Submitted:
11 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Canine visceral leishmaniasis immunology and currently available commercial vaccines
3. Development of transmission-blocking vaccines (TBVs): a strategy to interrupt pathogens transmission
4. Development of TBVs for the control of leishmaniasis
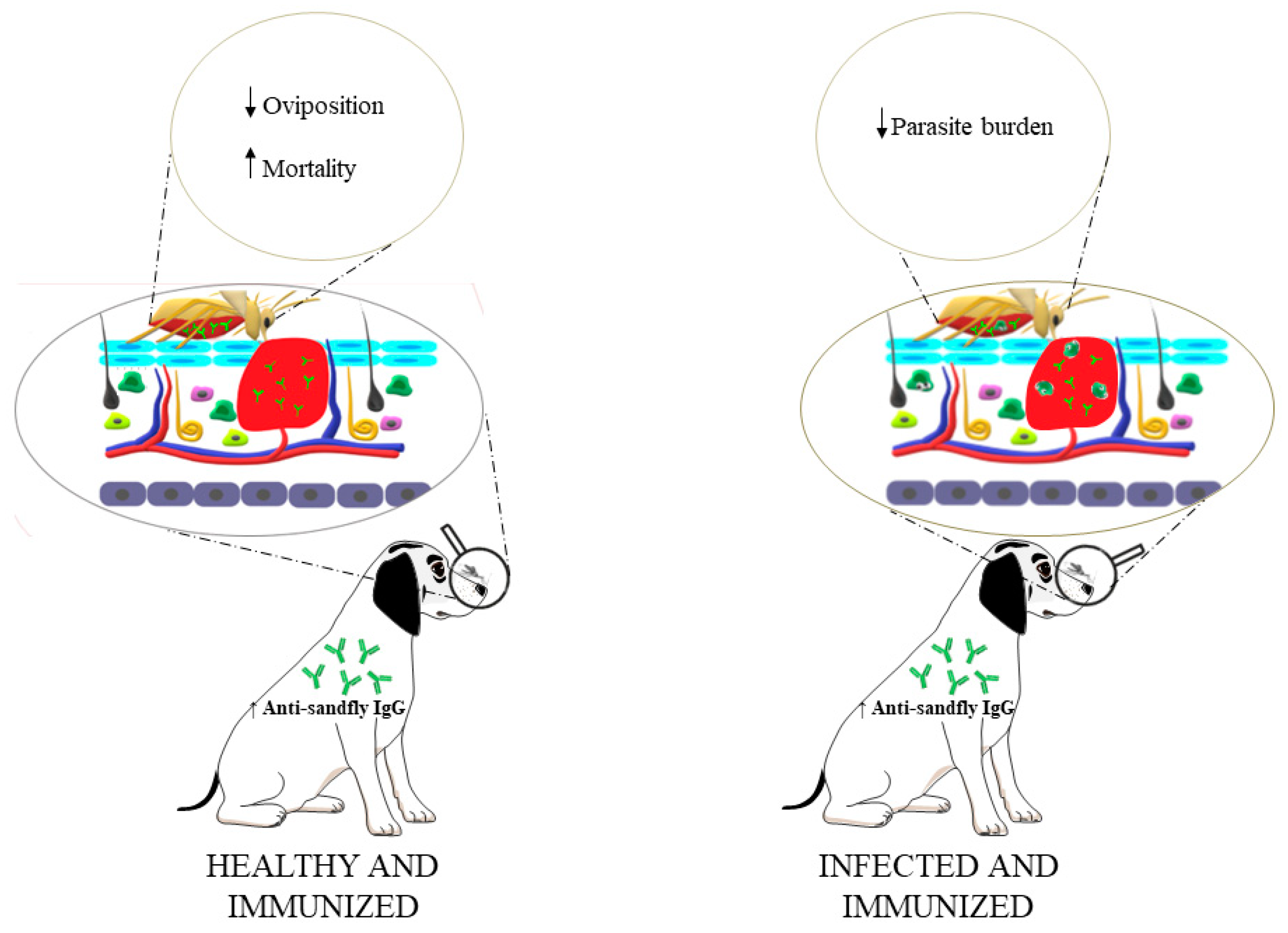
5. Conclusion: Future trends and perspectives of TBVs for visceral leishmaniasis control
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fraga,B.M.; Solcá, M.S.; Silva, V.M.; Borja, L.S.; Nascimento, E.G.; Oliveira, G.G.S.; Pontes-de-Carvalho, L.C.; Veras, P.S.T. S.; dos Santos, W.L.C. Temporal distribution of positive results of tests for detecting Leishmania infection in stray dogs of an endemic area of visceral leishmaniasis in the Brazilian tropics: A 13 years survey and association with human disease. Vet Parasitol 2012, 21, 591–594. [CrossRef]
- Cunha, A.M.; Chagas, E. Estudos sobre o parasito. In: Leishmaniose visceral americana, nova entidade mórbida do homem na América do Sul. Mem. Inst. Oswaldo Cruz 1937, 32, 329–337. [Google Scholar] [CrossRef]
- Maurício, I.L.; Stothard, J.R.; Miles, M.A. The strange case of Leishmania chagasi. Parasitol Today 2000, 16, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Vieira, C.P.; Dibo, M.R.; Guirado, M.M.; Rodas, L.A.C.; Chiaravalloti-Neto, F. Dispersal of Lutzomyia longipalpis and expansion of canine and human visceral leishmaniasis in São Paulo State, Brazil. Acta Trop 2016, 164, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; Neiva, A. Contribuição para o conhecimento das espécies do genero Phlebotomus existentes no Brazil. Memórias do Instituto Oswaldo Cruz 1912, 4, 84–95. [Google Scholar] [CrossRef]
- Soares, R.P.P.; Turco, S.J. Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae): A review. An Acad Bras Ciênc 2003, 75, 301–330. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Miró, G.; Bowman, D.D.; Gradoni, L.; Otranto, D. Culling Dogs for Zoonotic Visceral Leishmaniasis Control: The Wind of Change. Trends Parasitol 2019, 35, 97–101. [Google Scholar] [CrossRef]
- Ashford, D.A.; David, J.R.; Freire, M.S.; David, R.A.; Sherlock, Í.R. de A.; Eulálio, M. da C.; Sampaio, D.B.P.; Badaró, R.J. da S. Studies on Control of Visceral Leishmaniasis: Impact of Dog Control on Canine and Human Visceral Leishmaniasis in Jacobina, Bahia, Brazil. American Journal of Tropical Medicine and Hygiene 1998, 59, 53–57. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C.B.; Batista-de-Melo, L.M.; Borja-Cabrera, G.P.; Palatnik, M.; Lavor, C.C. Improving methods for epidemiological control of canine visceral leishmaniasis based on a mathematical model. Impact on the incidence of the canine and human disease. An Acad Bras Cienc 2004, 76, 583–593. [Google Scholar] [CrossRef]
- Nunes, C.M.; Pires, M.M.; da Silva, K.M.; Assis, F.D.; Filho,J. G.; Perri, S.H.V. Relationship between dog culling and incidence of human visceral leishmaniasis in an endemic area. Veterinary Parasitology 2010, 170, 131–133. [Google Scholar] [CrossRef]
- Romero, G.A.; Boelaert, M. Control of visceral leishmaniasis in latin america-a systematic review. PLoS Negl Trop Dis 2010, 19, e584. [Google Scholar] [CrossRef]
- Costa, C.H. How effective is dog culling in controlling zoonotic visceral leishmaniasis? A critical evaluation of the science, politics and ethics behind this public health policy. Rev Soc Bras Med Trop 2011, 44, 232–242. [Google Scholar] [CrossRef]
