1. Introduction
Colorectal cancer was the third most common disease and the second most common cause of death in the world in 2020 (1), and approximately 20% of cases are unresectable (2). Unresectable colorectal cancer is difficult to cure, but advances in drug therapy have led to a median survival of over 30 months (3, 4). Chemotherapy for unresectable colorectal cancer is expected to lead to long-term survival by a good sequential baton pass from first-line treatment to the later lines (5). Progression-free survival (PFS) in the first-line treatment for unresectable colorectal cancer is approximately 10 months, and PFS in the second-line treatment is reported to be about 6 months, with overall survival (OS) of 10-13 months, both of which are still unsatisfactory (6-8). Improving the outcome of second-line treatment is considered an important issue that contributes to the survival of patients with colorectal cancer.
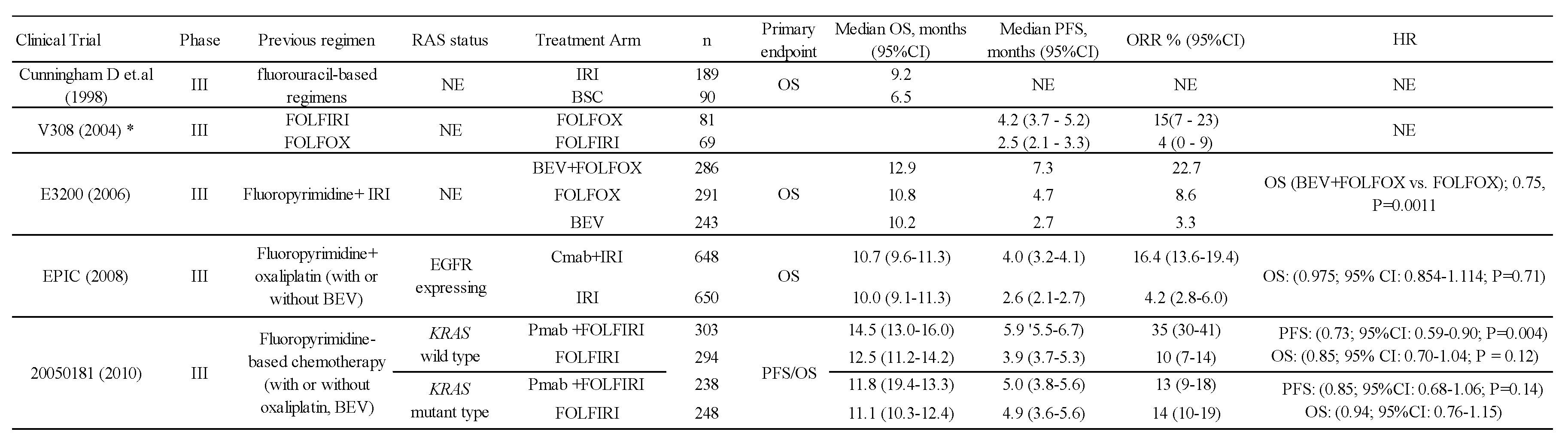
The history of second-line treatment for unresectable colorectal cancer dates back to a 1998 report (9). A comparison between irinotecan and best supportive care was made in patients with unresectable colorectal cancer who had failed fluorouracil. The primary endpoint, the 1-year survival rate, was significantly better in the irinotecan group than in the best supportive care group (36.2% vs. 13.8%), showing the survival benefit of second-line treatment for unresectable colorectal cancer. Subsequently, a comparative study investigating the order of administration of FOLFOX and FOLFIRI, which had been established as the standard first-line treatment regimen, was conducted. The results showed quite similar efficacy between FOLFOX followed by FOLFIRI and FOLFIRI followed by FOLFOX (5). This also demonstrated the usefulness of FOLFOX or FOLFIRI as second-line treatment. Next came molecularly targeted therapies such as bevacizumab (BEV), an angiogenesis inhibitor, and anti-EGFR antibodies. As second-line therapy, adding BEV to FOLFOX showed overall survival (OS) benefit compared with FOLFOX therapy (10). In terms of anti-EGFR antibodies, the EPIC trial failed to show the OS benefit of cetuximab (Cmab) plus IRI vs. IRI alone in patients with colorectal cancer expressing EGFR (11). On the other hand, the 20050181 study showed significant OS and PFS benefits of panitumumab (Pmab) plus FOLFIRI vs. FOLFIRI for KRAS-wild colorectal cancer, whereas no OS benefit was observed for KRAS-mutant colorectal cancer (12) (Table 1). These results showed that adding anti-EGFR antibodies to chemotherapy was beneficial for KRAS-wild colorectal patients even in the second-line setting.
Table 1.
Pivotal studies of second-line treatment for colorectal cancer.
Table 1.
Pivotal studies of second-line treatment for colorectal cancer.
In the current second-line treatment, in addition to RAS mutation and BRAF mutation, dMMR/MSI-H, HER2-statuses, and TMB-H statuses are also important for selecting treatment regimens, and the drug of choice depends on the regimen used in first-line treatment. In colorectal cancer, the incidence of RAS wild-type is reported to be 40%–50% (13-15), BRAF V600E mutation about 10% (16), dMMR/MSI about 15% (17), TMB-H about 11% (18), and HER2-positive 2%–11% (19). Depending on biomarker status, whether to select an anti-EGFR antibody, BRAF inhibitor, MEK inhibitor, HER2 inhibitor, or immune checkpoint inhibitor should be decided. In patients with RAS mutation, anti-EGFR antibody is not recommended by regional guidelines as one of the standard treatments (20-22). In patients with the BRAF V600E mutation, encorafenib plus binimetinib plus Cmab or encorafenib plus Cmab is recommended (23). In cases of HER2-positive (IHC 3+ or IHC2+/FISH-positive) colorectal cancer, trastuzumab plus pertuzumab therapy is recommended (24). In cases with TMB-H cancer, pembrolizumab is recommended (25), and in cases with dMMR/MSI-H, ipilimumab plus nivolumab therapy is recommended (26).
Currently, three anti-angiogenesis inhibitors are available for metastatic colorectal cancer in the second-line setting. BEV, ramucirumab (RAM), or aflibercept (AFL) can be used regardless of prior BEV use in second-line treatment. Based on the frequency of biomarkers, it is estimated that angiogenesis inhibitors are the most commonly used in second-line treatment. These three angiogenesis inhibitors are known to inhibit angiogenesis through different mechanisms of action. Previous biomarker studies suggested that angiogenesis-related factors such as VEGF-A and VEGF-D might be potential predictors of therapeutic efficacy of angiogenesis inhibitors (27-33). Currently, a prospective study to compare the safety and efficacy across these three angiogenesis inhibitors combined with FOLFIRI is ongoing in Japan, and it is exploring biomarkers that could help select an appropriate angiogenesis inhibitor to improve the outcomes of second-line treatment (34).
In this review, the second-line treatment for metastatic colorectal cancer is outlined with a focus on these angiogenesis inhibitors.
2. Second-Line Treatment with Angiogenesis Inhibitors for Unresectable Colorectal Cancer
2.1. Angiogenic Factors and Angiogenesis Inhibitors
Vascular endothelial growth factor (VEGF) is known to play an important physiological role in embryogenesis, wound healing, and angiogenesis, and it is associated with malignant tumor growth, invasion, and metastasis pathologically (35,36). Although the term VEGF usually refers to VEGF-A, there are other isoforms, such as VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF), which are collectively known as the VEGF family. These family members have been found to differ in expression patterns, receptor affinity, and biological functions (37).
VEGF-A is the most important angiogenic factor (38). Variants of VEGF121, VEGF145, VEGF148, VEGF165, VEGF183, VEGF189, and VEGF206 are known to exist due to selective splicing and have different affinities for the receptor (37). The most frequently expressed variants are VEGF121, 165, and 189, of which VEGF165 is the most frequently expressed and has the strongest angiogenic effect (39). VEGF-A binds to vascular endothelial growth factor receptor 1 (VEGFR-1) and VEGFR-2, and its affinity for VEGFR-1 is 10-fold stronger than that for VEGFR-2 (40).
VEGF-D is a protein similar to VEGF-C, it plays a central role in lymphangiogenesis, and it is also considered essential for angiogenesis. It is expressed at high levels in the lungs, the site of lymphatic vessel development in the fetus, as well as in adults, and it is highly expressed in the heart, lungs, skeletal muscle, and small intestine (41). It binds with strong affinity to VEGFR-2 and VEGFR-3. Although much of its function has not been elucidated, it has been reported to be associated with lymphangiogenesis and metastasis within tumors (42).
