Submitted:
10 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Sample collection and DNA extraction
2.2. Sequencing, assembling and analysis
2.3. Phylogenetic analysis
3. Results and discussion
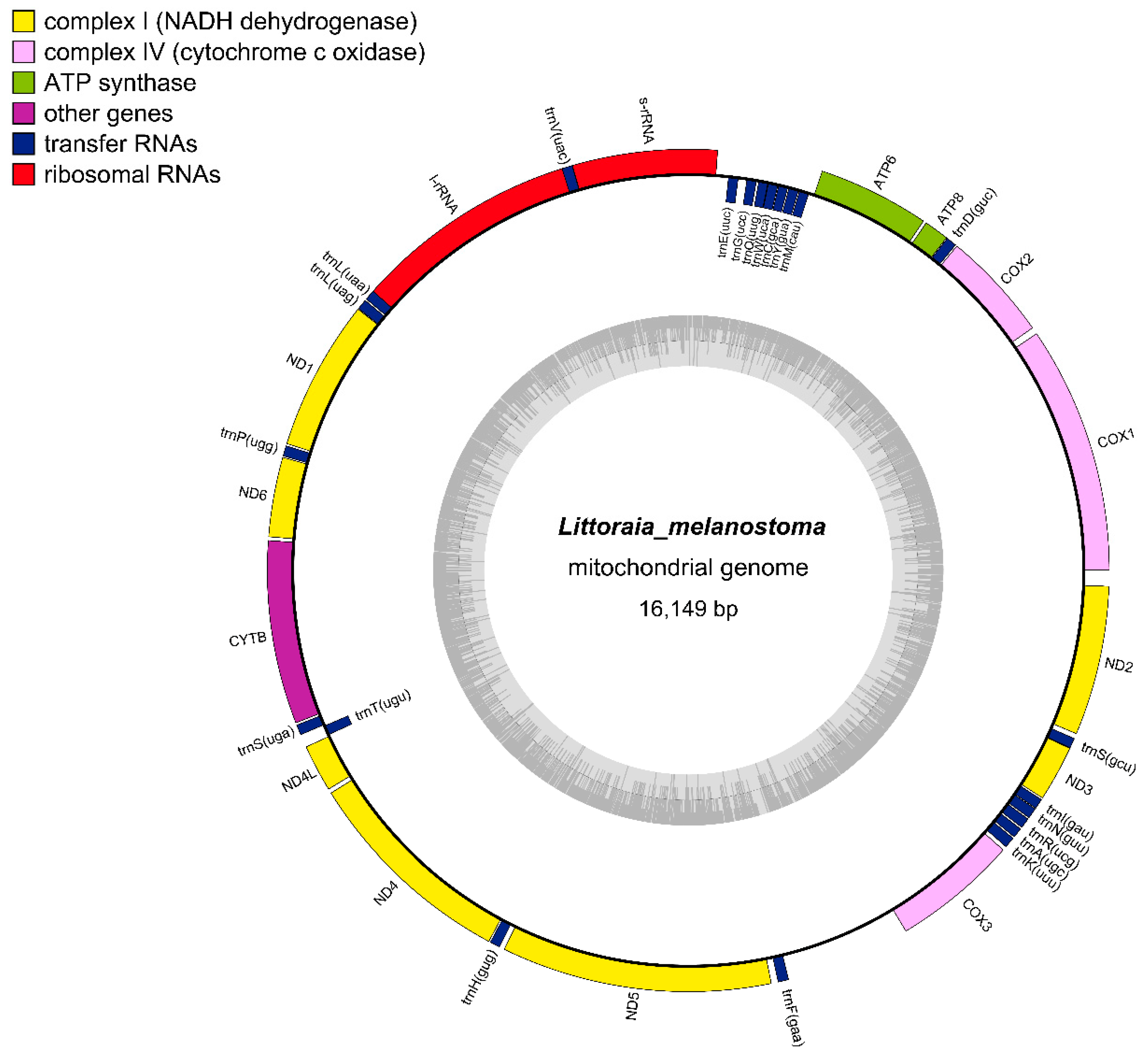
3.1. Genome structure and organization
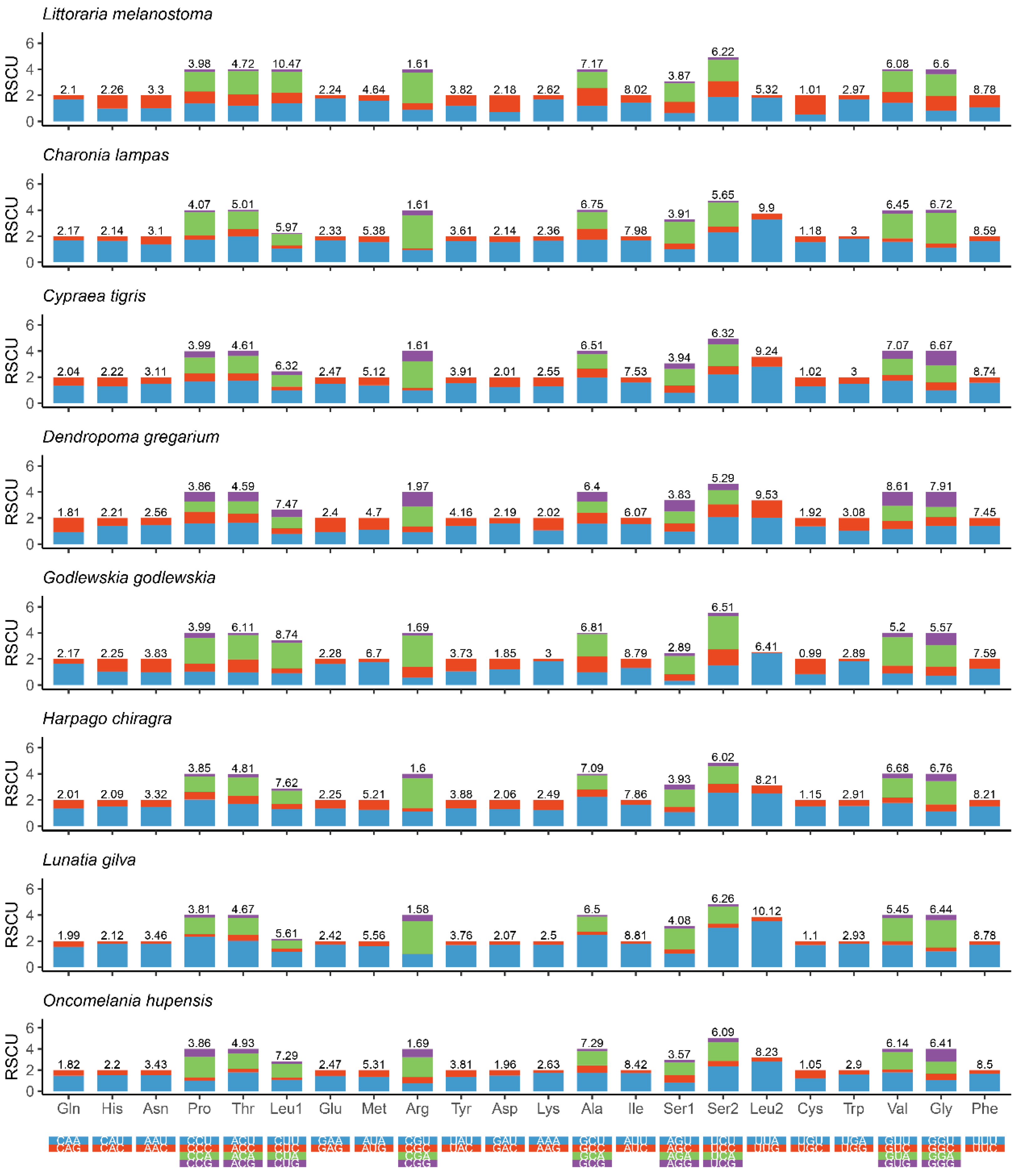
3.2. PCGs and codon usage
3.3. Ribosomal and transfer RNA genes
3.4. Intergenic spaces and overlapping sequences
3.5. Control regions
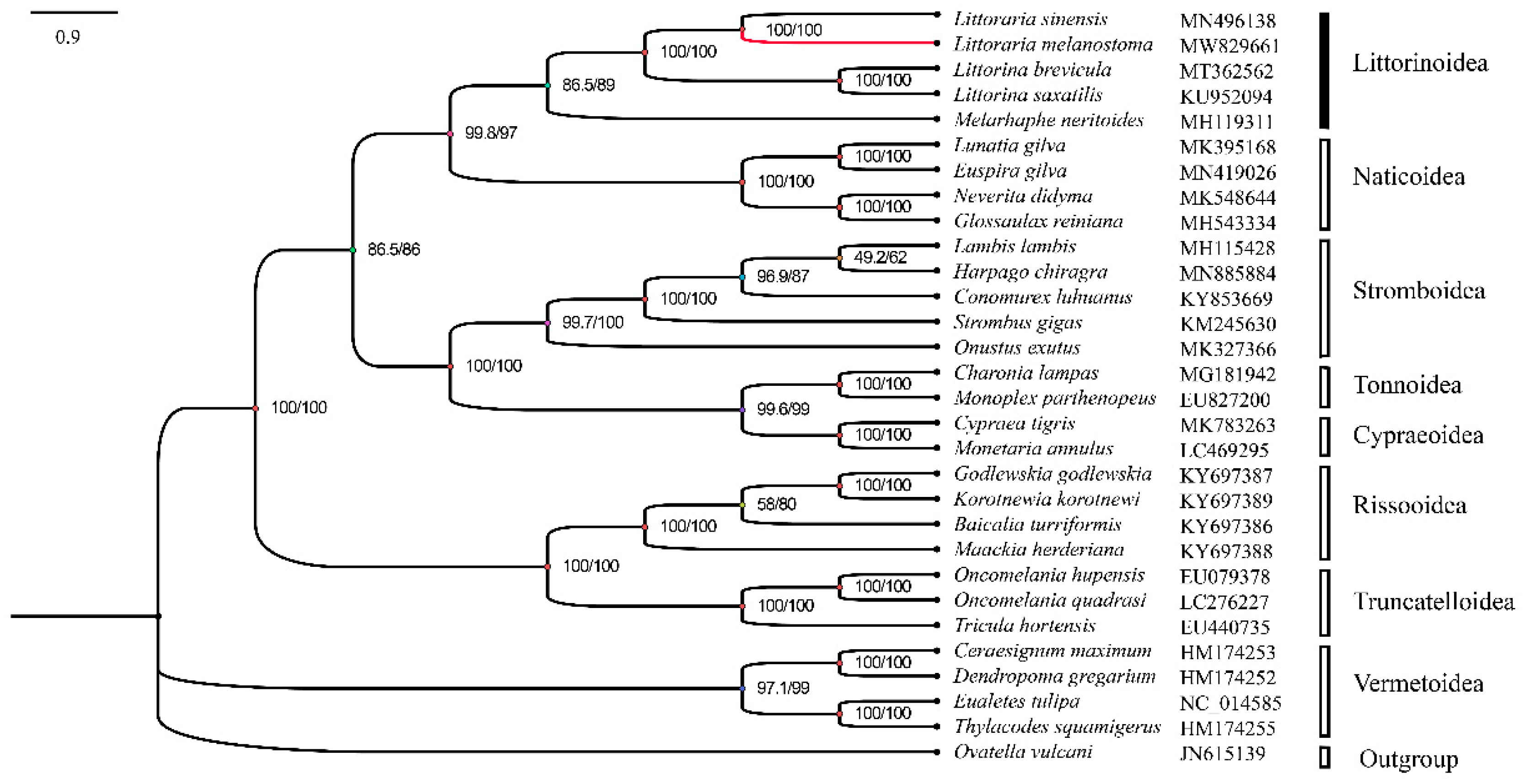
3.6. Phylogenetic analyses
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torres, P.; Alfiado, A.; Glassom, D.; Jiddawi, N.; Macia, A.; Reid, D.G.; Paula, J. Species composition, comparative size and abundance of the genus Littoraria (Gastropoda: Littorinidae) from different mangrove strata along the East African coast. Hydrobiologia 2008, 614, 339–351. [Google Scholar] [CrossRef]
- Berry, A.; Chew, E. Reproductive systems and cyclic release of eggs in Littorina melanostoma from Malayan mangrove swamps (Mollusca: Gastropoda). Journal of Zoology 1973, 171, 333–344. [Google Scholar] [CrossRef]
- Lee, O.H.; Williams, G.A.; Hyde, K.D. The diets of Littoraria ardouiniana and L. melanostoma in Hong Kong mangroves. Journal of the Marine Biological Association of the United Kingdom 2001, 81, 967–973. [Google Scholar] [CrossRef]
- Hamilton, P. Intertidal distribution and long-term movements of Littorina irrorata (Mollusca: Gastropoda). Marine Biology 1978, 46, 49–58. [Google Scholar] [CrossRef]
- Alfaro, A.C. Diet of Littoraria scabra, while vertically migrating on mangrove trees: Gut content, fatty acid, and stable isotope analyses. Estuarine, Coastal and Shelf Science 2008, 79, 718–726. [Google Scholar] [CrossRef]
- Little, C.; Stirling, P. Activation of a mangrove snail, Littorina scabra scabra (L.)(Gastropoda: Prosobranchia). Marine and Freshwater Research 1984, 35, 607–610. [Google Scholar] [CrossRef]