- Marcondes, M. , Day, M.J. (2019). Current status and management of canine leishmaniasis in Latin America. Res Vet Sci 2019, 123, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Vaz, T.P.; Gama-Melo, M.O.; Quaresma, P.F.; Gontijo, C.M.F.; Santos, G.; Barbosa, F.S.; Fontes, G. Evaluation of the Euthanasia of Seropositive Dogs for Canine Visceral Leishmaniasis as the Only Method of Controling the Disease in the Enzootic Area in the Midwestern Minas Gerais. Pesquisa Veterinaria Brasileira 2020, 40, 107–112. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C.B. Vaccines for Canine Leishmaniasis. Front Immunol 2012, 17, 3:69. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Jain, N.K. Vaccines for visceral leishmaniasis: A review. J. of Immunological Methods 2015, 422, 1–12. [Google Scholar] [CrossRef]
- McMahon-Pratt, D.; Alexander, J. (2004). Does the Leishmania major paradigm of pathogenesis and protection hold for new world cutaneous leishmaniases or the visceral disease? Immunol Rev 2004, 201, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.; Aguiar-Soares, R.D.O.; Coura-Vital, W.; Ker, H.G.; Moreira, N.D.; Vitoriano-Souza, J.; Giunchetti, R.C.; Carneiro, C.M.; Reis, A.B. Immunotherapy and immunochemotherapy in visceral leishmaniasis: Promising treatments for this neglected disease. Frontiers Immunology 13;5:272. 2014. [Google Scholar] [CrossRef]
- Giunchetti, R.C.; Silveira, P.; Resende, L.A.; Leite, J.C.; de Oliveira Melo-Júnior, O.A.; Rodrigues Alves, M.L.; Costa, L.M.; Lair, D.F.; Chaves, V.R.; Soares, I.S.; Mendonça, L.Z.; Lanna, M.F.; Ribeiro, H.S.; Maia-Gonçalves, A.A.; Santos, T.A.P.; Roatt, B.R.; Aguiar-Soares, R.D.O.; Vitoriano-Souza, J.; Moreira, N.D.; Mathias, F.A.S.; Cardoso, J.M.O.; Coura-Vital, W.; Galdino, A.S.; Viana, K.F.; Martins-Filho, O.A.; Silveira-Lemos, D.; Dutra, W.O.; Reis, A.B. Canine visceral leishmaniasis biomarkers and their employment in vaccines. Vet Parasitol 2019, 271, 87–97. [Google Scholar] [CrossRef]
- Pinelli, E.; Rutten, V.P.M.; Bruysters, M.; Moore, P.F.; Ruitemberg, J. Compensation for decreased expression of B7 molecules on Leishmania infantum-infected canine macrophages results in restoration of parasite-specific T-cell proliferation and gamma interferon production. Infect Immun 1999, 67, 237–243. [Google Scholar] [CrossRef]
- Pinelli, E.; Gonzalo, R.M.; Boog, C.J.; Rutten, V.P.; Gebhard, D.; del Real, G.; Ruitenberg, J. Leishmania infantum-specific T cell lines derived from asymptomatic dogs that lyse infected macrophages in a major histocompatibility complex-restricted manner. Eur J Immunol 1995, 25, 1594–1600. [Google Scholar] [CrossRef]
- Lage, R.S.; Oliveira, G.C.; Buzek, S.C.U.; Guerra, L.L.; Giunchetti, R.C.; Corrêa-Oliveira, R.; Reis, A.B. Analysis of the cytokine profile in spleen cells from dogs naturally infected by Leishmania chagasi. Vet Immunol Immunopathol 2007, 115, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Giunchetti, R.C.; Carrillo, E.; Martins-Filho, O.A.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Immunoparasitology series 2010, 26, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.A.; Alexandre, P.G.; Soares, C.M.; Marques, C.; Rodrigues, O.R.; Brito, T.V.; Fonseca, I.P.; Alves, L.C.; Santos-Gomes, G.M. Cytokine gene expression in the tissues of dogs infected by Leishmania infantum. J Comp Pathol 2011, 145, 336–344. [Google Scholar] [CrossRef]
