1. Introduction
Antiphospholipid syndrome (APS) is the most common type of acquired thrombophilia and also the most aggressive one. The revised Sapporo criteria for APS classification require at least one clinical criterion, vascular thrombosis or pregnancy morbidity, and one laboratory criterion, one of the antiphospholipid antibodies (aPL) that is persistently positive (at least 12 weeks apart), such as lupus anticoagulant (LA), anticardiolipin (aCL), and/or anti-β2-glycoprotein I (anti-β2GPI) antibodies) of the IgG and IgM isotypes [
1]
. A number of studies have suggested an association between APS and antiprothrombin antibodies, particularly those against the phosphatidylserine-prothrombin complex (aPS/PT), which have shown a strong association with LA activity and with the clinical manifestations of APS [
2,
3,
4].
In patients with APS, aPL are the main cause of thrombosis (arterial, venous, or microthrombosis) and pregnancy complications. Even in young people, if no other causes are found, aPL are the main cause of recurrent miscarriages, pregnancy losses and thrombosis. After APS diagnosis, therapy takes the form of lifelong oral anticoagulation or antiplatelet therapy, which in the most cases prevents recurrence of thrombosis. The incidence rate of APS in women and men is reported differently. According to one study, it is 5:1 [
5], while another study found a similar ratio between men and women [
6].
Regarding the pathology of thrombosis in APS, the so-called "two-trigger hypothesis" has been introduced in the recent decades, which states that the presence of infection or severe inflammation considerably increases the thrombogenic potential of aPL [
7]. According to this theory, COVID -19 could play the role of the "second trigger".
The single-stranded RNA virus SARS-CoV-2 of the Coronaviridae family caused a coronary pandemic (COVID -19) with a highly heterogeneous clinical course. The presence of aPL has been observed in patients with COVID -19 [
8,
9] and is thought to be associated with deep vein thrombosis, pulmonary embolism, and stroke in severe forms of the disease [
10,
11,
12].
Moreover, the catastrophic variant of APS is often fatal and shares some similarities with diffuse coagulopathy observed in patients with COVID-19 [
13].
Numerous studies have described high rates of aPL in patients hospitalized with COVID-19,
both with and without thrombosis [
11,
13,
14]
. The significance of these serologic markers in promoting thrombosis during COVID-19 remains unclear.
The purpose of this study was to evaluate the laboratory results of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete blood cell count (CBC) test, D-dimer, ferritin, troponin, procalcitonin, lactate dehydrogenase (LDH) and antiphospholipid antibodies (aPL): aCL, anti-β2GPI, and aPS/PT of the IgG, IgM, and IgA isotypes) obtained at 4 time points (hospital admission, worsening of COVID-19 disease, discharge from the hospital, and at 3-month follow-up) and compare the laboratory results of aPL with clinical data on arterial/venous thrombosis and pregnancy complications before (according to personal anamnesis) and during hospitalisation in patients with severe forms of COVID-19 hospitalised due to pneumonia.
2. Materials and Methods
2.1. Patients and Data Extraction
The study had randomly enrolled patients over 18 years of age with COVID -19 pneumonia who were admitted to the Intensive Care Unit (ICU) of the General Hospital Pančevo, Serbia, from March 2021 to May 2021.
Exclusion criteria were history of autoimmune diseases, APS, connective tissue diseases (systemic lupus erythematosus, systemic sclerosis, Sjögren`s disease, vasculitis, dermatomyositis) or inflammatory arthritis (rheumatoid arthritis and spondyloarthritis). In the ICU, continuous oxygenation was performed, and continuous noninvasive monitoring with pulse oximetry, electrocardiography and monitoring of blood pressure, pulse, and temperature were performed every 4 hours.
The history of clinical manifestations significant for APS was obtained from medical records and also monitored during the observation period. The study was conducted within the framework of the Slovenian national project P3-0314 according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Republic of Slovenia (#0120-7/2019/5, #0120-422/2020/6, and #0120-113/2021/4), the Ethics Committee of the Republic of Serbia (132/2, Jan 14, 2021), and the Ethics Committee of the General Hospital of Pančevo (#01-1492/21). Informed consent was obtained from all subjects participating in the study.
2.2. Study Endpoints and Follow-Up
Patients were monitored for clinical and laboratory features during hospitalization, i.e. at admission, at worsening of the COVID-19 disease (defined as cytokine storm, connection of the patient to the respirator and use of the anti-IL-6 drug – Tocilizumab), at hospital discharge and 3 months after discharge.
2.3. Blood Sampling and Antiphospholipid Antibody Measurment
At each time point, blood samples were routinely collected for determinatoion of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete blood cell count (CBC) test, D-dimer, ferritin, troponin, procalcitonin, lactate dehydrogenase (LDH) and determined in the Pancevo hospital.
