1. Introduction
Paternally expressed 10 (
PEG10) [
1] and
PEG11/Retrotransposon Gag-like 1(
RTL1) [
2], together with
syncytin [
3,
4]
, opened the door to a new field of research on retrovirus-derived genes in mammalian development and evolution. The fact that these are all essential endogenous genes despite their retroviral origin [
3,
4,
5,
6,
7,
8] has had a huge impact not only on genome biology but also on developmental and evolutionary biology, because retrotransposons, including endogenous retroviruses (ERVs), had long been construed to be “junk” in the mammalian genome.
The discovery of retroviral
ENV-derived
syncytin in primates stimulated the search for similar genes in primates as well as other lineages of eutherians and marsupials [
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. The discovery of essential functions for the retroviral
GAG and
POL-derived
PEG10 and
PEG11/RTL1 [
5,
6,
19] led to the further screening of RTL/sushi-ichi retrotransposon homolog (SIRH) genes in eutherians (
Figure 1) [
5,
20,
21,
22].
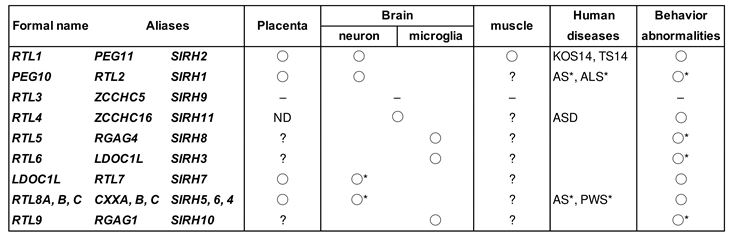
To date, 10 out of the 11 RTL/SIRH genes, including
PEG10 and
PEG11/RTL1, have been shown to play important roles in eutherian development, such as in the placenta, brain and innate immune system (
Table 1) [
23,
24,
25,
26,
27,
28]. In addition, important roles for
PEG11/RTL1 in muscle development [
29] and brain function [
30] as well as an important role for
PEG10 in the placenta in mid to late gestation [
31] have been elucidated.
PEG10 has also been implicated as the causative gene in Angelman syndrome [
32] and amyotrophic lateral sclerosis (ALS) [
33,
34]. In this review, we focus on the
PEG10,
PEG11/RTL1 and RTL/SIRH genes, summarizing recent advances in their investigation and discuss their roles in eutherian development and evolution.
2. Discovery of PEG10 and PEG11/RTL1 from genomic imprinting research
Genomic imprinting, a term which describes the functional differences between the paternally and maternally derived genomes, was discovered by pronuclear transplantation experiments in mice in 1984. Three groups independently demonstrated that parthenogenetic embryos with two maternally derived genomes exhibit early embryonic lethality due to severe placental defects, while androgenetic embryos with two paternally derived genomes exhibit severe embryonic growth retardation associated with an overgrown placenta [
35,
36,
37]. Extensive genetic analysis of mice with Robertsonian translocations of specific chromosomal loci also revealed functional differences between the parental chromosomes associated with early to late embryonic and postanal lethality as well as growth abnormalities [
38,
39]. The presence of imprinted genes with monoallelic paternal or maternal expression causes these genomic imprinting phenotypes [
40,
41,
42,
43,
44].
PEG10 and
PEG11/RTL1 have been identified as paternally expressed imprinted genes on human chromosome 7q21 and its orthologous mouse proximal chromosome 6 [
1,
45], and on the distal end of sheep chromosome 18 [
2], respectively. It is known that maternal duplication of proximal chromosome 6 results in early embryonic lethality [
39].
Peg10 is responsible for this early embryonic lethal phenotype as well as parthenogenetic death due to severe placental dysplasia [
5]. An inheritable form of muscular hypertrophy, called the callipyge phenotype, is mapped to the distal end of sheep chromosome 18 [
46]. In humans and mice, paternal and maternal duplication of its orthologous imprinted region, human chromosome 14 and mouse distal chromosome 12, cause Kagami-Ogata (KOS14) and Temple syndromes (TS14), two genomic imprinting disorders, and late embryonic/neonatal lethal phenotypes associated with growth abnormalities, respectively [
39,
47,
48,
49,
50,
51].
PEG11/RTL1, together with
Delta-like 1 homologue (
DLK1) are the major genes responsible for KOS14 and TS14 as well as the abnormal phenotypes in mice caused by paternal and maternal duplication of distal chromosome 12 [
6,
18,
29,
30,
52,
53,
54]. In sheep,
PEG11/RTL1 and
DLK1 also cause the callipyge phenotype [
55,
56]. Thus,
PEG10 and
PEG11/RTL1 are the genes responsible for certain abnormal imprinting phenotypes in eutherian mammals.
Both
PEG10 and
PEG11/RTL1 have homology to GAG and POL of the sushi-ichi long terminal repeat (LTR) retrotransposon [
1,
2,
57,
58,
59]. Therefore, they were originally thought to be derived from the sushi-ichi retrotransposon, and were thus named RTL and/or SIRH. However, it is reasonable to assume that they were originally derived from the GAG and POL of a certain extinct retrovirus having a high degree of homology to the suchi-ichi retrotransposon [
26,
60], since
PEG10 arose in a common therian ancestor and
PEG11/RTL1 and the other RTL/SIRH genes also arose in a common eutherian ancestor [
22,
61,
62], while the gypsy retrotransposon, which includes the sushi-ichi retrotransposon, is an infectious retrovirus in
Drosophila melanogaster [
63,
64].
2.1. Roles of PEG10 and PEG11/RTL1 in placental evolution in mammals
As we have already reviewed the essential roles of
PEG10 and
PEG11/RTL1 in the placenta elsewhere [
22,
40,
44,
60,
65], we here briefly summarize these points and focus on another placental role of
PEG10 as well as the possible interaction with
PEG11/RTL1, and discuss their roles in the evolution of the placenta in mammals.
The PEG10 protein is expressed all of the trophoblast cell lines in the placenta. Paternal transmission of the
Peg10 KO allele (hereafter referred to as
Peg10 KO) causes early embryonic lethality due to poor placental growth associated with a complete lack of the labyrinth and spongiotrophoblast layers, because only the paternal allele of
Peg10 is active, while its maternal allele is repressed by the genomic imprinting mechanism [
5]. As the labyrinth layer is an essential part of the placenta, where nutrient and gas exchange occur between fetal and maternal blood cells,
Peg10 KO embryos cannot grow beyond 9.5 days post coitus (dpc).
Paternal transmission of the
Peg11/RTL1 KO allele (hereafter referred to as
Peg11/Rtl1 Pat-KO) causes late fetal/neonatal lethality associated with late fetal growth retardation, while maternal transmission of the
Peg11/RTL1 KO allele (hereafter referred to as
Peg11/Rtl1 Mat-KO) causes neonatal lethality associated with abnormal fetal growth due to the overexpression of
Peg11/Rtl1 [
6,
52] This is because of the presence of maternally expressed
antiPeg11/antiRtl1, a non-coding RNA encoding 7 microRNAs (miRNAs) that target
Peg11/Rtl1 mRNA via an RNAi mechanism [
66,
67,
68]. The PEG11/RTL1 protein is restricted to expression in the endothelial cells of the fetal capillaries in the labyrinth layer of the placenta. Severe abnormalities of the fetal capillaries were observed in both the
Peg11/Rtl1 Pat- and Mat-KO placenta. In the former case, fetal capillary endothelial cells were clogged at many sites by an attack from surrounding trophoblast cells, while in the latter, the surrounding trophoblast cells were severely damaged, indicating that the PEG11/RTL1 protein plays an essential role in maintaining the feto-maternal interface of the placenta during pregnancy.
PEG10 retains the CCHC RNA-biding motif in GAG-like ORF1 and the DSG viral aspartic protease motif in POL-like ORF2. Unexpectedly, in contrast to the
Peg10 KO mice, the mice with the mutated DSG motif exhibit perinatal lethality (
Figure 2) [
31] like the
Peg11/Rtl1 Pat- and Mat-KO mice described above. A point mutation introduced into the PEG10 DSG motif by replacing aspartic acid (D) with an alanine (A) residue using the CRISPR-Cas9 system (hereafter referred to as
Peg10-ASG mutant) resulted in loss of the self-cleavage activity of PEG10. These
Peg10-ASG mutant mice exhibited embryonic and placental growth retardation from around 12.5 dpc, and about half of them died at 18.5 dpc (
Figure 2A). Severe inflammation was detected around the fetal vasculature in the labyrinth layer of the mutant placenta (
Figure 2B) [
31]. In the labyrinth layer, PEG10 is expressed in all three layers of trophoblast cells, the two layers of syncytiotrophoblast (SynT-I and II) cells and one layer of mononucleated sinusoidal trophoblast giant cells (s-TGCs) that surround the fetal capillary endothelial cells where PEG11/RTL1 is expressed [
6,
52].
These results demonstrate that not only the PEG11/RTL1 expression in endothelial cells but also the PEG10 expression in the surrounding trophoblast cells are essential for the maintenance of the fetal capillaries during mid to late gestation, although exactly what PEG10 and PEG11/RTL1 are doing there remains elusive at present. It is also apparent that
PEG10 has multiple essential functions in the placenta, and two of these functions, placental formation and maintenance of its fetal capillaries, were critical for the emergence of the chorioallantoic placenta in eutherian mammals.