- Gonçalves, A.A.M.; Leite, J.C.; Resende, L.A.; Mariano, R.M.S.; Silveira, P.; Melo-Júnior, O.A.O.; Ribeiro, H.S.; de Oliveira, D.S.; Soares, D.F.; Santos, T.A.P.; Marques, A.F.; Galdino, A.S.; Martins-Filho, O.A.; Dutra, W.O.; da Silveira-Lemos, D. , Giunchetti, R.C. An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front Cell Infect Microbiol 2019, 18, 9:427. [Google Scholar] [CrossRef]
- Leal, G.G.D.A.; Roatt, B.M.; Aguiar-Soares, R.D.O.; Carneiro, C.M.; Giunchetti, R.C.; Teixeira-Carvalho, A.; Martins-Filho,O. A.; Francisco, A.F.; Cardoso, J.M.; Mathias, F.A.S.; Correa-Oliveira, R.; Carneiro, M.; Coura-Vital, W.; Reis, A.B. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet Parasitol 2014, 205, 472–482. [Google Scholar] [CrossRef]
- Barbieri, C.L. Immunology of canine leishmaniasis. Parasite Immunol 2006, 28, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.F.; Moura, E.P.; Ribeiro, R.R.; Michalick, M.S.; Kalapothakis, E.; Bruna-Romero, O.; Tafuri, W.L.; Teixeira, M.M.; Melo, M.N. Expression of IFN-gamma, TNF-alpha, IL-10 and TGF-beta in lymph nodes associates with parasite load and clinical form of disease in dogs naturally infected with Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol 2009, 128, 349–358. [Google Scholar] [CrossRef]
- Do Nascimento,P. R.; Martins, D.R.; Monteiro,G.R.; Queiroz, P.V.; Freire-Neto, F.P.; Queiroz, J.W.; Lima, A.L.M.; Jeroimo, S.M.B. Association of pro-inflammatory cytokines and iron regulatory protein 2 (IRP2) with Leishmania burden in canine visceral leishmaniasis. PLoS ONE 2013, 11, e73873. [Google Scholar] [CrossRef]
- Moreira, M.L.; Costa-Pereira, C.; Alves, M.L.; Marteleto, B.H.; Ribeiro,V. M.; Peruhype-Magalhães, V.; Giunchetti, R.C.; Martins-Filho, O.A.; Araújo, M.S. Vaccination against canine leishmaniosis increases the phagocytic activity, nitric oxide production and expression of cell activation/migration molecules in neutrophils and monocytes. Vet Parasitol 2016, 15, 233–245. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Koutinas, C.K. Pathologic Mechanisms Underlying the Clinical Findings in Canine Leishmaniosis due to Leishmania infantum/chagasi. Vet Pathol 2014, 51, 527–538. [Google Scholar] [CrossRef]
- Carson, C.; Antoniou, M.; Ruiz-Argüello, M.B.; Alcami, A.; Christodoulou, V.; Messaritakis, I.; Blackwell, J.M.; Courtenay, O. A prime/boost DNA/ Modified vaccinia virus Ankara vaccine expressing recombinant Leishmania DNA encoding TRYP is safe and immunogenic in outbred dogs, the reservoir of zoonotic visceral leishmaniasis. Vaccine 2009, 27, 1080–1086. [Google Scholar] [CrossRef]
- Chaabouni, A.; Boubaker, E.R.; Mhadhbi, M.; Gharbi, M.; Sassi, A. Comparative analysis of the Leishmania infantum-specific antibody repertoires and the autoantibody repertoires between asymptomatic and symptomatic dogs. Vet Parasitol 2018, 15, 9–17. [Google Scholar] [CrossRef]
- De Freitas, J.C.; Lopes-Neto, B.E.; de Abreu, C.R.; Coura-Vital, W.; Braga, S.L.; Reis, A.B.; Nunes-Pinheiro, D.C. Profile of anti-Leishmania antibodies related to clinical picture in canine visceral leishmaniasis. Res Vet Sci 2012, 93, 705–709. [Google Scholar] [CrossRef]
- Srivastava, S.; Shankar, P.; Mishra, J.; Singh, S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors 2016, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Agricultura e Pecuária. Mapa suspende fabricação e venda e determina o recolhimento de lotes de vacina contra Leishmaniose. Available at: https://www.gov.br/agricultura/pt-br/assuntos/noticias/mapa-suspende-fabricacao-e-venda-e-determina-o-recolhimento-de-lotes-de-vacina-contra-leishmaniose-apos-fiscalizacao. Access in: 0731/2023.
