Submitted:
13 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
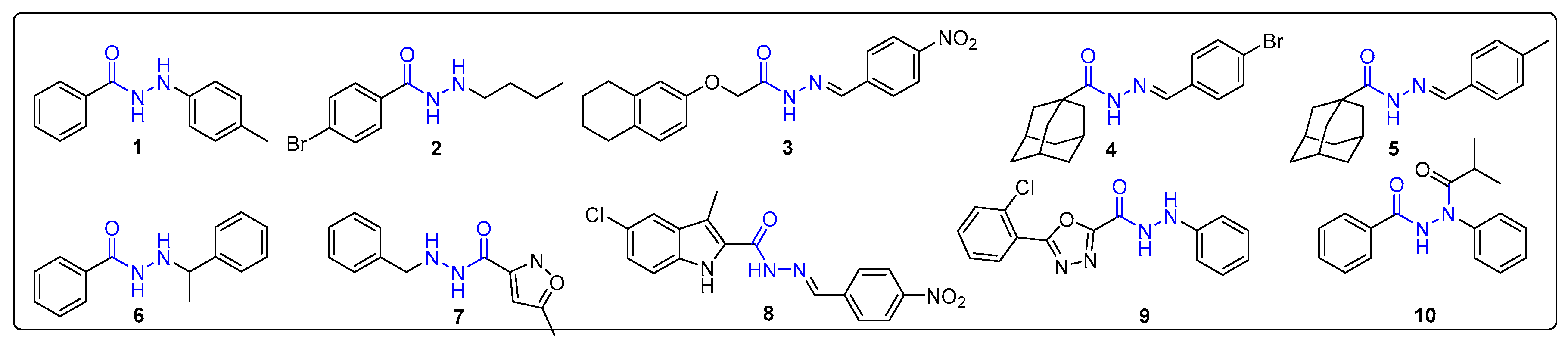
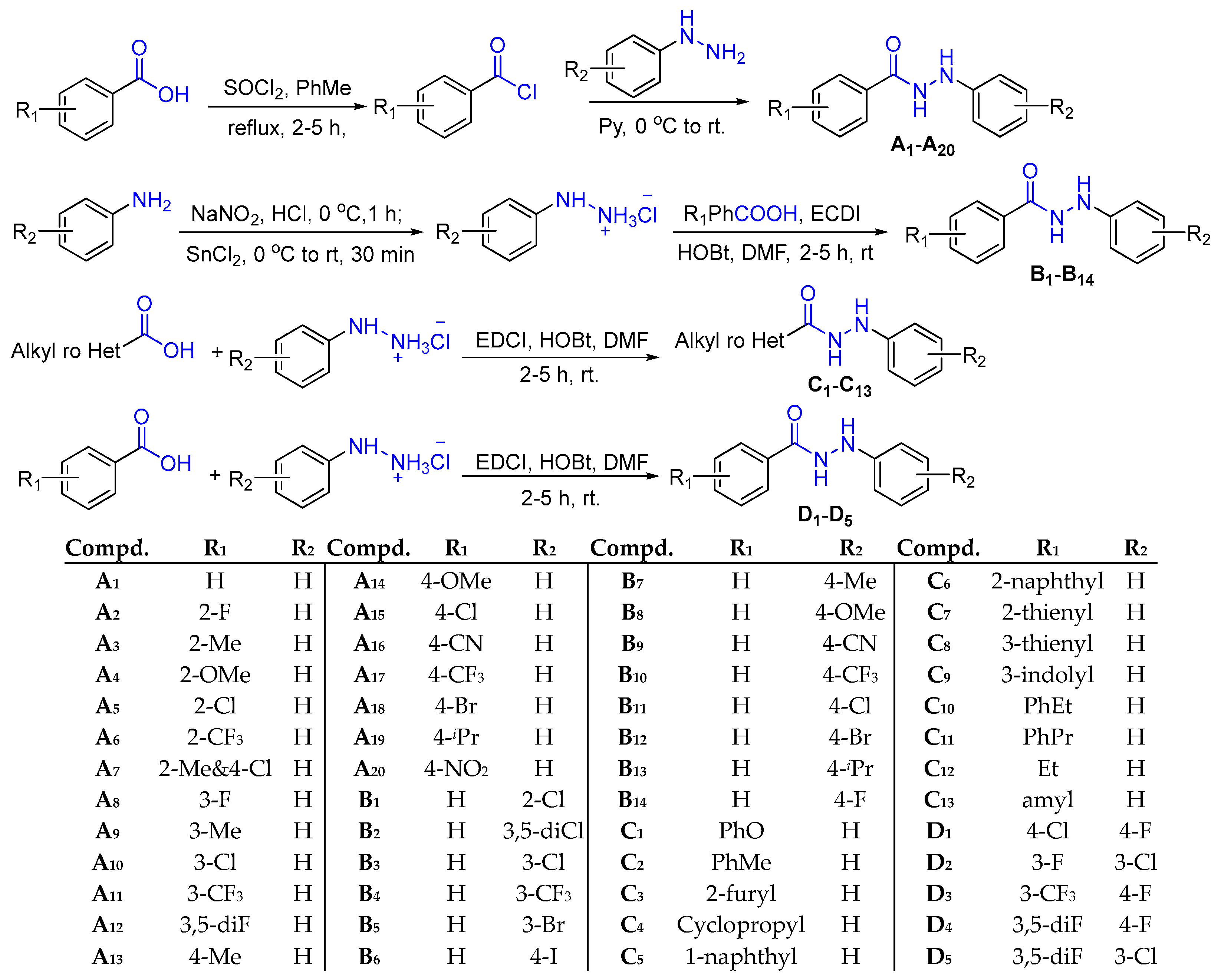
2.1. Design and Synthesis of N'-Phenylhydrazides
2.2. Evaluation of Antifungal Activity in Vitro
2.2.1. Antifungal Activity of Target Compounds
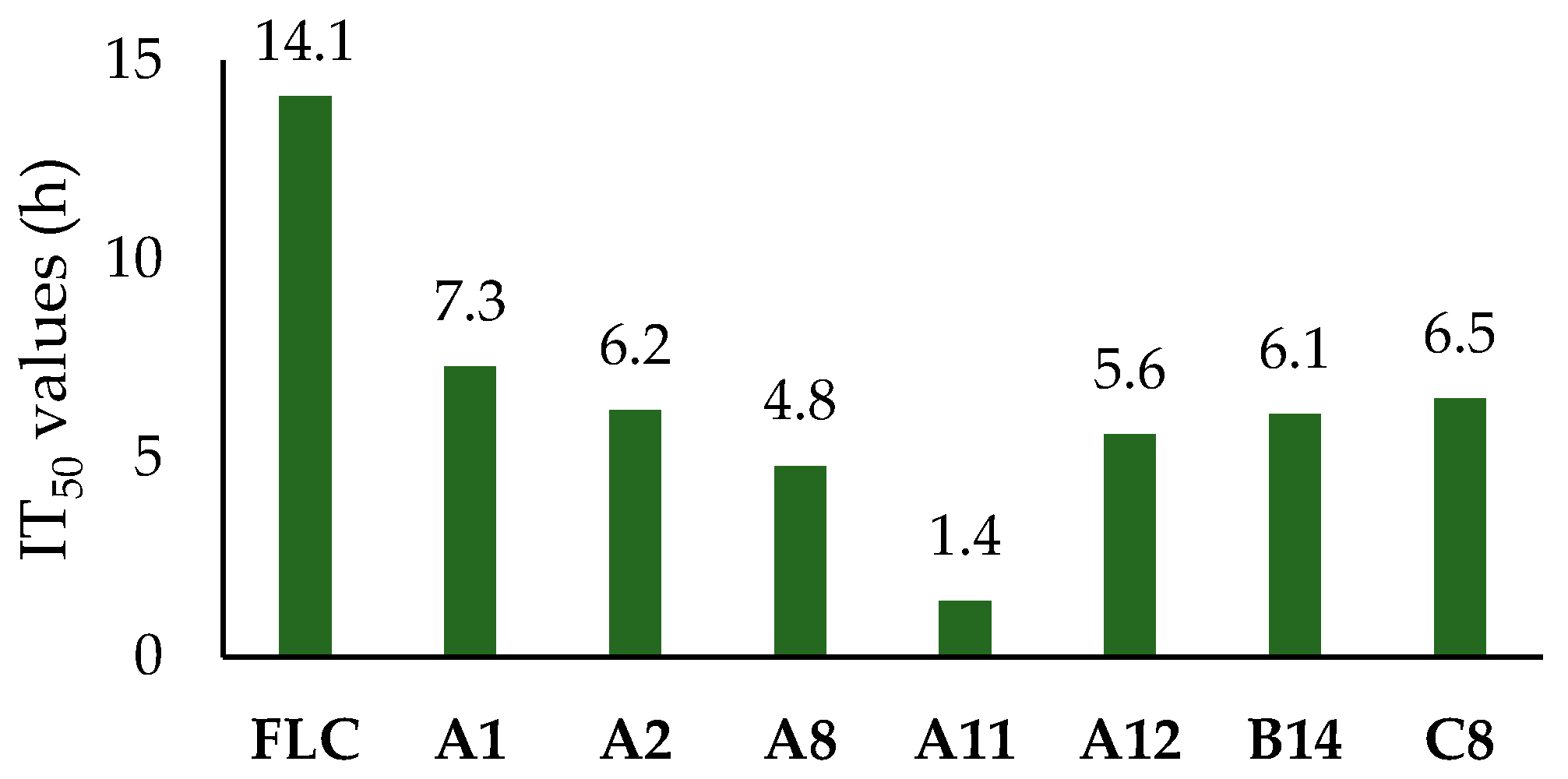
2.2.2. The Inhibitory Efficiency of Compounds with Higher Activity
2.2.3. The Analysis of Preliminary SARs
2.3. The Investigation of the Antifungal Mechanism
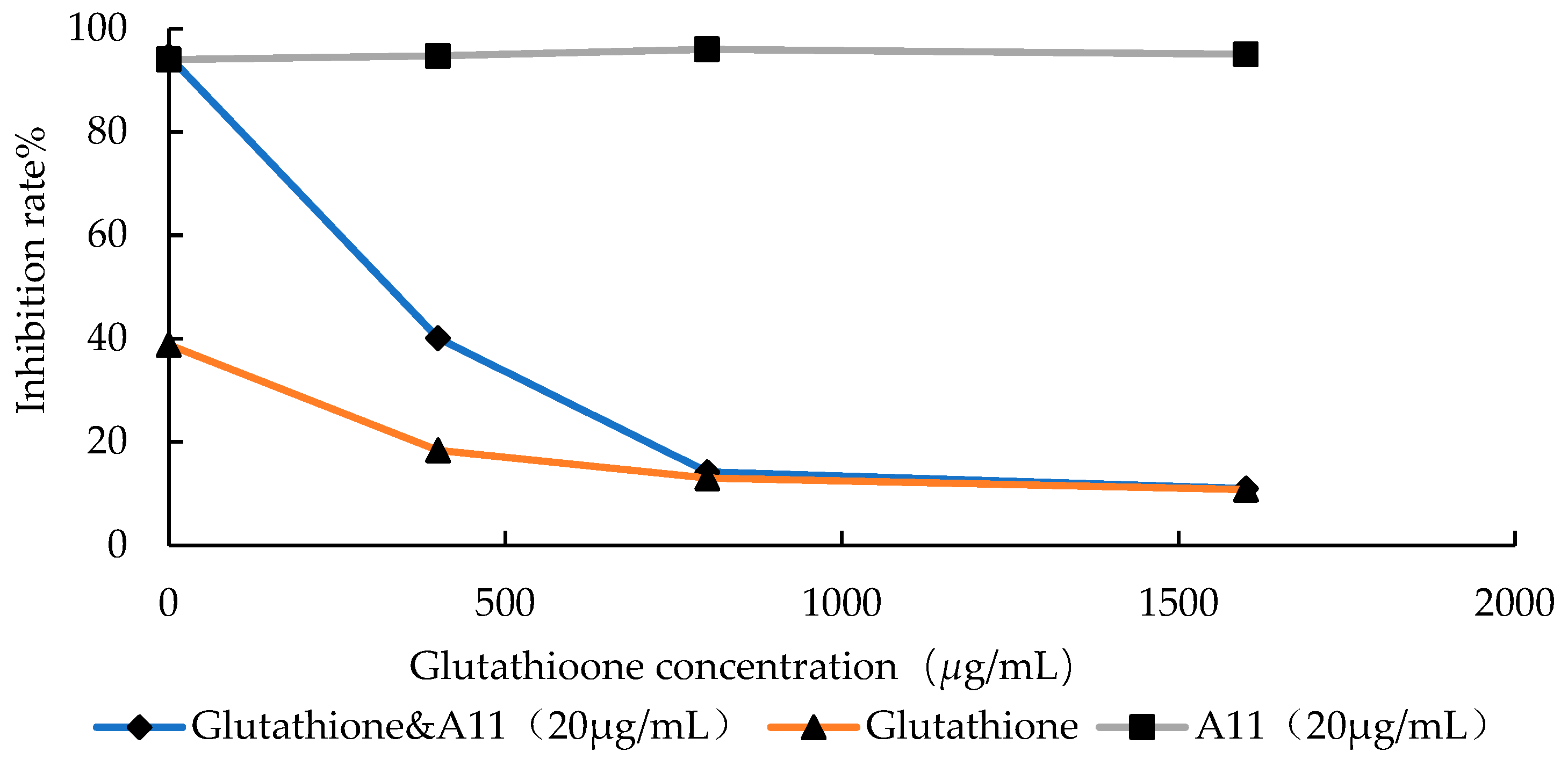
2.3.1. Assay of Free Radical Scavenging
2.3.2. Production of ROS
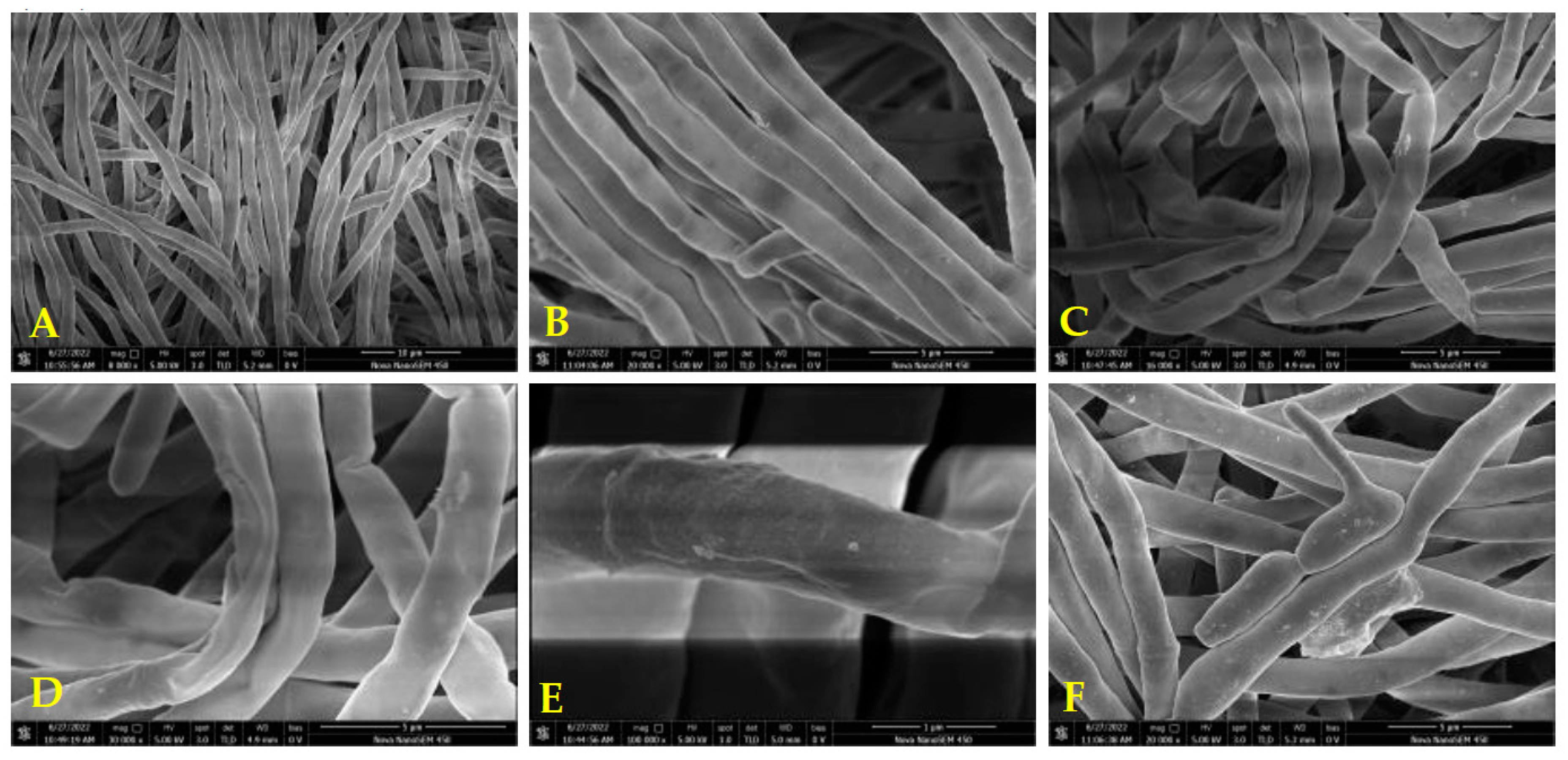
2.3.3. Effects of A11 on Hyphal Morphology
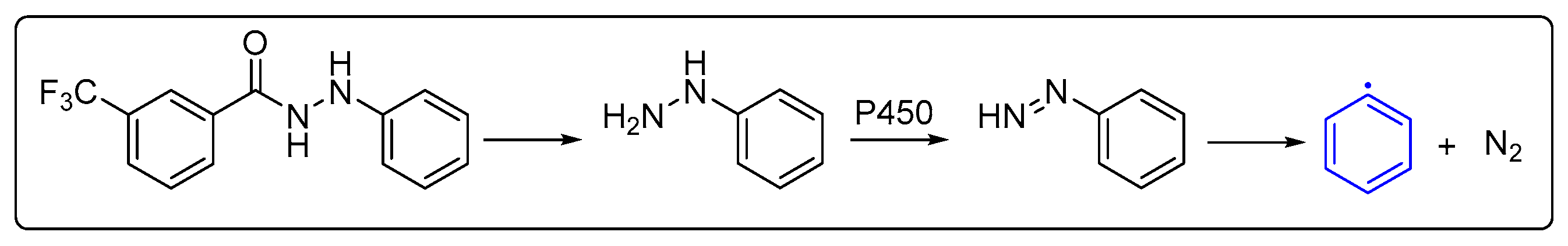
2.3.4. Preliminary Antifungal Mechanisms
3. Materials and Methods
3.1. Materials
3.2. The synthetic procedure
3.2.1. Synthesis of Substituted Phenylhydrazine Hydrochloride
3.2.2. Synthesis of N’-Phenylhydrazides
3.3. Antifungal Activity In Vitro
3.3.1. Determination of MIC80 value
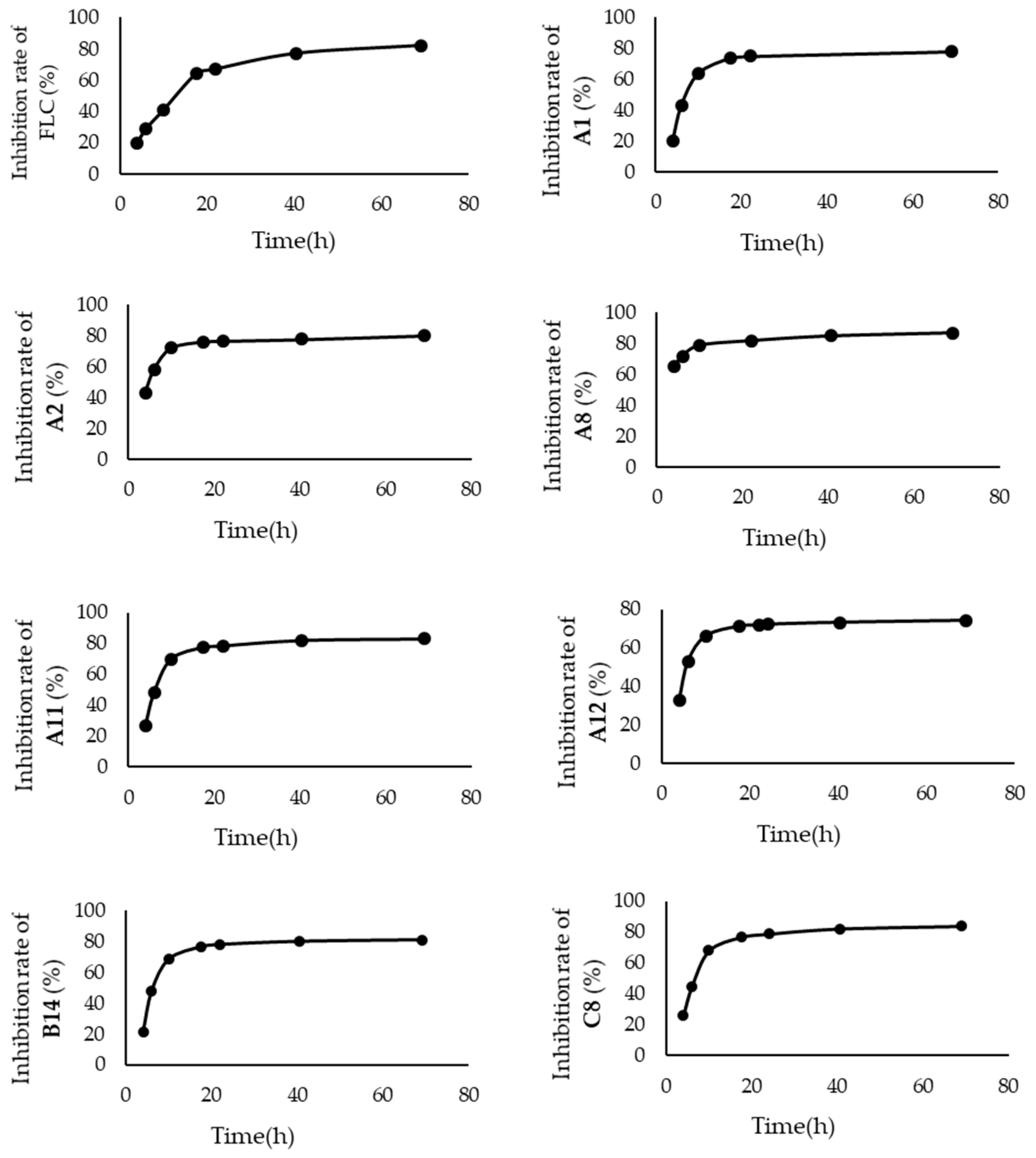
3.3.2. Time-Inhibition Rate Curves
3.4. The Investigation of the Antifungal Mechanism
3.4.1. Scavenging of Free Radical Generated from A11
3.4.2. Production of ROS
3.4.3. Analysis of the Mycelial Morphology
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Dartois, V.A.; Rubin, E.J. Anti-Tuberculosis Treatment Strategies and Drug Development: Challenges and Priorities. Nature Reviews Microbiology 2022, 20, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Chao, W.; Qin, Y.; Chen, J.; Wang, Y.; Liu, R.; Lv, Q.; Wang, J. Design, Synthesis and Antifungal Evaluation of Novel Pyrylium Salt in Vitro and in Vivo. Molecules 2022, 27, 4450. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed]