- Cook, L. Systematic effects on morph frequency in the polymorphic mangrove snail Littoraria pallescens. Heredity 1990, 65, 423–427. [Google Scholar] [CrossRef]
- Lee, O.H.; Williams, G.A. Spatial distribution patterns of Littoraria species in Hong Kong mangroves. Hydrobiologia 2002, 481, 137–145. [Google Scholar] [CrossRef]
- Blanco, J.F.; Cantera, J.R. The vertical distribution of mangrove gastropods and environmental factors relative to tide level at Buenaventura Bay, Pacific Coast of Colombia. Bulletin of marine science 1999, 65, 617–630. [Google Scholar]
- Reid, D.G. Habitat and zonation patterns of Littoraria species (Gastropoda: Littorinidae) in Indo-Pacific mangrove forests. Biological Journal of the Linnean Society 1985, 26, 39–68. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, B.; Liao, J.J.; Chen, G.L.; Huang, Y.; Chen, G.C. Composition and distribution pattern of Littorinid snails in young rehabilitated mangroves. Chinese Journal of Ecology 2017, 36, 460. [Google Scholar]
- Reid, D.G.; Dyal, P.; Williams, S.T. A global molecular phylogeny of 147 periwinkle species (Gastropoda, Littorininae). Zoologica Scripta 2012, 41, 125–136. [Google Scholar] [CrossRef]
- Lai, T.H; He, B.Y. Studies on the macrobenthos species diversity for Guangxi mangrove areas. Guangxi Sciences 1998, 5, 166–172. [Google Scholar]
- Chen, G.; Ye, Y. Restoration of Aegiceras corniculatum mangroves in Jiulongjiang Estuary changed macro-benthic faunal community. Ecological Engineering 2011, 37, 224–228. [Google Scholar] [CrossRef]