Also, at each time point, aPL (aCL, anti-β2GPI, and aPS/PT of isotypes IgG, IgM and IgA) were collected following the previously established protocol. Samples were allowed to clot for 30 to 60 minutes and then centrifuged at 1500×g for 15 minutes to separate clot and serum. The serum was transferred to 2 Eppendord Protien Low Bind Tubes and stored at -80°C until shipment on dry ice to prevent thawing. aPL were determined at the Department of Rheumatology, University Medical Centre Ljubljana (UMCL), Slovenia, using in-house enzyme-linked immunosorbent assays (ELISA) according to predefined protocols [
15,
16]. Values above the 99
th percentile of the healthy control population were considered positive, i.e., for aCL ≥ 11AU, for anti-β2GPI ≥ 2 AU, and for aPS/PT ≥ 5 AU.
2.4. Statistical Analyses
Frequencies, descriptive data, Wilcoxon signed rank test, Fishers exact test and paired samples t-test were performed using SPSS (version 21; IBM, Armonk, NY, USA). A p-value of 0.05 or less was considered statistically significant.
3. Results
3.1. Patient Baseline Characteristics
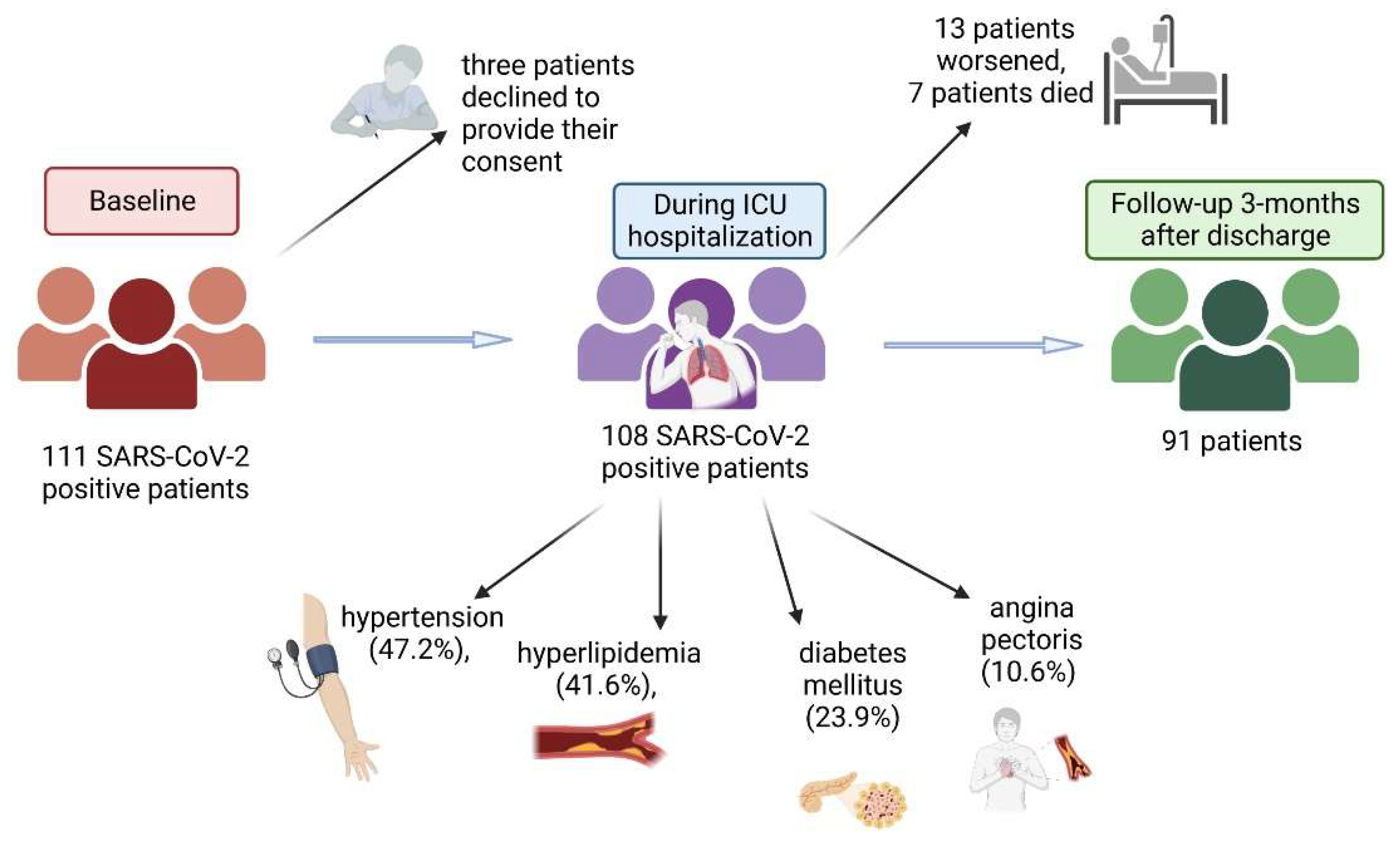
At baseline, 111 SARS-CoV-2-positive patients admitted to General Hospital Pančevo for COVID-19 pneumonia from March 2021 to May 2021 were included. Later, three patients declined to provide their consent to take part in the study (
Figure 1). The remaining 108 patients (67% men, mean age (SD) 59.4 (12.4) years (IQR 16 years) were monitored in the intensive care unit (ICU) and received treatment for pneumonia according to the established COVID-19 protocol, which includes administration of antibiotics, corticosteroids, anticoagulants and certain medications for comorbidities; patients who were hypoxic received oxygen support. Patients had hypertension (47.2%), hyperlipidemia (41.6%), diabetes mellitus (23.9%), and angina pectoris (10.6%) as comorbidities. During hospitalization, the condition of 13 patients worsened. Three patients died before laboratory test results were available. Of the remaining ten patients, six were treated with Tocilizumab and four with high oxygen flow. Despite therapy, four additional patients died. Follow up, performed 3 months after discharge, was achieved in 91 patients.
