Submitted:
12 August 2023
Posted:
14 August 2023
You are already at the latest version
Abstract
Keywords:
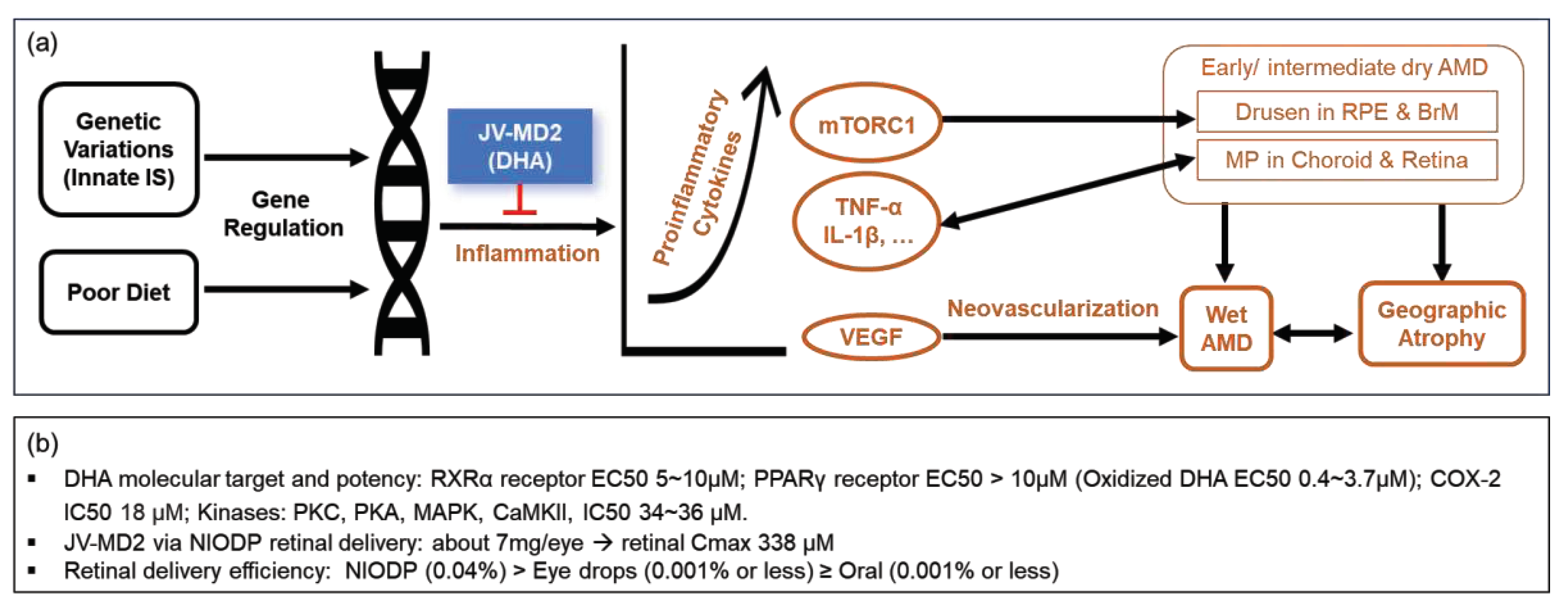
1. Introduction
2. Materials and Methods
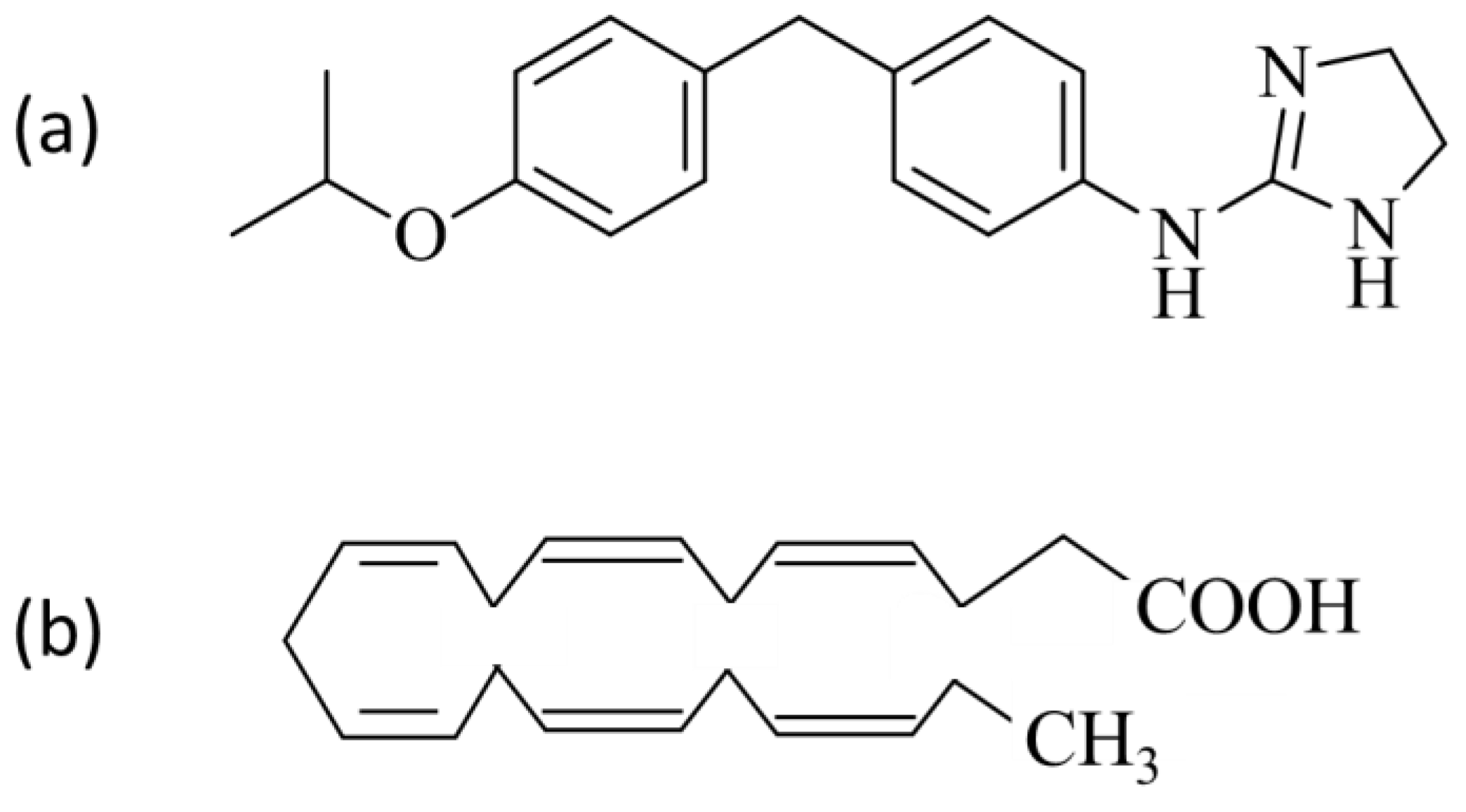
2.1. Compounds
2.2. Formulations
2.3. Choice of Animals for Eye Drop & NIODP Ocular Biodisposition Studies
2.4. Biodisposition of JV-DE1 Eye Drops in New Zealand Rabbits
2.5. Biodisposition of JV-DE1 and JV-MD2 Delivered via NIODP in Non-Human Primates
2.6. LC-MS/MS Methods for JV-DE1 in Rabbit Plasma and Ocular Tissues
2.7. LC-MS/MS Methods for JV-DE1 and JV-MD2 in Monkey Plasma and Ocular Tissues
3. Results
3.1. JV-DE1 Ocular and Plasma Bioavailability Delivered by Eye Drops in New Zealand Rabbits—The Evolution of Eye Drop Administration to Glaucoma Drug Periorbital Delivery, to Discovery of the Non-Invasive Ocular Delivery Platform (NIODP)
3.2. JV-DE1 Ocular and Plasma Bioavailability after a Single Dose NIODP Application in Non-Human Primates
3.3. JV-MD2 Ocular and Plasma Bioavailability via NIODP in Non-Human Primates
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Mosteller, M.W.; Gebhardt, B.M.; Hamilton, A.M.; Kaufman, H.E., Penetration of topical cyclosporine into the rabbit cornea, aqueous humor, and serum. Arch Ophthalmol 1985, 103, 101-2. [CrossRef]
- Said, T.; Dutot, M.; Christon, R.; Beaudeux, J.L.; Martin, C.; Warnet, J.M.; Rat, P., Benefits and side effects of different vegetable oil vectors on apoptosis, oxidative stress, and P2X7 cell death receptor activation. Invest Ophthalmol Vis Sci 2007, 48, 5000-6. [CrossRef]
- Agarwal, P.; Craig, J.P.; Rupenthal, I.D., Formulation Considerations for the Management of Dry Eye Disease. Pharmaceutics 2021, 13. [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K., A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res 2016, 6, 735-754. [CrossRef]