PEG10 is a therian-specific gene [
60] and therefore corresponds to the emergence of viviparity in therian mammals: Marsupials have a choriovitelline (yolk sac) placenta [
69], while eutherians have evolved a chorioallantoic placenta that allows for longer gestation via the generation of a novel feto-maternal interface, presumably through a collaborative interaction between PEG10 and the newly exapted eutherian-specific PEG11/RTL1.
2.2. Roles of PEG11/RTL1 in muscle development
PEG11/RTL1 is one of the major causative genes for the imprinting diseases KOS14 and TS14, which are caused by abnormal regulation of the imprinting region, that is, paternal and maternal disomy of human chromosome 14, respectively [
19,
47,
48,
49,
50,
51]. The former is characterized by neonatal lethality with respiratory failure, placentomegaly, polyhydramnios, developmental delay and/or intellectual disability as well as feeding difficulties [
48,
50], whereas the latter is characterized by prenatal and postnatal growth retardation, feeding difficulties, muscle hypotonia, motor delay, early onset of puberty and mild intellectual disability [
49,
51]. Their phenotypes are quite different, but their sites of damage are quite similar, i.e., in the muscle and brain as well as the placenta.
Importantly,
Peg11/Rtl1 Mat- and Pat-KO mice that respectively overexpress and lack PEG11/RTL1 expression, are very good models for KOS14 and TS14, not only in the placenta [
6,
19] but also in the muscle and brain as well (see next section) [
29,
30]. In skeletal muscle, expression of the PEG11/RTL1 protein is restricted to the late fetal and neonatal stages, and is therefore not detectable after 2 weeks of age, even in adults [
29]. In the neonatal stage,
Peg11/Rtl1 Mat-KO mice had a significantly larger muscle fiber size, while
Peg11/Rtl1 Pat-KO mice had significantly thinner muscle fibers. However, after fixation, the muscle fibers of the
Peg11/Rtl1 Mat-KO mice exhibited severe shrinkage and detachment from the extracellular matrix (ECM) muscle, indicating that it is immature and more fragile than normal muscle (
Figure 3A). In vivo experiments with cultured cells indicated that PEG11/RTL1 affects the proliferation of satellite cells (SCs) and the structural strength of SC-differentiated myoblasts, because myoblasts differentiated from SCs of Pat and Mat-KO mice clearly exhibited weak or low myoblast structural strength [
29]. This is consistent with the muscle-related defects in the KOS 14 and TS14 patients, such as respiratory failure and feeding difficulties in the former [
48,
50] and feeding difficulties, muscle hypotonia and motor delay in the latter [
49,
51].
In myocytes, PEG11/RTL1 partially colocalizes with DESMIN, a component of the sarcomere cytoskeleton that connects the sarcomere to membranes of the sarcolemma and the nucleus at the Z-disc (
Figure 3B) [
29], thus acting as the force-generating machinery in muscle. This suggests that PEG11/RTL1 plays some role in stabilizing the muscle contractile apparatus and/or regulating muscle contraction in the fetal/neonatal muscle fibers.
We assume that the intrinsically weak muscular strength and reduced movement of the fetus may be an adaptation to the long gestation period of the eutherian viviparous reproductive system, and is therefore a well conserved feature of eutherians, because ensuring a safe pregnancy is beneficial to both the mother and fetus. However, postnatal muscle PEG11 expression, which affects postnatal locomotor performance soon after birth, may be species-specific.
It should be noted that
PEG11/RTL1 and
DLK1 are the major genes that cause the sheep callipyge phenotype because
PEG11/RTL1 was first identified in the course of the sheep callipyge study [
2]. Both
PEG11/RTL1 and
DLK1 are critically involved in muscle development, and studies in transgenic mice also support this conclusion [
56,
70]. In sheep,
PEG11/RTL1 expression is relatively high until the late fetal stage, declines from just before birth, and is barely expressed after birth. The callipyge mutation recapitulates the normal fetal-like
PEG11/RTL1 expression program during postnatal development and this may contribute to the emergence of the muscle hypertrophy phenotype [
55].
2.3. PEG10 and PEG11/RTL1 in neurological disorders
KOS14 and TS14 patients exhibit certain neurodevelopmental disorders, such as developmental delay and/or intellectual disability and feeding difficulties in the former [
48,
50] and feeding difficulties, motor delay, early onset of puberty and mild intellectual disability in the latter [
49,
51].
DLK1 is critically involved in the early onset of puberty in TS14 [
53,
54], while
PEG11/RTL1 is responsible for the other neurodevelopmental phenotypes in these patients, as
Peg11/Rtl1 Mat- and Pat KO mice, which overexpress and lack PEG11/RTL1 expression, provide strong evidence for this conclusion [
30].
As in the case with muscle,
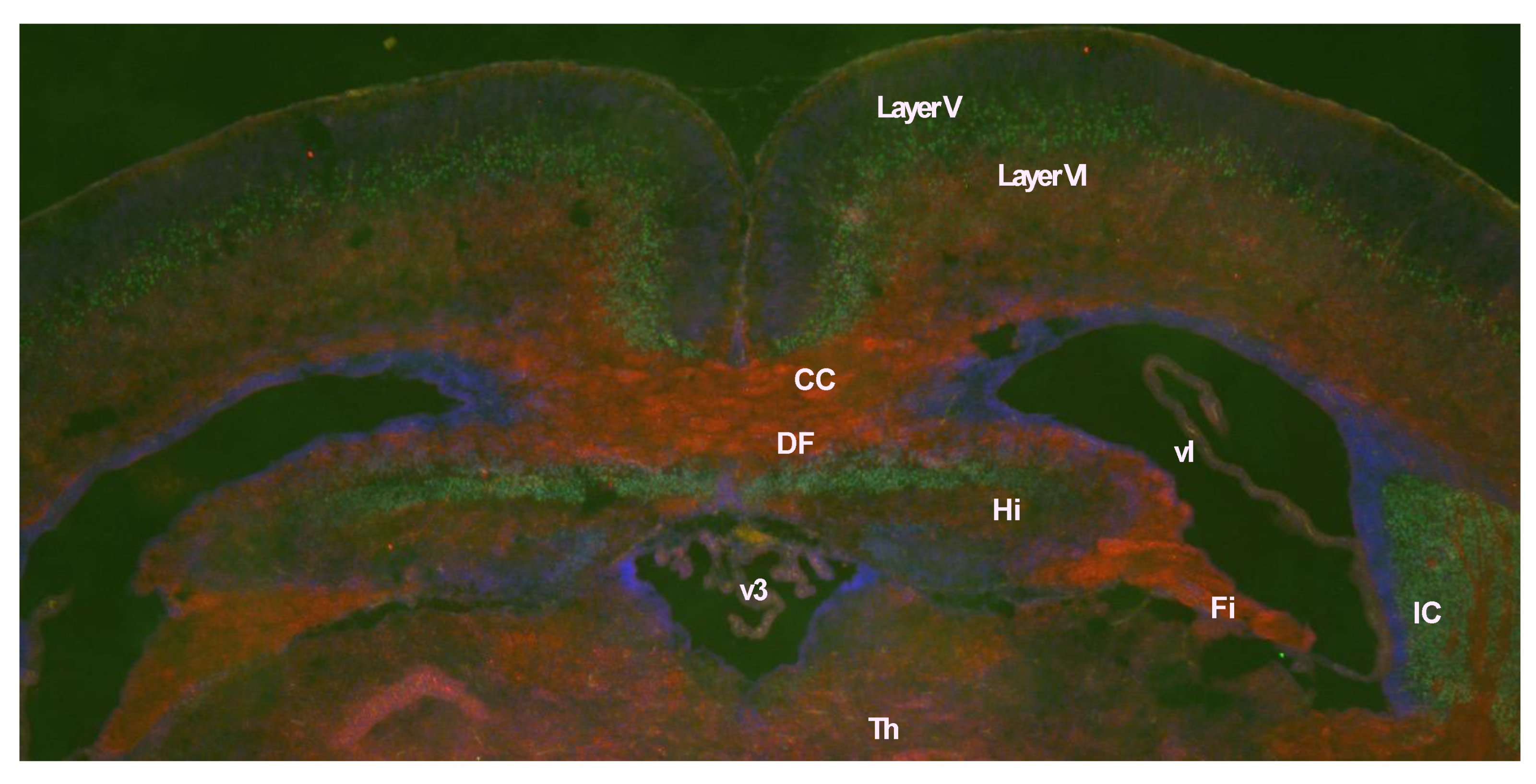
Peg11/Rtl1 mRNA expression in the central nervous system is restricted during the fetal to neonatal period, but at lower levels, and is barely detectable in adults. The PEG11/RTL1 protein is detected in the descending tracts, commissural fibers including the hippocampal commissure, corpus callosum as well as the limbic system, i.e., the hippocampal fimbria, fornix and medial amygdala nucleus (
Figure 4) [
30]. The corticospinal tract, one of the descending tracts, and the hippocampal commissureare mammalian-specific brain structures, whereas the corpus callosum is a eutherian-specific brain structure [
71,
72,
73]. The corticospinal tract runs from layer V of the neocortex to the brainstem and spinal cord and is responsible for fine voluntary skilled muscle movements of the limbs, while the hippocampal commissure is involved in hippocampal-dependent memory output [
30,
71,
74]. The corpus callosum is responsible for communication between the two hemispheres enabling faster transmission and integration of information from both sides [
71,
72,
73]. These results suggest that
PEG11/RTL1 is deeply involved in the functional evolution of the eutherian brain. A high expression level of PEG11/RTL1 is also reported in the locus coeruleus (LC), and decreased neuronal excitability and increased delay of action potential onset and inward currents in LC neurons have been reported in
Peg11/
Rtl1-deficient mice [
75].