3.2. Laboratory Values at 4-Time Points
Table 1 presents the laboratory parameters measured at 4 different time-points. Compared with admission, there was a significant decrease in ESR, CRP, number of leucocytes, neutrophils and platelets, as well as in ferritin, troponin and LDH levels, both at hospital discharge and at 3-months follow-up, whereas improvements in the erythrocyte number, hemoglobin, hematocrit, and D-dimer were seen only at 3-month follow-up. During hospitalization and at follow-up, procalcitonin levels remained unchanged.
The aPL levels were significantly higher at hospital discharge than at admission and follow-up, particularly aCL IgG, aCL IgM, and anti-β2GPI IgG (
Table 1).
3.3. Specific Subgroups of Patients
3.3.1. Patients Who Died during Hospitalization
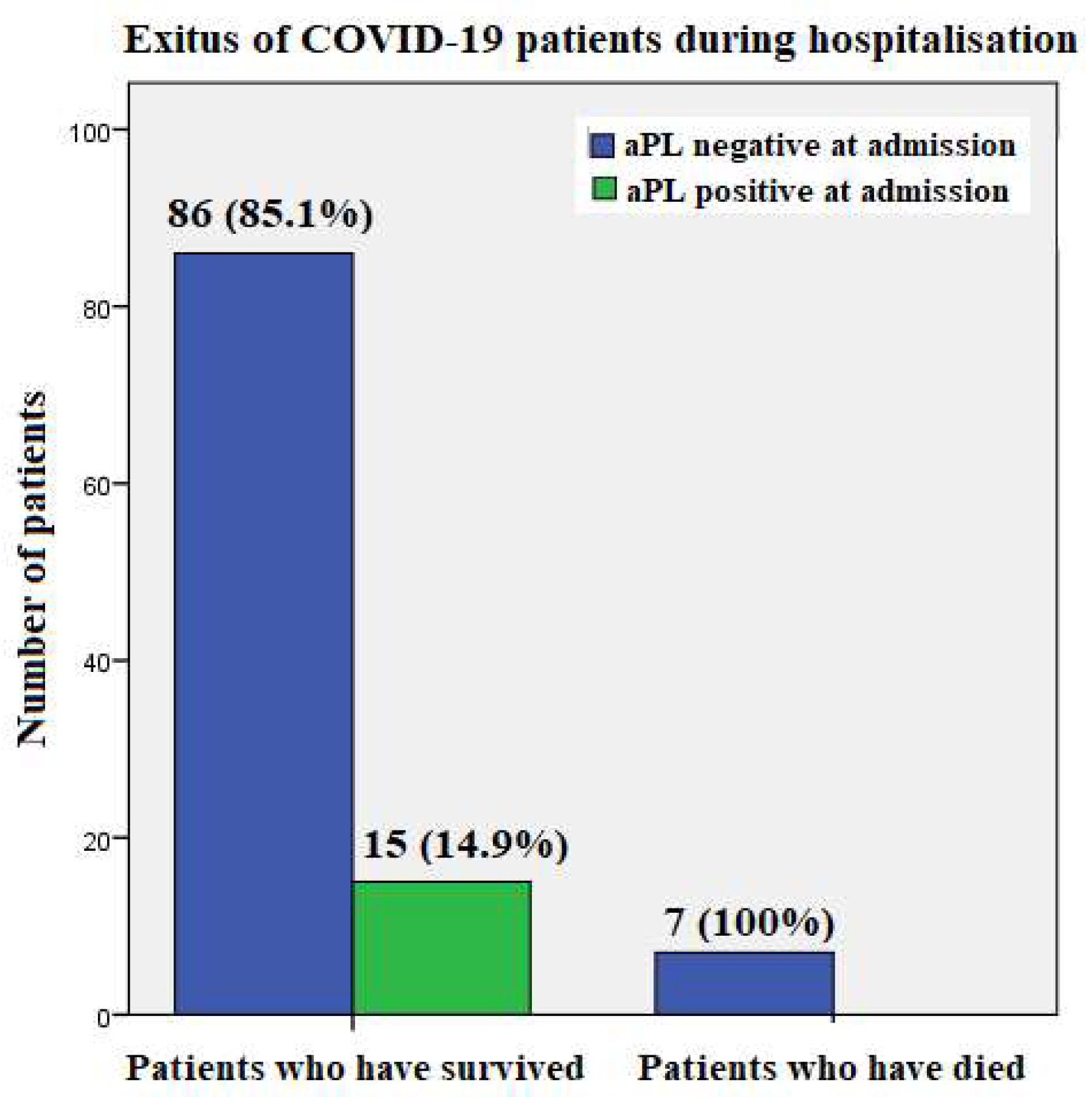
Seven patients (mean age 65.8 years, range 52 to 77 years) passed away during hospitalization, three of them from pulmonary artery thromboembolism, two from cytokine storm, and two on the respirator, although the exact cause of death was unknown in their case. Of those who died, two were treated with tocilizumab due to cytokine storm, two required respirator, and four received high oxygen flow. None of these patients tested positive for aPL on admission (
Figure 2).
3.3.2. Patients Who Have Experienced Thrombosis during Hospitalization
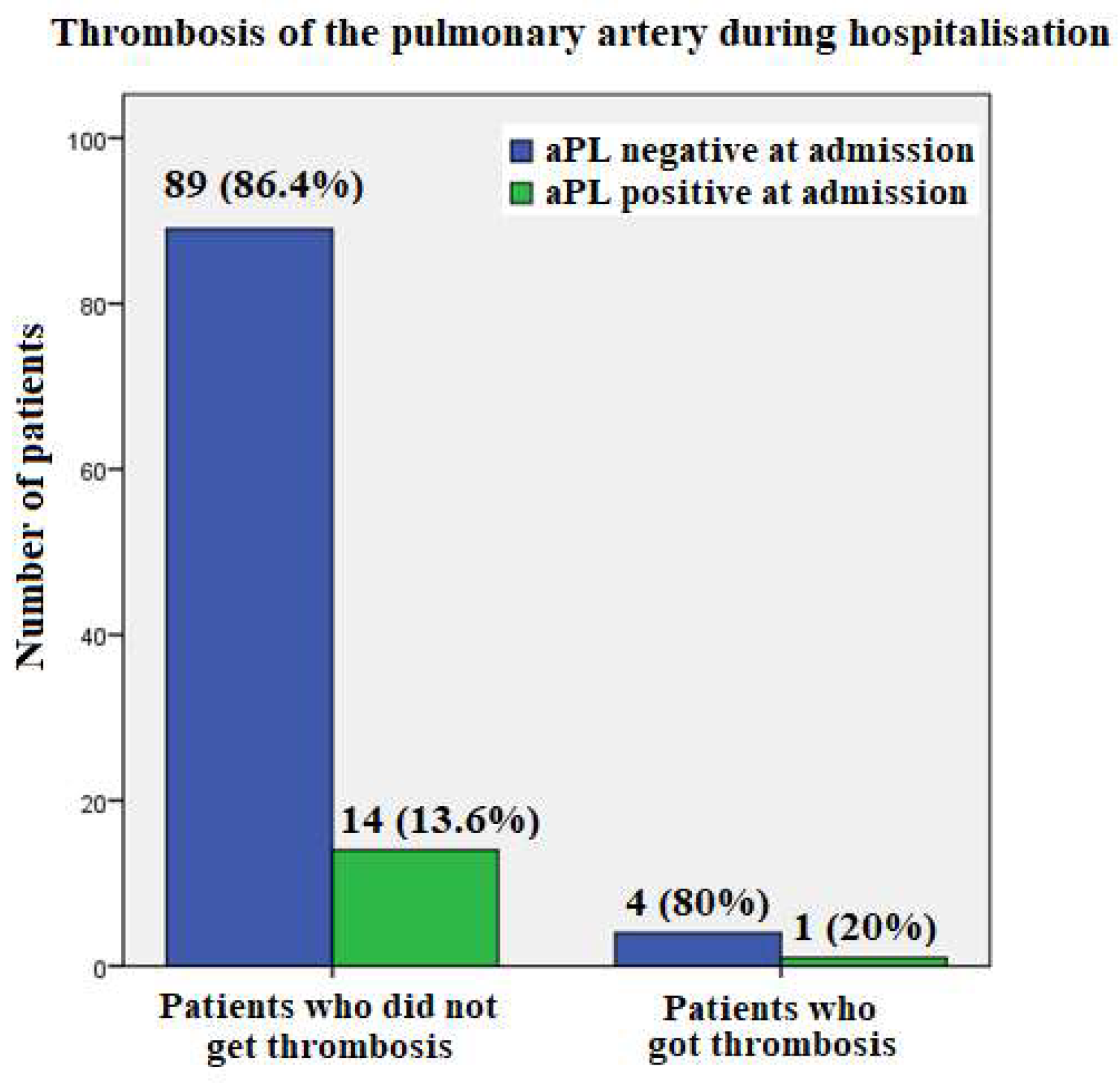
Five patients experienced pulmonary artery thrombosis during hospitalization, one had positive aPL at all time points and was therefore diagnosed with APS, while the others were negative (
Figure 3).