- Bai, J.; Guo, Y.; Feng, J.; Ye, Y.; Li, J.; Yan, C.; Mao, S. The complete mitochondrial genome and phylogenetic analysis of Littorina brevicula (Gastropoda, Littorinidea). Mitochondrial DNA Part B 2020, 5, 2280–2281. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.G; Dyal, P.; Williams, S. Global diversification of mangrove fauna: a molecular phylogeny of Littoraria (Gastropoda: Littorinidae). Molecular phylogenetics and evolution 2010, 55, 185–201. [Google Scholar] [CrossRef]
- Li, M. Y.; Li, Y. L.; Xing, T. F.; Liu, J. X. First mitochondrial genome of a periwinkle from the genus Littoraria: Littoraria sinensis. Mitochondrial DNA Part B 2019, 4, 4124–4125. [Google Scholar] [CrossRef]
- Irwin, A.R.; Strong, E.E.; Kano, Y.; Harper, E.M.; Williams, S.T. Eight new mitogenomes clarify the phylogenetic relationships of Stromboidea within the caenogastropod phylogenetic framework. Molecular Phylogenetics and Evolution 2021, 158, 107081. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. 1994, 3, 294–299. [Google Scholar]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic acids research 2017, 45, e18–e18. [Google Scholar]
- Meng, G.; Li, Y.; Yang, C.; Liu, S. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic acids research 2019, 47, e63–e63. [Google Scholar] [CrossRef] [PubMed]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: improved de novo metazoan mitochondrial genome annotation. Molecular phylogenetics and evolution 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular biology and evolution 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Kahlau, S.; Bock, R. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic acids research 2013, 41, W575–W581. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular ecology resources 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular biology and evolution 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular biology and evolution 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Marques, J.P.; Sotelo, G.; Larsson, T.; Johannesson, K.; Panova, M.; Faria, R. Comparative