- Lemesre, J.L.; Holzmuller, P.; Cavaleyra, M.; Gonçalves, R.B.; Hottin, G.; Papierok, G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine 2005, 23, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Vouldoukis, I.; Martin, V.; McGahie, D.; Cuisinier, A.M.; Gueguen, S. Use of a liesp/qa-21 vaccine (canileish) stimulates an appropriate th1-dominated cell-mediated immune response in dogs. PLoS Negl Trop Dis 2012, 6, e1683. [Google Scholar] [CrossRef]
- Holzmuller, P.; Cavaleyra, M.; Moreaux, J.; Kovacic, R.; Vincendeau, P.; Papierok, G.; Lemesre, J.L. Lymphocytes of dogs immunized with purified excreted-secreted antigens of Leishmania infantum co-incubated with Leishmania infected macrophages produce IFN gamma resulting in nitric oxide-mediated amastigote apoptosis. Vet Immunol Immunopathol 2005, 106, 247–257. [Google Scholar] [CrossRef]
- Lemesre, J.L.; Holzmuller, P.; Gonçalves, R.B.; Bourdoiseau, G.; Hugnet, C.; Cavaleyra, M.; Papierok, G. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: Double-blind randomised efficacy field trial. Vaccine 2007, 25, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Vouldoukis, I.; Moreno, J.; McGahie, D.; Gueguen, S.; Cuisinier, A.M. The protective immune response produced in dogs after primary vaccination with the LiESP/QA-21 vaccine (CaniLeish®) remains effective against an experimental challenge one year later. Vet Res 2014, 25, 45:69. [Google Scholar] [CrossRef]
- Oliva, G.; Nieto, J.; Foglia Manzillo, V.; Cappiello, S.; Fiorentino, E.; Di Muccio, T.; Scalone, A.; Moreno, J.; Chicharro, C.; Carrillo, E.; Butaud, T.; Guegand, L.; Martin, V.; Cuisinier, A.M.; McGahie, D.; Gueguen, S.; Cañavate, C.; Gradoni, L. (2014). A Randomised, Double-Blind, Controlled Efficacy Trial of the LiESP/QA-21 Vaccine in Naïve Dogs Exposed to Two Leishmania infantum Transmission Seasons. PLoS Negl Trop Dis Oct 2014, 9, e3213. [Google Scholar] [CrossRef]
- Regina-Silva, S.; Feres, A.M.L.T.; França-Silva, J.C.; Dias, E.S.; Michalsky, É.M.; de Andrade, H.M.; Coelho, E.A.F.; Ribeiro, G.M.; Fernandes, A.P.; Machado-Coelho, G.L.L. Field randomized trial to evaluate the efficacy of the Leish-Tec® vaccine against canine visceral leishmaniasis in an endemic area of Brazil. Vaccine 2016, 34, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Costa, M.M.S.; Coelho, E.A.F.; Michalick, M.S.M.; de Freitas, E.; Melo, M.N.; Tafuri, W.L.; Resende, D.M.; Hermont, V.; Abrantes, C.F.; Gazzinelli, R.T. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine 2008, 26, 5888–5895. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Teva, A.; Dos-Santos, C.B.; Santos, F.N.; Pinto, I.D.S.; Fux, B.; Leite, G.R.; Falqueto, A. Field trial of efficacy of the Leish-tec® vaccine against canine leishmaniasis caused by Leishmania infantum in an endemic area with high transmission rates. PLoS ONE 2017, 12, e0185438. [Google Scholar] [CrossRef]
- Aguiar-Soares, R.D. de O.; Roatt, B.M.; Mathias, F.A.S.; Reis, L.E.S.; Cardoso, J.M. de O.; Brito, R.C.F.; Ker, H.G.; Corrêa-Oliveira, R.; Giunchetti, R.C.; Reis, A.B. Phase I and II Clinical Trial Comparing the LBSap, Leishmune®, and Leish-Tec® Vaccines against Canine Visceral Leishmaniasis. Vaccines 2020, 8, 690. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, 2016. LetiFend_: Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/veterinary/003865/WC500207742.pdf (acessed ,2022). 18 May.
