1. Introduction
The interest in the importance and role of growth factors in schizophrenia has been steadily deepening. Their properties - influence on neuronal and glial growth, differentiation, migration, functioning and survival - seems to have scientific and clinical potential. It is known that changes in the cytoarchitecture of the nervous tissue, as well as cellular dysfunctions, e.g., changes in metabolism, morphology, interconnections, and receptors are of great importance for the onset and progression of schizophrenia and negative symptom in particular [
1,
2].
Epidermal growth factor (EGF) is widely expressed in the brain (e.g., in the prefrontal cortex, hippocampus, putamen, and amygdala) and can be found in neurons and astrocytic glia. Binding to its receptor (EGRF) located on the cell surface, EGF activates the insintric protein-tyrosine kinase activity of EGRF, which begins the cascade of signal transduction (an increase of cellular calcium levels, synthesis of protein, glycolysis, and gene expression) leading to cell proliferation [
3]. The literature on the relationship between EGF and schizophrenia is sparse, but authors indicate that EGF is associated with deficits in sensorimotor gating, latent inhibition, working memory, impaired social interaction and overall schizophrenia severity [
4,
5,
6]. What is particularly interesting, perinatal exposure to EGF in rodents results in an irreversible hyperdopaminergic state [
7]. For this to be the case, we should have more data on the involvement of these factors in the pathophysiological processes relevant to the various symptom dimensions of schizophrenia and other psychiatric disorders, meanwhile, as we noted above, current data on this subject are scarce. The results of several published studies do not provide clear conclusions. In older studies, serum EGF levels are elevated in schizophrenia [
5,
8,
9], whereas more recent studies indicate a reduction in EGF levels in first episode [
10] or in both first episode and chronic schizophrenia [
11]. It is worth noting, that EGF is utilized in clinical practice, for example in Parkinson's disease or diabetic foot ulcers, then might be a very attractive new therapeutic option in various neuropsychiatric conditions [
12,
13,
14,
15].
Excitatory glutamatergic and inhibitory GABA-ergic systems are involved in pathogenesis of negative, positive, cognitive, and affective symptoms in schizophrenia. In the study we assess effects of sarcosine (N-methylglycine), glycine transporter type I (GlyT1) inhibitor, located on astroglia [
16,
17]. Sarcosine causes increase of levels of co-agonist glycine around NMDA receptor (N-methyl-D-aspartate) and thus enhances activity of NMDA receptor especially on GABAergic inhibitory interneurons which function in schizophrenia is disturbed [
18,
19]. Previous sarcosine trials in schizophrenia showed its beneficial impact on negative symptoms severity and showed better results in this area than conventional therapy [
20,
21]. While negative and cognitive symptoms are closely linked with level of patients functioning and quality of life, we need options to help patients recover as much as possible. Data analysis in our population (the PULSAR study - Polish Sarcosine Study in Schizophrenia) indicates significant sarcosine-induced changes in different aspects of symptomatology (negative symptoms, general psychopathology and final score in Positive and Negative Syndrome Scale - PANSS) with simultaneous positive changes in neuronal or glial parameters in left dorsolateral prefrontal cortex, left hippocampus and left frontal white matter in magnetic resonance spectroscopy when compared to placebo [
22,
23,
24,
25].
Given our special interest in negative symptoms during the design of the study, we placed particular emphasis on examining the possible relationship between the severity of these symptoms (avolition, anhedonia, antisociality, flat affect, alogia) and the concentration of EGF in the blood. If EGF is involved in the mechanism of action of glutamatergic modulators (directly or indirectly), this could amend the picture of the pathomechanisms underlying schizophrenia and open new therapeutic possibilities. Both first- and second-generation antipsychotics are the recognized first-line treatment for patients with schizophrenia, but their efficacy is mainly limited to positive symptoms (delusions, hallucinations and thought disorders), while negative symptoms do not respond well to currently used drugs or forms of therapy. Stopping psychotic symptoms was previously considered a success, but there is a great need to improve other dimensions of psychosis as well (primary negative, cognitive, and affective symptoms) and thus overall quality of life and functioning.