BEV is a humanized anti-VEGF IgG1 monoclonal antibody based on murine anti-VEGF monoclonal antibody muMAb A4.6.1, which selectively binds to VEGF-A (43). VEGF-A is a major regulator of angiogenesis and is upregulated in most human tumors, contributing to tumor growth and metastasis. BEV selectively binds to VEGF-A, thereby blocking the binding of VEGF-A to its receptors (VEGFR-1 and VEGFR-2) expressed on vascular endothelial cells, blocking the VEGF signaling pathway and inhibiting VEGF-induced angiogenesis in tumor tissue (44-46). It is the first anti-cancer drug in the world to demonstrate clinical usefulness as an angiogenesis inhibitor (47,48).
RAM is a human anti-VEGFR-2 monoclonal antibody (IgG1b) against vascular endothelial growth factor receptor 2 (VEGFR-2a) that binds to VEGF-A, VEGF-C, and VEGF-D. In preclinical studies, RAM has been shown to bind specifically to human VEGFR-2 with high affinity, inhibiting the binding of VEGF ligands (VEGF-A, VEGF-C, and VEGF-D) to VEGFR-2 and blocking VEGFR-2 activation, thus suppressing tumor tissue angiogenesis (49-51).
AFL is a dimeric glycoprotein with a molecular weight of 97 kDa, produced by fusing the second immunoglobulin (Ig)-like C2 domain of human VEGFR-1 with the third Ig-like C2 domain of human VEGFR-2, which is then fused to the stationary region (Fc domain) of human IgG1 (52). It binds to VEFG-A, VEGF-B, and PlGF, leading to suppression of tumor growth by inhibiting vascularization.
2.2. Availability of Angiogenesis Inhibitors for Second-Line Treatment
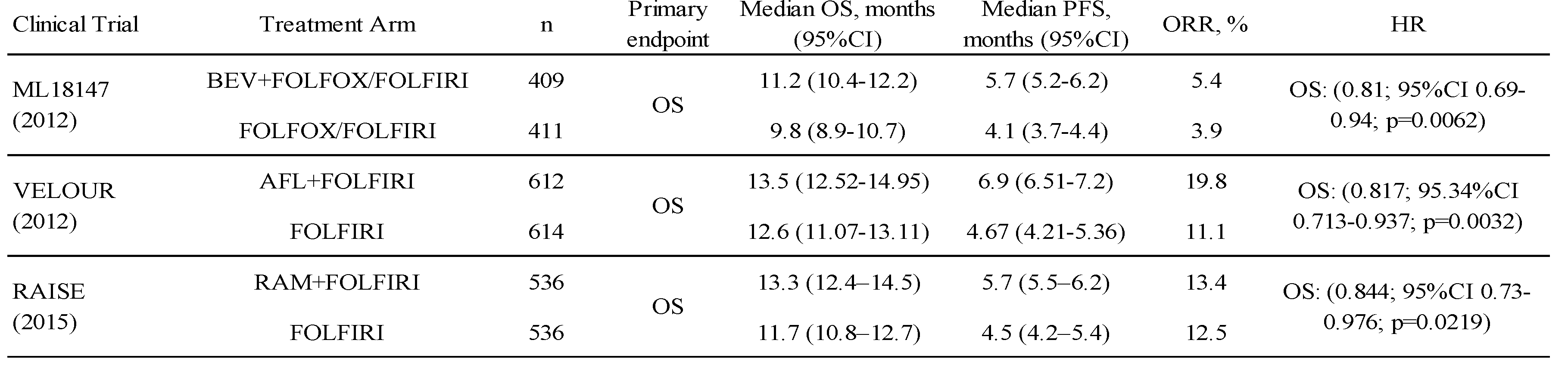
The triple combination therapy of BEV combined with oxaliplatin and fluoropyrimidine is one of the standard first-line treatments for unresectable colorectal cancer and is the most frequently used regimen worldwide (53). Following the approval of BEV, a large prospective, observational study in the USA identified continuous use of BEV as an independent factor prolonging survival in second-line colorectal cancer patients who experienced progression on first-line BEV treatment (54). Subsequently, a clinical trial was conducted to evaluate the benefit of continuous administration of BEV after failure of first-line BEV combination treatment. A phase III trial (ML18147) demonstrated the add-on effect of BEV to FOLFOX or FOLFIRI in colorectal cancer patients who failed the triplet therapy containing BEV, establishing it as the standard of care for second-line colorectal cancer therapy (6). Subsequently, the RAISE and VELOUR trials showed the add-on effects of RAM and AFL to FOLFIRI in the second-line setting, respectively (7,8). On the basis of these results, RAM plus FOLFIRI and AFL plus FOLFIRI were established as standard second-line treatments for colorectal cancer, as well as BEV plus FOLFIRI (20-22). The results of these three trials are shown in Table 2.
Table 2.
Pivotal studies of second-line treatment with angiogenesis inhibitors.
Table 2.
Pivotal studies of second-line treatment with angiogenesis inhibitors.
Notably, patients who failed in the early period of first-line treatment were excluded from the ML18147 study, and patients who had not received BEV in first-line treatment, accounting for 30% of all participants, were included in the VELOUR study. Although the eligibility criteria in each study were somewhat different, they all demonstrated the usefulness of an angiogenesis inhibitor as second-line treatment. However, no head-to-head randomized studies have been conducted to compare the efficacy and safety of these three angiogenesis inhibitors, and there is no evidence to support their choice in routine practice.
The 2019 edition of the Japanese guidelines for the treatment of colorectal cancer for physicians (22) strongly recommends the combination of FOLFIRI therapy and one of the angiogenesis inhibitors, BEV, RAM, or AFL, as standard second-line treatment after oxaliplatin combination therapy. The recommendations are comparable among the three angiogenesis inhibitors, and their selection should be based on the risk-benefit balance, such as toxicity profile and medical costs. The NCCN Guidelines version 1, 2022 (20) recommend the combination of FOLFIRI therapy and an angiogenesis inhibitor as second-line treatment after concurrent oxaliplatin, and when any of the three angiogenesis inhibitors is used, BEV is preferred in terms of toxicity and cost. Referring to the literature cited for cost (55), RAM was the most expensive in the USA, albeit as of 2015. Similarly, the ESMO guidelines (21) recommend the combination of FOLFIRI therapy and an angiogenesis inhibitor as standard second-line treatment after oxaliplatin combination therapy. For patients who have not received BEV as first-line treatment, the combination of BEV or AFL (limited to FOLFIRI) is recommended, and for patients who have received BEV as first-line treatment, all three drugs (BEV, RAM, and AFL) are recommended, with RAM and AFL being especially recommended in early refractory cases. These recommendations are thought to be based on the conditions of the subject populations in the three studies mentioned above, which demonstrated the benefit of each, but the medical biological reasons for these recommendations are not clear.