- Molano, L.; Alonso, G.M.; Mirón, C.; Redondo, E.; Requena, J.M.; Soto, M.; Cieto, C.G.; Alonso, C. A Leishmania infantum multi-component antigenic protein mixed with live BCG confers protection to dogs experimentally infected with L. infantum. Vet Immunol Immunopathol 2003, 92, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carcelén, J.; Iniesta, V.; Fernández-Cotrinaa, J.; Serrano, F.; Parejob, J.C.; Corralizac, I.; Gallardo-Solerc, A.; Marañnón, F.; Soto, M.; Alonsoe, C.; Gómez-Nietoa, C. The Chimerical Multi-Component Q protein from Leishmania in the absence of adjuvant protects dogs against an experimental Leishmania infantum infection. Vaccine 2009, 27, 5964–5973. [Google Scholar] [CrossRef]
- Cotrina, J.F.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend® against canine leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Kaye, P.M.; Cruz, I.; Picado, A.; Van Bocxlaer, K.; Croft, S.L. Leishmaniasis immunopathology—Impact on design and use of vaccines, diagnostics and drugs. Semin Immunopathol 2020, 42, 247–264. [Google Scholar] [CrossRef]
- Graciano, R.C.D.; Ribeiro, J.A.T.; Macedo, A.K.S.; de S Lavareda, J.P.; de Oliveira, P.R.; Netto, J.B.; Nogueira, L.M.; Machado, J.M.; Camposda-Paz, M.; Giunchetti, R.C.; Galdino, A.S. Recent Patents applications in red biotechnology: A mini-review. Recent Pat Biotechnol 2019, 13, 170–186. [Google Scholar] [CrossRef]
- Coutinho-Abreu, I.V.; Ramalho-Ortigao, M. Transmission blocking vaccines to control insect-borne diseases - A review. Mem Inst Oswaldo Cruz 2010, 105, 1–12. [Google Scholar] [CrossRef]
- Coelho, C.H.; Rappuoli, R.; Hotez, P.J.; Duffy, P.E. Transmission-Blocking Vaccines for Malaria: Time to Talk about Vaccine Introduction. Trends Parasitol 2019, 35, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Lê, H.G.; Kang, J.M.; Jun, H.; Lee, J.; Moe, M.; Thái, T.L.; Lin, K.; Myint, M.K.; Yoo, W.G.; Sohn, W.-M.; Kim, T.-S.; Na, B.K. Genetic diversity and natural selection of transmission-blocking vaccine candidate antigens Pvs25 and Pvs28 in Plasmodium vivax Myanmar isolates. Acta Trop 2019, 198, 105104. [Google Scholar] [CrossRef]
- Grotendorst, C.A.; Kumar, N.; Carter, R.; Kaushal, D.C. A surface protein expressed during the transformation of zygotes of Plasmodium gallinaceum is a target of transmission-blocking antibodies. Infect Immun 1984, 45, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Hisaeda, H.; Collins, W.E.; Saul, A.; Stowers, A.W. Antibodies to Plasmodium vivax transmission-blocking vaccine candidate antigens Pvs25 and Pvs28 do not show synergism. Vaccine 2001, 20, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.A.; Patterson, P.S.; Sacci, J.B.; Vaughan, J.A.; Paul, C.; Collins, W.E.; Wirtz, R.A.; Azad, A.F. Anti-mosquito midgut antibodies block development of Plasmodium falciparum and Plasmodium vivax in multiple species of Anopheles mosquitoes and reduce vector fecundity and survivorship. Proc Natl Acad Sci 2002, 98, 5228–5233. [Google Scholar] [CrossRef] [PubMed]
- Dinglasan, R.R.; Fields, I.; Shahabuddin, M.; Azad, A.F.; Sacci, J.B.Jr. Monoclonal antibody MG96 completely blocks Plasmodium yoelii development in Anopheles stephensi. Infect Immun 2003, 71, 6995–7001. [Google Scholar] [CrossRef]
- Dinglasan, R.R.; Kalume, D.E.; Kanzok, S.M.; Ghosh, A.K.; Muratova, O.; Pandey, A.; Jacobs-Lorena, M. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci 2007, 104, 13461–13466. [Google Scholar] [CrossRef]