3.3.3. Patients with a History of Thrombosis
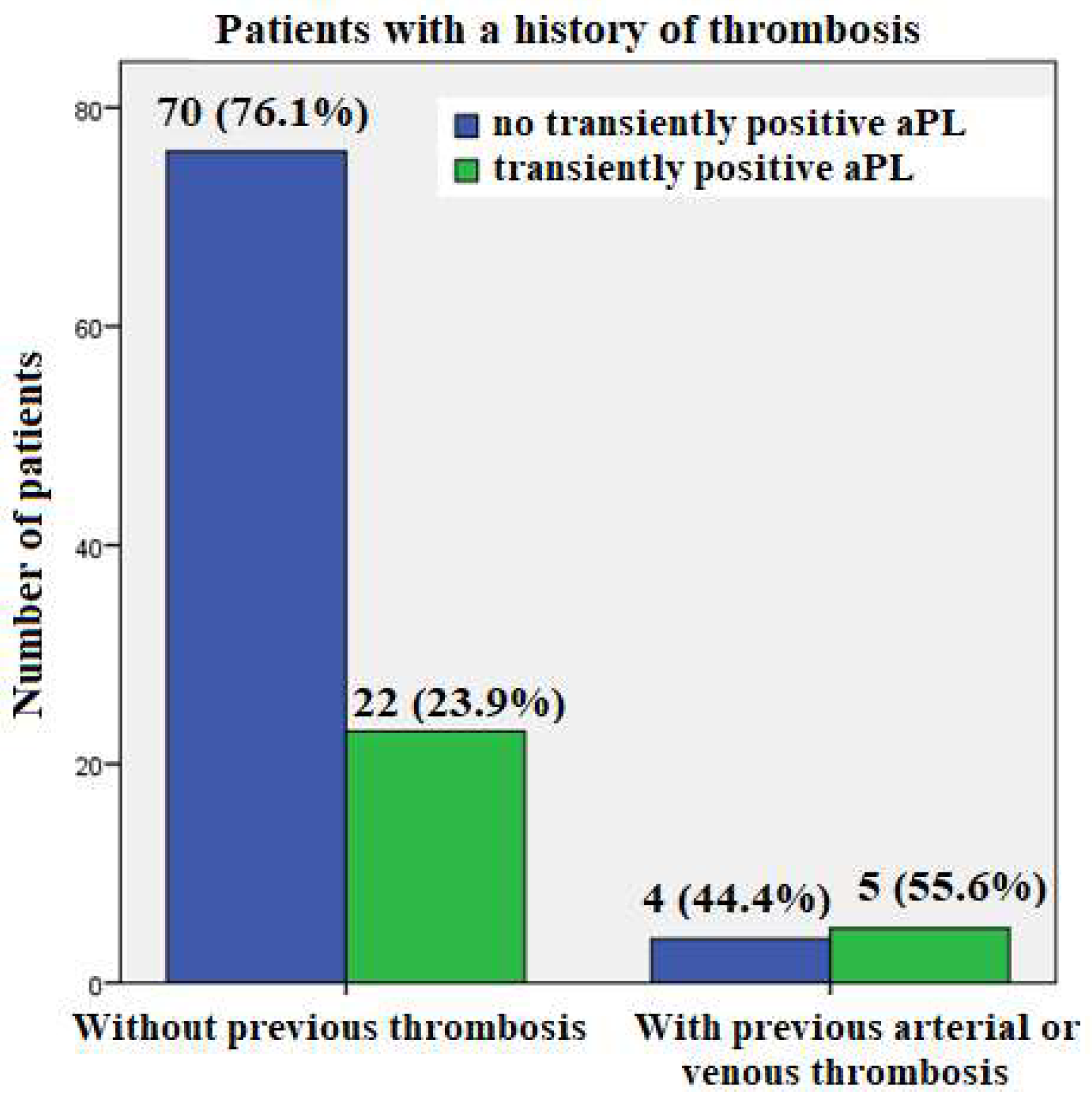
A history of thrombosis was found in 9/101 subjects, of whom five had arterial thrombosis (coronary and cerebral arteries) and four had venous thrombosis. Of the patients with arterial thrombosis, none had a positive aPL, either at admission or at follow-up. On the other hand, among the patients with a history of venous thrombosis, one tested positive for aPL at all follow up time points and was diagnosed with APS. The other three patients with VT had transiently positive aPL values (two at hospital discharge and one at admission).