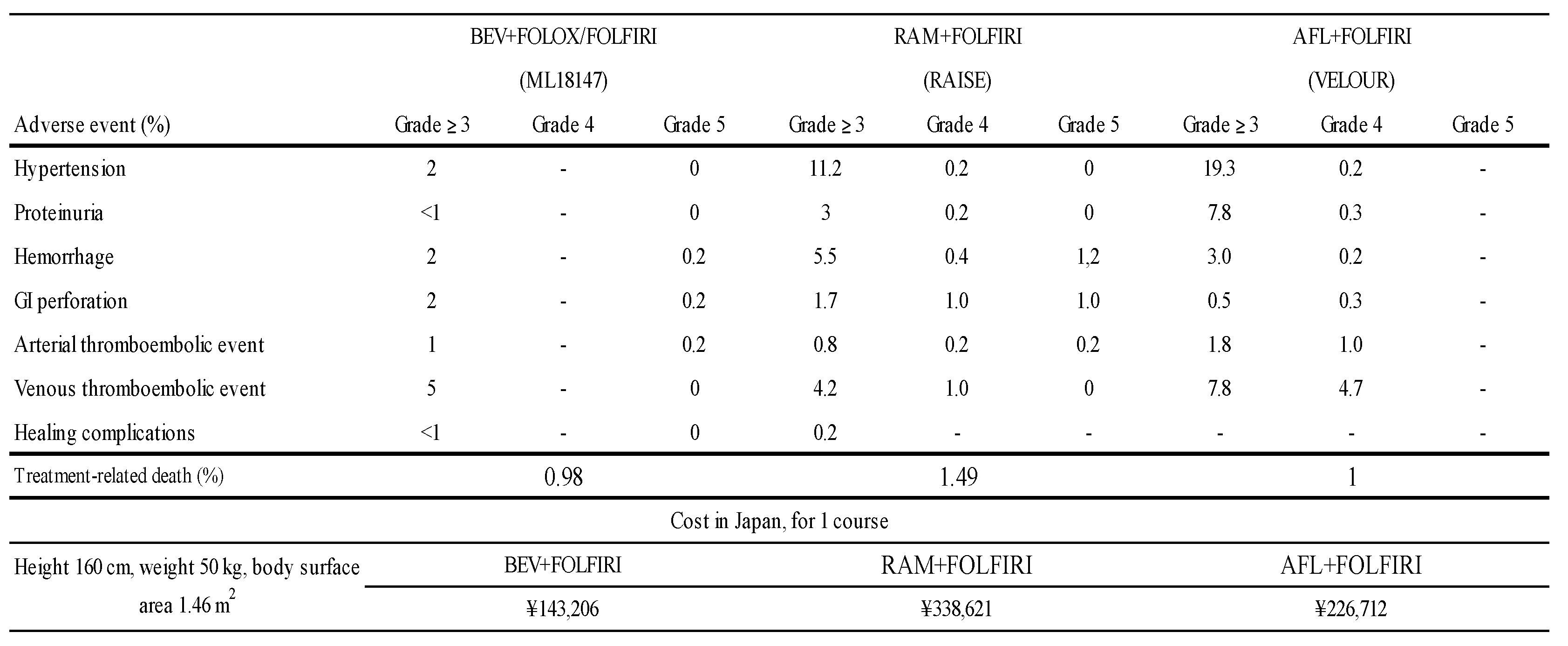
The toxicities from the three pivotal phase III studies are summarized in Table 3. The frequency of occurrence of these adverse events gives the impression that the toxicity of BEV is mild, but since the different trials are arranged side by side, the results should be interpreted with caution. Regarding the cost in Japan, the combination of RAM plus FOLFIRI is the most expensive when estimated based on the average body surface area of Japanese patients (Table 3.).
Table 3.
Toxicity and cost of second-line treatments with angiogenesis inhibitors.
Table 3.
Toxicity and cost of second-line treatments with angiogenesis inhibitors.
Thus, three angiogenesis inhibitors have demonstrated utility and are available for use as second-line treatment for colorectal cancer, but the strategy for choosing among them in terms of therapeutic efficacy remains unestablished.
2.3. Biomarkers for Angiogenesis Inhibitors
Recently, angiogenesis-related factors have been reported as possible biomarkers of efficacy for BEV, RAM, and AFL.
2.3.1. Biomarkers for BEV
A meta-analysis reported in 2013 analyzed clinical trials (1 report for colorectal cancer, 2 for lung cancer, 1 for renal cell carcinoma) in which VEGF-A was measured by ELISA in pretreatment plasma samples. The median baseline measurements were 44 pg/mL, 36 pg/mL, 45 pg/mL, and 55 pg/mL, respectively, in each study. In terms of distribution, almost half of the patients were in the 12.5–49 pg/mL range, with a measurable range of 12.5–889 pg/mL (56). This analysis showed that VEGF-A is a prognostic factor, but not a predictor of BEV efficacy. A 2013 report from the MD Anderson Cancer Center used a multiplex bead assay to measure VEGF-A levels in plasma samples from the untreated group, the progression group after chemotherapy without BEV, and the progression group after chemotherapy with BEV, with median values of 760 pg/mL, 534 pg/mL, and 1,740 pg/mL, respectively (57).
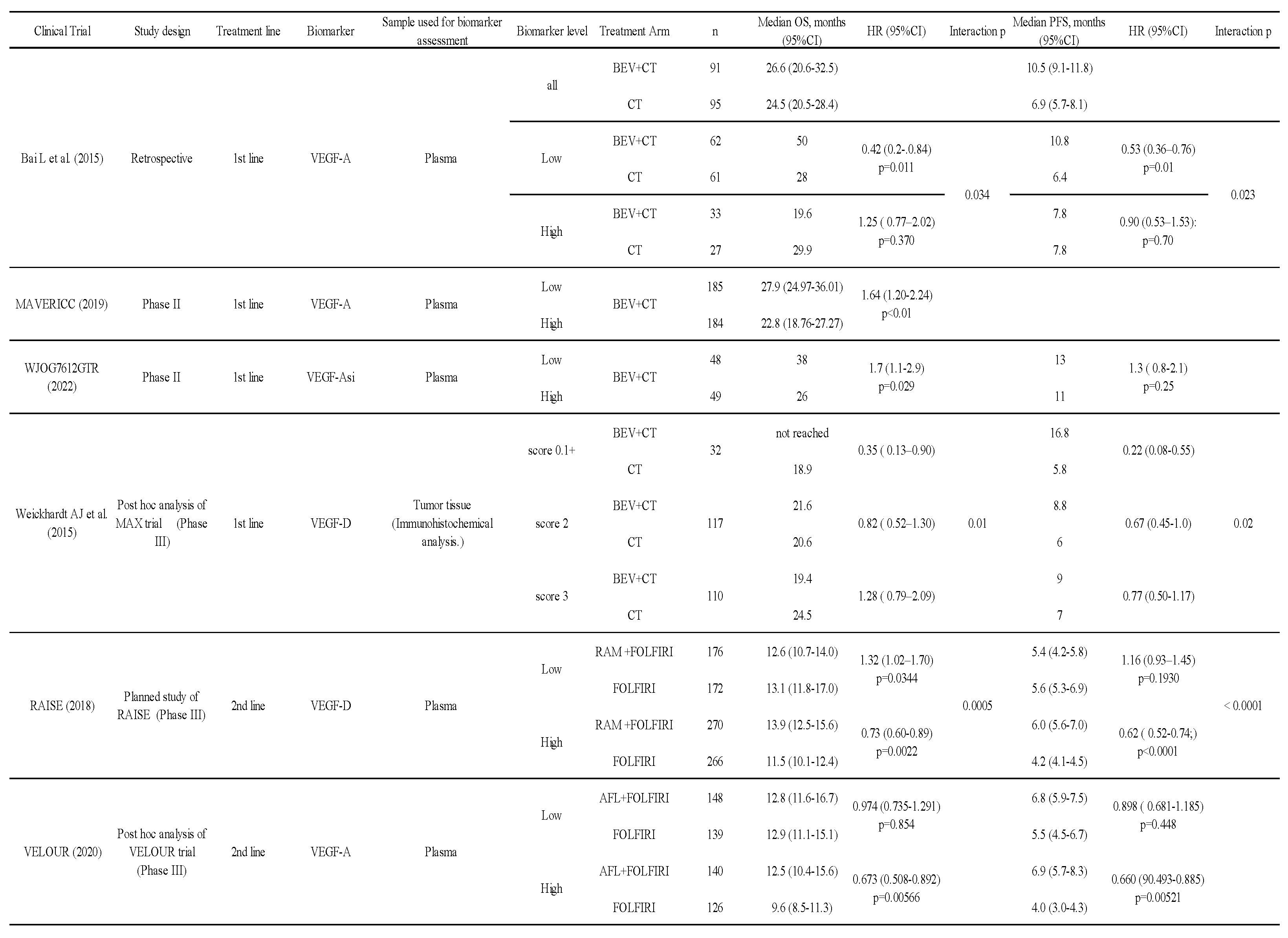
Bai et al. reported a retrospective study analyzing angiogenesis-related factors in pretreatment plasma of patients with unresectable colorectal cancer undergoing first-line treatment (27). The median PFS of the chemotherapy group with BEV vs. chemotherapy alone in the low VEGF-A group was 10.8 months vs. 6.4 months (hazard ratio [HR] 0.53, 95% confidence interval [CI] 0.36–0.76, P=0.01), and the median OS was 50.0 months vs. 28.0 months (HR 0.42, 95% CI 0.20–0.84, P=0.011). In the high VEGF-A group, median PFS was 7.8 months vs. 7.8 months (HR 0.90, 95% CI 0.53–1.53, P=0.70), and median OS was 19.6 months vs. 29.9 months (HR 1.25, 95% CI 0.77–2.02, P=0.37) in the chemotherapy with BEV group and chemotherapy alone group, respectively (Table 4). Even though this was a retrospective study, it suggested that lower levels of VEGF-A in the pre-treatment plasma may be a possible biomarker for treating with BEV.
Table 4.
Summary of biomarkers of angiogenesis inhibitors.
Table 4.
Summary of biomarkers of angiogenesis inhibitors.