- Lavazec, C.; Boudin, C.; Lacroix, R.; Bonnet, S.; Diop, A.; Thiberge, S.; Boisson, B.; Tahar, R.; Bourgouin, C. Carboxypeptidases B of Anopheles gambiae as targets for a Plasmodium falciparum transmission-blocking vaccine. Infect Immun 2007, 75, 1635–1642. [Google Scholar] [CrossRef]
- Ramasamy, M.S.; Sands, M.; Kay, B.H.; Fanning, I.D.; Lawrence, G.W.; Ramasamy, R. Anti-mosquito antibodies reduce the susceptibility of Aedes aegypti to arbovirus infection. Med Vet Entomol 1990, 4, 49–55. [Google Scholar] [CrossRef]
- Ramasamy, M.S.; Ramasamy, R. Effect of anti-mosquito antibodies on the infectivity of the rodent malaria parasite Plasmodium berghei to Anopheles farauti. Med Vet Entomol 1990, 4, 161–166. [Google Scholar] [CrossRef]
- Rodrigues-Alves, M.L.; Melo-Júnior, O.A.O.; Silveira, P.; Mariano, R.M.D. S.; Leite, J.C.; Santos, T.A.P.; Soares, I.S.; Lair, D.F.; Melo, M.M.; Resende, L.A.; da Silveira-Lemos, D.; Dutra, W.O.; Gontijo, N.F.; Araujo, R.N.; Sant'Anna, M.R.V.; Andrade, L.A.F.; da Fonseca, F.G.; Moreira, L.A.; Giunchetti, R.C. Historical Perspective and Biotechnological Trends to Block Arboviruses Transmission by Controlling Aedes aegypti Mosquitos Using Different Approaches. Front Med 2020, 23, 275. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Troupin, A.; Colpitts, T.M. Arbovirosis and potential transmission blocking vaccines. In Parasites and Vectors 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Ingonga, P.; Mbati, P.A.; Anjili, C.O.; Mutani, A.; Wishitemi, B.; Odongo, S.; Robert, L.L.; Githure, J.I. The effect of immune sera from hamsters immunized with sandfly gut and whole body extract antigens on the fecundity and mortality of Phlebotomus duboscqi (Diptera: Psychodidae). Acta Trop 1996, 60, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Tonui, W.K.; Mbati, P.A.; Anjili, C.O.; Orago, A.S.; Turco, S.J.; Githure, J.I.; Koech, D.K. Transmission Blocking Vaccine Studies in Leishmaniasis: I. Lipophosphoglycan Is a Promising Transmission Blocking Vaccine Molecule against Cutaneous Leishmaniasis. East Afr Med J 2001, 78, 84–89. [Google Scholar] [CrossRef]
- Kamhawi, S.; Ramalho-Ortigao, M.; Pham, V.M.; Kumar, S.; Lawyer, P.G.; Turco, S.J.; Barillas-Mury, C.; Sacks, D.L.; Valenzuela, J.G. A Role for Insect Galectins in Parasite Survival. Cell 2004, 119, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Vilela, M.L.; Souza, N.A.; Oliveira, S.M.P.; Costa-Pinto, D.; Cabello, P.H.; Rangel, E.F.; Traub-Cseko, Y.M. Considerations on the effect of anti-sandfly antibodies on biological parameters of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae). Braz J Biol. 2006, 66, 175–183. [Google Scholar] [CrossRef]
- Saraiva, E.M.; Barbosa, A.D.F.; Santos, F.N.; Borja-Cabrera, G.P.; Nico, D.; Souza, L.O.P.; Mendes-Aguiar, C.D.O.; De Souza, E.P.; Fampa, P.; Parra, L.E.; Menz, I.; Dias, J.G.; De Oliveira, S.M.; Palatnik-De-Sousa, C.B. The FML-vaccine (Leishmune®) against canine visceral leishmaniasis: A transmission blocking vaccine. Vaccine 2006, 24, 2423–2431. [Google Scholar] [CrossRef]
- MAPA, 2014. Ministério da Agricultura, Pecuária e Abastecimento. Nota técnica n°038/2014/DFIP/DAS. Suspensão da licença de fabricação e comercialização do produto Leishmune vacina contra leishmanioses visceral canina. Brasília. www.agricultura.gov.br/assuntos/politica-agricola/arquivos/notatecnica-dfip-38-14- leishmune.pdf/view (acessed ,2022). 14 May.
