription levels of p21 (CDKN1A) were analysed in order to confirm the functionality of the p53 protein.
HCT116 cells were treated with Doxorubicin and NV molecules, total mRNA was extracted and analyzed by Real-Time RT-PCR to determine p21 (
CDKN1A) mRNA expression levels (
Figure 5).
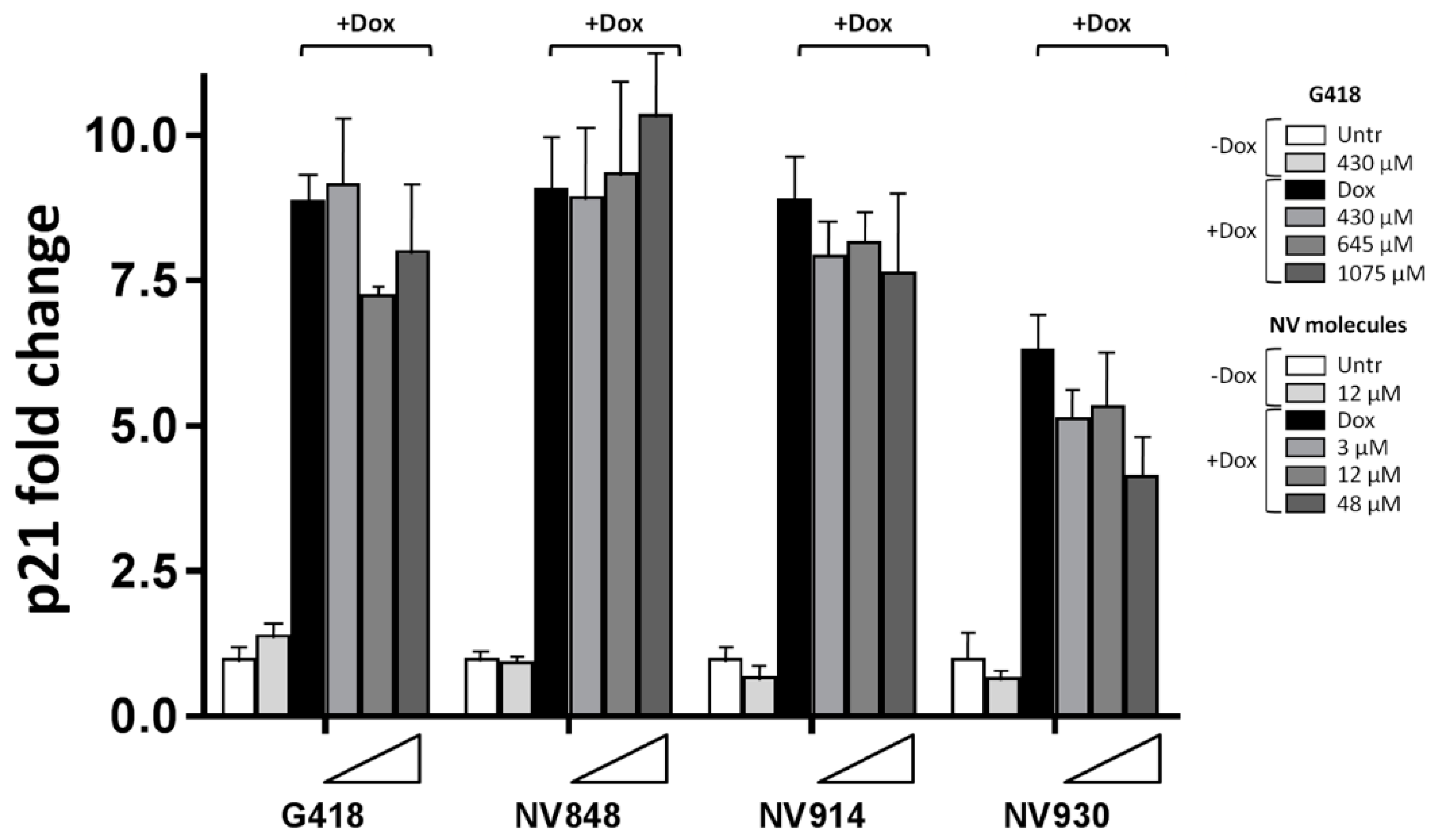
A sensible p21 mRNA reduction (around 16%) was observed in presence of DNA damage and high concentrations of the aminoglycoside G418 at 645 μM (Figure 5).
2.3. Molecular weight analysis of the two housekeeping proteins (Cystatin-C and β-2-microglobulin) after treatment with NV848, NV914, or NV930, to detect the possible NTCs readthrough miscoding
Recently, a new molecule named ELX-02 has been established to be effective to treat nonsense mutations and to be safe. Furthermore, it has been shown that its targets are specifically PTCs. In fact, to demonstrate all the above-mentioned characteristics of this TRID, Crawford et al. have developed a method similar to the one shown previously for the p53 protein [Crawford D.K., et al. 2020].
Precisely, they considered two housekeeping proteins, Cystatin-C and β-2-Microglobulin, that have respectively the molecular weight of 15 kDa and 13 kDa.
The rationale of the used method is the same as described in p53. Indeed, if TRIDs treatment induces readthrough of NTCs in Cystatin-C and β-2 Microglobulin mRNAs, a higher molecular weight can be detected by western blot analysis.
On the basis of the experiment performed by Crawford et al. human bronchial epithelial (16HBE) cells were treated with increasing concentrations of NV848, NV914, and NV930 molecules (
Figure 6) [
24]. The choice of this new cell model system was made on the basis that these cells are a model frequently used in cystic fibrosis research.