- Wijnants, S.; Vreys, J.; Van Dijck, P. Interesting Antifungal Drug Targets in the Central Metabolism of Candida Albicans. Trends in Pharmacological Sciences 2022, 43, 69–79. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida Albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Xiao, M.; Sun, Z.Y.; Kang, M.; Guo, D.W.; Liao, K.; Chen, S.C.; Kong, F.; Fan, X.; Cheng, J.W.; Hou, X.; et al. Five-Year National Surveillance of Invasive Candidiasis: Species Distribution and Azole Susceptibility from the China Hospital Invasive Fungal Surveillance Net (Chif-Net) Study. J Clin Microbiol 2018, 56. [Google Scholar] [CrossRef]
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N.A.R. Candida Albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition. Frontiers in Immunology 2017, 8. [Google Scholar] [CrossRef]
- Tits, J.; Cammue, B.P.A.; Thevissen, K. Combination Therapy to Treat Fungal Biofilm-Based Infections. Int. J. Mol. Sci. 2020, 21, 8873. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Eltanahy, A.; Omran, A.; Mansour, A.T.; Safhi, F.A.; Alwutayd, K.M.; Khamis, T.; Husseiny, W.A.; Ismail, S.H.; Yousefi, M.; et al. Chitosan Nanogel Aqueous Treatment Improved Blood Biochemicals, Antioxidant Capacity, Immune Response, Immune-Related Gene Expression and Infection Resistance of Nile Tilapia. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2024, 269, 110876. [Google Scholar] [CrossRef]
- Li, J.; Buchner, J. Structure, Function and Regulation of the Hsp90 Machinery. Biomed J 2013, 36, 106–117. [Google Scholar] [PubMed]
- Lee, K.-H.; Park, S.J.; Choi, S.J.; Park, J.Y. Proteus Vulgaris and Proteus Mirabilis Decrease Candida Albicans Biofilm Formation by Suppressing Morphological Transition to Its Hyphal Form. Yonsei Med. J. 2017, 58, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Dhillon, R.; Hughes, H.; Wise, M.P.; Backx, M. Covid-19 and Fungal Infection: The Need for a Strategic Approach. The Lancet Microbe 2020, 1, e196. [Google Scholar] [CrossRef] [PubMed]