Our aim is to determine whether EGF levels in peripheral blood are associated with the severity of schizophrenia symptoms and whether modulation of glutamatergic transmission have impact on symptomatology and EGF concentrations.
Based on our results and the available literature, sarcosine reduces the severity of negative symptoms. Therefore, as primary outcomes, we want to observe whether EGF concentrations change during the study and whether they are correlated with PANSS negative subscale scores during the 6-month administration of the amino acid. We preliminarily assume that they should increase during sarcosine administration.
As secondary outcomes, we also want to evaluate possible correlations of EGF concentrations with the severity of positive dimensions, overall psychopathology, total PANSS score, and affective symptoms assessed with the Calgary Depression Scale for Schizophrenia (CDSS).
Previous studies have considered the severity of all schizophrenia symptoms and have not answered the question of whether EGF levels are related to the severity of positive, negative, and affective dimensions. To provide more accurate measurements, biochemical measurements and detailed anthropometric measurements were combined with body composition analysis, determined by bioelectrical impedance analysis (BIA), which provides accurate measurements of body fat and lean mass.
To the best of our knowledge, this is the first study to investigate a similar combination of parameters in subjects with schizophrenia.
2. Methods
2.1. Participants and Study Design
All participants were European Caucasian patients aged 18-60 years diagnosed with paranoid schizophrenia (295.30, according to DSM-IV, F20.0 according to ICD-10). Recruitment was conducted in psychiatric outpatient clinics. Patients in stable physical, neurological and endocrine state with laboratory values in normal range (blood routine tests, biochemical tests incl. TSH, liver and kidney parameters, ECG) were eligible to enrollment. Prior to all procedures, after precise information about aims and methods of the study, all patients signed the informed consent form, then they underwent a structured interview in accordance with criteria of schizophrenia defined in ICD-10 and DSM-IV. For the study we enrolled patients in stable mental state with predominant negative symptoms (scored a minimum of 3 points in each item in PANSS negative symptoms subscale) and without signs of psychotic exacerbation (maximum of 3 points in each item in positive symptoms subscale). To evaluate the influence of sarcosine as precisely as possible we required stable treatment dosage for at least 3 months prior to the enrollment. Patients with exacerbated psychosis, declaring suicide risk, or taking clozapine (combining glutamatergic modulators such as glycine or sarcosine with clozapine was not effective or led to mental worsening of mental state by increasing severity of positive symptoms), did not enter the trial [
26,
27].
The PULSAR project was designed as a 6-months parallel group, randomized, double blind and placebo-controlled study. Participants were assigned in random order to sarcosine (n = 30) or placebo (n = 30) groups in a 1:1 ratio using the protocol obtained from a dedicated website (
http://www.randomization.com), accessed on October 6, 2012. The randomization process was performed by a person not involved in the examination of the patients. The randomization code was not broken until all patients had completed the study. We added both sarcosine and placebo to ongoing and stable antipsychotic treatment. All patients received plastic capsules containing 2 grams of amino acid or microcrystalline cellulose as placebo. Then participants were trained on how to open the container, dissolve the contents in water, and drink the dissolved powder from one capsule once a day in the morning.
Protocol of the study was approved by the Bioethics Committee of the Medical University of Łódź (permission number and date: RNN/153/08/KE, 15 July 2008). There was no financial industry involvement. For further details of Polish Sarcosine Study in Schizophrenia (PULSAR) please see ClinicalTrials.gov site, study identifier: NCT01503359.
2.2. Measurements
All measurements were performed at least twice – on the visit before beginning sarcosine or placebo use and after 6 months – after last dose of investigated treatment. In 14 subjects from the placebo group and in 15 subjects from the sarcosine group additional EGF assessments were performed after 6 weeks.
2.2.1. Clinical Assessments
Raters assessed symptoms of schizophrenia with PANSS scale (total score and its positive, negative, and general symptomatology subscales), severity of depressive symptomatology with the Calgary Depression Scale for Schizophrenia (CDSS). For each patient all ratings with all scales were conducted constantly by one trained rater. CDSS score over 6 points classified patients as depressed.