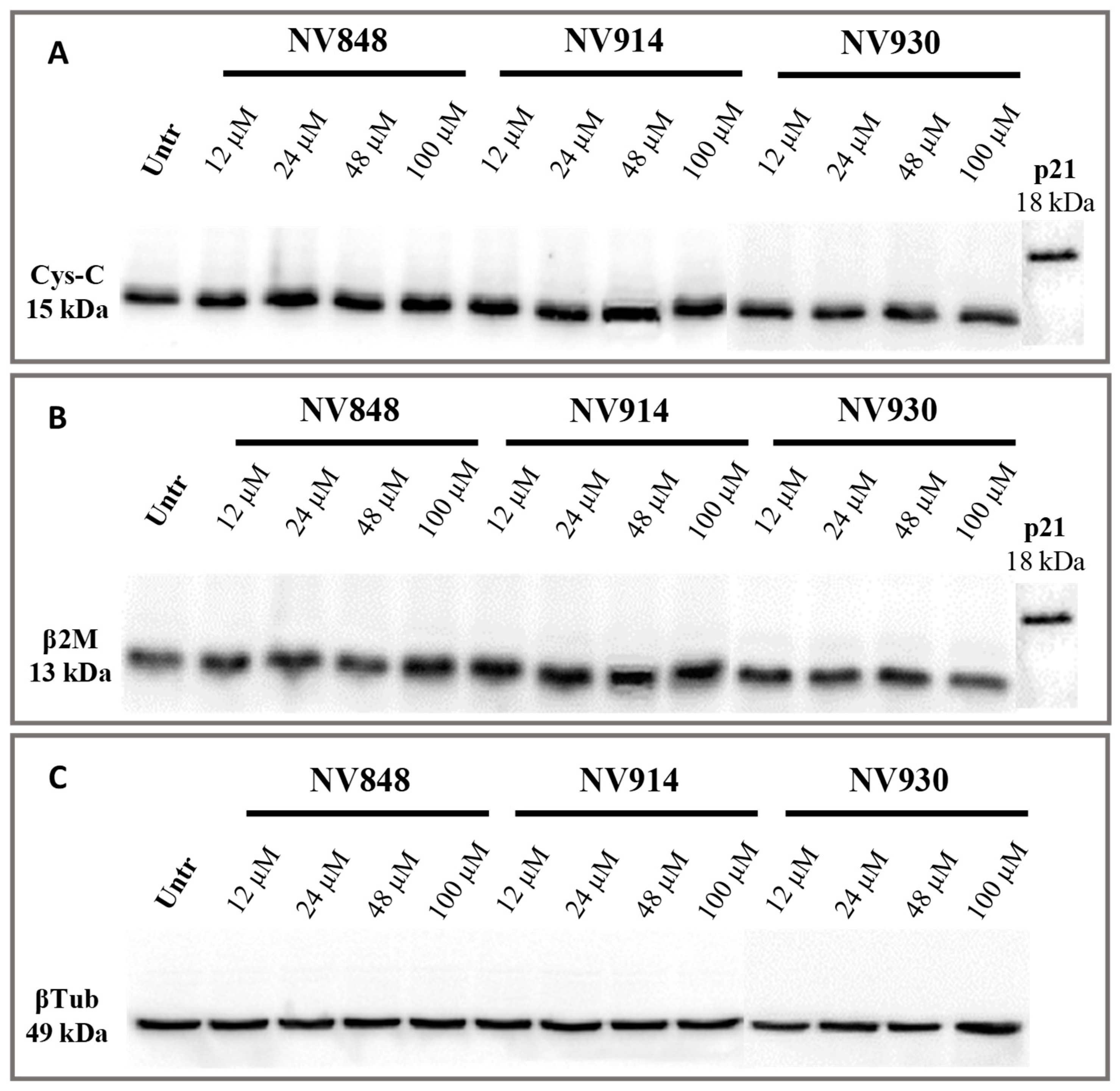
In particular, cells were treated for 24 hours at 12, 24, 48, or 100 µM, and then proteins were extracted and separated by western blot analysis. The p21 protein detection was used as an internal control of expected high molecular weight, evaluating the hypothetical molecular weight of the two proteins if the natural stop codon was translated (
Figure 7).
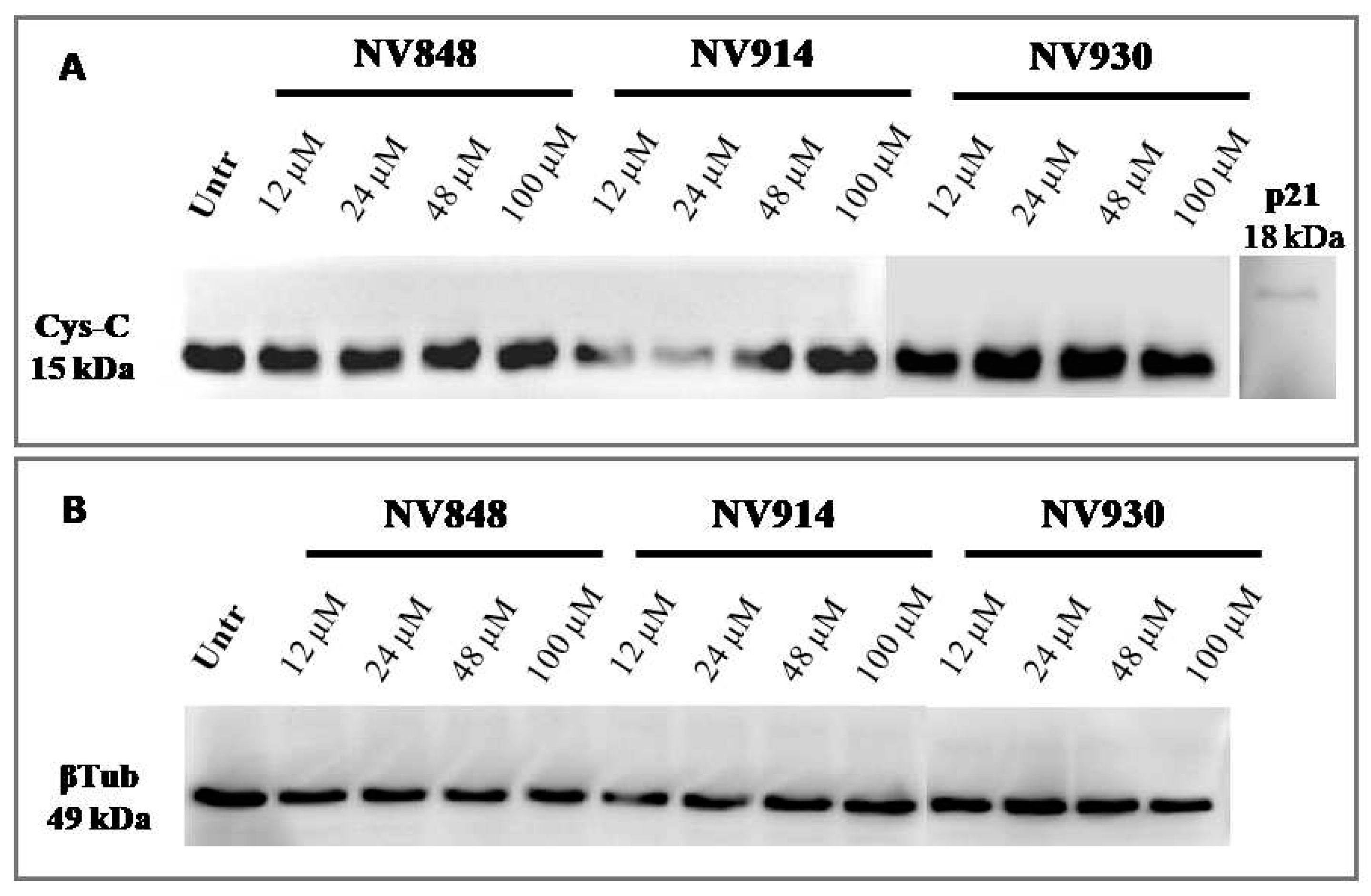
In addition, the same experiment was performed with prolonged (72 hours) treatment with NV molecules to validate the absence of NTCs readthrough (
Figure 8).
No bands relative to the cystatin-C proteins with higher or abnormal molecular weight are visible, confirming the data obtained after 24 hours of treatment with NV molecules.
These experiments confirm that NV molecules do not induce significant readthrough on natural stop codons.
3. Discussion
The three new TRIDs NV848, NV914, and NV930 result to be good compounds in order to induce translational readthrough in cystic fibrosis cellular systems and are well tolerated
in vivo [
21,
22]. According to the experimental evidence, NV molecules restore CFTR protein expression after treatment of
in vitro cystic fibrosis model systems [
21] and Shwachman-Diamond model systems [
11].
Advanced studies about the main off-target effects of readthrough-inducing compounds are proposed in this work. The specificity of NV848, NV914, and NV930 against PTCs and the possibility of NTCs readthrough are respectively fundamental and limiting factors for the TRID’s efficiency.
We used two different approaches to investigate the possible NV molecules NTCs readthrough:
The p53 protein analyses after DNA-damage induction by Doxorubicin, are considered a temporal-induced activation of the p53 protein translation [
29]. According to the experimental hypothesis, if the combined treatment with Doxorubicin and NV848, NV914, or NV930 would have induced p53 NTCs readthrough, we could expect p53 molecular weight alterations. However, the western blot analyses do not show any p53 protein form with increased molecular weight.
In order to exclude the possibility of p53 C-terminal functional domain alterations, the protein localization and transcriptional ability investigation after DNA-damage induction were investigated. No significant changes were detected relative to p53 nuclear localization and CDKN1A (p21) induction.