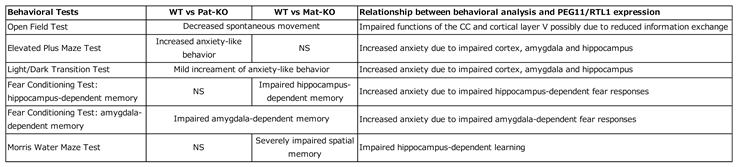
Peg11/Rtl1 Mat- and Pat-KO mice exhibit neurodevelopmental abnormalities corresponding to these expression sites, such as decreased spontaneous movement, increased anxiety-like behavior, and learning and memory impairments (
Table 2) [
30]. These symptoms suggest impairment of the corticospinal tract involved in trunk and limb movement, and/or the corpus callosum, hippocampal commissure and medial amygdala nucleus. It is likely that they are also associated with the developmental delay and intellectual disability observed in KOS14 and TS14 patients [
30]. It should be noted that maternally expressed
antiPeg11/antiRtl1 is also an important factor because it encodes seven miRNAs that regulate the
Peg11/Rtl1 mRNA levels via an RNAi mechanism [
66,
67,
76].
Recently, PEG10 was implicated in a certain neurological disorder, Angelman syndrome (AS) [
32]. Pandya et al. demonstrated that the PEG10 protein accumulates in the neurons induced from AS patient iPS cells. AS is a severe neurodevelopmental genomic imprinting disorder, which is characterized by developmental delay, intellectual disability, severe language impairment, ataxia and other symptoms [
77,
78], and caused by the paternal uniparental disomy of chromosome 15 and/or the mutations of maternally expressed
ubiquitin-protein ligase E3A (
UBE3A). They identified PEG10 as a target of UBE3A and suggested that
PEG10 may be critically involved in the pathophysiology of AS, although further work will be required to determine how PEG10 is mechanistically involved in AS.
PEG10 has also been implicated in another neurological disorder, amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease characterized by progressive loss of motor function, typically in middle age [
79].
UBQLN2, a member of the ubiquilin family involved in proteasomal degradation, is the gene responsible for familial ALS [
80]. Whiteley and colleagues demonstrated that UBQLN2 facilitates the proteasome-dependent degradation of the PEG10-ORF1/2 protein, and that PEG10-ORF1/2 is specifically upregulated in the spinal cord of ALS patients compared to healthy controls [
33,
34]. In addition, changes in gene expression in axon remodeling are induced by a nuclear-localized PEG10 fragment excised by its own self-cleavage activity in POL-like PEG10-ORF2. These results suggest that PEG10-ORF1/2 accumulation is an important contributor to ALS disease progression. Intriguingly, UBQLN2 is a marker protein of stress granules, where PEG10 localizes under the stress conditions observed in the AS study [
32]. Therefore, it is possible that the pathogenic mechanism of AS and ALS may partially overlap and that PEG10 accumulation may also be a common mechanism causing other neuronal defects, including neurological disorders in which PEG10 has not yet been formally shown to be involved.
3. Retrovirus-derived RTL/SIRH genes as eutherian-specific genes
The demonstration of essential roles for
PEG10 and
PEG11/RTL1 stimulated the screening of similar retrovirus-derived genes, and as a result, in eutherians and marsupials 9 and 1 RTL/SIRH genes were respectively identified [
5,
20,
21,
81] (
Figure 1). Except for therian-specific
PEG10, all of the other SIRH/RTL, including
PEG11/RTL1, are eutherian-specific genes. It is conceivable that all of the SIRH/RTL genes originated from the same retrovirus, because the encoded proteins exhibit 20~30 % homology to the sushi-ichi retrotransposon GAG. It is also possible that eutherian-specific SIRH/RTL arose from cDNA retrotransposition from the PEG10 ORF1 (and sometimes ORF1/2) transcript because it is the oldest among the RTL/SIRH genes [
61]. Alternatively, some could have arisen from
PEG11/RTL1, because it might have emerged in a common therian ancestor like PEG10, but was lost in the marsupial lineage [
62].
However, as shown in
Figure 1, each protein has a unique amino acid sequence and length, and the SIRH/RTL genes have diverse functions. To date, 10 out of the 11 RTL/SIRH genes have been found to have essential and/or important functions in the current eutherian developmental system (Table 1).
3.1. RTL7/SIRH7 in the placenta
In addition to maternal-fetal exchange and maternal tolerance of fetopaternal antigens, the placenta is a major endocrine organ during pregnancy [
82].
RTL7/SIRH7 (formal name:
Leucine Zipper Down-Regulated in Cancer 1(
LDOC1)) is another essential placental gene like
PEG10 and
PEG11/RTL1. It is expressed in trophoblast lineages in early placental development and regulates trophoblast differentiation (
Figure 5A-J), and thus deeply involved in several types of hormone production in trophoblast cells during pregnancy [
83,
84].
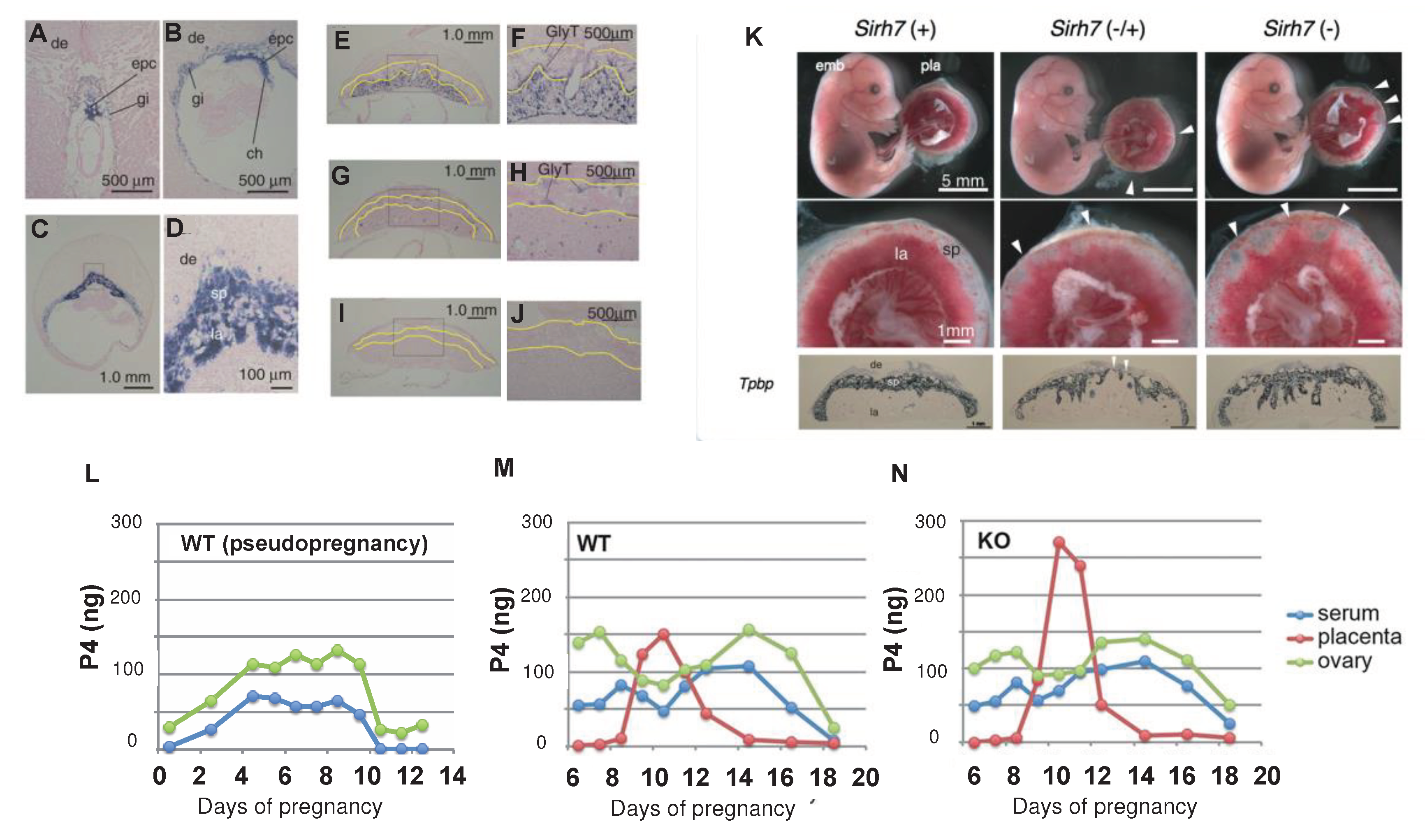
Rtl7/Sirh7 KO placentas have an irregular boundary between the spongiotrophoblast and labyrinth layers, as well as a decreased number of SpTs, although the fetuses appear normal (
Figure 5K). Female
Rtl7/Sirh7 KO exhibit delayed parturition due to residual progesterone (P4) in the serum on 18.5 dpc, one day before the parturition, and pups die due to inadequate maternal care [
23]. Since all pups are viable when foster mothers are used, it is indicated that the problems are due to the KO mothers.