2.2.2. Blood
Blood samples were collected and then immediately transferred to our central laboratory between 7 am and 8 am, after at least 8 h fasting. Blood was centrifuged at 3500 rpm at 22°C for 10 minutes. Serum levels of EGF was measured according to the kit's instructions using a commercially available high sensitivity ELISA plates (Diaclone, Besancon Cedex, France), intra-assay CV < 4.5%, inter-assay CV < 9.2%. Before ELISA assessments serum samples were stored at -80 °C. The optical density of wells was measured in Central Scientific Laboratory, Medical University of Łódź, with an automated microplate reader (Emax; Molecular Devices, USA). Serum glucose and lipids levels were analyzed by Dirui CS-400 equipment (Dirui, China).
2.2.3. Anthropometry
The patients' height was measured to the nearest 0.5 cm with a wall-mounted height measure. Weight was measured (and recorded to the nearest 0.5 kg) in light clothing without shoes with a spring balance placed on a horizontal firm surface. Body mass index (BMI) was calculated as body weight [kg]/height [m]2. We also measured waist, abdominal, and hip circumferences with a non-stretchable measuring tape.
2.2.4. Body Composition
We used Maltron BF-906 Body Fat Analyzer (Maltron, UK), a single-frequency bioelectrical impedance analyzer (BIA) to measure total body fat and lean body mass and determine resistance and reactance at 50 Hz. The BIA assessments were made by trained staff. In brief, BIA determines the electrical impedance, or opposition to the flow of an electric current through body tissues enabling estimations of total body water, and then (with Maltron proprietary equations) fat-free body mass and body fat.
2.2.5. Determination of Metabolic Syndrome and Other Measurements
We used International Diabetes Foundation (IDF) criteria for metabolic syndrome and abdominal obesity. Impaired fasting glucose was defined as fasting plasma glucose ≥ 100 mg/dL. We diagnosed dyslipidemia when triglycerides (TGA) levels were ≥ 150 mg/dL and/or total cholesterol (TC) ≥ 200 mg/dL and/or reduced HDL cholesterol level < 40 mg/dL for men and < 50 mg/dL for women and/or raised LDL cholesterol level ≥ 135 mg/dL. Waist-to-hip ratio (WHR) was calculated as waist circumference divided by hip circumference. Fat mass index (FMI) was estimated as total body fat divided by the height in meters squared (kg/m2).
2.3. Statistical Analysis
The randomization code was not broken until we collected all data in the study. We performed statistical procedures using R 4.1.3 software (R Foundation for Statistical Computing, Vienna, Austria). We calculated simple descriptive statistics (means and standard deviations) for all continuous variables. For discrete variables, we gave the number of patients and percentages. Shapiro-Wilk test was used to check the normality of distribution, then we applied T-test for variables with normal distribution, while in other situations, we employed the Wilcoxon rank-sum test. For analysis of the difference between proportions, we used Fisher’s exact. Spearman's rank correlation coefficient was employed to describe associations between EGF serum level and clinical symptoms. The significance level was set at p < 0.05 (two-sided).