mitogenomic analysis of three species of periwinkles: Littorina fabalis, L. obtusata and L. saxatilis. Marine genomics 2017, 32, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Song, W.T.; Gao, X.G.; Li, Y.F.; Liu, W.D.; Liu, Y.; He, C.B. Comparison of mitochondrial genomes of bivalves. Hereditas 2009, 31, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.J.; Boore, J.; Brown, W. A novel mitochondrial genome organization for the blue mussel, Mytilus edulis. Genetics 1992, 131, 397–412. [Google Scholar] [CrossRef] [PubMed]

| Taxonomy | Species | bp | Accession number |
| Littorinoidea | Littoraria melanostoma | 16,149 | NC064398 |
| Littorina brevicula | 16,356 | MT362562 | |
| Littorina saxatilis | 16,887 | KU952094 | |
| Littoraria sinensis | 16,420 | MN496138 | |
| Melarhaphe neritoides | 15,676 | MH119311 | |
| Stromboidea | Harpago chiragra | 16,404 | MN885884 |
| Lambis lambis | 15,481 | MH115428 | |
| Conomurex luhuanus | 15,799 | KY853669 | |
| Strombus gigas | 15,461 | KM245630 | |
| Cypraeoidea | Cypraea tigris | 16,177 | MK783263 |
| Monetaria annulus | 16,087 | LC469295 | |
| Naticoidea | Lunatia gilva | 16,139 | MK395168 |
| Euspira gilva | 16,119 | MN419026 | |
| Neverita didyma | 15,252 | MK548644 | |
| Glossaulax reiniana | 15,254 | MH543334 | |
| Xenophoroidea | Onustus exutus | 16,043 | MK327366 |
| Vermetoidea | Dendropoma gregarium | 15,641 | HM174252 |
| Ceraesignum maximum | 15,578 | HM174253 | |
| Thylacodes squamigerus | 15,544 | HM174255 | |
| Eualetes tulipa | 15,078 | NC_014585 | |
| Tonnoidea | Charonia lampas | 15,405 | MG181942 |
| Monoplex parthenopeus | 15,270 | EU827200 | |
| Truncatelloidea | Oncomelania hupensis | 15,191 | EU079378 |
| Oncomelania quadrasi | 15,184 | LC276227 | |