Of nine individuals with a history of arterial or venous thrombosis, 5/9 (55.6%) had transiently positive aPL result only at hospital discharge, whereas a much smaller proportion of patients without a history of thrombosis were transiently aPL positive only at hospital discharge: 22/92 (23.9%) (p=0.05) (
Figure 4).
3.3.4. Patients with a History of Pregnancy Morbidity
Regarding history of pregnancy morbidity, two patients had an abortion after the 10th week of gestation, but none of them tested positive aPL at any time point. In our cohort, there were no patients who had delivered before the 34th week of gestation or who had 3 or more abortions before the 10th week of gestation.
4. Discussion
The present study of antiphospholipid antibodies (aPL) and thrombosis in patients with the severe forms of COVID-19 has several clear long term applicable findings.
First, none of the patients who were hospitalized for COVID-19 pneumonia in our study and died had positive aPL at the time of admission, although three of them had pulmonary artery thrombosis. Also, of the 5 patients who developed pulmonary artery thromboembolism during hospitalization, only one was aPL-positive at all time points and was therefore diagnosed with APS; the others were tested aPL negative.
Second, two patients with COVID-19 pneumonia developed arterial thrombosis during hospitalization - one developed cerebral artery thrombosis and had triple-positivity of aPL (aCL IgG, anti-β2GPI IgM and aPS/PT IgM) on admission and the other developed coronary artery thrombosis with myocardial infarction and was aPL negative on admission. In addition, five patients developed microthrombosis during hospitalization; one was aPL positive (aCL IgG and anti-β2GPI IgM) on admission and developed pulmonary microthrombosis; the other four were aPL negative (they had pulmonary microthrombosis and coronary artery microthrombosis). Of the patients who developed arterial thrombosis or microthrombosis during hospitalization, none had a transiently positive aPL at hospital discharge compared with admission and follow-up.
Third, comparing patients with and without a history of thrombosis, the first group had a significantly higher transient increase in aPL (aCL IgG, aCL IgM, and anti-β2GPI IgG) at hospital discharge compared to admission and 3-months follow-up.
Many COVID-19 patients, with and without thrombosis, have been found to have elevated aPL levels [
4,
17,
18]. However, the significance of these serological markers for thrombogenic potential in COVID-19 is unclear. It is suggested that aPL may contribute to the mechanism of hypercoagulability, but it may also be an epiphenomenon associated with COVID-19 infection. The process of hypercoagulability might be related to aPL, whereas it is also possible that this epiphenomenon could be caused by the COVID-19 infection itself.
On the other hand, whether aPL are permanently or transiently elevated during COVID-19 and what types of aPL exist have not been fully investigated. Given that the most severe forms of COVID-19 infection are associated with cardiovascular and neurological consequences, the dosage and duration of anticoagulant therapy could be determined by the extent of the aPL presence in these patients.
The exact molecular basis of the SARS-CoV-2 infection's disproportionately promoting impact on the coagulation system is mainly unknown. Platelet activation, coagulopathy and venous thromboembolism are likely to be significantly affected by SARS-CoV-2-induced endotheliopathy and production of cytokines and growth factors (GFs) [
19]. In order to follow the various COVID-19 outcomes , many studies have investigated the induction of aPL in this disease. It has been documented that COVID-19 infection may be the only identified trigger for multi-organ thrombosis [
13]. Furthermore, injection of IgG purified from COVID-19 patient serum into mice accelerated venous thrombosis in two mouse models [
4].
In the present study, we have found significantly higher transiently elevation of aCL IgG, aCL IgM, and anti-β2GPI IgG at hospital discharge (compared with admission and follow-up) in patients with previous thrombosis, but none of these patients experienced thrombosis during hospitalization.
According to published studies, 50% of hospitalized COVID-19 patients develop at least transient positivity of aPL antibodies, and some investigators suggested that these autoantibodies may be harmful [
4,
11,
12]. However, various studies have yielded conflicting results, making it difficult to draw conclusions about the relationship and correlation between aPL positivity and COVID-19 disease outcomes such as thrombosis or mortality [14, 20].
In a review of 163 published cases of virus-associated aPL, thrombotic events were noted in 116 cases, although the clinical implications of transient virus-associated aPL antibodies have not been fully elucidated yet [
21].