- Sarkadi-Nagy, E.; Wijendran, V.; Diau, G.Y.; Chao, A.C.; Hsieh, A.T.; Turpeinen, A.; Nathanielsz, P.W.; Brenna, J.T., The influence of prematurity and long chain polyunsaturate supplementation in 4-week adjusted age baboon neonate brain and related tissues. Pediatr Res 2003, 54, 244-52. [CrossRef]
- Sarkadi-Nagy, E.; Wijendran, V.; Diau, G.Y.; Chao, A.C.; Hsieh, A.T.; Turpeinen, A.; Lawrence, P.; Nathanielsz, P.W.; Brenna, J.T., Formula feeding potentiates docosahexaenoic and arachidonic acid biosynthesis in term and preterm baboon neonates. J Lipid Res 2004, 45, 71-80. [CrossRef]
- Sripetch, S.; Loftsson, T., Topical drug delivery to the posterior segment of the eye: Thermodynamic considerations. Int J Pharm 2021, 597, 120332. [CrossRef]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G., Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev 2005, 57, 2010-32. [CrossRef]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L., Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int J Mol Sci 2018, 19. [CrossRef]
- Lakhani, P.; Patil, A.; Majumdar, S., Recent advances in topical nano drug-delivery systems for the anterior ocular segment. Ther Deliv 2018, 9, 137-153. [CrossRef]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M., Anterior eye segment drug delivery systems: current treatments and future challenges. J Ocul Pharmacol Ther 2013, 29, 92-105. [CrossRef]
- Varela-Fernandez, R.; Diaz-Tome, V.; Luaces-Rodriguez, A.; Conde-Penedo, A.; Garcia-Otero, X.; Luzardo-Alvarez, A.; Fernandez-Ferreiro, A.; Otero-Espinar, F.J., Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12. [CrossRef]
- Rodrigues, G.A.; Lutz, D.; Shen, J.; Yuan, X.; Shen, H.; Cunningham, J.; Rivers, H.M., Topical Drug Delivery to the Posterior Segment of the Eye: Addressing the Challenge of Preclinical to Clinical Translation. Pharm Res 2018, 35, 245. [CrossRef]