- Maubon, D.; Garnaud, C.; Calandra, T.; Sanglard, D.; Cornet, M. Resistance of Candida Spp. To Antifungal Drugs in the Icu: Where Are We Now? Intensive Care Medicine 2014, 40, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A. Caspofungin Acetate: An Antifungal Agent. Am. J. Health-Syst. Pharm. 2001, 58, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Cooper, K.; Marriott, M.S.; Tarbit, M.H.; Troke, P.F.; Whittle, P.J. Discovery of Fluconazole, a Novel Antifungal Agent. Rev Infect Dis 1990, 12 Suppl 3, S267–S271. [Google Scholar] [CrossRef]
- Boken, D.J.; Swindells, S.; Rinaldi, M.G. Fluconazole-Resistant Candida Albicans. Clin Infect Dis 1993, 17, 1018–1021. [Google Scholar] [CrossRef]
- Pasquale, T.; Tomada, J.R.; Ghannoun, M.; Dipersio, J.; Bonilla, H. Emergence of Candida Tropicalis Resistant to Caspofungin. J. Antimicrob. Chemother. 2008, 61, 219. [Google Scholar] [CrossRef]
- Sawant, B.; Khan, T. Recent Advances in Delivery of Antifungal Agents for Therapeutic Management of Candidiasis. Biomed. Pharmacother. 2017, 96, 1478–1490. [Google Scholar] [CrossRef]
- Carmo, A.; Rocha, M.; Pereirinha, P.; Tomé, R.; Costa, E. Antifungals: From Pharmacokinetics to Clinical Practice. Antibiotics 2023, 12. [Google Scholar] [CrossRef]
- Wang, D.; Zetterström, C.E.; Gabrielsen, M.; Beckham, K.S.H.; Tree, J.J.; Macdonald, S.E.; Byron, O.; Mitchell, T.J.; Gally, D.L.; Herzyk, P.; et al. Identification of Bacterial Target Proteins for the Salicylidene Acylhydrazide Class of Virulence-Blocking Compounds*. Journal of Biological Chemistry 2011, 286, 29922–29931. [Google Scholar] [CrossRef] [PubMed]