As
Rtl7/Sirh7 is an X-linked gene, maternal transmission of the
Rtl7/Sirh7 KO allele results in a null phenotype in the placenta due to imprinted X-inactivation in the mouse placenta [
85,
86]. In
Rtl7/Sirh7 KO conceptuses, their placentas overproduce placental P4 (
Figure 5I-K), leading to a delayed transition from placental lactogen (PL)1 to PL2 in the giant trophoblast cells in the KO placenta, which presumably in turn leads to delayed downregulation of maternal ovarian P4 production in the late pregnancy, resulting in delayed parturition.
Although the ovary was thought to be the major P4 producing organ in in rodents throughout gestation [
82], P4 production is observed in rodent placenta during 9.5 to 11.5 dpc when a temporal reduction of serum P4 level occurs due to a shift from the corpus luteum of pseudopregnancy to pregnancy (
Figure 5L-N) [
87,
88], strongly indicating that placental P4 plays an important role in the maintenance of gestation at this critical stage and that
Rtl7/Sirh7 plays an important role in placental P4 production [
23]. In eutherians, the P4 production in the corpus luteum is regulated by the pituitary and/or placenta, whereas it is autonomous in marsupials and monotremes [
89]. Therefore, it will be of interest to elucidate how
Rtl7/Sirh7 functions in these processes at the molecular level because
Rtl7/Sirh7 is a eutherian-specific gene.
3.2. RTL4/SIRH11 in the brain.
RTL4/SIRH11 (aka
Zinc Finger CCHC Domain-Containing Protein 16 (
ZCCHC16) is a causative gene in autism spectrum disorders (ASD) [
90]. Lim et al. performed a comprehensive screening of patients with ASD and identified a family with a rare nonsense
ZCCHC16 mutation leading to ASD in a male proband and his male sibling, as it is an X-linked gene [
90].
In mice,
Sirh11/Zcchc16 KO mice exhibit increased impulsivity and decreased spatial memory, presumably due to low recovery of noradrenaline in the frontal cortex although they do not exhibit lethality or growth abnormalities [
24].
They do not adapt to routine processes and tend to exhibit extreme behavior when transferred to a new environment. They display agitated movements in their cages when staff personnel enter the breeding room, and sometimes jump out when the cages are changed, even after a long breeding period. In particular, they remained hyperactive for 5 consecutive nights in the home cage activity test, while normal control mice gradually settled to lower levels of activity (
Figure 6A). In the Light/Dark transition test, the latency before entering into the light chamber was significantly decreased, while the number of transitions was significantly increased, suggesting a reduced attention and/or enhanced impulsivity (
Figure 6B). In the Y-maze test, KO mice exhibited a lower success rate, suggesting that they have a poor working memory (
Figure 6C). This is likely due to low noradrenaline (NA) recovery in the frontal cortex (
Figure 6D), because the locus coeruleus (LC) NA neurons has been reported to play important roles in attention, behavioral flexibility and modulation of cognition [
91,
92,
93] andtheir activation occurs in concert with the cognitive shifts that facilitate dynamic reorganization of target neural networks, allowing rapid behavioral adaptation to the demands of changing environmental demands [
94], indicating that all of the behavioral defects of the
Sirh11/Zcchc16 KO mice are somehow related to a dysregulation of the noradrenergic system in the brain [
24].
RTL4/SIRH11 is a very important gene in neurodevelopment, and it is likely that it confers a critically important advantage both in the competition that occurs in daily life and in the evolution of the eutherian brain. However, because RTL4/SIRH11 expression is very low in the brain in both humans and mice, it remains unclear exactly where the RTL4/SIRH11 protein is expressed and what its function is.
3.3. RTL8A, 8B, 8C/SIRH5, 6, 4 in the brain
RTL8A, B, C/SIRH5, 6, 4 are triplet genes that encode almost identical proteins of 112 to 113 amino acids (aa). Their number (2-4, mostly 3, excluding pseudogenes) and aa sequence are well conserved in eutherians, suggesting they confer an evolutionary advantage. Therefore, it is of interest to know why they exist multiple genes and what their function is. However, in most cases the RTL8A-C/SIRH5, 6, 4 genes within the same species exhibit higher homology to each other than other species, suggesting that they are not in a precise orthologous relationship in eutherians, presumably due to independent gene conversion events in each species.
It was recently reported that the RTL8A, B, C
/SIRH5, 6, 4 proteins accumulated together with the PEG10 protein in the neuronal cells differentiated from iPS cells of AS patients (see also section 1.3) [
32]. AS is a neurodevelopmental genomic imprinting disorder which is characterized by delayed development, intellectual disability, severe speech impairment, ataxia and other symptoms [
77,
78]. It is caused by paternal uniparental disomy of chromosome 15 and/or mutations of a maternally expressed
UBE3A gene. This implies that the RTL8A, B, C/SIRH5, 6, 4 and PEG10 proteins are directly targeted by UBE3A in neuronal cells.
Rtl8a, b/Sirh5, 6 double KO mice exhibit late onset obesity and depression-like behavior, demonstrating they are also important brain genes [
27]. These phenotypes correlate well with their protein expression sites, such as the hypothalamus and prefrontal cortex, the control centers for appetite [
95,
96,
97] and depression [
98,
99,
100], respectively. It is clear that decreased expression of RTL8A, B, C/SIRH5, 6, 4 proteins causes neurodevelopmental disorders, and it is likely that overexpression of these proteins also plays some role in AS. Since no such behavioral abnormalities are observed in
Rtl8c/Sirh4 single KO mice, it is likely that they have a gene dosage effect on neuronal development. Ongoing analysis of the
Rtl8a, b, c/Sirh5, 6, 4 triple KO mice should provide an answer to this question.
3.4. RTL6/SIRH3, RTL5/SIRH8 and RTL9/SIRH10 are microglial genes in the brain.
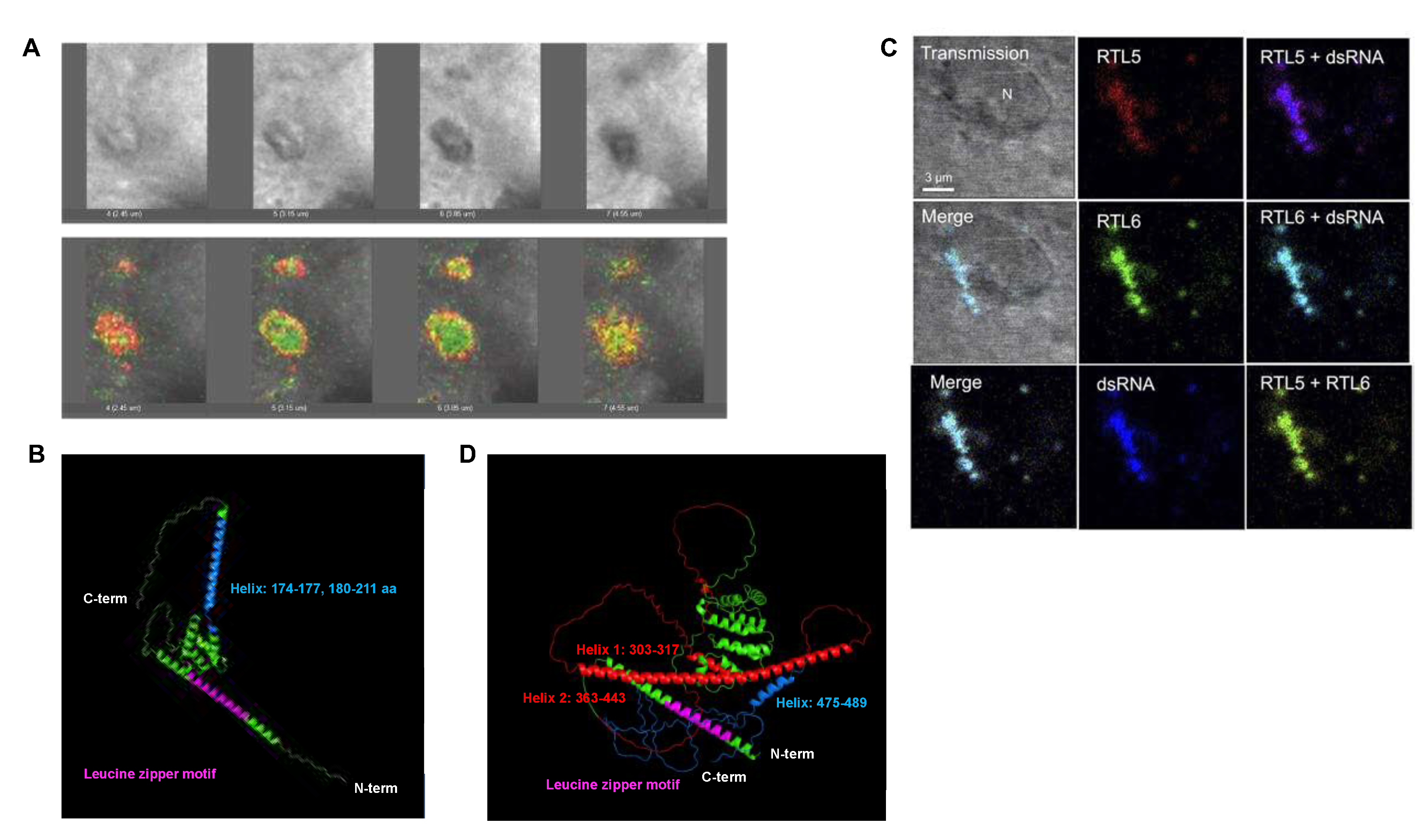
RTL6/SIRH3 (aka
LDOCKL), which encodes an extremely basic protein (pI=11.15), is the most conserved gene among the RTL/SIRH genes, with a non-synonymous/synonymous (dn/ds) rate of less than 0.1. Despite its evolutionary importance, it has been very difficult to identify the RTL6/SIRH3 protein because of the lack of effective antibodies. Analysis of
Rtl6-CV KI mice, in which a Venus ORF is integrated into the endogenous
Rtl6/Sirh3 locus immediately after the C-terminus, demonstrated that the RTL6/SIRH3 protein is expressed in the central nervous system during development and that it is secreted by microglia and responds to LPS (
Figure 7A). It was subsequently demonstrated in
Rtl6/Sirh3 KO mice that the RTL6/SIRH3 protein functionally protects against bacteria by removing lipopolysaccharide (LPS) [
26]. Thus, the
Rtl6/Sirh3 gene plays an important role in the innate immune system in the brain. Because LPS is a highly acidic substance and an extremely dangerous pathogen, this explains why
RTL6/SIRH3 is extremely basic and highly conserved in eutherians (
Figure 7B), because the role of
RTL6/SIRH3 in LPS removal is critical.