In addition, in a phase II study comparing BEV plus FOLFOX with BEV plus FOLFIRI in first-line treatment, both groups were divided into high and low pretreatment plasma VEGF-A groups. The OS was significantly worse in the high group than in the low VEGF-A group (28). Another phase II study showed whether VEGF-A short isoform (VEGF-Asi) could be a predictor of BEV efficacy in BEV plus FOLFOX/XELOX in first-line treatment (29). Pre-treatment plasma samples were collected, and the pVEGF-Asi level was divided according to the median pVEGF-Asi value of 37 (range 6.5–262) pg/mL. The HR of the high pVEGF-Asi group vs. the low group for PFS was 1.3 (95% CI 0.8–2.1). These results of biomarker analyses from Phase II trials are consistent with previous results showing that lower levels of VEGF-A are associated with benefits from BEV.
The MAX trial, a phase III study, demonstrated the benefit of BEV in the first-line treatment of unresectable colorectal cancer (58). In a post hoc analysis of this trial, angiogenic factor expression in tumor tissue (immunohistochemistry) and the therapeutic effect were examined (30). In the group with weak expression of VEGF-D (score 0, 1+), the median OS in the BEV with chemotherapy group vs. the chemotherapy alone group was ‘not reached’ vs. 18.9 months (HR 0.35, 95% CI 0.13–0.90). In the moderate expression (score 2+) group, median OS was 21.6 vs. 20.6 months (HR 0.82, 95% CI 0.52–1.30). In the strong expression (score 3+) group, median OS was 19.4 vs. 24.5 months (HR 1.28, 95% CI 0.79–2.09), respectively, indicating that the therapeutic effect of BEV was decreased as the expression of VEGF-D increased (interaction P=0.01) (Table 4).
These results suggest that the therapeutic effect of BEV may be poor in the high VEGF-A and high VEGF-D expression groups before treatment, and good in the low VEGF-A and VEGF-D expression groups.
2.3.2. Biomarkers for RAM
Biomarker analyses in the RAISE study examined plasma VEGF-C, VEGF-D, sVEGFR-1, sVEGFR-2, sVEGFR-3, and VEGFR-2. In the analysis of the RAISE study (31), a cutoff value of 115 pg/mL (median 135 pg/mL) was set by multivariate analysis in a predefined exploratory set of populations, and efficacy was confirmed in a validation set. Finally, analysis of the full translational research set (exploratory set plus validation set) was reported. OS of the chemotherapy with RAM group vs. chemotherapy alone group in the high VEGF-D level was significantly better in the combined RAM group. In contrast, in the lower VEGF-D group, OS was worse in the combined RAM group. Analysis of OS also showed an interaction between high and low VEGF-D levels. The same results were observed for PFS (Table 4). There were no significant interactions of other factors.
These results suggest that a high plasma VEGF-D level is a potential predictor of RAM efficacy; it is interesting that the effect is opposite to the trend for BEV.
2.3.3. Biomarkers for AFL
In the biomarker analysis of the VELOUR study, VEGF-A, PlGF, endoglin, T-cadherin, VEGFR-3, serum amyloid P-component, vitamin D binding protein, neuropilin-1, C-reactive protein, IL-8, MIF, EoTaxin-1, VEGFR2, Hepsin, and SPD were examined (32-33). The results showed that OS in the high plasma VEGF-A group was better in the AFL group than in the placebo group. On the other hand, there was no clear difference in the low VEGF-A group, suggesting that a high plasma VEGF-A level may be a predictor of treatment response to AFL, with an opposite effect to the trend for BEV in VEGF-A. The overall median baseline VEGF-A level in the VELOUR trial was 144 pg/mL, which was used as a cutoff to compare efficacy (Table 4).
2.3.4. Other Biomarkers thought to be Associated with Angiogenesis Inhibitors
PlGF, a member of the VEGF family, is a 25-kDa cytokine discovered in human placenta that binds to VEGFR-1 and is known to be involved in angiogenesis (59). Although PlGF as a biomarker has been studied in a phase II trial of BEV plus FOLFIRI as first-line treatment for unresectable colorectal cancer, its potential as a predictive biomarker was not shown. (60). In the biomarker analysis of the VELOUR study described above, OS in patients with high plasma PlGF levels was better in the AFL group than in the placebo group (12.2 months vs. 9.7 months, HR 0.586, P = 0.00429). On the other hand, OS in patients with low plasma PlGF levels was comparable between the AFL and placebo groups (12.8 months vs. 11.7 months, HR 0.947, P=0.653), indicating that PlGF may be a predictor of response (33).
IL-8 is a chemokine that was first discovered as a neutrophil migration factor, but it has since been found to be associated with angiogenesis and tumor growth (61). In the aforementioned phase II trial of BEV plus FOLFIRI, when pretreatment plasma IL-8 levels were divided into high and low, OS was better in patients with a low level vs. a high level (11.0 months vs. 15.1 months, HR 2.05, P=0.037), suggesting that IL-8 might be a possible prognostic factor (60). A post hoc analysis of the biomarker in the VELOUR trial was reported at ASCO-GI in 2015. The plasma IL-8 level was shown to be a possible prognostic factor, with OS worse in the high IL-8 group than in the low IL-8 group, regardless of the AFL combination. In the high IL-8 group alone, median OS was 9.4 months in the AFL plus FOLFIRI group and 8.0 months in the FOLFIRI group, significantly better in the AFL combination group (HR 0.632, 95% CI 0.489–0.817, P=0.0006). In the low IL-8 group, there was no clear difference between patients with and without concomitant AFL (18.8 months vs. 19.8 months, HR 0.957, P=0.782). These results suggest that IL-8 may be a possible predictor of AFL efficacy (32). The RAINBOW study, which investigated the additive effects of RAM for advanced gastric cancer, showed that IL-8 might be a possible prognostic factor for RAM in patients with unresectable gastric cancer, although the cancer types were different, with lower pretreatment IL-8 levels reported to be associated with a better prognosis (62).
3. Angiogenesis Inhibitors and anti-EGFR Antibodies
3.1. Second-Line Treatment with Anti-EGFR Antibodies
Although anti-EGFR antibodies are likely to be used in the first-line treatment of RAS wild-type, left-sided colorectal cancer, no conclusion has been reached as to whether the primary site influences the effect of anti-EGFR antibodies in previously treated patients.
A small number of retrospective studies has been reported. Reports from Taiwan of KRAS wild-type colorectal cancer refractory to 2 or 3 regimens (63) and from Italy of RAS/BRAF wild-type colorectal cancer refractory to 2 or 3 regimens (64) both suggested that the therapeutic effect of anti-EGFR antibodies is better in left-sided colorectal cancer. However, these were retrospective reports of a small number of cases and cannot be considered concrete evidence. The NCCN guidelines state that the use of anti-EGFR antibodies in previously treated cases can be considered regardless of the localization of the primary site (20).
The frequency of RAS mutations in colorectal cancer has been reported to be 50%–60% for KRAS, NRAS, and HRAS (13-15). Around 80% of RAS wild-type patients have left-sided disease (65). It is thought that anti-EGFR antibody would be selected for these patients in first-line treatment. Thus, the use of anti-EGFR antibodies in second-line treatment is expected to be limited to approximately 10% of colorectal cancers.
As a second-line treatment for RAS/BRAF wild-type colorectal cancer, treatment regimens using anti-EGFR antibodies are recommended by regional guidelines as one of the standard treatments (20-22). The approved anti-EGFR antibodies are Cmab, a chimeric antibody, and Pmab, a fully human antibody.
In 2008, Sobrero et al. reported the EPIC trial comparing Cmab plus irinotecan vs. irinotecan alone in patients with colorectal cancer who were refractory to the combination of fluoropyrimidine and oxaliplatin (with or without BEV) (11). Although the primary endpoint of OS did not show superiority, it was considered to be influenced by the fact that 47% of patients in the irinotecan monotherapy group received Cmab as subsequent treatment, and the usefulness of Cmab as second-line treatment was taken to have been demonstrated.
For second-line treatment with Pmab, a comparative study that tested the superiority of Pmab plus FOLFIRI over FOLFIRI in metastatic colorectal cancer that was refractory to fluoropyrimidine-based first-line treatment was reported (12). The primary endpoints were PFS and OS by KRAS status. KRAS testing was performed after completion of patient enrollment and just prior to the interim OS analysis. PFS of Pmab plus FOLFIRI was better compared with FOLFIRI in the wild-type KRAS population, indicating the superiority of Pmab. OS in the wild-type KRAS was not shown to be statistically significantly different between the Pmab combined group and FOLFIRI alone group. The number of patients receiving BEV in first-line treatment was about 20% in both groups, and subgroup analysis showed that prior BEV treatment did not affect PFS (Table 1).