- Tirapegui, C.; Acevedo-Fuentes, W.; Dahech, P.; Torrent, C.; Barrias, P.; Rojas-Poblete, M.; Mascayano, C. Easy and Rapid Preparation of Benzoylhydrazides and Their Diazene Derivatives as Inhibitors of 15-Lipoxygenase. Bioorganic & Medicinal Chemistry Letters 2017, 27, 1649–1653. [Google Scholar]
- Kozlov, M.V.; Konduktorov, K.A.; Shcherbakova, A.S.; Kochetkov, S.N. Synthesis of N′-Propylhydrazide Analogs of Hydroxamic Inhibitors of Histone Deacetylases (Hdacs) and Evaluation of Their Impact on Activities of Hdacs and Replication of Hepatitis C Virus (Hcv). Bioorganic & Medicinal Chemistry Letters 2019, 29, 2369–2374. [Google Scholar]
- Turan-Zitouni, G.; Altintop, M.D.; Ozdemir, A.; Demirci, F.; Abu Mohsen, U.; Kaplancikli, Z.A. Synthesis and Antifungal Activity of New Hydrazide Derivatives. J Enzyme Inhib Med Chem 2013, 28, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Hydrazide-Hydrazones with the 1-Adamantyl-Carbonyl Moiety. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Nam, G.; Suh, J.-M.; Yi, Y.; Lim, M.H. Drug Repurposing: Small Molecules against Cu(Ii)–Amyloid-Β and Free Radicals. Journal of Inorganic Biochemistry 2021, 224, 111592. [Google Scholar] [CrossRef]
- Ganrot, P.O.; Rosengren, E.; Gottfries, C.G. Effect of Iproniazid on Monoamines and Monamine Oxidase in Human Brain. Experientia 1962, 18, 260–261. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Drewe, J.; Tseng, B.; Kasibhatla, S.; Cai, S.X. Discovery and Sar of Indole-2-Carboxylic Acid Benzylidene-Hydrazides as a New Series of Potent Apoptosis Inducers Using a Cell-Based Hts Assay. Bioorganic & Medicinal Chemistry 2004, 12, 3649–3655. [Google Scholar]