Interestingly, the fluorescence intensity analyses of the p53 protein after DNA-damage induction and G418 treatment revealed an intensity signal comparable to the controls without doxorubicin treatment in some cells. This data could suggest that G418 NTCs readthrough altered partially the localization of p53 during the DNA-damage response.
Molecular weight studies of the two housekeeping proteins Cystatin-C and β-2-Microglobulin represent known methodologies to analyze NTCs readthrough by TRIDs [
24].
We included for these analyses the sense-codons present between the NTC of the Cystatin-C and β-2-Microglobulin and the next stop codon inside UTR regions.
After 24 hours, and 72 hours treatments with NV848, NV914, or NV930 in a normal cellular system (16HBE) do not induce any molecular weight increase.
These two presented methodologies, especially the studies conducted on p53 protein expression and functionality, are alternative investigations for the evaluation of protein alterations mediated by compounds with the possible ability to induce NTCs readthrough. In these experiments, we analyzed the protein samples and their alterations in contrast to ribosome profiling in which the ribosome position is evaluated [
17]. Moreover, the protein analyses in combination with ribosome profiling could give complementary information regarding possible NTCs readthrough mediated by TRIDs.
4. Conclusions
The data shown in this study demonstrate that NV848, NV914, and NV930 do not induce any p53 molecular weight alterations after the combined treatment with Doxorubicin. In addition, the p53-mediated DNA-damage response (p53 nuclear localization and CDKN1A expression) does not seem to be altered by NV molecules treatments.
The results of the molecular weight analyses of two housekeeping proteins (Cystatin-C and β-2-Microglobulin) after NV848, NV914, or NV930 treatments confirm the absence of elongated protein products in a normal cellular system as 16HBE cells.
In conclusion, our collected evidence about the possible off-target effects of NV848, NV914, and NV930 suggested that these TRIDs do not have appreciable NTCs readthrough effects and in consequence, showed a specific action for the PTCs.
5. Materials and Methods
5.1. Compounds
Compounds were prepared and purchased as reported on
Table 1:
5.2. Cell culture and conditions
All cells were cultured in a humidified incubator with an atmosphere of 5% CO2 at 37°C.