- Kim, H.M.; Woo, S.J., Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13. [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D., Ocular Drug Delivery: Present Innovations and Future Challenges. J Pharmacol Exp Ther 2019, 370, 602-624. [CrossRef]
- Nomoto, H.; Shiraga, F.; Kuno, N.; Kimura, E.; Fujii, S.; Shinomiya, K.; Nugent, A.K.; Hirooka, K.; Baba, T., Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci 2009, 50, 4807-13. [CrossRef]
- Davis, B.M.; Normando, E.M.; Guo, L.; Turner, L.A.; Nizari, S.; O'Shea, P.; Moss, S.E.; Somavarapu, S.; Cordeiro, M.F., Topical delivery of Avastin to the posterior segment of the eye in vivo using annexin A5-associated liposomes. Small 2014, 10, 1575-84. [CrossRef]
- de Cogan, F.; Hill, L.J.; Lynch, A.; Morgan-Warren, P.J.; Lechner, J.; Berwick, M.R.; Peacock, A.F.A.; Chen, M.; Scott, R.A.H.; Xu, H.; Logan, A., Topical Delivery of Anti-VEGF Drugs to the Ocular Posterior Segment Using Cell-Penetrating Peptides. Invest Ophthalmol Vis Sci 2017, 58, 2578-2590. [CrossRef]
- Sigurdsson, H.H.; Konraethsdottir, F.; Loftsson, T.; Stefansson, E., Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol Scand 2007, 85, 598-602. [CrossRef]
- Hartman, R.R.; Kompella, U.B., Intravitreal, Subretinal, and Suprachoroidal Injections: Evolution of Microneedles for Drug Delivery. J Ocul Pharmacol Ther 2018, 34, 141-153. [CrossRef]
- Del Amo, E.M.; Rimpela, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; Subrizi, A.; Turunen, T.; Reinisalo, M.; Itkonen, J.; Toropainen, E.; Casteleijn, M.; Kidron, H.; Antopolsky, M.; Vellonen, K.S.; Ruponen, M.; Urtti, A., Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res 2017, 57, 134-185. [CrossRef]
- Karti, O.; Saatci, A.O., Intravitreal Dexamethasone Implant in the Treatment of Non-Infectious Uveitic Macular Edema. Med Hypothesis Discov Innov Ophthalmol 2018, 7, 169-175.