- Coutinho-Abreu, I.V.; Sharma, N.K.; Robles-Murguia, M.; Ramalho-Ortigao, M. Targeting the Midgut Secreted Ppchit1 Reduces Leishmania Major Development in Its Natural Vector, the Sand Fly Phlebotomus Papatasi. PLoS Neglected Tropical Diseases 2010, 4. [Google Scholar] [CrossRef]
- Bongiorno, G.; Paparcone, R.; Manzillo, V.F.; Oliva, G.; Cuisinier, A.M.; Gradoni, L. Vaccination with LiESP/QA-21 (CaniLeish®) Reduces the Intensity of Infection in Phlebotomus Perniciosus Fed on Leishmania Infantum Infected Dogs-A Preliminary Xenodiagnosis Study. Veterinary Parasitology 2013, 197, 691–695. [Google Scholar] [CrossRef]
- Rios-Barros, L.V.; Silva-Moreira, A.L.; Horta, M.F.; Gontijo, N.F.; Castro-Gomes, T. How to get away with murder: The multiple strategies employed by pathogenic protozoa to avoid complement killing. Mol Immunol 2022, 149, 27–38. [Google Scholar] [CrossRef]
- Secundino, N.; Kimblin, N.; Peters, N. C.; Lawyer, P.; Capul, A. A.; Beverley, S. M.; Turco, S. J.; Sacks, D. Proteophosphoglycan confers resistance of Leishmania major to midgut digestive enzymes induced by blood feeding in vector sand flies. Cellular Microbiology, 2010, 12, 7, 906–918, Petitdidier, E.; Pagniez, J.; Pissarra, J.; Holzmuller, P.; Papierok, G.; Vincendeau, P.; Lemesre, J.L.; Bras-Gonçalves, R. Peptide-based vaccine successfully induces protective immunity against canine visceral leishmaniasis. npj Vaccines 2019, 4, 1–9. [CrossRef]
- Petitdidier, E.; Pagniez, J.; Pissarra, J.; Holzmuller, P.; Papierok, G.; Vincendeau, P.; Lemesre, J.L.; Bras-Gonçalves, R. Peptide-based vaccine successfully induces protective immunity against canine visceral leishmaniasis. npj Vaccines 2019, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lawyer, P.; Killick-Kendrick, M.; Rowland, T.; Rowton, E.; Volf, P. Laboratory colonization and mass rearing of phlebotomine sand flies (Diptera, Psychodidae). Parasite 2017, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Pereira, D.F.S.; Melo-Junior, O.; Mariano, R.M. da S.; Leite, J.C.; Silva, A.V. da, Oliveira, D.S. de, Gonçalves, A.A.M.; Lair, D.F.; Soares, I. dos S.; Santos, T.A.P.; Galdino, A.S.; Silveira-Lemos, D. da, Paes, P.R. de O.; Melo, M.M.; Dutra, W.O.; Araujo, R.N.; Giunchetti, R.C. Vaccine approaches applied to controlling dog ticks. Ticks and Tick-borne Diseases 2021, 12. [Google Scholar] [CrossRef]
- Giunchetti, R.C.; Filho, O. A. M.; Mendonça, L.Z.; Gontijo, N.F.; Bartholomeu, D.C.; Borges, W.C. Imunobiológico para controle do vetor da Leishmaniose, processos de obtenção e uso. Brazil Patent BR 10 2013 02 2805 2, 2013.