- Wu, Y.-Y.; Shao, W.-B.; Zhu, J.-J.; Long, Z.-Q.; Liu, L.-W.; Wang, P.-Y.; Li, Z.; Yang, S. Novel 1,3,4-Oxadiazole-2-Carbohydrazides as Prospective Agricultural Antifungal Agents Potentially Targeting Succinate Dehydrogenase. Journal of Agricultural and Food Chemistry 2019, 67, 13892–13903. [Google Scholar] [CrossRef]
- Clements, J.S.; Islam, R.; Sun, B.; Tong, F.; Gross, A.D.; Bloomquist, J.R.; Carlier, P.R. N′-Mono- and N, N′-Diacyl Derivatives of Benzyl and Arylhydrazines as Contact Insecticides against Adult Anopheles Gambiae. Pesticide Biochemistry and Physiology 2017, 143, 33–38. [Google Scholar] [CrossRef]
- de Sousa, E.S.O.; Cortez, A.C.A.; de Souza Carvalho Melhem, M.; Frickmann, H.; de Souza, J.V.B. Factors Influencing Susceptibility Testing of Antifungal Drugs: A Critical Review of Document M27-A4 from the Clinical and Laboratory Standards Institute (Clsi). Brazilian Journal of Microbiology 2020, 51, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Lv, Q.-Y.; Fu, J.; Zhang, T.-Y.; Du, Y.-S.; Yang, X.-J.; Zhou, L. New 7-Chloro-9-Methyl-2-Phenyl-3,4-Dihydro-Β-Carbolin-2-Iums as Promising Fungicide Candidates: Design, Synthesis, and Bioactivity. Journal of Agricultural and Food Chemistry 2022, 70, 4256–4266. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Gardner, I.; Obach, R.S.; Shaffer, C.L.; Callegari, E.; Henne, K.R.; Mutlib, A.E.; Dalvie, D.K.; Lee, J.S.; Nakai, Y.; et al. A Comprehensive Listing of Bioactivation Pathways of Organic Functional Groups. Curr Drug Metab 2005, 6, 161–225. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, T.; Abe, H.; Kotouge, Y.; Matsubuchi, M.; Sugou, M.; Honma, C.; Tsukuta, K.; Satoh, S.; Shioya, T.; Nakamura, H.; et al. Live-Cell Imaging of Septins and Cell Polarity Proteins in the Growing Dikaryotic Vegetative Hypha of the Model Mushroom Coprinopsis Cinerea. Scientific Reports 2023, 13, 10132. [Google Scholar] [CrossRef]