- Villegas, V.M.; Gold, A.S.; Wildner, A.; Latiff, A.; Murray, T.G., Intravitreal triamcinolone acetonide: a "real world" analysis of visual acuity, pressure and outcomes. Int J Ophthalmol 2016, 9, 789-91.
- Cao, L.; Weetall, M.; Bombard, J.; Qi, H.; Arasu, T.; Lennox, W.; Hedrick, J.; Sheedy, J.; Risher, N.; Brooks, P.C.; Trifillis, P.; Trotta, C.; Moon, Y.C.; Babiak, J.; Almstead, N.G.; Colacino, J.M.; Davis, T.W.; Peltz, S.W., Discovery of Novel Small Molecule Inhibitors of VEGF Expression in Tumor Cells Using a Cell-Based High Throughput Screening Platform. PLoS One 2016, 11, e0168366.
- Roskoski, R., Jr., The role of small molecule platelet-derived growth factor receptor (PDGFR) inhibitors in the treatment of neoplastic disorders. Pharmacol Res 2018, 129, 65-83. [CrossRef]
- Kaiser, P.K. Retina Pipeline 2021 Ongoing Innovation Wet AMD. https://retinatoday.com/images/retina-pipeline/retina-pipeline-2021/pdfs-for-download/Wet-AMD-Retina-Pipeline-Poster.pdf (accessed 1-20-2022).
- Kaiser, P.K. Retina Pipeline 2021 Ongoing Innovation Dry AMD. . https://retinatoday.com/images/retina-pipeline/retina-pipeline-2021/pdfs-for-download/Dry-AMD-Retina-Pipeline-Poster.pdf (accessed 1-20-2022).
- Woodward, D.F.; Wang, J.W.; Coleman, R.A.; Woodrooffe, A.J.; Clark, K.L.; Stamer, W.D.; Tao, G.; Fan, S.; Toris, C.B., A Highly Effective and Ultra-Long-Acting Anti-Glaucoma Drug, with a Novel Periorbital Delivery Method. J Ocul Pharmacol Ther 2019, 35, 265-277. [CrossRef]
- Dilbeck, M.D.; Spahr, Z.R.; Nanjappa, R.; Economides, J.R.; Horton, J.C., Columnar and Laminar Segregation of Retinal Input to the Primate Superior Colliculus Revealed by Anterograde Tracer Injection Into Each Eye. Invest Ophthalmol Vis Sci 2022, 63, 9. [CrossRef]
- Zarbin, M.A.; Novack, G., N-of-1 Clinical Trials: A Scientific Approach to Personalized Medicine for Patients with Rare Retinal Diseases Such as Retinitis Pigmentosa. J Ocul Pharmacol Ther 2021, 37, 495-501. [CrossRef]
- Zernii, E.Y.; Baksheeva, V.E.; Iomdina, E.N.; Averina, O.A.; Permyakov, S.E.; Philippov, P.P.; Zamyatnin, A.A.; Senin, II, Rabbit Models of Ocular Diseases: New Relevance for Classical Approaches. CNS Neurol Disord Drug Targets 2016, 15, 267-91. [CrossRef]
- Bley, K.R.; Bhattacharya, A.; Daniels, D.V.; Gever, J.; Jahangir, A.; O'Yang, C.; Smith, S.; Srinivasan, D.; Ford, A.P.; Jett, M.F., RO1138452 and RO3244794: characterization of structurally distinct, potent and selective IP (prostacyclin) receptor antagonists. Br J Pharmacol 2006, 147, 335-45. [CrossRef]
- Watsky, M.A.; Jablonski, M.M.; Edelhauser, H.F., Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res 1988, 7, 483-6. [CrossRef]
- NanoSense Lesson 2: Scale of Objects ...Student Materials. https://nanosense.sri.com/activities/sizematters/sizeandscale/SM_Lesson2Student.pdf.