HCT116 cells were cultured in DMEM (Dulbecco’s modified eagle medium, Gibco) supplemented with 10% FBS (fetal bovine serum, Gibco) and 1% Streptomycin and Penicillin antibiotics (Corning). Antibiotics were removed 24 hours before treatments.
Human bronchial epithelial cells (16HBE) were cultured in MEM (minimum essential medium, Gibco) supplemented with 10% FBS (fetal bovine serum, Gibco) and 1% Streptomycin and Penicillin antibiotics (Corning). Before plating, every plate was coated with rat tail collagen (1:100). Antibiotics were removed 24 hours before treatments.
5.3. Western blotting
Proteins samples were extracted from cellular pellets using RIPA buffer (ThermoScientific) and protease cocktail inhibitor (1:100, ThermoScientific) at 4°C. After extraction, proteins were quantified by the Bradford assay method (Coomassie blue dye, Thermo Scientific) and samples were compared respect to a BSA (bovine serum albumin) protein standard curve at known concentrations.
For the analysis of p53, Cystatin-C (Cys-C) and β-2-Microglobulin (β2M), proteins (20 μg) were separated in 12% SDS-PAGE gel and transferred to a PVDF transfer membrane, overnight at 4°C and 12 V (constant voltage). Blotted membranes were blocked with non-fat dry milk 5% (1 hour at room temperature) and after that, membranes were incubated with primary antibody anti-p53 (mouse, p53 DO-1, Santa Cruz Biotechnology, 1:2000), primary antibody anti-Cystatin-C (rabbit, Cell Signaling Biotechnology, 1:1000) or primary antibody anti-β-2-Microglobulin (rabbit, Cell Signaling Biotechnology, 1:1000), overnight at 4°C. Anti-β Tubulin primary antibody (mouse, Sigma Aldrich, 1:5000) was used to detect β-tubulin (βTub) as a loading control to normalize the protein bands.
After three washes (15 min on shaker) with TBS-TweenTM-20 1X (ThermoScientific), membranes were incubated with anti-mouse (Invitrogen, 1:5000) or anti-rabbit (Promega, 1:2500) HRP-conjugated secondary antibodies for 1 hour. After incubation, the membranes were washed three times (15 min on a shaker) with TBS-TweenTM-20 1X (ThermoScientific). The detection of the bands was performed by SuperSignal® West Femto kit (ThermoScientific) and images were acquired by ChemiDoc MP imaging system (Bio-Rad). Gel bands were quantified by ImageJ software.
5.4. Immunofluorescence microscopy
HCT116 cells were grown on round glass coverslips in 12-well plates with 1 ml of medium (without antibiotics). After removing the medium and one wash in DPBS 1X (Dulbecco’s phosphate buffer saline, GIBCO), cells were fixed with cold methanol for 1 min and treated with Triton-X 0,01% for 10 min at room temperature. After washing, fixed cells were blocked in BSA (bovine serum albumin) 0,1% for 1 hour and incubated with primary antibody anti-p53 (mouse, p53 DO-1, Santa Cruz Biotechnology, 1:2000) overnight at 4°C. Coverslips were then incubated with a goat polyclonal to mouse Alexa Fluor-488 (Abcam, 1:1000) secondary antibody for 1 hour at room temperature.
Nuclei were stained with ProLongTM Gold antifade mounting medium with DAPI (Invitrogen). Cells were observed using a Zeiss Axioskop microscope equipped for fluorescence. Fluorescence signals were quantified by ImageJ software.
5.5. Real-time RT-PCR
Total RNA was extracted from the cellular pellet by using the RNeasy® Mini Kit (QIAGEN) according to the manufacturer’s instructions and samples were purified by DNase Max® kit (QIAGEN). RNA was reverse transcribed in a final volume of 50 μl using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). For each sample, 2 μl of cDNA, corresponding to 100 ng of reverse transcribed RNA, was analyzed by real-time RT-PCR (95°C for 15 s, 60°C for 60 s, repeated for 40 cycles) in triplicate, using AB PRISM 7300 instrument (Applied Biosystems). Real-time RT-PCR was performed in a final volume of 25 μl comprising 1X Master Mix SYBR Green (Applied Biosystems) and 1 μM of forward and reverse primers, which are the following:
-p21 Fwd= 5’-CTG GAG ACT CTC AGG GTC GA-3’,
Rev: 5’-CGG ATT AGG GCT TCC TCT TG-3’;
-GAPDH Fwd= 5’-CTC ATG ACC ACA GTC CAT GCC-3’,
Rev= 5’-GCC ATC CAC AGT CTT CTG GGT-3’.
Data were analyzed using triplicates values of Ct (cycle threshold). Levels of RNA were determined by using the SDS software version (Applied Biosystems) according to the 2-ΔΔ Ct method and Ct values were normalized to the internal control GAPDH.
5.6. Statistics
All data are expressed as mean values ± standard error of the mean (SEM). Statistical analysis was performed by Student’s t-test and one-way ANOVA when appropriate by GraphPad Prism software (Inc., La Jolla, CA, USA) version 7.0.0 for Windows. A probability value (p) of less than 0.05 was regarded as significant and indicated in relevant graphs as one symbol (*) for p< 0.05, two symbols (**) for p< 0.01, three symbols (***) for p< 0.001, and four symbols (****) for p< 0.0001.
6. Patents
Pibiri, A. Pace, M. Tutone, L. Lentini, R. Melfi, A. Di Leonardo, Oxadiazole Derivatives For The Treatment Of Genetic Diseases Due To Nonsense Mutations, PCT Int. Appl. (2019), WO 2019/101709 A1 20190531.