Thrombosis is also thought to be induced by an increase in total IgA and aPL IgA antibodies, which have been found to be significantly associated with severe disease (12). However, none of the patients who developed pulmonary artery thrombosis or arterial, venous, or microthrombosis in our study had an increase in aPL of IgA type (aCL, anti-β2GPI, or aPS/PT).
Positive aPL were previously observed in several cohorts of severely ill patients, including those with COVID-19, and there was an independent association between aCL IgG
and more severe disease regardless of COVID-19 status. Furthermore, there were no discernible differences in platelet counts, platelet-to- neutrophil ratios, or the need for therapeutic anticoagulation in aCL IgG positive patients [
11].
The association between elevated aPL and severe COVID-19 has been suggested previously [
22]. Our study confirmed a transient increase in aPL in patients with COVID-19 pneumonia at the time of hospital discharge compared with admission and follow-up. Moreover, transiently positive aPL were significantly more prevalent in patients with previous thrombosis (55.6%) than in patients without thrombosis (26.1%).
The major strength of this study is the longitudinal comparison of clinical data regarding thromboses (both actual and in personal history) with aPL findings during hospitalization and 3 months later at follow-up.
5. Conclusions
Although aPL would be expected to be associated with vascular thrombosis in severe forms of COVID-19 infection, all individuals with pneumonia in our cohort who passed away were aPL negative.
The novelty of this study is that among patients hospitalized for COVID-19 pneumonia with a history of arterial or venous thrombosis, more than 50% had positive aPL at hospital discharge, which was later found to be negative at the 3-month follow-up. Similarly, of the patients who developed arterial thrombosis or microthrombosis during hospitalization, none had a transiently positive aPL at hospital discharge, compared with admission and follow-up. This finding may help us in recommending anticoagulation therapy after hospitalization and medical follow-up.
Author Contributions
Conceptualization, M.Z.Š., M.Z. and G.R..; methodology, M.Z.Š. and M.Z.; software, M.O. and M.Z.Š.; database, M.O. and A.R.; investigation, M.O and S.Č.; writing—original draft preparation, MZŠ.; writing— review and editing, PŽ, M.O. and SČ; project administration, MZŠ and PŽ. All authors have read and approve the submitted version of the manuscript.
Funding
This work was done under the Slovenian National Research Program: Sistemske avtoimunske bolezni (Sistemic autoimmune diseases) P3-0314.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Republic of Slovenia (#0120-7/2019/5, #0120-422/2020/6 and #0120-113/2021/4), Ethics Committee of the Republic of Serbia (132/2, 14.01.2021) and Ethics Committee of the General Hospital Pančevo (#01-1492/21).
Informed Consent Statement
Informed consent was obtained from all of the subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons.
Acknowledgments
The authors would like to thank Dr. Mirjana Karličić, an internal medicine specialist in the Pančevo hospital, for her engagement in data collection. They also thank Dr. Aleksandar Radivčev for his work with the database input. Authors also appreciate the time and engagement of all health care professionals in General Hospital (GH) Pančevo, Serbia, the Institute of rheumatology in Belgrade, Serbia, the University hospital medical center Bežanijska Kosa in Belgrade, Serbia and the Department of Rheumatology, University Medical Centre Ljubljana, Slovenia.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Miyakis, S; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J.Thromb.Haemost. 2006; 4:295-306. [CrossRef]
- Žigon, P.; Podovšovnik, A.; Ambrožič, A.; Tomšič, M.; Hočevar, A.; Gašperšič, N.; Rotar, Ž.; Praprotnik, S.; Šemrl, S.S.; Čučnik, S. Added value of non-criteria antiphospholipid antibodies for antiphospholipid syndrome: lessons learned from year-long routine measurements. Clinical rheumatology 2019; 38: 371-378.