RTL5/SIRH8 (aka
Retrotransposon Gag Domain-Containing Protein(
RGAG4)) is phylogenetically related to
RTL6/SIRH3 and well conserved in eutherians, despite there being some exceptions. It encodes a larger protein which covers the entire RTL6/SIRH3 and is strongly acidic (pI=4.39), and functions as another microglial gene in the innate immune system against viruses by removing double-stranded RNA from the brain [
26]. Analysis of
Rtl5-CmC KI mice in which an mCherry ORF is integrated into the endogenous
Rtl5/Sirh8 locus immediately after the C-terminus demonstrated that the RTL5/SIRH8 protein is also expressed in the brain and that the RTL5/SIRH8 protein is likewise secreted by microglia and responds to double-stranded (ds) RNA (
Figure 7C). Subsequently, it was demonstrated in
Rtl5/Sirh8 KO mice that the RTL5/SIRH8 protein functions against viruses by removing dsRNA [
26]. This explains why
RTL5/SIRH8 is strongly acidic and well conserved in eutherians (
Figure 7D), because the role of
RTL5/SIRH8 in dsRNA removal is also of critical importance.
Using the same approach of combining Venus KI mice and KO mice,
RTL9/SIRH10 (aka
RGAG1) was demonstrated to be another microglial gene that is actively protective against fungi by reacting to zymosan, the cell wall of fungi [
28]. It encodes a large protein comprising two herpes virus-derived domains in addition to the GAG-like domain. The role of the first two regions remains unknown, but the latter is essential for zymosan removal. Unlike RTL6/SIRH3 and RTL5/SIRH8, RTL9/SIRH10 is restricted to the lysosomes of microglia, where the zymosan is ultimately taken up and degraded. Thus, at least three RLT/SIRH genes are involved in the clearance of bacterial, viral and fungal pathogens from the brain, suggesting that these genes must have critically contributed to the evolution of the innate immune system in eutherians [
26,
28].
All of these studies clearly demonstrate that domestication of retrovirus-derived genes made important contributions to the generation of eutherian-specific features in the placenta and brain. Microglia originate from the yolk sac during early development and eventually become permanently resident in the brain. Therefore, it is of interest to notice that the placenta and yolk sac, the extraembryonic tissues in intrauterine development, evidently serve as origin sites for the incubation of such retrovirus-derived genes, including both
PEG10 and
PEG11/RTL1, in the course of mammalian evolution [
22,
26,
28,
43].
4. Two types of exapted genes: acquired RTL/SIRH genes and captured syncytin genes
As mentioned in the introductory session,
PEG10,
PEG11/RTL1 and other RTL/SIRH genes are thought to be derived from GAG and POL of an extinct retrovirus.
PEG10 emerged between 164 and 148 MYA, after the diversification from monotremes and before the split between eutherians and marsupials [
61], while
PEG11/RTL1 and the other RTL/SIRH genes emerged between 148 and 120 MYA, after the diversification from marsupials and before the emergence of common eutherian ancestor [
22,
62]. They completely lost LTR sequences at both ends and also an ENV gene, which were present at the time of the original retroviral insertions. Their encoded proteins share 20 to 30 % homology to the sushi-ichi retrotransposon, but are completely different proteins from the original GAG and POL, each with its own unique aa sequence and novel function. Hence, they are referred to as genes acquired from a retrovirus [
22].
In contrast,
ENV-derived
syncytins were sequentially domesticated in a lineage-specific manner after the establishment of the eutherians. For example,
syncytin-1 and
syncytin-2 emerged 40 and 20 MYA in primates from different retroviruses, respectively [
3,
4,
9]. They retain almost all of the ENV sequences of the original retrovirus and therefore have fusion activity, which is important for syncytiotrophoblast cell fusion in the placenta. The LTRs, GAG and POL sequences often persist in a remnant form due to severe mutations. Therefore, it is hypothesized that the
syncytin gene was replaced several times in each eutherian lineage by a newly integrated
ENV gene having superior fusion activity [
101,
102]. Therefore, they are called captured genes [
101]. Thus, there are two distinct ways to domesticate retrovirus-derived genes.
5. Conclusions and future prospects
The RTL/SIRH genes contributed to evolutionary modification, such as the emergence of the placenta, the development of highly organized brain functions and the improvement of the brain’s innate immune system in therian- and/or eutherian-specific ways. However, this may be just the tip of the iceberg, and more diverse functions will be elucidated in the future in other as-yet-to-be-identified retrovirus-derived genes. The concept of retrovirus-derived genes will also be important in determining how many protein-coding genes exist in humans. It is likely that there are many more primate- or human-specific genes derived from retroviruses and that they may play an important role in certain primate- and/or human-specific traits.
The exaptation of retrovirus-derived genes implies the robustness and/or plasticity of the organism. The fact that many retrovirus-derived genes have been identified in eutherians suggests that eutherians may have greater robustness and/or plasticity than other vertebrates, or may simply reflect the fact that the eutherian genome has been more extensively analyzed than the others. It is likely that extraembryonic tissues, such as the placenta and yolk sac, served as origin site for the retrovirus-derived genes because they have lower levels of DNA methylation, which represses the expression of endogenous retroviruses and retrotransposons. This may have increased the chances for exaptation of retrovirus-derived genes in eutherians.
It is of considerable interest to establish how the retrovirus-derived genes function and why the GAG proteins are selected for such diverse roles. What is the function of their CCHC RNA-binding motif and DSG protease motif? Do they function as enzymes or as structural proteins, or as virus-like particles (VLPs)? Recently, PEG10 and other GAG-like proteins have been shown to self-assemble into VLPs [
103,
104]. Taking advantage of this property, Segel et al. developed a novel specific RNA deliver system called selective endogenous encapsidation for cellular delivery (SEND) [
104]. However, caution should be exercised in its use because overexpression of PEG10 itself exerts untoward effects, as seen in AS and ALS. The history of how they became mutated and were selected is also of great interest, as is the question of how they made an evolutionary contribution. In conclusion, the genes derived from the retroviruses will open a new window on the relationship between development and evolution in organisms, the question of how ontogeny and phylogeny are interrelated.
Author Contributions
FI and TK-I wrote the manuscript.