- Triastuti, A.; Vansteelandt, M.; Barakat, F.; Amasifuen, C.; Jargeat, P.; Haddad, M. Untargeted Metabolomics to Evaluate Antifungal Mechanism: A Study of Cophinforma Mamane and Candida Albicans Interaction. Natural Products and Bioprospecting 2023, 13, 1. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Yang, S.-S.; Zhang, Q.; Zhang, T.-T.; Zhang, T.-Y.; Zhou, B.-H.; Zhou, L. Discovery of N-Phenylpropiolamide as a Novel Succinate Dehydrogenase Inhibitor Scaffold with Broad-Spectrum Antifungal Activity on Phytopathogenic Fungi. Journal of Agricultural and Food Chemistry 2023, 71, 3681–3693. [Google Scholar] [CrossRef]
- Jacob, N.; Guillemard, L.; Wencel-Delord, J. Highly Efficient Synthesis of Hindered 3-Azoindoles Via Metal-Free C-H Functionalization of Indoles. Synthesis 2020, 52, 574–580. [Google Scholar] [CrossRef]
- Afri, M.; Frimer, A.A.; Cohen, Y. Active Oxygen Chemistry within the Liposomal Bilayer Part Iv: Locating 2',7'-Dichlorofluorescein (Dcf), 2',7'-Dichlorodihydrofluorescein (Dcfh) and 2',7'-Dichlorodihydrofluorescein Diacetate (Dcfh-Da) in the Lipid Bilayer. Chem. Phys. Lipids 2004, 131, 123–133. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Jia, X.; Xin, L.; Zhai, H. Antifungal Effects and Potential Mechanism of Essential Oils on Collelotrichum Gloeosporioides in Vitro and in Vivo. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]

| Compd. | R1 | R2 | C. alb.SC5314 | C. alb.4395 | C. alb.5122 | C. alb.5172 | C. alb.5272 | TAI | ||||
| A1 | H | H | 26.0 | 15.0 | 0.9 | >64.0 | >64.0 | <1.76 | ||||
| A2 | 2-F | H | 13.0 | 4.1 | 8.5 | 6.7 | 12.1 | 1.79 | ||||
| A3 | 2-Me | H | 49.3 | >64.0 | 13.3 | 21.2 | 53.0 | N. A.d | ||||
| A4 | 2-OMe | H | 14.7 | 31.0 | 5.7 | 7.8 | 6.4 | 1.61 | ||||