| Continent/ Country |
Product | Company | Vaccine composition | Efficacy biomarkers | References |
|---|---|---|---|---|---|
| Europe | CaniLeish® | Virbac (France) | Purified excreted-secreted proteins of L. infantum (LiESP) associated with the adjuvant muramyl dipeptide (MDP) | ↑IFN-γ and nitrite; 75% of vaccinated dogs showed in vitro leishmanicidal effect | Holzmuller et al., 2005 [39] |
| ↑IFN-γ and nitrite; 100% efficacy (bone marrow culture) | Lemesre et al., 2005 [37] | ||||
| ↑IFN-γ and nitrite; 92% efficacy (bone marrow PCR) | Lemesre et al., 2007 [40] | ||||
| ↑IFN-γ, iNOS and nitrite; ↑ in vitro leishmanicidal effect | Moreno et al., 2012 [38] | ||||
| ↑IFN-γ, iNOS and nitrite; parasites were detected in bone marrow in 3/10 vaccinated dogs (qPCR) | Martin et al., 2014 [41] | ||||
| ↑IgG2; 68.4% efficacy (PCR and culture, serological and clinical examinations) | Oliva et al., 2014 [42] | ||||
| Brazil | Leish-Tec® | CEVA (France) | Recombinant A2 (rA2) protein with the adjuvant saponin | ↑IgG, IgG2, IFN-γ and IL-10; parasites detected in 57.14% (bone marrow culture) and 28.5% (blood PCR) in vaccinated dogs | Fernandes et al., 2008 [44] |
| ↑IgG, IgG2 and IgG1; 58.1% efficacy (bone marrow culture + xenodiagnoses) | Regina-Silva et al., 2016 [43] | ||||
| ↑IgG and ↑IgG2; 43% of vaccinated dogs developed the disease |
Grimaldi et al., 2017 [45] | ||||
| ↑ CD8+IFN-γ+ | Aguiar-Soares et al., 2020 [46] | ||||
| Europe | LetiFend® | LETI Laboratories (Spain) | Protein Q – a genetic fusion of five antigenic fragments from four L. infantum proteins, named acidic ribosomal proteins Lip2a, Lip2b, LiP0, and the histone H2A | ↑ DTH (9/10 vaccinated dogs); 90% of vaccinated dogs remain healthy (lymph nodes culture, clinical and anatomic-pathologic analysis) | Molano et al., 2003 [48] |
| ↑ NO production and DTH; parasites detected in vaccinated dogs (single dose): 1/7, 1/7 and 0/ 7 (PCR of skin, lymph node and spleen, respectively); parasites detected in vaccinated dogs (two doses): 4/7, 1/7 and 2/ 7 (PCR of skin, lymph node and spleen, respectively) | Carcelén et al., 2009 [49] | ||||
| ↑IgG2 anti-Protein Q; 72% efficacy (lymph nodes or bone marrow PCR and smear) | Cotrina et al., 2018 [50] |
| TBV composition | Vector / Parasite | Vaccination schedule/ Animals/ Artificial or in vivo feeding | Evaluated parameters | Main findings | Reference |
|---|---|---|---|---|---|
| Sandfly gut antigens | Phlebotomus duboscqi | 1 IM dose, followed by 2 SC doses (14th and 21th day) / 24 hamsters / in vivo feeding | Humoral response; Survival and fecundity of sandflies | ↑ P. duboscqi-specific IgG; ↓ survival; egg production and egg hatching | Ingonga et al., 1996 [66] |
| Crude whole Leishmania major parasites or rgp63 or LPG or rgp63/LPG | Phlebotomus duboscqi / Leishmania major | 4 IV doses, at 7-day interval or 3 IV doses, at 14-day interval / BALB/c mice, posteriorly infected with L. major/ in vivo feeding | Humoral response; Infection rate in sandfly; Promastigote forms presented after blood meal; Histopathology of midgut | ↑ IgG anti-soluble L. major antigen; ↓ infection rate; ↓ infective metacyclic forms | Tonui et al., 2001 [67] |
| PpGalec | Phlebotomus papatasi / Leishmania major | 5 doses / BALB/c / Artificial feeding | Infection rate by ex vivo and in vivo analyses in sandflies | ↓ infection rate | Kamhawi et al., 2004 [68] |
| Repeated sandfly bites | Lutzomyia longipalpis | Repeated bites of 100-120 females / Rabits / in vivo feeding | Humoral response; sandfly survival and oviposition analysis | ↑ IgG anti-sandfly; ↑ mortality; ↓ oviposition | Vilela et al., 2006 [69] |
| Leishmune® | Lutzomyia longipalpis / Leishmania chagasi | 3 SC doses at 20-day interval / mongrel dogs / Artificial feeding | Infection rate (in vitro and in vivo analysis) in sandflies | ↑ L. chagasi binding to sandfly midguts; ↓ infection rate | Saraiva et al., 2006 [70] |
| PpChit1 | Phlebotomus papatasi /Leishmania major | 3 SC doses at 14-day interval / BALB/c / Artificial feeding | Infection rate in sandflies | ↓ infection rate | Coutinho-Abreu et al., 2010 [72] |
| CaniLeish® | Phlebotomus perniciosus / Leishmania infantum | 3 doses / beagle dogs, natural infected after vaccination / in vivo infection | Infection rate in sandflies | ↓ infection rate | Bongiorno et al., 2013 [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