Funding
This work was supported by a funding program for Next Generation World-Leading Researchers (NEXT Program LS112) and Grants-in-Aid for Scientific Research (C) (17K07243 and 21K06127 to T.K.-I.) from the Japan Society for the Promotion of Science (JSPS), by Grants-in-Aid for Scientific Research (S) (23221010 to F.I.) and (A) (16H02478 and 19H00978 to F.I.) from JSPS, and by the Nanken Kyoten Program, Medical Research Institute, Tokyo Medical and Dental University to T.K.-I. and F.I. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank M Kitazawa-Fukuda for her help making Tables and all the collaborators and colleagues concerning researches on genomic imprinting and retrovirus-derived genes.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ono, R.; Kobayashi, S.; Wagatsuma, H.; Aisaka, K.; Kohda, T.; Kaneko-Ishino, T.; Ishino, F. A Retrotransposon-Derived Gene, PEG10, Is a Novel Imprinted Gene Located on Human Chromosome 7q21. Genomics 2001, 73, 232–237. [Google Scholar] [CrossRef]
- Charlier, C.; Segers, K.; Wagenaar, D.; Karim, L.; Berghmans, S.; Jaillon, O.; Shay, T.; Weissenbach, J.; Cockett, N.; Gyapay, G.; et al. Human–Ovine Comparative Sequencing of a 250-kb Imprinted Domain Encompassing the Callipyge (clpg) Locus and Identification of Six Imprinted Transcripts: DLK1, DAT, GTL2, PEG11, antiPEG11, and MEG8. Genome Res. 2001, 11, 850–862. [Google Scholar] [CrossRef]
- Blond, J.-L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.-L. An Envelope Glycoprotein of the Human Endogenous Retrovirus HERV-W Is Expressed in the Human Placenta and Fuses Cells Expressing the Type D Mammalian Retrovirus Receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.-P.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.-Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef]
- Ono, R.; Nakamura, K.; Inoue, K.; Naruse, M.; Usami, T.; Wakisaka-Saito, N.; Hino, T.; Suzuki-Migishima, R.; Ogonuki, N.; Miki, H.; et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat. Genet. 2005, 38, 101–106. [Google Scholar] [CrossRef]
- Sekita, Y.; Wagatsuma, H.; Nakamura, K.; Ono, R.; Kagami, M.; Wakisaka, N.; Hino, T.; Suzuki-Migishima, R.; Kohda, T.; Ogura, A.; et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat. Genet. 2008, 40, 243–248. [Google Scholar] [CrossRef]
- Dupressoir, A.; Vernochet, C.; Bawa, O.; Harper, F.; Pierron, G.; Opolon, P.; Heidmann, T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA 2009, 106, 12127–12132. [Google Scholar] [CrossRef]
- Dupressoir, A.; Vernochet, C.; Harper, F.; Guegan, J.; Dessen, P.; Pierron, G.; Heidmann, T. A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast. Proc. Natl. Acad. Sci. USA 2011, 108, E1164–E1173. [Google Scholar] [CrossRef]
- Blaise, S.; de Parseval, N.; Bénit, L.; Heidmann, T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. 2003, 100, 13013–13018. [Google Scholar] [CrossRef]
- Mallet, F.; Bouton, O.; Prudhomme, S.; Cheynet, V.; Oriol, G.; Bonnaud, B.; Lucotte, G.; Duret, L.; Mandrand, B. The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. Proc. Natl. Acad. Sci. 2004, 101, 1731–1736. [Google Scholar] [CrossRef]
- Heidmann, O.; Vernochet, C.; Dupressoir, A.; Heidmann, T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: a new "syncytin" in a third order of mammals. Retrovirology 2009, 6, 107–107. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Heidmann, O.; Degrelle, S.A.; Vernochet, C.; Lavialle, C.; Letzelter, C.; Bernard-Stoecklin, S.; Hassanin, A.; Mulot, B.; Guillomot, M.; et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc. Natl. Acad. Sci. USA 2013, 110, E828–E837. [Google Scholar] [CrossRef]
- Cornelis, G.; Vernochet, C.; Malicorne, S.; Souquere, S.; Tzika, A.C.; Goodman, S.M.; Catzeflis, F.; Robinson, T.J.; Milinkovitch, M.C.; Pierron, G.; et al. Retroviral envelope syncytin capture in an ancestrally diverged mammalian clade for placentation in the primitive Afrotherian tenrecs. Proc. Natl. Acad. Sci. 2014, 111, E4332–E4341. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Vernochet, C.; Carradec, Q.; Souquere, S.; Mulot, B.; Catzeflis, F.; Nilsson, M.A.; Menzies, B.R.; Renfree, M.B.; Pierron, G.; et al. Retroviral envelope gene captures and syncytin exaptation for placentation in marsupials. Proc. Natl. Acad. Sci. 2015, 112, 201417000–96. [Google Scholar] [CrossRef]
- Nakaya, Y.; Koshi, K.; Nakagawa, S.; Hashizume, K.; Miyazawa, T. Fematrin-1 Is Involved in Fetomaternal Cell-to-Cell Fusion in Bovinae Placenta and Has Contributed to Diversity of Ruminant Placentation. J. Virol. 2013, 87, 10563–10572. [Google Scholar] [CrossRef]
- Sugimoto, J.; Sugimoto, M.; Bernstein, H.; Jinno, Y.; Schust, D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci. Rep. 2013, 3, 01462. [Google Scholar] [CrossRef]
- Sugimoto, J.; Schust, D.J.; Kinjo, T.; Aoki, Y.; Jinno, Y.; Kudo, Y. Suppressyn localization and dynamic expression patterns in primary human tissues support a physiologic role in human placentation. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kitao, K.; Shoji, H.; Miyazawa, T.; Nakagawa, S. Dynamic Evolution of Retroviral Envelope Genes in Egg-Laying Mammalian Genomes. Mol. Biol. Evol. 2023, 40. [Google Scholar] [CrossRef]
- Kagami, M.; Sekita, Y.; Nishimura, G.; Irie, M.; Kato, F.; Okada, M.; Yamamori, S.; Kishimoto, H.; Nakayama, M.; Tanaka, Y.; et al. Deletions and epimutations affecting the human 14q32.2 imprinted region in individuals with paternal and maternal upd(14)-like phenotypes. Nat. Genet. 2008, 40, 237–242. [Google Scholar] [CrossRef]
- Brandt, J.; Schrauth, S.; Veith, A.-M.; Froschauer, A.; Haneke, T.; Schultheis, C.; Gessler, M.; Leimeister, C.; Volff, J.-N. Transposable elements as a source of genetic innovation: expression and evolution of a family of retrotransposon-derived neogenes in mammals. Gene 2005, 345, 101–111. [Google Scholar] [CrossRef]
- Youngson, N.A.; Kocialkowski, S.; Peel, N.; Ferguson-Smith, A.C. A Small Family of Sushi-Class Retrotransposon-Derived Genes in Mammals and Their Relation to Genomic Imprinting. J. Mol. Evol. 2005, 61, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kaneko-Ishino, T.; Ishino, F. The role of genes domesticated from LTR retrotransposons and retroviruses in mammals. Front. Microbiol. 2012, 3, 262. [Google Scholar] [CrossRef]
- Naruse, M.; Ono, R.; Irie, M.; Nakamura, K.; Furuse, T.; Hino, T.; Oda, K.; Kashimura, M.; Yamada, I.; Wakana, S.; et al. Sirh7/Ldoc1knockout mice exhibit placental P4 overproduction and delayed parturition. Development 2014, 141, 4763–4771. [Google Scholar] [CrossRef]
- Irie, M.; Yoshikawa, M.; Ono, R.; Iwafune, H.; Furuse, T.; Yamada, I.; Wakana, S.; Yamashita, Y.; Abe, T.; Ishino, F.; et al. Cognitive Function Related to the Sirh11/Zcchc16 Gene Acquired from an LTR Retrotransposon in Eutherians. PLOS Genet. 2015, 11, e1005521. [Google Scholar] [CrossRef]
- Irie, M.; Koga, A.; Kaneko-Ishino, T.; Ishino, F. An LTR Retrotransposon-Derived Gene Displays Lineage-Specific Structural and Putative Species-Specific Functional Variations in Eutherians. Front. Chem. 2016, 4, 26. [Google Scholar] [CrossRef]
- Irie, M.; Itoh, J.; Matsuzawa, A.; Ikawa, M.; Kiyonari, H.; Kihara, M.; Suzuki, T.; Hiraoka, Y.; Ishino, F.; Kaneko-Ishino, T. Retrovirus-derived RTL5 and RTL6 genes are novel constituents of the innate immune system in the eutherian brain. Development 2022, 149. [Google Scholar] [CrossRef]
- Fujioka, Y. , Shiura, H., Ishii, M., Ono, R., Endo, T., Kiyonari, H., et al. (2023). Targeting retrovirus-derived Rtl8a and Rtl8b causes late onset obesity and neurodevelopmental defects like Prader-Willi syndrome. bioRxiv. [CrossRef]
- Ishino, F. , Itoh, J., Irie, M., Matsuzawa, A., Naruse, M., Suzuki, T. et al. (2023). Retrovirus-derived RTL9 plays an important role in innate antifungal immunity in the eutherian brain. bioRxiv. [CrossRef]