3. Results
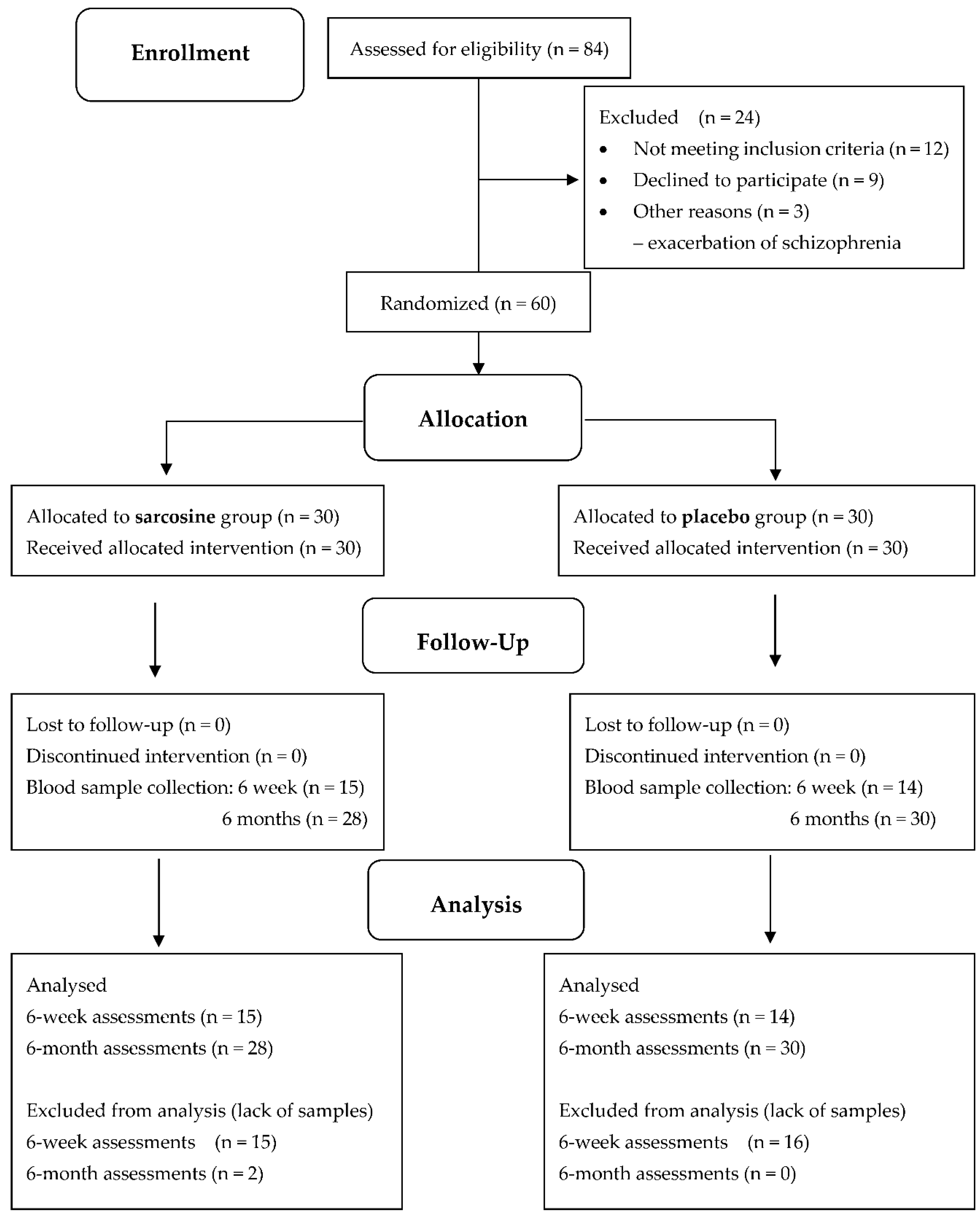
Sixty subjects were randomized to receive either sarcosine (n =30) or a matching placebo (n = 30) and completed a 6-month, double-blind, placebo-controlled study. Two patients in the sarcosine group did not complete the blood tests for EGF. Therefore, 58 patients with diagnosis of schizophrenia, including 28 patients taking sarcosine, were subjected to the analysis (
Figure 1).
Both groups were very comparable in terms of demographic, clinical, therapeutic, anthropometric, and metabolic parameters (
Table 1).
All participants remained on their stable antipsychotic treatment for the duration of the study. Clinically, there were no differences between initial PANSS scores in both subgroups. However, patients in the sarcosine group achieved significant improvement in PANSS scores at the end of the study compared to the placebo group (reduction in negative subscore: -6.7 ± 3.5 vs. -0.8 ± 1.7, p < 0.001; reduction in general subscore: -5.7 ± 5.7 vs. -1.4 ± 5.6, p=0.006; reduction in the total score: -13.2± 8.3 vs. -2.3 ± 8.7, p<0.001). There was no significant change in PANSS positive score (-0.6 ± 2.7 vs. -0.1 ± 3.4, p = 0.4) and in CDSS score between both groups (sarcosine: -0.2 ± 1.8, placebo: 0.9 ± 3.3, p = 0.2). At the end of the study, there were no significant changes in any of the analyzed cardiometabolic and body composition parameters in both groups.