| A5 | 2-Cl | H | 25.3 | 52.5 | 10.8 | 13.8 | 13.0 | 1.19 | ||||
| A6 | 2-CF3 | H | 11.7 | 4.5 | 6.3 | 12.0 | 22.1 | 1.66 | ||||
| A7 | 2-Me&4-Cl | H | 26.0 | >64.0 | >64.0 | 23.4 | 63.0 | N. A. | ||||
| A8 | 3-F | H | 3.4 | 9.7 | 0.7 | >64.0 | 27.0 | N. A. | ||||
| A9 | 3-Me | H | 25.3 | 61.4 | 10.5 | 14.7 | 17.8 | 1.13 | ||||
| A10 | 3-Cl | H | 6.9 | 6.6 | 15.0 | 12.3 | 59.0 | 1.44 | ||||
| A11 | 3-CF3 | H | 1.9 | 4.0 | 2.8 | 7.4 | 3.7 | 2.71 | ||||
| A12 | 3,5-diF | H | 3.6 | 25.0 | 1.6 | >64.0 | >64.0 | N. A. | ||||
| A13 | 4-Me | H | 13.9 | 31.0 | 5.0 | 14.6 | 27.0 | 1.35 | ||||
| A14 | 4-OMe | H | 5.9 | 32.0 | 1.5 | >64.0 | >64.0 | N. A. | ||||
| A15 | 4-Cl | H | 5.9 | 32.0 | 1.5 | >64.0 | >64.0 | N. A. | ||||
| A16 | 4-CN | H | 47.5 | 15.0 | 6.3 | 5.8b | 7.2 | 1.6 | ||||
| A17 | 4-CF3 | H | >64.0 | 15.0 | 4.6 | 9.6 | 9.3 | N. A. | ||||
| A18 | 4-Br | H | 24.4 | >64.0 | 7.4 | 41.4 | >64.0 | N. A. | ||||
| A19 | 4-iPr | H | >64.0 | 61.3 | >64.0 | >64.0 | 13.5 | N. A. | ||||
| A20 | 4-NO2 | H | 6.7 | 16 | 0.8 | >64.0 | >64.0 | N. A. | ||||
| B1 | H | 2-Cl | 26.2 | 52.3 | 13.0 | 14.0 | 12.5 | 1.16 | ||||
| B2 | H | 3,5-diCl | 45.3 | >64.0 | 32.2 | 45.0 | >64.0 | N. A. | ||||
| B3 | H | 3-Cl | 6.9 | 19.0 | 0.7 | >64.0 | >64.0 | N. A. | ||||
| B4 | H | 3-CF3 | >64.0 | >64.0 | 31.5 | 58.6 | >64.0 | N. A. | ||||
| B5 | H | 3-Br | 24.6 | 60.0 | 3.6 | 13.9 | 6.3 | 1.52 | ||||
| B6 | H | 4-I | 16.7 | >64.0 | 31.8 | 30.0 | 19.0 | N. A. | ||||
| B7 | H | 4-Me | 13.4 | 32.0 | 21.5 | 25.0 | 13.2 | 1.14 | ||||
| B8 | H | 4-OMe | 9.2 | 22.0 | 0.8 | >64.0 | >64.0 | N. A. | ||||
| B9 | H | 4-CN | >64.0 | >64.0 | >64.0 | >64.0 | 62.6 | N. A. | ||||
| B10 | H | 4-CF3 | >64.0 | >64.0 | >64.0 | >64.0 | >64.0 | N. A. | ||||
| B11 | H | 4-Cl | 10.7 | 12.3 | 4.8 | 7.1 | 57 | 1.56 | ||||