- Zohoury, N.; Bertolaccini, M.L.; Rodriguez-Garcia, J.L.; Shums, Z.; Ateka-Barrutia, O.; Sorice, M.; Norman, G.L.; Khamashta, M. Closing the Serological Gap in the Antiphospholipid Syndrome: The Value of "Non-criteria" Antiphospholipid Antibodies. The Journal of rheumatology 2017; 44: 1597-1602. [CrossRef]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G; Gockman, K; Madison, J.A.; Zuo, M; Yadav, V; Wang, J; Woodard, W; Lezak, SP; Lugogo, NL; Smith, SA; Morrissey, JH; Kanthi, Y; Knight, JS. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 2020;12(570):eabd3876. [CrossRef]
- Cervera, R.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S. et al. Euro-Phospholipid Project Group (European Forum on Antiphospholipid Antibodies). Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2009; 68: 1428-32. [CrossRef]
- Duarte-Garcia, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.J.; Matteson, E.L. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019;71(9):1545-1552. [CrossRef]
- Shoenfeld, Y.; Blank, M.; Cervera, R.; Font, J.; Raschi, E ; Meron, P.L. Infectious origin of the antiphospholipid syndrome. Ann Rheum Dis 2006; 65:2–6. [CrossRef]
- Stelzer, M.; Henes, J; Saur, S. The Role of Antiphospholipid Antibodies in COVID-19. Curr Rheumatol Rep. 2021;23(9):72-4. [CrossRef]
- Taha, M.; Samavati, L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open 2021;7:e001580. [CrossRef]
- Gkrouzman, E.; Barbhaiya, M.; Erkan, D.; Lockshin, M.D. Reality Check on Antiphospholipid Antibodies in COVID-19-Associated Coagulopathy. Arthritis & rheumatology (Hoboken, N.J.) 2021; 73: 173-174. [CrossRef]
- Trahtemberg, U.; Rottapel, R.; Dos Santos, C.C.; Slutsky, A.S.; Baker, A.; Fritzler, M.J. Anticardiolipin and other antiphospholipid antibodies in critically ill COVID-19 positive and negative patients. Ann Rheum Dis. 2021;80:1236-1240. [CrossRef]
- Hasan Ali, O.; Bomze, D.; Risch, L.; Brugger, S.D.; Paprotny, M.; Weber, M.; Thiel, S.; Kern, L.; Albrich, W.C.; Kohler, P.; Kahlert, C.R.; Vernazza, P.; Bühler, P.K.;, Schüpbach, R.A.; Gómez-Mejia, A.; Popa, A.M.; Bergthaler, A.; Penninger, J.M.; Flatz, L. Severe Coronavirus Disease 2019 (COVID-19) is Associated With Elevated Serum Immunoglobulin (Ig) A and Antiphospholipid IgA Antibodies. Clin Infect Dis. 2021;73(9):e2869-e2874. [CrossRef]
- Bitterman, L.; Solhjoo, M.; Shah, V.; Kwon, S.M.; Torralba, K.; Kazbour, H. Catastrophic Antiphospholipid Syndrome as a Complication of COVID-19 Infection. J Investig Med High Impact Case Rep. 2023;11:23247096231165736. [CrossRef]
- Stelzer, M.; Henes, J.; Saur, S. The Role of Antiphospholipid Antibodies in COVID-19. Curr Rheumatol Rep. 2021;23(9):72. [CrossRef]
- Božič, B.; Kveder, T.; Stegnar, M.; Morosini-Berus, E.; Kos-Golja, M.; Peternel, P.; Rozman, B. Influence of degraded phosphatidylserine on binding of antiphospholipid antibodies. Int. Arch. Allergy Immunol. 1997; 112: 19–26. [CrossRef]
- Žigon, P.; Ambrožic, A.; Cucnik, S.; Kveder, T.; Rozman, B.; Božic, B. Modified phosphatidylserine-dependent antiprothrombin ELISA enables identification of patients negative for other antiphospholipid antibodies and also detects low avidity antibodies. Clin. Chem. Lab. Med. 2011;49:1573. [CrossRef]
- Mendoza-Pinto, C.; Escárcega, R.O.; García-Carrasco, M.; Bailey, D.J.O.; Gálvez-Romero, J.L.; Cervera, R. Viral infections and their relationship with catastrophic antiphospholipid syndrome: a possible pathogenic mechanism of severe COVID-19 thrombotic complications. J Intern Med. 2020;288:737-739. [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; Wang, C.; Zhao, J.; Sun, X.; Tian, R.; Wu, W.; Wu, D.; Ma. J.; Chen, Y.; Zhang, D.; Xie, J.; Yan, X.; Zhou, X.; Liu, Z.; Wang, J.; Du, B.; Qin, Y.; Gao, P.; Qin, X.; Xu, Y.; Zhang, W.; Li, T.; Zhang, F.; Zhao, Y.; Li, Y.; Zhang, S. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382(17):e38. [CrossRef]
- Ahmad, F.; Kannan, M.; Ansari, A.W.; Role of SARS-CoV-2 -induced cytokines and growth factors in coagulopathy and thromboembolism. Cytokine Growth Factor Rev. 2022;63:58-68.
- Butt, A.; Erkan, D.; Lee, A.I.; COVID-19 and antiphospholipid antibodies. Best Pract Res Clin Haematol. 2022;35(3):101402. [CrossRef]
- Abdel-Wahab, N.; Lopez-Olivo, M. A.; Pinto-Patarroyo, G. P.; Suarez-Almazor, M. E. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus 2016; 25: 1520–1531. [CrossRef]
- Zhang, Y.; Cao, W.; Jiang, W. Xiao, M.; Li, Y.; Tang, N.; Liu, Z.; Yan, X.; Zhao, Y.; Li, T.; Zhu, T. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):580-586.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).