Figure 2 shows EGF levels in the study groups at three times points (initially, after six weeks, and after six months). At the beginning of the study, EGF level was comparable in both study groups (sarcosine: 235.1 ± 165.0 pg/mL, placebo: 315.5 ± 226.4 pg/mL, p = 0.2). There was a significant difference in EGF levels (sarcosine: 200.7 ± 117.9 pg/mL, placebo: 116.1 ± 72.6 pg/mL, p = 0.03) after six weeks, but not at six months (sarcosine: 288.1 ± 231.3 pg/mL, placebo: 182.1 ± 135.8 pg/mL, p = 0.1).
Next, we analyzed changes in EGF levels between the study groups at each of the three-time points, see
Figure 3.
These changes were found to be non-significant for initial, 6-week, and 6-month time points. There was no significant change in EGF after six weeks or six months in the sarcosine group. Interestingly, there was a significant and progressing decrease between baseline EGF levels after six weeks (p = 0.02) and after six months (p = 0.01) in the placebo group. Initial EGF levels were correlated with levels at six weeks (rho = 0.38, p = 0.04), change after six weeks (rho = -0.56, p = 0.001), and after six months (rho = -0.59, p < 0.001). There were no correlations between initial EGF level and PANSS or CDSS scores/subscores including negative symptoms PANSS subscale. Also, there were no correlations between changes (initial vs. after six months) in EGF level and changes in PANSS or CDSS scores/subscores. Initial EGF levels were not correlated with the previous clinical course of schizophrenia and its treatment (treatment duration, number of hospitalizations, antipsychotics dose), nor with cardiometabolic parameters. Also, there was no difference in initial or change (initial vs. six months) levels of EGF between patients whose scores in PANSS total, P/N/G subscores, and CDSS score improved or not.
4. Discussion
This is the first randomized placebo-controlled study examining the impact of sarcosine on serum EGF concentration in schizophrenia. The primary study objectives were 1) to assess changes of EGF serum levels during antipsychotic treatment augmentation with sarcosine and 2) test correlations of EGF concentrations with PANSS negative subscale scores in study group.
- (1)
EGF levels were stable throughout the project in the sarcosine group, while there was a significant decrease in EGF levels in the control group despite no differences between the two groups at baseline.
- (2)
there were no correlations between initial EGF level and negative symptoms PANSS subscale scores, there were also no correlations between changes (initial vs. after six months) in EGF level and changes in negative symptoms PANSS subscale scores.
The secondary study objective was to evaluate whether EGF levels correlate with severity of positive symptoms, overall psychopathology, total PANSS score, and affective symptoms of schizophrenia and its changes.
- (1)
there were no correlations between initial EGF level and PANSS total/positive/general psychopathology and CDSS scores. Also, there were no correlations between changes (initial vs. after six months) in EGF level and changes in PANSS total/positive/general psychopathology or CDSS scores.
Our study outcomes showed, that EGF does not seem to have a direct relationship with the symptoms' severity and improvement in PANSS (total and subscale scores) and CDSS scales during sarcosine use. We conclude that the clinical effect achieved during sarcosine treatment is not mediated by EGF, at least not as indicated by changes in peripheral blood concentrations. The clinical difference is not due to mental deterioration in the control group, which could link negative symptomatology to EGF levels. We assume that sarcosine and potentiation of NMDA receptor function also have no direct effect on changes in peripheral EGF concentrations.
However, observations on EGF concentrations in the sarcosine and placebo groups may indicate that the neuroprotective effect of sarcosine (by normalizing glutamatergic transmission and reducing its excitotoxic effects) may induce EGF production or inhibit the decline in EGF concentrations that progressed in the placebo group. EGF is probably the only growth factor that also has neuroprotective effects (including protecting dopaminergic neurons from the excitotoxic effects of elevated glutamate concentrations [
28,
29] and inducing reparative processes [
30,
31]. In brain injury, EGF promotes the production of the glycine transporter GlyT-1 to prevent high concentrations of glutamate and subsequent cell death via an excitotoxicity mechanism [
32]. The bioavailability of EGF in the brain is ensured by production in glial cells and neurons and by uptake from the peripheral circulation [
3], indicating that peripheral concentrations may be somewhat related to concentrations within the brain, although this has yet to be described in detail. It may, of course, affect the interpretation of our results, especially since EGF is not specific to neural tissue and is found, for example, in the skin, salivary glands, and platelets [
33,
34].