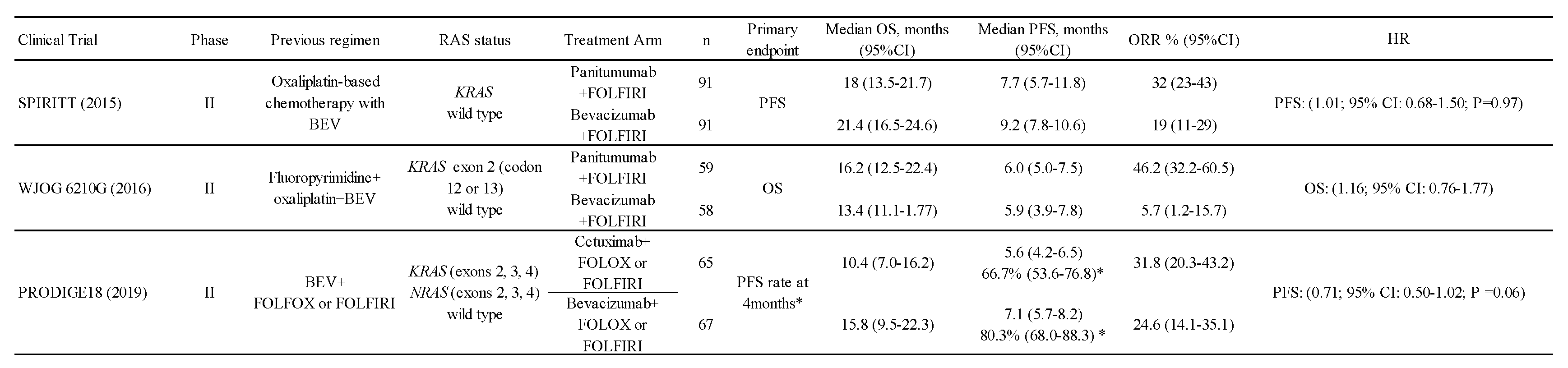
3.2. Angiogenesis Inhibitors vs. Anti-EGFR Antibody with Bevacizumab beyond Progression
As presented in 3.1, anti-EGFR antibodies have shown utility in second-line treatment. In the same way, angiogenesis inhibitors have also shown usefulness in second-line treatment, as mentioned in 2.2. Which of these molecularly targeted agents should we use in second-line treatment after BEV use? Several clinical trials were conducted to address this question (Table 5).
Table 5.
Comparative study of anti-EGFR antibodies and BEV after BEV failure.
Table 5.
Comparative study of anti-EGFR antibodies and BEV after BEV failure.
Hecht et al. reported the results of a randomized, phase II trial comparing Pmab plus FOLFIRI with Bev plus FOLFIRI in patients with KRAS wild-type colorectal cancer after failure of a BEV plus oxaliplatin-based chemotherapy regimen (66). Although the response rate was better in the Pmab group, the primary endpoint, which was PFS, was not met. Shitara et al. compared Pmab plus FOLFIRI with BEV plus FOLFIRI as second-line treatment in patients with KRAS exon 2 (codon 12, codon 13) wild-type colorectal cancer who had failed first-line treatment including fluoropyrimidine, oxaliplatin, and BEV (67). This was a randomized, phase II trial with a primary endpoint of OS. The trial was originally set up to investigate the superiority of BEV plus FOLFIRI over Pmab plus FOLFIRI, but it was changed to investigate similar OS in both arms based on the results of several randomized trials published after the start of this trial. The HR for the primary endpoint of OS was 1.16 (95% CI 0.76–1.77), suggesting similar treatment efficacy. Bennouna et al. conducted a phase II trial comparing chemotherapy with BEV with Cmab after failure of chemotherapy with BEV for KRAS/NRAS wild-type unresectable colorectal cancer (68). The primary endpoint of 4-month PFS was 80.3% (95% CI 68.0%–88.3%) in the BEV chemotherapy arm and 66.7% (95% CI 53.6%–76.8%) in the Cmab arm.
These trials have not shown significant differences in primary endpoints (PFS or OS) between chemotherapy with anti-EGFR antibody and chemotherapy with BEV after failure of BEV combination chemotherapy. However, these were phase II trials with a small number of patients and cannot be considered definitive. More to the point, whether BEV is appropriate to compare to anti-EGFR antibody in those situation is also inconclusive.
3.3. Treatment with Angiogenesis Inhibitors after Use of an Anti-EGFR Antibody
Although anti-EGFR antibodies are likely to be used in the first-line treatment of RAS wild-type, left-sided colorectal cancer, there is no clear evidence for the efficacy of angiogenesis inhibitors in subsequent second-line treatment.
An Italian, multicenter, retrospective study analyzed 277 colorectal cancer cases treated with anti-EGFR antibodies in first-line treatment and angiogenesis inhibitors in second-line treatment (69). Of the 277 patients, 228 (82%) were treated with BEV and 49 (18%) with AFL. Although it is impossible to say anything definite due to the retrospective nature of the study and variability in the number of patients, a trend toward longer PFS for BEV was shown.
A retrospective report from Japan was also published (70). Analysis of 1,163 patients with wild-type RAS who were given anti-EGFR antibodies in first-line treatment was performed. Of the 1,163 cases, 83% involved the left-sided colon, and 84% were given Pmab plus FOLFOX as the first-line treatment regimen. Angiogenesis inhibitors used in second-line treatment included BEV (63%), RAM (27%), and AFL (10%). This study analyzed treatment durations (from the start of second-line treatment to the end of antitumor drug therapies), rather than PFS. Median PFS was 12.5 months (95% CI 11.2–14.0 months) for the BEV group, 12.5 months (95% CI 11.2–14.8 months) for the RAM group, and 14.0 months (95% CI 10.4–17.0 months) for the AFL group. The results were similar in each group.
A prospective, phase II trial from Japan was reported at ASCO-GI in 2022. The efficacy and safety of RAM plus FOLFIRI in second-line treatment after first-line use of anti-EGFR antibody were evaluated, and PFS was reported to be 7 months (95% CI 5.7–7.6 months) (71).
Cases in which patients received anti-EGFR antibodies in the first-line treatment are expected to receive angiogenesis inhibitors in second-line treatment. Two retrospective studies and one prospective study involving such patients described above seem to suggest the usefulness of angiogenesis inhibitors (69-71). Historically, the results of these studies are similar to the data of BEV beyond progression (6-8). However, it is unclear which angiogenesis inhibitor should be selected in this setting.
4. New Angiogenesis Inhibitors and Regimens
In the area of angiogenesis inhibitors, a new drug, fluquintinib, has also been developed in the later line (72,73). In addition, a phase II study of fluquintinib in combination with capecitabine as maintenance therapy after first-line treatment comparing it to BEV with capecitabine is ongoing (74).
There are also a number of ongoing clinical trials using immune-checkpoint inhibitors for the second-line treatment of colorectal cancer, including a phase III study of chidamide, the histone deacetylase inhibitor, and sintilimab, the anti-PD-1 antibody, in combination with BEV plus FOLFIRI, the standard of care for second-line treatment (75).
Even in the development of these new angiogenesis inhibitors and new regimens, BEV is often the drug of choice for concomitant use. This may change as the usefulness of the aforementioned biomarkers is demonstrated.
5. Conclusions
The second-line treatment for unresectable colorectal cancer, in particular, with angiogenesis inhibitors, was reviewed. As discussed in detail in the section on angiogenesis inhibitors, there is currently no clear rationale for choosing which angiogenesis inhibitor to use in second-line treatment. This is a very troublesome situation in clinical practice. We are faced with this problem in various situations, whether it is failure to respond to BEV regimens, anti-EGFR antibody regimens, or postoperative adjuvant chemotherapy. More recently, as reported in the SUNLIGHT trial (76), the value of concomitant use of BEV with trifluridine/tipiracil has been demonstrated in third-line treatment and beyond. In this case, too, it is unclear whether the BEV combination is better than RAM, AFL, or the same.