Author Contributions
Conceptualization, Ivana Pibiri and Laura Lentini; Data curation, Pietro Salvatore Carollo and Laura Lentini; Formal analysis, Riccardo Perriera, Emanuele Vitale and Davide Ricci; Funding acquisition, Ivana Pibiri and Laura Lentini; Methodology, Riccardo Perriera, Emanuele Vitale, Pietro Salvatore Carollo and Ignazio Fiduccia; Validation, Federica Corrao, Maria Grazia Zizzo and Marco Tutone; Writing – original draft, Riccardo Perriera; Writing – review & editing, Riccardo Perriera, Ivana Pibiri, Pietro Salvatore Carollo, Federica Corrao, Ignazio Fiduccia, Raffaella Melfi, Maria Grazia Zizzo, Andrea Pace and Laura Lentini.
Funding
The research leading to these results has received funding from the European Union - NextGenerationEU through the Italian Ministry of University and Research under PNRR - M4C2-I1.3 Project PE_00000019 "HEAL ITALIA" to Ivana Pibiri and Laura Lentini CUP B73C22001250006 (Dip. STEBICEF-University of Palermo), and by Italian Cystic Fibrosis Research Foundation with FFC#06/2020 grant to Laura Lentini and Ivana Pibiri.
The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palma M, Lejeune F. Deciphering the molecular mechanism of stop codon readthrough. Biol Rev Camb Philos Soc. 2021 Feb;96(1):310-329. Epub 2020 Oct 22. [CrossRef] [PubMed]
- Beißel C, Neumann B, Uhse S, Hampe I, Karki P, Krebber H. Translation termination depends on the sequential ribosomal entry of eRF1 and eRF3. Nucleic Acids Res. 2019 May 21;47(9):4798-4813. PMID: 30873535; PMCID: PMC6511868. [CrossRef]
- Neu-Yilik G, Raimondeau E, Eliseev B, Yeramala L, Amthor B, Deniaud A, Huard K, Kerschgens K, Hentze MW, Schaffitzel C, Kulozik AE. Dual function of UPF3B in early and late translation termination. EMBO J. 2017 Oct 16;36(20):2968-2986. Epub 2017 Sep 12. PMID: 28899899; PMCID: PMC5641913. [CrossRef]
- Yamashita A, Takeuchi O. Translational control of mRNAs by 3'-Untranslated region binding proteins. BMB Rep. 2017 Apr;50(4):194-200. PMID: 28287067; PMCID: PMC5437963. [CrossRef]
- Wangen JR, Green R. Stop codon context influences genome-wide stimulation of termination codon readthrough by aminoglycosides. Elife. 2020 Jan 23;9:e52611. PMID: 31971508; PMCID: PMC7089771. [CrossRef]
- Morais P, Adachi H, Yu YT. Suppression of Nonsense Mutations by New Emerging Technologies. Int J Mol Sci. 2020 Jun 20;21(12):4394. PMID: 32575694; PMCID: PMC7352488. [CrossRef]
- Laselva O, Guerra L, Castellani S, Favia M, Di Gioia S, Conese M. Small-molecule drugs for cystic fibrosis: Where are we now?Pulm Pharmacol Ther. 2022 Feb;72:102098. Epub 2021 Nov 15. [CrossRef] [PubMed]
- Morkous, SS. Treatment with Ataluren for Duchene Muscular Dystrophy. Pediatr Neurol Briefs. 2020 Dec 4;34:12. PMID: 33304086; PMCID: PMC7718099. [CrossRef]
- Sanchez-Alcudia R, Garcia-Hoyos M, Lopez-Martinez MA, Sanchez-Bolivar N, Zurita O, Gimenez A, Villaverde C, Rodrigues-Jacy da Silva L, Corton M, Perez-Carro R, Torriano S, Kalatzis V, Rivolta C, Avila-Fernandez A, Lorda I, Trujillo-Tiebas MJ, Garcia-Sandoval B, Lopez-Molina MI, Blanco-Kelly F, Riveiro-Alvarez R, Ayuso C. A Comprehensive Analysis of Choroideremia: From Genetic Characterization to Clinical Practice. PLoS One. 2016 Apr 12;11(4):e0151943. PMID: 27070432; PMCID: PMC4829155. [CrossRef]
- Lopez-Herrera G, Tampella G, Pan-Hammarström Q, Herholz P, Trujillo-Vargas CM, Phadwal K, Simon AK, Moutschen M, Etzioni A, Mory A, Srugo I, Melamed D, Hultenby K, Liu C, Baronio M, Vitali M, Philippet P, Dideberg V, Aghamohammadi A, Rezaei N, Enright V, Du L, Salzer U, Eibel H, Pfeifer D, Veelken H, Stauss H, Lougaris V, Plebani A, Gertz EM, Schäffer AA, Hammarström L, Grimbacher B. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012 Jun 8;90(6):986-1001. Epub 2012 May 17. PMID: 22608502; PMCID: PMC3370280. [CrossRef]