Our study has its limitations: relatively small study groups including only patients with predominant negative symptoms, heterogeneous antipsychotic treatment across the study groups. On the other hand, in our both study groups anthropometric and metabolic parameters were comparable, limiting potential impact of these co-variables on EGF levels. The exception to this is tobacco smoking (the percentage of smokers was significantly higher in the placebo group), and this may be of potential importance, as smoking is known to reduce EGF expression. However, the above findings relate to salivary glands, but there are no data on levels in the brain [
35].
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, D.S.; methodology, D.S., A.W.; software, A.W.; validation, A.P., D.S. and A.W.; formal analysis, A.P., D.S. and A.W.; investigation, A.P. and D.S.; resources, D.S.; data curation, D.S., A.W.; writing—original draft preparation, A.P, B.K, D.S. and A.W.; writing—review and editing, A.P., D.S.; visualization, A.W.; supervision, D.S.; project administration, D.S.; funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are (partially) supported by Polish Ministry of Science and Higher Education (grant N402 268836).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the Medical University of Łódź (permission number and date: RNN/153/08/KE, 15 July 2008).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study before all procedures.
Data Availability Statement
Raw data will be provided upon request.
Acknowledgments
We would like to thank all patients participating in the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galvez-Contreras, A.Y.; Campos-Ordonez, T.; Lopez-Virgen, V.; Gomez-Plascencia, J.; Ramos-Zuniga, R.; Gonzalez-Perez, O. Growth Factors as Clinical Biomarkers of Prognosis and Diagnosis in Psychiatric Disorders. Cytokine Growth Factor. Rev. 2016, 32, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, M.G.; Scassellati, C.; Cattane, N.; Riva, M.A.; Cattaneo, A. Neurotrophic Factors, Childhood Trauma and Psychiatric Disorders: A Systematic Review of Genetic, Biochemical, Cognitive and Imaging Studies to Identify Potential Biomarkers. J. Affect. Disord. 2022, 308, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Plata-Salamán, C.R. Epidermal Growth Factor and the Nervous System. Peptides 1991, 12, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Oyagi, A.; Hara, H. Essential Roles of Heparin-Binding Epidermal Growth Factor-like Growth Factor in the Brain. CNS Neurosci. Ther. 2012, 18, 803–810. [Google Scholar] [CrossRef]
- Futamura, T.; Toyooka, K.; Iritani, S.; Niizato, K.; Nakamura, R.; Tsuchiya, K.; Someya, T.; Kakita, A.; Takahashi, H.; Nawa, H. Abnormal Expression of Epidermal Growth Factor and Its Receptor in the Forebrain and Serum of Schizophrenic Patients. Mol. Psychiatry 2002, 7, 673–682. [Google Scholar] [CrossRef]
- Sotoyama, H.; Namba, H.; Chiken, S.; Nambu, A.; Nawa, H. Exposure to the Cytokine EGF Leads to Abnormal Hyperactivity of Pallidal GABA Neurons: Implications for Schizophrenia and Its Modeling. J. Neurochem. 2013, 126, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Nawa, H.; Sotoyama, H.; Iwakura, Y.; Takei, N.; Namba, H. Neuropathologic Implication of Peripheral Neuregulin-1 and EGF Signals in Dopaminergic Dysfunction and Behavioral Deficits Relevant to Schizophrenia: Their Target Cells and Time Window. Biomed. Res. Int. 2014, 2014, 697935. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, W.; Chen, K.Y.; Zhao, Y.; Ye, F.; Tang, X.; Du, X. Decreased Serum EGF in First-Episode and Chronic Schizophrenia Patients: Negative Correlation with Psychopathology. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, W.; Chen, K.; Zhao, Y.; Ye, F.; Tang, X.; Du, X. Serum Epidermal Growth Factor Is Low in Schizophrenia and Not Affected by Antipsychotics Alone or Combined With Electroconvulsive Therapy. Front. Psychiatry 2020, 11, 104. [Google Scholar] [CrossRef]