Each of the three angiogenesis inhibitors has a slightly different biological mechanism of action. This raises the question of whether there might be some difference in the effectiveness of treatment. To address the confusion and biological questions in clinical practice, the authors initiated a prospective clinical trial, the JCOG2004 trial, which is a randomized, phase II trial comparing BEV plus FOLFIRI, with RAM plus FOLFIRI and AFL plus FOLFIRI in patients with unresectable colorectal cancer who have failed or not tolerated first-line treatment with fluoropyrimidine and oxaliplatin, and exploring biomarkers useful for treatment selection (jRCTs031220058). As mentioned in the angiogenesis inhibitor section, we expect VEGF-D and VEGF-A to be useful biomarkers and will compare the therapeutic effects of high and low VEGF-D and VEGF-A using the Angiogenesis Panel test (77). Even if we refer to the ASCO annual meeting 2023 or Clinical Trials.gov, there are no clinical trials comparing these three angiogenesis inhibitors, and JCOG2004 is the first randomized trial in the world. We believe that, if we complete this clinical trial, we may be getting closer to a method for differentiating the use of angiogenesis inhibitors in the future.
As we have shown, in unresectable advanced colorectal cancer, inhibition of angiogenesis is undoubtedly an important therapeutic strategy in all lines of treatment. It is anticipated that angiogenesis inhibitors will be used in combination with newer agents. In this important therapeutic strategy, it is vital to elucidate the predictors of therapeutic efficacy and to select the appropriate drug from among the many angiogenesis inhibitors.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O'Neil, B.H.; Atkins, J.N.; Berry, S.; Polite, B.N.; O'Reilly, E.M.; Goldberg, R.M.; Hochster, H.S.; Schilsky, R.L.; Bertagnolli, M.M.; El-Khoueiry, A.B.; Watson, P.; Benson 3rd, A.B.; Mulkerin, D.L.; Mayer, R.J.; Blanke, C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Muro, K.; Shitara, K.; Yamazaki, K.; Shiozawa, M.; Ohori, H.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; Ojima, H.; Yuasa, Y.; Miwa, K.; Yasui, H.; Oki, E.; Sato, T.; Naitoh, T.; Komatsu, Y.; Kato, T.; Hihara, M.; Soeda, J.; Misumi, T.; Yamamoto, K.; Akagi, K.; Ochiai, A.; Uetake, H.; Tsuchihara, K.; Yoshino, T. Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2023 Apr 18;329(15):1271-1282. 1: 18;329(15).
- Grothey, A.; Sargent, D.; Goldberg, R.M.; Schmoll, H.J. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004, 22, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; Steffens, C.C.; Alonso-Orduña, V.; Schlichting, C.; Reyes-Rivera, I.; Bendahmane, B.; André, T.; Kubicka, S. ; ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; Prausová, J.; Garcia-Alfonso, P.; Yamazaki, K.; Clingan, P.R.; Lonardi, S.; Kim, T.W.; Simms, L.; Chang, S.C.; Nasroulah, F.; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol, 2015, 16, 499-508.
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; McKendrick, J.; Polikoff, J.; Tellier, A.; Castan, R.; Allegra, C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Cunningham, D.; Pyrhönen, S.; James, R.D.; Punt, C.J.; Hickish, T.F.; Heikkila, R.; Johannesen, T.B.; Starkhammar, H.; Topham, C.A.; Awad, L.; Jacques, C.; Herait, P. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998, 352, 1413–1418. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O'Dwyer, P.J.; Mitchell, E.P. : Alberts, S.R.; Schwartz, M.A., Ed.; Benson, A.B.3rd.; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007, 25: 1539-1544. [Google Scholar]
- Sobrero, A.F.; Maurel, J.; Fehrenbacher, L.; Scheithauer, W.; Abubakr, Y.A.; Lutz, M.P.; Vega-Villegas, M.E.; Eng, C.; Steinhauer, E.U.; Prausova, J.; Lenz, H.J.; Borg, C.; Middleton, G.; Kröning, H.; Luppi, G.; Kisker, O.; Zubel, A.; Langer, C.; Kopit, J.; Burris, H. A 3rd. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008, 26, 2311–2319. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.; Strickland, A.H.; Wilson, G.; Ciuleanu, T.E.; Roman, L.; Van Cutsem, E.; Tzekova, V.; Collins, S.; Oliner, K.S.; Rong, A.; Gansert, J. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010, 28, 4706–4713. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; Rivera, F.; Kocákova, I.; Ruff, P.; Błasińska-Morawiec, M.; Šmakal, M.; Canon, J.L.; Rother, M.; Williams, R.; Rong, A.; Wiezorek, J.; Sidhu, R.; Patterson, S.D. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013, 369, 1023–1034. [Google Scholar] [CrossRef]
- Peeters, M.; Oliner, K.S.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.; Strickland, A.H.; Wilson, G.; Ciuleanu, T.E; Roman, L.; Van Cutsem, E.; He, P.; Yu, H.; Koukakis, R.; Terwey, J.H.; Jung, A.S.; Sidhu, R.; Patterson, S.D. Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin Cancer Res 2015, 21, 5469–5479. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Oliner, K.S.; Parker, A.; Siena, S.; Van Cutsem, E.; Huang, J.; Humblet, Y.; Van Laethem, J.L.; André, T.; Wiezorek, J.; Reese, D.; Patterson, S.D. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res 2013, 19, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; de la Fouchardiere, C.; Rivera, F.; Elez, E.; Bendell, J.; Le, D.T.; Yoshino, T.; Van Cutsem, E.; Yang, P.; Farooqui, M.Z.H.; Marinello, P.; Diaz, L. A Jr.; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Yoshino, T.; Tukachinsky, H.; Lee, J.K.; Sokol, E.; Pavlick, D.C.; Aiyer, A.; Fabrizio, D.; Venstrom, J.M.; Mishima, S.; Nakamura, Y.; Oxnard, G.R. Genomic immunotherapy (IO) biomarkers detected on comprehensive genomic profiling (CGP) of tissue and circulating tumor DNA (ctDNA). J Clin Oncol, 2021, 39, no. 15_suppl, 2541-2541.
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol 2018, 29, 1108–1119. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer Version 1.2022 — February 25, 2022. 25 February.
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; Ciardiello, F.; D'Hoore, A.; Diaz-Rubio, E.; Douillard, J.Y.; Ducreux, M.; Falcone, A.; Grothey, A.; Gruenberger, T.; Haustermans, K.; Heinemann, V.; Hoff, P.; Köhne, C.H.; Labianca, R.; Laurent-Puig, P.; Ma, B.; Maughan, T.; Muro, K.; Normanno, N.; Österlund, P.; Oyen, W.J.; Papamichael, D.; Pentheroudakis, G.; Pfeiffer, P.; Price, T.J. ; Punt, C,; Ricke, J.; Roth, A.; Salazar, R.; Scheithauer, W.; Schmoll, H.J.; Tabernero, J.; Taïeb, J.; Tejpar, S.; Wasan, H.; Yoshino, T.; Zaanan, A.; Arnold, D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; Ishihara, S.; Kanemitsu, Y.; Kinugasa, Y.; Murofushi, K.; Nakajima, T.E.; Oka, S.; Tanaka, T.; Taniguchi, H. Tsuji, A.; Uehara, K.; Ueno, H.; Yamanaka, T.; Yamazaki, K.; Yoshida, M.; Yoshino, T.; Itabashi, M.; Sakamaki, K.; Sano, K.; Shimada, Y.; Tanaka, S.; Uetake, H.; Yamaguchi, S.; Yamaguchi, N.; Kobayashi, H.; Matsuda, K.; Kotake, K.; Sugihara, K,; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Onco 2020, 25, 1–42. [Google Scholar]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; Steeghs, N.; Guren, T.K.; Arkenau, H.T.; Garcia-Alfonso, P.; Pfeiffer, P.; Orlov, S.; Lonardi, S.; Elez, E.; Kim, T.W.; Schellens, J.H.M.; Guo, C.; Krishnan, A.; Dekervel, J.; Morris, V.; Calvo Ferrandiz, A.; Tarpgaard, L.S.; Braun, M.; Gollerkeri, A.; Keir, C.; Maharry, K.; Pickard, M.; Christy-Bittel, J.; Anderson, L.; Sandor, V.; Tabernero, J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; Sawada, K.; Taniguchi, H.; Fuse, N.; Nomura, S.; Fukui, M.; Matsuda, S.; Sakamoto, Y.; Uchigata, H.; Kitajima, K.; Kuramoto, N.; Asakawa, T.; Olsen, S.; Odegaard, J.I.; Sato, A.; Fujii, S.; Ohtsu, A.; Yoshino, T. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med, 2021, 27, 1899–1903. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W. H Jr. ; Italiano, A.; Kao, S.; Piha-Paul, S.A.; Delord, J.P.; McWilliams, R.R.; Fabrizio, D.A.; Aurora-Garg, D.; Xu, L.; Jin, F.; Norwood, K.; Bang, Y.J. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol, 2020, 21, 1353–1365. [Google Scholar]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; Sawyer, M.B.; Hendlisz, A.; Neyns, B.; Svrcek, M.; Moss, R.A.; Ledeine, J.M.; Cao, Z.A.; Kamble, S.; Kopetz, S.; André, T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, F.; Zhang, D.S.; Li, C.; Jin, Y.; Wang, D.S.; Chen, D.L.; Qiu, M.Z.; Luo, H.Y.; Wang, Z.Q.; Li, Y.H.; Wang, F.H.; Xu, R.H. A plasma cytokine and angiogenic factor (CAF) analysis for selection of bevacizumab therapy in patients with metastatic colorectal cancer. Sci Rep 2015, 5, 17717. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Lee, F.C.; Yau, L.; Koh, H.; Knost, J.; Mitchell, E.P.; Bosanac, I.; Choong, N.; Scappaticci, F.; Mancao, C.; Lenz, H.J. MAVERICC, a Randomized, Biomarker-stratified, Phase II Study of mFOLFOX6-Bevacizumab versus FOLFIRI-Bevacizumab as First-line Chemotherapy in Metastatic Colorectal Cancer. Clin Cancer Res 2019, 25, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, W.; Sakai, K.; Makiyama, A.; Yamamoto, Y.; Shitara, K. ; Denda. T.; Izawa, N.; Nakano, Y.; Nishina, T.; Esaki, T.; Hara, H.; Miura, Y.; Boku, N.; Yamazaki, K.; Hironaka, S.; Misumi, T.; Hyodo, I.; Muro, K.; Nishio, K. A phase II study to explore biomarkers for the use of mFOLFOX6/XELOX plus bevacizumab as a first-line chemotherapy in patients with metastatic colorectal cancer (WJOG7612GTR). ESMO Open. 2022, 7, 100592. [Google Scholar]
- Weickhardt, A.J.; Williams, D.S.; Lee, C.K.; Chionh, F.; Simes, J.; Murone, C.; Wilson, K.; Parry, M.M.; Asadi, K.; Scott, A.M.; Punt, C.J.; Nagtegaal, I.D.; Price, T.J.; Mariadason, J.M.; Tebbutt, N.C. Vascular endothelial growth factor D expression is a potential biomarker of bevacizumab benefit in colorectal cancer. Br J Cancer 2015, 113, 37–45. [Google Scholar] [CrossRef]
- Tabernero, J.; Hozak, R.R.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Prausová, J.; Muro, K.; Siegel, R.W.; Konrad, R.J.; Ouyang, H.; Melemed, S.A.; Ferry, D.; Nasroulah, F.; Van Cutsem, E. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann Oncol 2018, 29, 602–609. [Google Scholar] [CrossRef]
- Sims, T.N.; Gao, B.; Phillips, R.; Lowy, I. Potential predictive and prognostic biomarkers identified in baseline plasma samples from the VELOUR trial. J Clin Oncol, 2015, 33, no. 3_suppl, 638-638.
- Van Cutsem, E.; Paccard, C.; Chiron, M.; Tabernero, J. Impact of Prior Bevacizumab Treatment on VEGF-A and PlGF Levels and Outcome Following Second-Line Aflibercept Treatment: Biomarker Post Hoc Analysis of the VELOUR Trial. Clin Cancer Res 2020, 26, 717–725. [Google Scholar] [CrossRef]
- Hashimoto, T.; Otsu, S.; Hironaka, S.; Takashima, A.; Mizusawa, J.; Kataoka, T.; Fukuda, H.; Tsukamoto, S.; Hamaguchi, T.; Kanemitsu, Y.; Colorectal Cancer Study Group of the Japan Clinical Oncology Group. Phase II biomarker identification study of anti-VEGF agents with FOLFIRI for pre-treated metastatic colorectal cancer in press.
- Tischer, E.; Gospodarowicz, D.; Mitchell, R.; Silva, M.; Schilling, J.; Lau, K.; Crisp, T.; Fiddes, J.C.; Abraham, J.A. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun 1989, 165, 1198–1206. [Google Scholar] [CrossRef]
- Pàez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Viñals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell, 2009, 15, 220–31. [Google Scholar] [CrossRef]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med 2012, 2, a006502. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev, 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Arcondéguy, T.; Lacazette, E.; Millevoi, S.; Prats, H.; Touriol, C. VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res, 2013, 41, 7997–8010. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr Rev 1997, 18, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 2018, 59, 455–467. [Google Scholar] [PubMed]
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R. : Jackson, D.G.; Nishikawa, S.; Kubo, H.; Achen, M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001, 7, 186–191. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun, 2005, Jul, 333, 328-335.
- Muller, Y.A.; Chen, Y.; Christinger, H.W.; Li, B.; Cunningham, B.C.; Lowman, H.B.; de Vos, A.M. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure 1998, 6, 1153–1167. [Google Scholar] [CrossRef]
- Angelucci, A.; Delle Monache, S.; Cortellini, A.; Di Padova, M.; Ficorella, C. "Vessels in the Storm": Searching for Prognostic and Predictive Angiogenic Factors in Colorectal Cancer. Int J Mol Sci 2018, 19, 299. [Google Scholar] [CrossRef]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef]
- Kabbinavar, F.; Hurwitz, H.I.; Fehrenbacher, L.; Meropol, N.J.; Novotny, W.F.; Lieberman, G.; Griffing, S.; Bergsland, E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003, 21, 60–65. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; Ferrara, N.; Fyfe, G.; Rogers, B.; Ross, R.; Kabbinavar, F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Lu, D.; Jimenez, X.; Zhang, H.; Bohlen, P.; Witte, L.; Zhu, Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002, 97, 393–399. [Google Scholar] [CrossRef]
- Rockwell, P.; Neufeld, G.; Glassman, A.; Caron, D.; Goldstein, N. In vitro neutralization of vascular endothelial growth factor activation of Flk-1 by a monoclonal antibody. Mol Cell Differ 1995, 3, 91–109. [Google Scholar]
- Spratlin, J.L.; Cohen, R.B.; Eadens, M.; Gore, L.; Camidge, D.R.; Diab, S.; Leong, S.; O'Bryant, C.; Chow, L.Q.; Serkova, N.J.; Meropol, N.J.; Lewis, N.L.; Chiorean, E.G.; Fox, F.; Youssoufian, H.; Rowinsky, E.K.; Eckhardt, S.G. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010, 28, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; Ioffe, E.; Huang, T.; Radziejewski, C.; Bailey, K.; Fandl, J.P.; Daly, T.; Wiegand, S.J.; Yancopoulos, G.D.; Rudge, J.S. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 2002, 99, 11393–11398. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; Couture, F.; Sirzén, F.; Cassidy, J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sugrue, M.M.; Purdie, D.M.; Dong, W.; Sargent, D.; Hedrick, E.; Kozloff, M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 2008, 26, 5326–5334. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.A.; El-Rayes, B.F. Considering Efficacy and Cost, Where Does Ramucirumab Fit in the Management of Metastatic Colorectal Cancer? Oncologist 2015, 20, 981–982. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Jubb, A.M.; Chen, D.; Li, N.F.; Meng, Y.G.; Bernaards, C.; Elliott, R.; Scherer, S.J.; Chen, D.S. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res 2013, 19, 929–937. [Google Scholar] [CrossRef]
- Lieu, C.H.; Tran, H.; Jiang, Z.Q.; Mao, M.; Overman, M.J.; Lin, E.; Eng, C.; Morris, J.; Ellis, L.; Heymach, J.V.; Kopetz, S. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One 2013, 8, e77117. [Google Scholar] [CrossRef]