| B12 | H | 4-Br | 47.5 | 14.7 | 50.4 | 30 | 13.1 | 1.00 | ||||
| B13 | H | 4-iPr | 14.3 | >64.0 | 21.0 | 52.7 | >64.0 | N. A. | ||||
| B14 | H | 4-F | 12.2 | 4.0 | 3.5 | 3.3 | 14.7 | 2.13 | ||||
| C1 | PhO | H | 31.7 | 14.3 | 11 | 6.7 | 7.2 | 1.50 | ||||
| C2 | PhMe | H | 3.4 | 42 | 1.7 | >64 | >64 | N. A. | ||||
| C3 | 2-furyl | H | 17.5 | 45.8 | 2.7 | 7.3 | 13 | 1.64 | ||||
| C4 | Cyclopropyl | H | 58.9 | 8.9 | 7.7 | 3.9 | 3.8 | 1.85 | ||||
| C5 | 1-naphthyl | H | >64.0 | >64.0 | 62.0 | >64.0 | >64.0 | N. A. | ||||
| C6 | 2-naphthyl | H | 14.6 | >64.0 | 51.8 | 58.7 | >64.0 | N. A. | ||||
| C7 | 2-thienyl | H | 14.2 | 60.0 | 7.0 | 16.5 | 42.0 | 1.17 | ||||
| C8 | 3-thienyl | H | 13.9 | 22.6 | 11.6 | 14.7 | >64.0 | N. A. | ||||
| C9 | 3-indolyl | H | >64.0 | >64.0 | >64.0 | 1.4 | >64.0 | N. A. | ||||
| C10 | PhEt | H | 51.8 | 16.1 | 15.0 | >64.0 | 11.0 | N. A. | ||||
| C11 | PhPr | H | >64.0 | >64.0 | 22.8 | >64.0 | 15.2 | N. A. | ||||
| C12 | Et | H | >64.0 | >64.0 | 13.8 | >64.0 | 12.1 | N. A. | ||||
| C13 | amyl | H | >64.0 | 42.6 | 34.4 | >64.0 | 28.5 | N. A. | ||||
| D1 | 4-Cl | 4-F | 12.2 | 5.5 | 6.4 | 3.7 | 29.5 | 1.81 | ||||
| D2 | 3-F | 3-Cl | >64.0 | 13.8 | 9.6 | >64.0 | 7.6 | N. A. | ||||
| D3 | 3-CF3 | 4-F | 63.6 | >64 | 3.8 | >64.0 | 7.6 | N. A. | ||||
| D4 | 3,5-diF | 4-F | 30.4 | 13.5 | 6.3 | >64.0 | 7.6 | N. A. | ||||
| D5 | 3,5-diF | 3-Cl | 64 | 29.3 | 2.2 | 2.7 | 2.4 | 2.25 | ||||
| FLC | 1.8a | >128.0 | 58.0 | >64.0 | >128.0 | <1.19c | ||||||
| Compd. | SC5314 | 4395 | 5122 | 5172 | 5272 |
|---|---|---|---|---|---|
| A | 6.4 | 5.4 | 11.6 | 5.8 | 5.2 |
| B | 5.0 | 4.8 | 12.0 | 4.8 | 5.0 |
| C | 5.0 | 4.2 | 6.4 | 7.8 | 5.8 |
| D | 3.4 | 5.8 | 9.2 | 11.2 | 7.6 |
| FLC | 14.8 | <1.8a | 2.6 | <2.6 | <1.8a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