- Chiang, B.; Jung, J.H.; Prausnitz, M.R., The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev 2018, 126, 58-66. [CrossRef]
- Tornquist, P.; Alm, A.; Bill, A., Permeability of ocular vessels and transport across the blood-retinal-barrier. Eye (Lond) 1990, 4 ( Pt 2), 303-9. [CrossRef]
- Hammadi, S.; Tzoumas, N.; Ferrara, M.; Meschede, I.P.; Lo, K.; Harris, C.; Lako, M.; Steel, D.H., Bruch's Membrane: A Key Consideration with Complement-Based Therapies for Age-Related Macular Degeneration. J Clin Med 2023, 12. [CrossRef]
- Clark, S.J.; McHarg, S.; Tilakaratna, V.; Brace, N.; Bishop, P.N., Bruch's Membrane Compartmentalizes Complement Regulation in the Eye with Implications for Therapeutic Design in Age-Related Macular Degeneration. Front Immunol 2017, 8, 1778. [CrossRef]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D., Immunology of age-related macular degeneration. Nat Rev Immunol 2013, 13, 438-51. [CrossRef]
- Cheng, S.Y.; Cipi, J.; Ma, S.; Hafler, B.P.; Kanadia, R.N.; Brush, R.S.; Agbaga, M.P.; Punzo, C., Altered photoreceptor metabolism in mouse causes late stage age-related macular degeneration-like pathologies. Proc Natl Acad Sci U S A 2020, 117, 13094-13104. [CrossRef]
- Casciano, F.; Zauli, E.; Rimondi, E.; Mura, M.; Previati, M.; Busin, M.; Zauli, G., The role of the mTOR pathway in diabetic retinopathy. Front Med (Lausanne) 2022, 9, 973856. [CrossRef]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M., Ageing: from inflammation to cancer. Immun Ageing 2018, 15, 1. [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X., mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci 2020, 10, 31. [CrossRef]
- Li, X.; Gao, S.; Zhang, Y.; Xin, M.; Zuo, C.; Yan, N.; Xia, Q.; Zhang, M., Dihydroartemisinin Inhibits Laser-Induced Choroidal Neovascularization in a Mouse Model of Neovascular AMD. Front Pharmacol 2022, 13, 838263. [CrossRef]
- Gutierrez, S.; Svahn, S.L.; Johansson, M.E., Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci 2019, 20. doi:10.3390/ijms20205028.
- sccessed 7/27).
- Georgiou, T.; Prokopiou, E., The New Era of Omega-3 Fatty Acids Supplementation: Therapeutic Effects on Dry Age-Related Macular Degeneration. J Stem Cells 2015, 10, 205-15.
- Prokopiou, K.; Kolovos, P.; Tsangari, H.; Bandello, F.; Rossetti, L.M.; Mastropasqua, L.; Mohand-Said, S.; Georgiou, T., A prospective, multicentre, randomised, double-blind study designed to assess the potential effects of omega-3 fatty acids supplementation in dry age-related macular degeneration or Stargardt disease. Investigative Ophthalmology & Visual Science 2022, 63.
- Souied, E.H.; Delcourt, C.; Querques, G.; Bassols, A.; Merle, B.; Zourdani, A.; Smith, T.; Benlian, P.; Nutritional, A.M.D.T.S.G., Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology 2013, 120, 1619-31.