- Tebbutt, N.C.; Wilson, K.; Gebski, V.J.; Cummins, M.M.; Zannino, D.; van Hazel, G.A.; Robinson, B.; Broad, A.; Ganju, V.; Ackland, S.P.; Forgeson, G.; Cunningham, D.; Saunders, M.P.; Stockler, M.R.; Chua, Y.; Zalcberg, J.R.; Simes, R.J.; Price, T.J. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol 2010, 28, 3191–3198. [Google Scholar] [CrossRef]
- Albonici, L.; Giganti, M.G.; Modesti, A.; Manzari, V.; Bei, R. Multifaceted Role of the Placental Growth Factor (PlGF) in the Antitumor Immune Response and Cancer Progression. Int J Mol Sci 2019, 20, 2970. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Hoff, P.M.; Morris, J.S.; Wolff, R.A.; Eng, C.; Glover, K.Y.; Adinin, R.; Overman, M.J.; Valero, V.; Wen, S.; Lieu, C.; Yan, S.; Tran, H.T.; Ellis, L.M.; Abbruzzese, J.L.; Heymach, J.V. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010, 28, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin Cancer Res 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Muro, K.; Cunningham, D.; Bodoky, G.; Sobrero, A.; Cascinu, S.; Ajani, J.; Oh, S.C.; Al-Batran, S.E.; Wainberg, Z.A.; Wijayawardana, S.R.; Melemed, S.; Ferry, D.; Hozak, R.R.; Ohtsu, A. ; RAINBOW Investigators. Biomarker analyses of second-line ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. Eur J Cancer 2020, 127, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Shao, Y.Y.; Chen, H.M.; Lin, Y.L.; Lin, Z.Z.; Lai, M.S.; Cheng, A.L.; Yeh, K.H. Primary tumor site is a useful predictor of cetuximab efficacy in the third-line or salvage treatment of KRAS wild-type (exon 2 non-mutant) metastatic colorectal cancer: a nationwide cohort study. BMC Cancer 2016, 16, 327. [Google Scholar] [CrossRef]
- Moretto, R.; Cremolini, C.; Rossini, D.; Pietrantonio, F.; Battaglin, F.; Mennitto, A.; Bergamo, F.; Loupakis, F.; Marmorino, F.; Berenato, R.; Marsico, V.A.; Caporale, M.; Antoniotti, C.; Masi, G.; Salvatore, L.; Borelli, B.; Fontanini, G.; Lonardi, S.; De Braud, F.; Falcone, A. Location of Primary Tumor and Benefit From Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies in Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer. Oncologist 2016, 21, 988–994. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; Cervantes, A.; Ciardiello, F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Hecht, J.R.; Cohn, A.; Dakhil, S.; Saleh, M.; Piperdi, B.; Cline-Burkhardt, M.; Tian, Y.; Go, W.Y. SPIRITT: A Randomized, Multicenter, Phase II Study of Panitumumab with FOLFIRI and Bevacizumab with FOLFIRI as Second-Line Treatment in Patients with Unresectable Wild Type KRAS Metastatic Colorectal Cancer. Clin Colorectal Cancer 2015, 14, 72–80. [Google Scholar] [CrossRef]
- Shitara, K.; Yonesaka, K.; Denda, T.; Yamazaki, K.; Moriwaki, T.; Tsuda, M.; Takano, T.; Okuda, H.; Nishina, T.; Sakai, K.; Nishio, K.; Tokunaga, S.; Yamanaka, T.; Boku, N.; Hyodo, I.; Muro, K. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci 2016, 107, 1843–1850. [Google Scholar] [CrossRef]
- Bennouna, J.; Hiret, S.; Bertaut, A.; Bouché, O.; Deplanque, G.; Borel, C.; François, E.; Conroy, T.; Ghiringhelli, F.; des Guetz, G.; Seitz, J.F.; Artru, P.; Hebbar, M.; Stanbury, T.; Denis, M.G.; Adenis, A.; Borg, C. Continuation of Bevacizumab vs Cetuximab Plus Chemotherapy After First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER PRODIGE18 Randomized Clinical Trial. JAMA Oncol 2019, 5, 83–90. [Google Scholar] [CrossRef]
- Parisi, A.; Cortellini, A.; Cannita, K.; Venditti, O.; Camarda, F.; Calegari, M.A.; Salvatore, L.; Tortora, G.; Rossini, D.; Germani, M.M.; Boccaccino, A.; Dell'Aquila, E.; Fulgenzi, C.; Santini, D.; Tursi, M.; Tinari, N.; Marino, P.D.; Lombardi, P.; Keränen, S.R.; Álvaro, M.H.; Zurlo, I.V.; Corsi, D.C.; Emiliani, A.; Zanaletti, N.; Troiani, T.; Vitale, P.; Giampieri, R.; Merloni, F.; Occhipinti, M.A.; Marchetti, P.; Roberto, M.; Mazzuca, F.; Ghidini, M.; Indini, A.; Garajova, I.; Zoratto, F.; Monache, S.D.; Porzio, G.; Ficorella, C. Evaluation of Second-line Anti-VEGF after First-line Anti-EGFR Based Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Multicenter "SLAVE" Study. Cancers (Basel) 2020, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Kagawa, Y.; Shinozaki, E.; Tanizawa, Y.; Jin, L.; Cai, Z.; Makiyama, A. Real-World Data Analysis of Second-Line Antiangiogenic Targeted Treatments Following Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies and First-Line FOLFOX for Patients with Metastatic Colorectal Cancer. Adv Ther 2022, 39, 2596–2613. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Tsuji, A.; Okita, Y.; Matsumoto, T.; Sagawa, T.; Watanabe, T,; Kataoka, K.; Manaka, D.;
Shiraishi, K.; Akazawa, N.; Okuno, T.; Shimura, T.; Shiozawa, M.; Noura, S.; Sunakawa, Y.; Akiyama, Y.;
Ota, H.; Takeuchi, M.; Ichikawa, W.; Fujii. M. A multicenter phase 2 trial of ramucirumab plus FOLFIRI as
second-line treatment for patients with RAS wild-type metastatic colorectal cancer previously treated with
combination chemotherapy with anti-EGFR antibody: JACCRO CC-16. J Clin Oncol, 2022, 40, no. 4_suppl,
112-112.
- Li, J.; Qin, S.; Xu, R.H.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.D.; Zhong, H.; Pan, H.; Guo, W.; Shu, Y.; Yuan, Y.; Zhou, J.; Xu, N.; Liu, T.; Ma, D.; Wu, C.; Cheng, Y.; Chen, D.; Li, W.; Sun, S.; Yu, Z.; Cao, P.; Chen, H.; Wang, J.; Wang, S.; Wang, H.; Fan, S.; Hua, Y.; Su, W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018, 319, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Lonardi, S.; Garcia-Carbonero, R.; Elez, E.; Yoshino, T.; Sobrero, A.; Yao, J.; García-Alfonso, P.;
Kocsis, J.; Cubillo, Gracian.A.; Sartore-Bianchi, A.; Satoh, T.; Randrian, V.; Tomasek, J.; Chong, G.; Paulson,
A.S.; Masuishi, T.; Jones, J.; Csőszi, T.; Cremolini, C.; Ghiringhelli, F.; Shergill, A.; Hochster, H.S.; Krauss,
J.; Bassam, A.; Ducreux, M.; Elme, A.; Faugeras, L.; Kasper, S.; Van, Cutsem.E.; Arnold, D.; Nanda, S.;
Yang, Z.; Schelman, W.R.; Kania, M.; Tabernero, J.; Eng, C.; FRESCO-2 Study Investigators. Fruquintinib
versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international,
multicentre, randomised, double-blind, phase 3 study. Lancet, 2023, 402, 41-53.
- Fruquintinib Plus Capecitabine Versus Bevacizumab Plus Capecitabine as Maintenance Therapy Following First-line Treatment for Metastatic Colorectal Cancer. https://www.clinicaltrials.gov/study/NCT04733963?term=Fruquintinib%20Plus%20Capecitabine&rank=2.
- Comparing Chidamide+Sintilimab+Bev With Standard Second-line FOLFIRI+Bev in Advanced MSS/pMMR mCRC. https://www.clinicaltrials.gov/study/NCT05768503?term=Chidamide%2BSintilimab%2BBev%20With%20Standard%20Second-line%20FOLFIRI%2BBev%20&rank=1.
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F. ; Van, Cutsem.E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; Poureau, P.G.; Liposits, G.; Cremolini, C.; Bondarenko, I.; Modest, D.P.; Benhadji, K.A.; Amellal, N.; Leger, C.; Vidot, L.; Tabernero, J.; SUNLIGHT Investigators. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N Engl J Med 2023, 388, 1657–1667. [Google Scholar] [PubMed]
- Taniguchi, H.; Yuki, S.; Shiozawa, M.; Masuishi, T.; Nishina, T.; Kagawa, Y.; Takahashi, T.; Yasui, H.; Denda, T.; Sunakawa, Y.; Yamazaki, K.; Esaki, T.; Kawakami, H.; Kato, T.; Yoshida, K.; Takashima, A.; Ohmiya, H.; Nomura, S.; Ohtsu, A.; Yoshino, T. Plasma VEGF-D and PlGF levels according to prior use of biologics among metastatic colorectal cancer: Preliminary results from GI-SCREEN CRC-Ukit study. J Clin Oncol 2020, 38 no. 4_suppl, 178. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).