- Bezzerri V, Lentini L, Api M, Busilacchi EM, Cavalieri V, Pomilio A, Diomede F, Pegoraro A, Cesaro S, Poloni A, Pace A, Trubiani O, Lippi G, Pibiri I, Cipolli M. Novel Translational Read-through-Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome. Biomedicines. 2022 Apr 12;10(4):886. PMID: 35453634; PMCID: PMC9024944. [CrossRef]
- Chu D, Wei L. Nonsynonymous, synonymous and nonsense mutations in human cancer-related genes undergo stronger purifying selections than expectation. BMC Cancer. 2019 Apr 16;19(1):359. PMID: 30991970; PMCID: PMC6469204. [CrossRef]
- Campofelice A, Lentini L, Di Leonardo A, Melfi R, Tutone M, Pace A, Pibiri I. Strategies against Nonsense: Oxadiazoles as Translational Readthrough-Inducing Drugs (TRIDs). Int J Mol Sci. 2019 Jul 6;20(13):3329. PMID: 31284579; PMCID: PMC6651739. [CrossRef]
- 14. Burke JF, Mogg AE. Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucleic Acids Res. 1985 Sep 11;13(17):6265-72, PMID: 2995924; PMCID: PMC321951. [CrossRef]
- Leubitz A, Frydman-Marom A, Sharpe N, van Duzer J, Campbell KCM, Vanhoutte F. Safety, Tolerability, and Pharmacokinetics of Single Ascending Doses of ELX-02, a Potential Treatment for Genetic Disorders Caused by Nonsense Mutations, in Healthy Volunteers. Clin Pharmacol Drug Dev. 2019 Nov;8(8):984-994. Epub 2019 Jan 16. [CrossRef] [PubMed]
- Roy B, Friesen WJ, Tomizawa Y, Leszyk JD, Zhuo J, Johnson B, Dakka J, Trotta CR, Xue X, Mutyam V, Keeling KM, Mobley JA, Rowe SM, Bedwell DM, Welch EM, Jacobson A. Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12508-12513. Epub 2016 Oct 4. PMID: 27702906; PMCID: PMC5098639. [CrossRef]
- Prokhorova I, Altman RB, Djumagulov M, Shrestha JP, Urzhumtsev A, Ferguson A, Chang CT, Yusupov M, Blanchard SC, Yusupova G. Aminoglycoside interactions and impacts on the eukaryotic ribosome. Proc Natl Acad Sci U S A. 2017 Dec 19;114(51):E10899-E10908. Epub 2017 Dec 5. PMID: 29208708; PMCID: PMC5754804. [CrossRef]
- Huang S, Bhattacharya A, Ghelfi MD, Li H, Fritsch C, Chenoweth DM, Goldman YE, Cooperman BS. Ataluren binds to multiple protein synthesis apparatus sites and competitively inhibits release factor-dependent termination. Nat Commun. 2022 May 6;13(1):2413. PMID: 35523781; PMCID: PMC9076611. [CrossRef]
- Lentini, L.; Melfi, R.; Di Leonardo, A.; Spinello, A.; Barone, G.; Pace, A.; Palumbo Piccionello, A.; Pibiri, I. Towards a rationale for the PTC124 (Ataluren) promoted read-through of premature stop codons: A computational approach and GFP- reporter cell-based assay. Mol. Pharm. 2014, 11, 653–664. [Google Scholar] [CrossRef]
- Pibiri I, Melfi R, Tutone M, Di Leonardo A, Pace A, Lentini L. Targeting Nonsense: Optimization of 1,2,4-Oxadiazole TRIDs to Rescue CFTR Expression and Functionality in Cystic Fibrosis Cell Model Systems. Int J Mol Sci. 2020 Sep 3;21(17):6420. PMID: 32899265; PMCID: PMC7504161. [CrossRef]
- Corrao F, Zizzo MG, Tutone M, Melfi R, Fiduccia I, Carollo PS, Leonardo AD, Caldara G, Perriera R, Pace A, Belmonte B, Sammataro S, Pibiri I, Lentini L. Nonsense codons suppression. An acute toxicity study of three optimized TRIDs in murine model, safety and tolerability evaluation. Biomed Pharmacother. 2022 Oct 18;156:113886. Epub ahead of print. [CrossRef] [PubMed]