| Tricula hortensis | 15,179 | EU440735 | |
| Rissooidea | Godlewskia godlewskia | 15,224 | KY697387 |
| Baicalia turriformis | 15,127 | KY697386 | |
| Korotnewia korotnewi | 15,171 | KY697389 | |
| Maackia herderiana | 15,154 | KY697388 | |
| Outgroup | Ovatella vulcani | 14,274 | JN615139 |
| Littoraria melanostoma | ||||||||
| A % | T % | G % | C % | AT % | GC % | AT-Skew | GC-Skew | |
| Mitogenome | 29.79 | 34.37 | 14.66 | 21.18 | 64.16 | 35.84 | -0.071 | -0.182 |
| All PCGS | 27.77 | 34.62 | 15.15 | 22.46 | 62.39 | 37.61 | -0.110 | -0.194 |
| COX1 | 26.43 | 34.18 | 17.71 | 21.68 | 60.61 | 39.39 | -0.128 | -0.101 |
| COX2 | 28.97 | 30.57 | 17.76 | 22.71 | 59.53 | 40.47 | -0.027 | -0.122 |
| ATP8 | 32.70 | 36.48 | 10.69 | 20.13 | 69.18 | 30.82 | -0.055 | -0.306 |
| ATP6 | 26.44 | 36.06 | 13.22 | 24.28 | 62.50 | 37.50 | -0.154 | -0.295 |
| ND1 | 26.09 | 34.50 | 15.23 | 24.17 | 60.60 | 39.40 | -0.139 | -0.227 |
| ND6 | 27.31 | 34.94 | 13.05 | 24.70 | 62.25 | 37.75 | -0.123 | -0.309 |
| CYTB | 25.70 | 33.07 | 15.09 | 26.14 | 58.77 | 41.23 | -0.125 | -0.268 |
| ND4L | 25.70 | 33.07 | 15.09 | 26.14 | 68.01 | 31.99 | -0.125 | -0.268 |
| ND4 | 28.56 | 37.21 | 13.57 | 20.66 | 65.77 | 34.23 | -0.132 | -0.207 |
| ND5 | 30.15 | 33.13 | 13.35 | 23.36 | 63.29 | 36.71 | -0.047 | -0.273 |
| COX3 | 25.90 | 32.18 | 19.36 | 22.56 | 58.08 | 41.92 | -0.108 | -0.076 |
| ND3 | 27.35 | 39.32 | 15.10 | 18.23 | 66.67 | 33.33 | -0.180 | -0.094 |
| ND2 | 28.89 | 37.38 | 14.39 | 19.33 | 66.27 | 33.73 | -0.128 | -0.147 |
| Position | Size(bp) | Intergenic nucleotides | Codon | Strand | ||||
| gene | From | To | Start | Stop | ||||
| Littoraia melanostoma | ||||||||
| 1 | COX1 | 1 | 1536 | 1536 | ATG | TAA | H | |
| 2 | COX2 | 1575 | 2261 | 687 | 38 | ATG | TAA | H |
| 3 | trnD(guc) | 2268 | 2336 | 69 | 6 | H | ||