- Koido, K.; Innos, J.; Haring, L.; Zilmer, M.; Ottas, A.; Vasar, E. Taurine and Epidermal Growth Factor Belong to the Signature of First-Episode Psychosis. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Haring, L.; Koido, K.; Vasar, V.; Leping, V.; Zilmer, K.; Zilmer, M.; Vasar, E. Antipsychotic Treatment Reduces Psychotic Symptoms and Markers of Low-Grade Inflammation in First Episode Psychosis Patients, but Increases Their Body Mass Index. Schizophr. Res. 2015, 169, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.; Dagher, A.; Hong, M.; Hebb, A.; Gaudet, P.; Law, A.; Weerasinghe, S.; King, D.; Desrosiers, J.; Darvesh, S.; et al. Enhancement of Survival of Stored Dopaminergic Cells and Promotion of Graft Survival by Exposure of Human Fetal Nigral Tissue to Glial Cell Line- Derived Neurotrophic Factor in Patients with Parkinson’s Disease. J. Neurosurg. 2000, 92. [Google Scholar] [CrossRef] [PubMed]
- Buytaert-Hoefen, K.A.; Alvarez, E.; Freed, C.R. Generation of Tyrosine Hydroxylase Positive Neurons from Human Embryonic Stem Cells after Coculture with Cellular Substrates and Exposure to GDNF. Stem Cells 2004, 22. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, J.; Fernández, J.I.; López, E.; López, P.A. ; del Río, A.; Valenzuela, C.; Baldomero, J.; Muzio, V.; Raíces, M.; Silva, R.; et al. Heberprot-P: A Novel Product for Treating Advanced Diabetic Foot Ulcer. MEDICC Rev. 2013, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Geng, Z.; Ma, K.; Sun, X.; Fu, X. Efficacy of Topical Recombinant Human Epidermal Growth Factor for Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. Int. J. Low. Extrem. Wounds 2016, 15, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.C.; Luo, D.Z.; Gau, S.S.; Chang, C.Y.; Lai, W.S. Directly and Indirectly Targeting the Glycine Modulatory Site to Modulate NMDA Receptor Function to Address Unmet Medical Needs of Patients With Schizophrenia. Front. Psychiatry 2021, 12, 742058. [Google Scholar] [CrossRef] [PubMed]
- Zakowicz, P.; Pawlak, J. Glycine Transporters in Schizophrenia. A New Hope or Informational Noise? Psychiatr. Pol. 2022, 56, 217–228. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Tang, K. Interneuron Development and Dysfunction. FEBS J. 2022, 289, 2318–2336. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The Origin of NMDA Receptor Hypofunction in Schizophrenia. Pharmacol. Ther. 2020, 205, 107426. [Google Scholar] [CrossRef]
- Marchi, M.; Galli, G.; Magarini, F.M.; Mattei, G.; Galeazzi, G.M. Sarcosine as an Add-on Treatment to Antipsychotic Medication for People with Schizophrenia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Expert. Opin. Drug Metab. Toxicol. 2021, 17. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, C.H.; Liu, C.Y.; Chen, S.J.; Lane, H.Y. Efficacy and Cognitive Effect of Sarcosine (N-Methylglycine) in Patients with Schizophrenia: A Systematic Review and Meta-Analysis of Double-Blind Randomised Controlled Trials. J. Psychopharmacol. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, D.; Podgórski, M.; Kałużyńska, O.; Stefańczyk, L.; Kotlicka-Antczak, M.; Gmitrowicz, A.; Grzelak, P. Adding Sarcosine to Antipsychotic Treatment in Patients with Stable Schizophrenia Changes the Concentrations of Neuronal and Glial Metabolites in the Left Dorsolateral Prefrontal Cortex. Int. J. Mol. Sci. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, D.; Podgórski, M.; Kałużyńska, O.; Gawlik-Kotelnicka, O.; Stefańczyk, L.; Kotlicka-Antczak, M.; Gmitrowicz, A.; Grzelak, P. Supplementation of Antipsychotic Treatment with Sarcosine—GlyT1 Inhibitor—Causes Changes of Glutamatergic 1NMR Spectroscopy Parameters in the Left Hippocampus in Patients with Stable Schizophrenia. Neurosci. Lett. 2015, 606, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, D.; Podgórski, M.; Kałużyńska, O.; Gawlik-Kotelnicka, O.; Stefańczyk, L.; Kotlicka-Antczak, M.; Gmitrowicz, A.; Grzelak, P. Supplementation of Antipsychotic Treatment with the Amino Acid Sarcosine Influences Proton Magnetic Resonance Spectroscopy Parameters in Left Frontal White Matter in Patients with Schizophrenia. Nutrients 2015, 7. [Google Scholar] [CrossRef]
- Strzelecki, D.; Kałuzyńska, O.; Wysokiński, A. BDNF Serum Levels in Schizophrenic Patients during Treatment Augmentation with Sarcosine (Results of the PULSAR Study). Psychiatry Res. 2016, 242. [Google Scholar] [CrossRef] [PubMed]
- Veerman, S.R.T.; Schulte, P.F.J.; Begemann, M.J.H.; Engelsbel, F.; de Haan, L. Clozapine Augmented with Glutamate Modulators in Refractory Schizophrenia: A Review and Metaanalysis. Pharmacopsychiatry 2014, 47. [Google Scholar] [CrossRef]
- Lane, H.Y.; Huang, C.L.; Wu, P.L.; Liu, Y.C.; Chang, Y.C.; Lin, P.Y.; Chen, P.W.; Tsai, G. Glycine Transporter I Inhibitor, N-Methylglycine (Sarcosine), Added to Clozapine for the Treatment of Schizophrenia. Biol. Psychiatry 2006, 60. [Google Scholar] [CrossRef]
- Perez-Saad, H.; Subiros, N.; Berlanga, J.; Aldana, L.; Garcia del Barco, D. Neuroprotective Effect of Epidermal Growth Factor in Experimental Acrylamide Neuropathy: An Electrophysiological Approach. J. Peripher. Nerv. Syst. 2017, 22. [Google Scholar] [CrossRef]
- Subirós, N.; Pérez-Saad, H.; Aldana, L.; Gibson, C.L.; Borgnakke, W.S.; Garcia-del-Barco, D. Neuroprotective Effect of Epidermal Growth Factor plus Growth Hormone-Releasing Peptide-6 Resembles Hypothermia in Experimental Stroke. Neurol. Res. 2016, 38. [Google Scholar] [CrossRef]
- Casper, D.; Blum, M. Epidermal Growth Factor and Basic Fibroblast Growth Factor Protect Dopaminergic Neurons from Glutamate Toxicity in Culture. J. Neurochem. 1995, 65, 1016–1026. [Google Scholar] [CrossRef]
- Honegger, P.; Guentert-Lauber, B. Epidermal Growth Factor (EGF) Stimulation of Cultured Brain Cells. I. Enhancement of the Developmental Increase in Glial Enzymatic Activity. Dev. Brain Res. 1983, 11, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Figiel, M.; Maucher, T.; Rozyczka, J.; Bayatti, N.; Engele, J. Regulation of Glial Glutamate Transporter Expression by Growth Factors. Exp. Neurol. 2003, 183, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Custo, S.; Baron, B.; Felice, A.; Seria, E. A Comparative Profile of Total Protein and Six Angiogenically-Active Growth Factors in Three Platelet Products. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2022, 11. [Google Scholar] [CrossRef]
- Venturi, S.; Venturi, M. Iodine in Evolution of Salivary Glands and in Oral Health. Nutr. Health 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, V.F.; Gleber-Netto, F.O.; Sousa, S.F.; Silva, T.A.; Abreu, M.H.N.G.; Aguiar, M.C.F. EGF in Saliva and Tumor Samples of Oral Squamous Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2011, 19. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).