- Carollo PS, Tutone M, Culletta G, Fiduccia I, Corrao F, Pibiri I, Di Leonardo A, Zizzo MG, Melfi R, Pace A, Almerico AM, Lentini L. Investigating the Inhibition of FTSJ1, a Tryptophan tRNA-Specific 2'-O-Methyltransferase by NV TRIDs, as a Mechanism of Readthrough in Nonsense Mutated CFTR. Int J Mol Sci. 2023 Jun 1;24(11):9609. PMID: 37298560; PMCID: PMC10253411. [CrossRef]
- Crawford DK, Mullenders J, Pott J, Boj SF, Landskroner-Eiger S, Goddeeris MM. Targeting G542X CFTR nonsense alleles with ELX-02 restores CFTR function in human-derived intestinal organoids. J Cyst Fibros. 2021 May;20(3):436-442. Epub 2021 Feb 5. [CrossRef] [PubMed]
- Tanaka T, Watanabe M, Yamashita K. Potential therapeutic targets of TP53 gene in the context of its classically canonical functions and its latest non-canonical functions in human cancer. Oncotarget. 2018 Mar 23;9(22):16234-16247. PMID: 29662640; PMCID: PMC5882331. [CrossRef]
- Gencel-Augusto J, Lozano G. p53 tetramerization: at the center of the dominant-negative effect of mutant p53. GenesDev. 2020 Sep 1;34(17-18):1128-1146. PMID: 32873579; PMCID: PMC7462067. [CrossRef]
- Rizzotto D, Englmaier L, Villunger A. At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity. Int J Mol Sci. 2021 Oct 8;22(19):10883. PMID: 34639222; PMCID: PMC8509445. [CrossRef]
- 27. Georgakilas AG, Martin OA, Bonner WM. p21: A Two-Faced Genome Guardian. Trends Mol Med. 2017 Apr;23(4):310-319, Epub 2017 Mar 7. [CrossRef] [PubMed]
- Grover R, Candeias MM, Fåhraeus R, Das S. p53 and little brother p53/47: linking IRES activities with protein functions. Oncogene. 2009 Jul 30;28(30):2766-72. Epub 2009 Jun 1. [CrossRef] [PubMed]
- 29. Hu J, Cao J, Topatana W, Juengpanich S, Li S, Zhang B, Shen J, Cai L, Cai X, Chen M. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J HematolOncol. 2021 Sep 28;14(1):157, PMID: 34583722; PMCID: PMC8480024. [CrossRef]
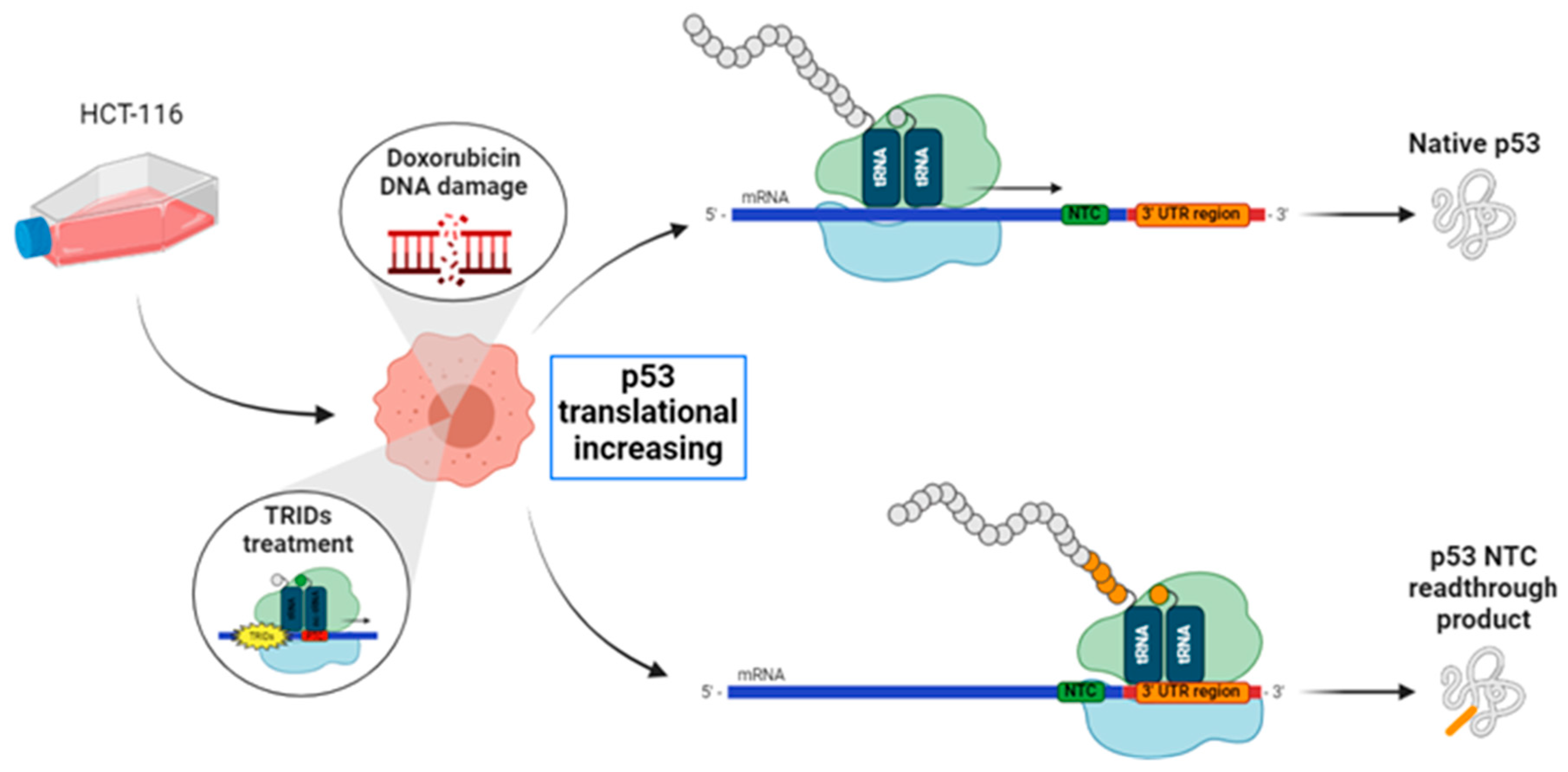
Figure 1.
Experimental scheme. Doxorubicin-DNA damage induces p53 expression in HCT116 cells. NV molecules could generate translational readthrough of the natural mRNA termination codons (NTCs), even on p53 NTC. This miscoding error could result in alterations in protein expression, nuclear localization, and/or DNA/protein interaction.
Figure 1.
Experimental scheme. Doxorubicin-DNA damage induces p53 expression in HCT116 cells. NV molecules could generate translational readthrough of the natural mRNA termination codons (NTCs), even on p53 NTC. This miscoding error could result in alterations in protein expression, nuclear localization, and/or DNA/protein interaction.
Figure 2.
(A) Western blot analysis to detect p53 in HCT116 cells 24 hours after treatment with G418 (430 μM, 645 μM, and 1075 μM) or NV molecules (3 μM, 12 μM, and 48 μM) at increasing concentrations, in combination with Doxorubicin (Dox, 0,2 μg/ml). Only compound indicated the treatment without DNA damage induction (-Dox). NV or G418 samples indicated the treatment with different TRID concentrations (triangles) in combination with Doxorubicin (+Dox). Beta-Tubulin (β-Tub) was used as a loading control. (B) Western blot band quantification was performed by ImageJ software.
Figure 2.
(A) Western blot analysis to detect p53 in HCT116 cells 24 hours after treatment with G418 (430 μM, 645 μM, and 1075 μM) or NV molecules (3 μM, 12 μM, and 48 μM) at increasing concentrations, in combination with Doxorubicin (Dox, 0,2 μg/ml). Only compound indicated the treatment without DNA damage induction (-Dox). NV or G418 samples indicated the treatment with different TRID concentrations (triangles) in combination with Doxorubicin (+Dox). Beta-Tubulin (β-Tub) was used as a loading control. (B) Western blot band quantification was performed by ImageJ software.
Figure 3.