| 4 | ATP8 | 2338 | 2496 | 159 | 1 | ATG | TAA | H |
| 5 | ATP6 | 2512 | 3207 | 696 | 15 | ATG | TAA | H |
| 6 | trnM(cau) | 3240 | 3306 | 67 | 32 | L | ||
| 7 | trnY(gua) | 3310 | 3377 | 68 | 3 | L | ||
| 8 | trnC(gca) | 3382 | 3446 | 65 | 4 | L | ||
| 9 | trnW(uca) | 3448 | 3514 | 67 | 1 | L | ||
| 10 | trnQ(uug) | 3514 | 3578 | 65 | -1 | L | ||
| 11 | trnG(ucc) | 3590 | 3656 | 67 | 11 | L | ||
| 12 | trnE(uuc) | 3710 | 3777 | 68 | 53 | L | ||
| 13 | s-rRNA | 3856 | 4756 | 901 | 78 | H | ||
| 14 | trnV(uac) | 4754 | 4822 | 69 | -3 | H | ||
| 15 | l-rRNA | 4801 | 6219 | 1419 | -22 | H | ||
| 16 | trnL(uaa) | 6210 | 6277 | 68 | -10 | H | ||
| 17 | trnL(uag) | 6284 | 6352 | 69 | 6 | H | ||
| 18 | ND1 | 6353 | 7291 | 939 | 0 | ATG | TAA | H |
| 19 | trnP(ugg) | 7301 | 7369 | 69 | 9 | H | ||
| 20 | ND6 | 7374 | 7871 | 498 | 4 | ATG | TAA | H |
| 21 | CYTB | 7890 | 9029 | 1140 | 18 | ATG | TAA | H |
| 22 | trnS(uga) | 9040 | 9107 | 68 | 10 | H | ||
| 23 | trnT(ugu) | 9111 | 9178 | 68 | 3 | L | ||
| 24 | ND4L | 9185 | 9481 | 297 | 6 | ATG | TAG | H |
| 25 | ND4 | 9505 | 10845 | 1341 | 23 | ATT | TAA | H |
| 26 | trnH(gug) | 10852 | 10918 | 67 | 6 | H | ||
| 27 | ND5 | 10947 | 12624 | 1678 | 28 | ATT | CTT | H |
| 28 | trnF(gaa) | 12663 | 12732 | 70 | 38 | H | ||
| CR | 12733 | 13505 | 773 | 0 | ||||
| 29 | COX3 | 13506 | 14285 | 780 | 773 | ATG | TAA | H |
| 30 | trnK(uuu) | 14307 | 14378 | 72 | 21 | H | ||
| 31 | trnA(ugc) | 14385 | 14451 | 67 | 6 | H | ||
| 32 | trnR(ucg) | 14459 | 14527 | 69 | 7 | H | ||
| 33 | trnN(guu) | 14533 | 14602 | 70 | 5 | H | ||
| 34 | trnI(gau) | 14604 | 14671 | 68 | 1 | H | ||
| 35 | ND3 | 14679 | 15029 | 351 | 7 | ATA | TAA | H |
| 36 | trnS(gcu) | 15029 | 15095 | 67 | -1 | H | ||
| 37 | ND2 | 15123 | 16053 | 931 | 27 | ATG | AAT | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