- Souied, E.H.; Aslam, T.; Garcia-Layana, A.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C., Omega-3 Fatty Acids and Age-Related Macular Degeneration. Ophthalmic Res 2015, 55, 62-9. [CrossRef]
- Edelhauser, H.F.; Rowe-Rendleman, C.L.; Robinson, M.R.; Dawson, D.G.; Chader, G.J.; Grossniklaus, H.E.; Rittenhouse, K.D.; Wilson, C.G.; Weber, D.A.; Kuppermann, B.D.; Csaky, K.G.; Olsen, T.W.; Kompella, U.B.; Holers, V.M.; Hageman, G.S.; Gilger, B.C.; Campochiaro, P.A.; Whitcup, S.M.; Wong, W.T., Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci 2010, 51, 5403-20. [CrossRef]
- Khoo, H.E.; Ng, H.S.; Yap, W.S.; Goh, H.J.H.; Yim, H.S., Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants (Basel) 2019, 8. [CrossRef]
- Querques, G.; Forte, R.; Souied, E.H., Retina and omega-3. J Nutr Metab 2011, 2011, 748361.
- Calder, P.C., Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355-74. doi:10.3390/nu2030355.
- German, O.L.; Monaco, S.; Agnolazza, D.L.; Rotstein, N.P.; Politi, L.E., Retinoid X receptor activation is essential for docosahexaenoic acid protection of retina photoreceptors. J Lipid Res 2013, 54, 2236-2246. [CrossRef]
- Lengqvist, J.; Mata De Urquiza, A.; Bergman, A.C.; Willson, T.M.; Sjovall, J.; Perlmann, T.; Griffiths, W.J., Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol Cell Proteomics 2004, 3, 692-703. doi:10.1074/mcp.m400003-mcp200.
- Itoh, T.; Yamamoto, K., Peroxisome proliferator activated receptor gamma and oxidized docosahexaenoic acids as new class of ligand. Naunyn Schmiedebergs Arch Pharmacol 2008, 377, 541-7.
- Calder, P.C., Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013, 75, 645-62.
- Li, X.; Yu, Y.; Funk, C.D., Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J 2013, 27, 4987-97. [CrossRef]
- Iverson, C.; Bacong, A.; Liu, S.; Baumgartner, S.; Lundstrom, T.; Oscarsson, J.; Miner, J.N., Omega-3-carboxylic acids provide efficacious anti-inflammatory activity in models of crystal-mediated inflammation. Sci Rep 2018, 8, 1217. [CrossRef]
- Mirnikjoo, B.; Brown, S.E.; Kim, H.F.; Marangell, L.B.; Sweatt, J.D.; Weeber, E.J., Protein kinase inhibition by omega-3 fatty acids. J Biol Chem 2001, 276, 10888-96.
- Djebli, N.; Khier, S.; Griguer, F.; Coutant, A.L.; Tavernier, A.; Fabre, G.; Leriche, C.; Fabre, D., Ocular Drug Distribution After Topical Administration: Population Pharmacokinetic Model in Rabbits. Eur J Drug Metab Pharmacokinet 2017, 42, 59-68. [CrossRef]
- Bos, J.D.; Meinardi, M.M., The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol 2000, 9, 165-9. [CrossRef]
- Schramlova, J.; Blazek, K.; Bartackova, M.; Otova, B.; Mardesicova, L.; Zizkovsky, V.; Hulinska, D., Electron microscopic demonstration of the penetration of liposomes through skin. Folia Biol (Praha) 1997, 43, 165-9.