Immunofluorescence analysis of HCT116 to visualize the localization of p53 (green) 24 hours after DNA damage induction by Doxorubicin (0,2 μg/ml) and after 24 hours of treatment with G418, NV848, NV914, or NV930 at the indicated concentrations. p53 protein (green) was revealed by a specific primary antibody and a fluorochrome-conjugated secondary antibody (Alexa-488). Nuclei (blue) were stained with DAPI (4′,6-diamidino-2-phenylindole). G418 was used as a positive control of NTCs readthrough.
Figure 3.
Immunofluorescence analysis of HCT116 to visualize the localization of p53 (green) 24 hours after DNA damage induction by Doxorubicin (0,2 μg/ml) and after 24 hours of treatment with G418, NV848, NV914, or NV930 at the indicated concentrations. p53 protein (green) was revealed by a specific primary antibody and a fluorochrome-conjugated secondary antibody (Alexa-488). Nuclei (blue) were stained with DAPI (4′,6-diamidino-2-phenylindole). G418 was used as a positive control of NTCs readthrough.
Figure 4.
Quantification of the p53 signal relative to the immunofluorescence analysis. The single shapes indicate the amount of fluorescence of a single cell (arbitrary units). Fluorescence intensity quantification was performed by ImageJ software. Samples were analyzed compared to DNA damage controls only treated with Doxorubicin (Dox). Data were analyzed by GraphPad Prism 7 software. A probability value (p): two symbols (**) for p < 0.01, four symbols (****) for p < 0.0001.
Figure 4.
Quantification of the p53 signal relative to the immunofluorescence analysis. The single shapes indicate the amount of fluorescence of a single cell (arbitrary units). Fluorescence intensity quantification was performed by ImageJ software. Samples were analyzed compared to DNA damage controls only treated with Doxorubicin (Dox). Data were analyzed by GraphPad Prism 7 software. A probability value (p): two symbols (**) for p < 0.01, four symbols (****) for p < 0.0001.
Figure 5.
Real-Time RT-PCR of the p21 (CDKN1A) mRNA in HCT116 cells untreated (Untr) or treated, with G418 (430 μM, 645 μM, 1075 μM) or NV molecules (3 μM, 12 μM, 48 μM) after DNA damage induction by Doxorubicin (Dox, 0,2μg/ml). Only compound indicated the treatment without DNA damage induction (-Dox). NV or G418 samples indicated the treatment with different TRID concentrations (triangles) in combination with Doxorubicin (+Dox). Analyses were performed 24 hours after treatment conditions.
Figure 5.
Real-Time RT-PCR of the p21 (CDKN1A) mRNA in HCT116 cells untreated (Untr) or treated, with G418 (430 μM, 645 μM, 1075 μM) or NV molecules (3 μM, 12 μM, 48 μM) after DNA damage induction by Doxorubicin (Dox, 0,2μg/ml). Only compound indicated the treatment without DNA damage induction (-Dox). NV or G418 samples indicated the treatment with different TRID concentrations (triangles) in combination with Doxorubicin (+Dox). Analyses were performed 24 hours after treatment conditions.
Figure 6.
Western blot analysis in 16HBE cells untreated (Untr; negative control) or treated after 24 hours with NV848, NV914, and NV930 at indicated concentrations. Images show the molecular weights of two housekeeping proteins, Cystatin-C (Cys-C; A) and β-2-microglobulin (β2M; B). p21 protein was used as an internal control of expected high molecular weight. β-tubulin (βTub) was included as a loading control (C). Images were derived from different membranes, and they were placed according to molecular weight markers migration.
Figure 6.
Western blot analysis in 16HBE cells untreated (Untr; negative control) or treated after 24 hours with NV848, NV914, and NV930 at indicated concentrations. Images show the molecular weights of two housekeeping proteins, Cystatin-C (Cys-C; A) and β-2-microglobulin (β2M; B). p21 protein was used as an internal control of expected high molecular weight. β-tubulin (βTub) was included as a loading control (C). Images were derived from different membranes, and they were placed according to molecular weight markers migration.
Figure 7.
In silico translation of Cystatin-C (Cys-C) and β-2-microglobulin (β2M) mRNAs. Open riding frames are highlighted in red.
Figure 7.
In silico translation of Cystatin-C (Cys-C) and β-2-microglobulin (β2M) mRNAs. Open riding frames are highlighted in red.
Figure 8.
Western blot analysis in 16HBE cells untreated (Untr; negative control) or treated after 72 hours with NV848, NV914, and NV930 at indicated concentrations. Every 24 hours the treatments were refreshed. Images show the molecular weights of cystatin-C (Cys-C; A). p21 protein was used as an internal control of expected high molecular weight. Β-tubulin (βTub) was included as a loading control (B). Images were derived from different membranes and they were placed according to molecular weight markers migration.
Figure 8.
Western blot analysis in 16HBE cells untreated (Untr; negative control) or treated after 72 hours with NV848, NV914, and NV930 at indicated concentrations. Every 24 hours the treatments were refreshed. Images show the molecular weights of cystatin-C (Cys-C; A). p21 protein was used as an internal control of expected high molecular weight. Β-tubulin (βTub) was included as a loading control (B). Images were derived from different membranes and they were placed according to molecular weight markers migration.
Table 1.
Compounds were dissolved in DMSO (dimethyl sulfoxide, Sigma Aldrich) or sterile water (H
2O) at the indicated stock concentrations. All compound stocks were stored at -20°C. ( *) TRIDs are produced at the STEBICEF Department, University of Palermo, Palermo, according to the procedure reported in Pibiri I., et al. 2020 [
20].
Table 1.
Compounds were dissolved in DMSO (dimethyl sulfoxide, Sigma Aldrich) or sterile water (H
2O) at the indicated stock concentrations. All compound stocks were stored at -20°C. ( *) TRIDs are produced at the STEBICEF Department, University of Palermo, Palermo, according to the procedure reported in Pibiri I., et al. 2020 [
20].
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).