- Kitazawa, M.; Hayashi, S.; Imamura, M.; Takeda, S.; Oishi, Y.; Kaneko-Ishino, T.; Ishino, F. Deficiency and overexpression of Rtl1 in the mouse cause distinct muscle abnormalities related to Temple and Kagami-Ogata syndromes. Development 2020, 147. [Google Scholar] [CrossRef]
- Kitazawa, M.; Sutani, A.; Kaneko-Ishino, T.; Ishino, F. The role of eutherian-specific RTL1 in the nervous system and its implications for the Kagami-Ogata and Temple syndromes. Genes Cells 2021, 26, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Shiura, H.; Ono, R.; Tachibana, S.; Kohda, T.; Kaneko-Ishino, T.; Ishino, F. PEG10 viral aspartic protease domain is essential for the maintenance of fetal capillary structure in the mouse placenta. Development 2021, 148. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.J.; Wang, C.; Costa, V.; Lopatta, P.; Meier, S.; Zampeta, F.I.; Punt, A.M.; Mientjes, E.; Grossen, P.; Distler, T.; et al. Secreted retrovirus-like GAG-domain-containing protein PEG10 is regulated by UBE3A and is involved in Angelman syndrome pathophysiology. Cell Rep. Med. 2021, 2, 100360. [Google Scholar] [CrossRef]
- Whiteley, A.M.; Prado, M.A.; de Poot, S.A.; Paulo, J.A.; Ashton, M.; Dominguez, S.; Weber, M.; Ngu, H.; Szpyt, J.; Jedrychowski, M.P.; et al. Global proteomics of Ubqln2-based murine models of ALS. J. Biol. Chem. 2021, 296, 100153. [Google Scholar] [CrossRef]
- Black, H.H.; Hanson, J.L.; E Roberts, J.; Leslie, S.N.; Campodonico, W.; Ebmeier, C.C.; Holling, G.A.; Tay, J.W.; Matthews, A.M.; Ung, E.; et al. UBQLN2 restrains the domesticated retrotransposon PEG10 to maintain neuronal health in ALS. eLife 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Surani, M.A.H.; Barton, S.C.; Norris, M.L. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 1984, 308, 548–550. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Solter, D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 1984, 37, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.R.; Lovell-Badge, R.H. Inviability of parthenogenones is determined by pronuclei, not egg cytoplasm. Nature 1984, 310, 66–67. [Google Scholar] [CrossRef]
- Cattanach, B.M.; Kirk, M. Differential activity of maternally and paternally derived chromosome regions in mice. Nature 1985, 315, 496–498. [Google Scholar] [CrossRef]
- Cattanach, B.M.; Beechey, C.V. Autosomal and X-chromosome imprinting. Development 1990, 108, 63–72. [Google Scholar] [CrossRef]
- Kaneko-Ishino, T.; Kohda, T.; Ishino, F. The regulation and biological significance of genomic imprinting in mammals. J. Biochem. 2003, 133, 699–711. [Google Scholar] [CrossRef]
- Bartolomei, M.S.; Ferguson-Smith, A.C. Mammalian Genomic Imprinting. Cold Spring Harb. Perspect. Biol. 2011, 3, a002592–a002592. [Google Scholar] [CrossRef]
- Barlow, D.P.; Bartolomei, M.S. Genomic Imprinting in Mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a018382–a018382. [Google Scholar] [CrossRef]
- Kaneko-Ishino, T.; Ishino, F. Mammalian-specific genomic functions: Newly acquired traits generated by genomic imprinting and LTR retrotransposon-derived genes in mammals. Proc. Jpn. Acad. Ser. B 2015, 91, 511–538. [Google Scholar] [CrossRef] [PubMed]
- Kaneko-Ishino, T.; Ishino, F. Evolution of viviparity in mammals: what genomic imprinting tells us about mammalian placental evolution. Reprod. Fertil. Dev. 2019, 31, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Shiura, H.; Aburatani, H.; Kohda, T.; Kaneko-Ishino, T.; Ishino, F. Identification of a Large Novel Imprinted Gene Cluster on Mouse Proximal Chromosome 6. Genome Res. 2003, 13, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- E Cockett, N.; Jackson, S.P.; Shay, T.L.; Nielsen, D.; Moore, S.S.; Steele, M.R.; Barendse, W.; Green, R.D.; Georges, M. Chromosomal localization of the callipyge gene in sheep (Ovis aries) using bovine DNA markers. Proc. Natl. Acad. Sci. 1994, 91, 3019–3023. [Google Scholar] [CrossRef] [PubMed]
- Kotzot, D. Maternal uniparental disomy 14 dissection of the phenotype with respect to rare autosomal recessively inherited traits, trisomy mosaicism, and genomic imprinting. 47. [CrossRef]
- Kagami, M.; Nishimura, G.; Okuyama, T.; Hayashidani, M.; Takeuchi, T.; Tanaka, S.; Ishino, F.; Kurosawa, K.; Ogata, T. Segmental and full paternal isodisomy for chromosome 14 in three patients: Narrowing the critical region and implication for the clinical features. Am. J. Med Genet. Part A 2005, 138A, 127–132. [Google Scholar] [CrossRef]
- Ioannides, Y.; Lokulo-Sodipe, K.; Mackay, D.J.G.; Davies, J.H.; Temple, I.K. Temple syndrome: improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: an analysis of 51 published cases. J. Med Genet. 2014, 51, 495–501. [Google Scholar] [CrossRef]
- Kagami, M.; Kurosawa, K.; Miyazaki, O.; Ishino, F.; Matsuoka, K.; Ogata, T. Comprehensive clinical studies in 34 patients with molecularly defined UPD(14)pat and related conditions (Kagami–Ogata syndrome). Eur. J. Hum. Genet. 2015, 23, 1488–1498. [Google Scholar] [CrossRef]
- Kagami, M.; Nagasaki, K.; Kosaki, R.; Horikawa, R.; Naiki, Y.; Saitoh, S.; Tajima, T.; Yorifuji, T.; Numakura, C.; Mizuno, S.; et al. Temple syndrome: comprehensive molecular and clinical findings in 32 Japanese patients. Anesthesia Analg. 2017, 19, 1356–1366. [Google Scholar] [CrossRef]
- Kitazawa, M.; Tamura, M.; Kaneko-Ishino, T.; Ishino, F. Severe damage to the placental fetal capillary network causes mid- to late fetal lethality and reduction in placental size inPeg11/Rtl1KO mice. Genes Cells 2017, 22, 174–188. [Google Scholar] [CrossRef]
- Dauber, A.; Cunha-Silva, M.; Macedo, D.B.; Brito, V.N.; Abreu, A.P.; Roberts, S.A.; Montenegro, L.R.; Andrew, M.; Kirby, A.; Weirauch, M.T.; et al. Paternally Inherited DLK1 Deletion Associated With Familial Central Precocious Puberty. J. Clin. Endocrinol. Metab. 2017, 102, 1557–1567. [Google Scholar] [CrossRef]
- Macedo, D.B.; Kaiser, U.B. DLK1, Notch Signaling and the Timing of Puberty. Semin. Reprod. Med. 2019, 37, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.; Colgrave, M.L.; Vuocolo, T.; Pearson, R.; Bidwell, C.A.; Cockett, N.E.; Lynn, D.J.; Fleming-Waddell, J.N.; Tellam, R.L. The Imprinted Retrotransposon-Like Gene PEG11 (RTL1) Is Expressed as a Full-Length Protein in Skeletal Muscle from Callipyge Sheep. PLOS ONE 2010, 5, e8638–e8638. [Google Scholar] [CrossRef]
- Xu, X.; Ectors, F.; Davis, E.E.; Pirottin, D.; Cheng, H.; Farnir, F.; Hadfield, T.; Cockett, N.; Charlier, C.; Georges, M.; et al. Ectopic Expression of Retrotransposon-Derived PEG11/RTL1 Contributes to the Callipyge Muscular Hypertrophy. PLOS ONE 2015, 10, e0140594. [Google Scholar] [CrossRef] [PubMed]
- Poulter, R.; Butler, M. A retrotransposon family from the pufferfish (fugu) Fugu rubripes. Gene 1998, 215, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Goodwin, T.; Simpson, M.; Singh, M.; Poulter, R. Vertebrate LTR Retrotransposons of the Tf1/Sushi Group. J. Mol. Evol. 2001, 52, 260–274. [Google Scholar] [CrossRef]
- Volff, J.-N.; Körting, C.; Schartl, M. Ty3/Gypsy Retrotransposon Fossils in Mammalian Genomes: Did They Evolve into New Cellular Functions? Mol. Biol. Evol. 2001, 18, 266–270. [Google Scholar] [CrossRef]
- Imakawa, K.; Kusama, K.; Kaneko-Ishino, T.; Nakagawa, S.; Kitao, K.; Miyazawa, T.; Ishino, F. Endogenous Retroviruses and Placental Evolution, Development, and Diversity. Cells 2022, 11, 2458. [Google Scholar] [CrossRef]
- Suzuki, S.; Ono, R.; Narita, T.; Pask, A.J.; Shaw, G.; Wang, C.; Kohda, T.; E Alsop, A.; Graves, J.A.M.; Kohara, Y.; et al. Retrotransposon Silencing by DNA Methylation Can Drive Mammalian Genomic Imprinting. PLOS Genet. 2007, 3, e55–e55. [Google Scholar] [CrossRef]
- A Edwards, C.; Mungall, A.J.; Matthews, L.; Ryder, E.; Gray, D.J.; Pask, A.J.; Shaw, G.; Graves, J.A.; Rogers, J.; Dunham, I.; et al. The Evolution of the DLK1-DIO3 Imprinted Domain in Mammals. PLOS Biol. 2008, 6, e135. [Google Scholar] [CrossRef]
- Kim, A.; Terzian, C.; Santamaria, P.; Pélisson, A.; Purd'Homme, N.; Bucheton, A. Retroviruses in invertebrates: the gypsyretrotransposon is apparently an infectious retrovirus of Drosophilamelanogaster. Proc. Natl. Acad. Sci. 1994, 91, 1285–1289. [Google Scholar] [CrossRef]
- Song, S.U.; Gerasimova, T.; Kurkulos, M.; Boeke, J.D.; Corces, V.G. An env-like protein encoded by a Drosophila retroelement: evidence that gypsy is an infectious retrovirus. J. Bone Jt. Surg. 1994, 8, 2046–2057. [Google Scholar] [CrossRef]
- Kaneko-Ishino, T.; Ishino, F. The Evolutionary Advantage in Mammals of the Complementary Monoallelic Expression Mechanism of Genomic Imprinting and Its Emergence From a Defense Against the Insertion Into the Host Genome. Front. Genet. 2022, 13, 832983. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Youngson, N.; Lin, S.-P.; Dalbert, S.; Paulsen, M.; Bachellerie, J.-P.; Ferguson-Smith, A.C.; Cavaillé, J. Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene. Nat. Genet. 2003, 34, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Caiment, F.; Tordoir, X.; Cavaillé, J.; Ferguson-Smith, A.; Cockett, N.; Georges, M.; Charlier, C. RNAi-Mediated Allelic trans-Interaction at the Imprinted Rtl1/Peg11 Locus. Curr. Biol. 2005, 15, 743–749. [Google Scholar] [CrossRef]
- Ito, M.; Sferruzzi-Perri, A.N.; Edwards, C.A.; Adalsteinsson, B.T.; Allen, S.E.; Loo, T.-H.; Kitazawa, M.; Kaneko-Ishino, T.; Ishino, F.; Stewart, C.L.; et al. A trans-homologue interaction between reciprocally imprinted miR-127 and Rtl1 regulates placenta development. Development 2015, 142, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Renfree, M.B. Review: Marsupials: Placental Mammals with a Difference. Placenta 2010, 31, S21–S26. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Ditzel, N.; Mahmood, A.; Isa, A.; A Traustadottir, G.; Schilling, A.F.; Ruiz-Hidalgo, M.-J.; Laborda, J.; Amling, M.; Kassem, M. DLK1 is a novel regulator of bone mass that mediates estrogen deficiency-induced bone loss in mice. J. Bone Miner. Res. 2011, 26, 1457–1471. [Google Scholar] [CrossRef]
- Mihrshahi, R. The corpus callosum as an evolutionary innovation. J. Exp. Zoöl. Part B: Mol. Dev. Evol. 2006, 306B, 8–17. [Google Scholar] [CrossRef]
- Suã¡Rez, R.; Gobius, I.; Richards, L.J. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front. Hum. Neurosci. 2014, 8, 497. [Google Scholar] [CrossRef]
- Fame, R.M.; MacDonald, J.L.; Macklis, J.D. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011, 34, 41–50. [Google Scholar] [CrossRef]
- Aboitiz, F.; Montiel, J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz. J. Med Biol. Res. 2003, 36, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-Y.; Hu, M.-C.; Chen, P.-Y.; Hsu, C.-L.; Lin, T.-Y.; Tan, M.-J.; Lee, C.-Y.; Kuo, M.-F.; Huang, P.-H.; Wu, V.-C.; et al. RTL1/PEG11 imprinted in human and mouse brain mediates anxiety-like and social behaviors and regulates neuronal excitability in the locus coeruleus. Hum. Mol. Genet. 2022, 31, 3161–3180. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-P.; Youngson, N.; Takada, S.; Seitz, H.; Reik, W.; Paulsen, M.; Cavaille, J.; Ferguson-Smith, A.C. Asymmetric regulation of imprinting on the maternal and paternal chromosomes at the Dlk1-Gtl2 imprinted cluster on mouse chromosome 12. Nat. Genet. 2003, 35, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, R.D.; Knepper, J.L. Genome Organization, Function, and Imprinting in Prader-Willi and Angelman Syndromes. Annu. Rev. Genom. Hum. Genet. 2001, 2, 153–175. [Google Scholar] [CrossRef]
- Buiting, K.; Williams, C.; Horsthemke, B. Angelman syndrome — insights into a rare neurogenetic disorder. Nat. Rev. Neurol. 2016, 12, 584–593. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Deng, H.X.; Chen, W.; Hong, S.T.; Boycott, K.M.; Gorrie, G.H.; Siddique, N.; Yang, Y.; Fecto, F.; Shi, Y.; Zhai, H.; et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 2011, 477, 211–215. [Google Scholar] [CrossRef]
- Ono, R.; Kuroki, Y.; Naruse, M.; Ishii, M.; Iwasaki, S.; Toyoda, A.; Fujiyama, A.; Shaw, G.; Renfree, M.B.; Kaneko-Ishino, T.; et al. Identification of tammar wallaby SIRH12, derived from a marsupial-specific retrotransposition event. DNA Res. 2011, 18, 211–219. [Google Scholar] [CrossRef]
- Malassine, A.; Frendo, J.L.; Evain-Brion, D. A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Updat. 2003, 9, 531–539. [Google Scholar] [CrossRef]
- Arensburg, J.; Payne, A.H.; Orly, J. Expression of Steroidogenic Genes in Maternal and Extraembryonic Cells During Early Pregnancy in Mice1. Endocrinology 1999, 140, 5220–5232. [Google Scholar] [CrossRef]
- Simmons, D.G.; Rawn, S.; Davies, A.; Hughes, M.; Cross, J.C. Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genom. 2008, 9, 1–20. [Google Scholar] [CrossRef]
- Lyon, M.F. Gene Action in the X-chromosome of the Mouse (Mus musculus L.). Nature 1961, 190, 372–373. [Google Scholar] [CrossRef]
- Takagi, N.; Sasaki, M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 1975, 256, 640–642. [Google Scholar] [CrossRef]
- Murr, S.M.; Stabenfeldt, G.H.; Bradford, G.E.; Geschwind, I.I. Plasma Progesterone During Pregnancy in the Mouse1. Endocrinology 1974, 94, 1209–1211. [Google Scholar] [CrossRef]
- Virgo, B.B.; Bellward, G.D. Serum Progesterone Levels in the Pregnant and Postpartum Laboratory Mouse. Endocrinology 1974, 95, 1486–1490. [Google Scholar] [CrossRef]
- Tyndale-Biscoe, H.; Renfree, M. Ovarian function and control. In Reproductive Physiology of Marsupials; Chapter 6; Cambridge University Press: Cambridge, UK, 1987; pp. 203–203. [Google Scholar] [CrossRef]
- Lim, E.T.; Raychaudhuri, S.; Sanders, S.J.; Stevens, C.; Sabo, A.; MacArthur, D.G.; Neale, B.M.; Kirby, A.; Ruderfer, D.M.; Fromer, M.; et al. Rare Complete Knockouts in Humans: Population Distribution and Significant Role in Autism Spectrum Disorders. Neuron 2013, 77, 235–242. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Rajkowski, J.; Cohen, J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 1999, 46, 1309–1320. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Bouret, S.; Sara, S.J. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005, 28, 574–582. [Google Scholar] [CrossRef]
- Lanfray, D.; Richard, D. Emerging Signaling Pathway in Arcuate Feeding-Related Neurons: Role of the Acbd7. Front. Neurosci. 2017, 11, 328. [Google Scholar] [CrossRef]
- Seabrook, L.T.; Borgland, S.L. The orbitofrontal cortex, food intake and obesity. J. Psychiatry Neurosci. 2020, 45, 304–312. [Google Scholar] [CrossRef]
- Yousefvand, S.; Hamidi, F. Role of Paraventricular Nucleus in Regulation of Feeding Behaviour and the Design of Intranuclear Neuronal Pathway Communications. Int. J. Pept. Res. Ther. 2019, 26, 1231–1242. [Google Scholar] [CrossRef]
- Szczepanski, S.M.; Knight, R.T. Insights into Human Behavior from Lesions to the Prefrontal Cortex. Neuron 2014, 83, 1002–1018. [Google Scholar] [CrossRef] [PubMed]
- Hare, B.D.; Duman, R.S. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol. Psychiatry 2020, 25, 2742–2758. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Roberts, A.C. Correction: Prefrontal cortex and depression. Neuropsychopharmacology 2021, 47, 609–609. [Google Scholar] [CrossRef]
- Lavialle, C.; Cornelis, G.; Dupressoir, A.; Esnault, C.; Heidmann, O.; Vernochet, C.; Heidmann, T. Paleovirology of ‘ syncytins ’, retroviral env genes exapted for a role in placentation. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120507. [Google Scholar] [CrossRef]
- Imakawa, K.; Nakagawa, S.; Miyazawa, T. Baton pass hypothesis: successive incorporation of unconserved endogenous retroviral genes for placentation during mammalian evolution. Genes Cells 2015, 20, 771–788. [Google Scholar] [CrossRef]
- Abed, M.; Verschueren, E.; Budayeva, H.; Liu, P.; Kirkpatrick, D.S.; Reja, R.; Kummerfeld, S.K.; Webster, J.D.; Gierke, S.; Reichelt, M.; et al. The Gag protein PEG10 binds to RNA and regulates trophoblast stem cell lineage specification. PLOS ONE 2019, 14, e0214110. [Google Scholar] [CrossRef]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).