- Souto, E.B.; Macedo, A.S.; Dias-Ferreira, J.; Cano, A.; Zielinska, A.; Matos, C.M., Elastic and Ultradeformable Liposomes for Transdermal Delivery of Active Pharmaceutical Ingredients (APIs). Int J Mol Sci 2021, 22. [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D., Nanocarrier mediated retinal drug delivery: overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2018, 10. [CrossRef]
| Items | Detail Information | |||||||
|---|---|---|---|---|---|---|---|---|
| (a) | ||||||||
| Column | Waters BEH C18 1.7μm (50×2.10mm) | |||||||
| Column temperature | 50 °C | |||||||
| Auto-sampler temperature | 4 °C | |||||||
| Mobile Phase | MPA: 0.1% Formic Acid (FA) in water MPB: 0.1% Formic Acid (FA) in acetonitrile (ACN) |
|||||||
| Gradient elution | Flow rate | 0.6ml/min | ||||||
| Time/min | MPA (%) | MPB (%) | ||||||
| Initial | 75.0 | 25.0 | ||||||
| 1.30 | 5.0 | 95.0 | ||||||
| 1.75 | 5.0 | 95.0 | ||||||
| 1.76 | 75.0 | 25.0 | ||||||
| 2.00 | 75.0 | 25.0 | ||||||
| Injection Volume | 3μL | |||||||
| (b) | ||||||||
| Column | Waters BEH C18 1.7μm (50×2.10mm) | |||||||
| Column temperature | 50 °C | |||||||
| Auto-sampler temperature | 4 °C | |||||||
| Mobile Phase | MPA: 0.1% FA and 1mM NH4Ac in H2O/ACN (95:5, v:v) MPB: 0.1% FA and 1mM NH4Ac in H2O/ACN (5:95, v:v) |
|||||||
| Gradient elution | Flow rate | 0.6ml/min | ||||||
| Time/min | MPA (%) | MPB (%) | ||||||
| Initial | 60.0 | 40.0 | ||||||
| 1.00 | 2.0 | 98.0 | ||||||
| 1.30 | 2.0 | 98.0 | ||||||
| 1.31 | 60.0 | 40.0 | ||||||
| 1.50 | 60.0 | 40.0 | ||||||
| Injection Volume Valco Valve |
2μL 0.3min: switch into mass, 0.8min: switch into waste |
|||||||
| (c) | ||||||||
| Ion Source | Ion Spray Voltage: 5500V CAD: 10 |
|||||||
| CUR: 40psi | ||||||||
| Gas1: 55psi | ||||||||
| Gas2: 55psi | ||||||||
| Temperature: 450°C (plasma); 550°C (eye tissue and aqueous humor) | ||||||||
| Resolution Q1: unit | ||||||||
| Resolution Q3: unit | ||||||||
| MR Pause: 5.007msec | ||||||||
| Model | ESI, Positive, MRM | |||||||
| Mass Parameters (plasma) |
Analyte and IS | Q1/Q3 | Dwell time (ms) | DP (v) |
EP (v) |
CE (v) |
CXP (v) |
|
| JV-DE1 | 309.8/266.3 | 150 | 150 | 10 | 46 | 13 | ||
| Tolbutamide | 271.1/172.0 | 150 | 70 | 10 | 18 | 13 | ||
| Mass Parameters (eye tissue and aqueous humor) |
Analyte and IS | Q1/Q3 | Dwell time (ms) | DP (v) |
EP (v) |
CE (v) |
CXP (v) |
|
| JV-DE1 | 309.8/266.3 | 80 | 150 | 10 | 46 | 13 | ||
| Tolbutamide | 271.1/172.0 | 80 | 70 | 10 | 18 | 13 | ||
| Time/min | Flow Rate (mL/min) | A% | B% |
|---|---|---|---|
| Initial | 0.6 | 75 | 25 |
| 1.30 | 0.6 | 5 | 95 |
| 1.75 | 0.6 | 5 | 95 |
| 1.76 | 0.6 | 75 | 25 |
| 2.00 | 0.6 | 75 | 25 |
| Time/min | Flow Rate (mL/min) | A% | B% |
|---|---|---|---|
| Initial | 0.5 | 40 | 60 |
| 2.20 | 0.5 | 10 | 90 |
| 2.50 | 0.5 | 10 | 90 |
| 2.51 | 0.5 | 40 | 60 |
| 2.90 | 0.5 | 40 | 60 |
| Test Article | JV-DE1 | |||||
|---|---|---|---|---|---|---|
| Dosing site | Topical eye drop (OD) | |||||
| Rabbit ID. | All | 2036281 | 2036282 | 2036283 | 2036281 | |
| Time (h) | 0 | 0.5 | 3 | 6 | 24 | |
| Dose Level (μg/eye) | 0 | 120 | 120 | 120 | 120 | |
| Drug in Plasma (μg/mL) | BLQ | 0.0010 | 0.0003 | 0.0001 | BLQ | |
| Drug in Vitreous humor (μg/mL) | NF | 0.0026 | 0.0017 | 0.0012 | 0.0004 | |
| Drug in OD Tissues (μg/g) |
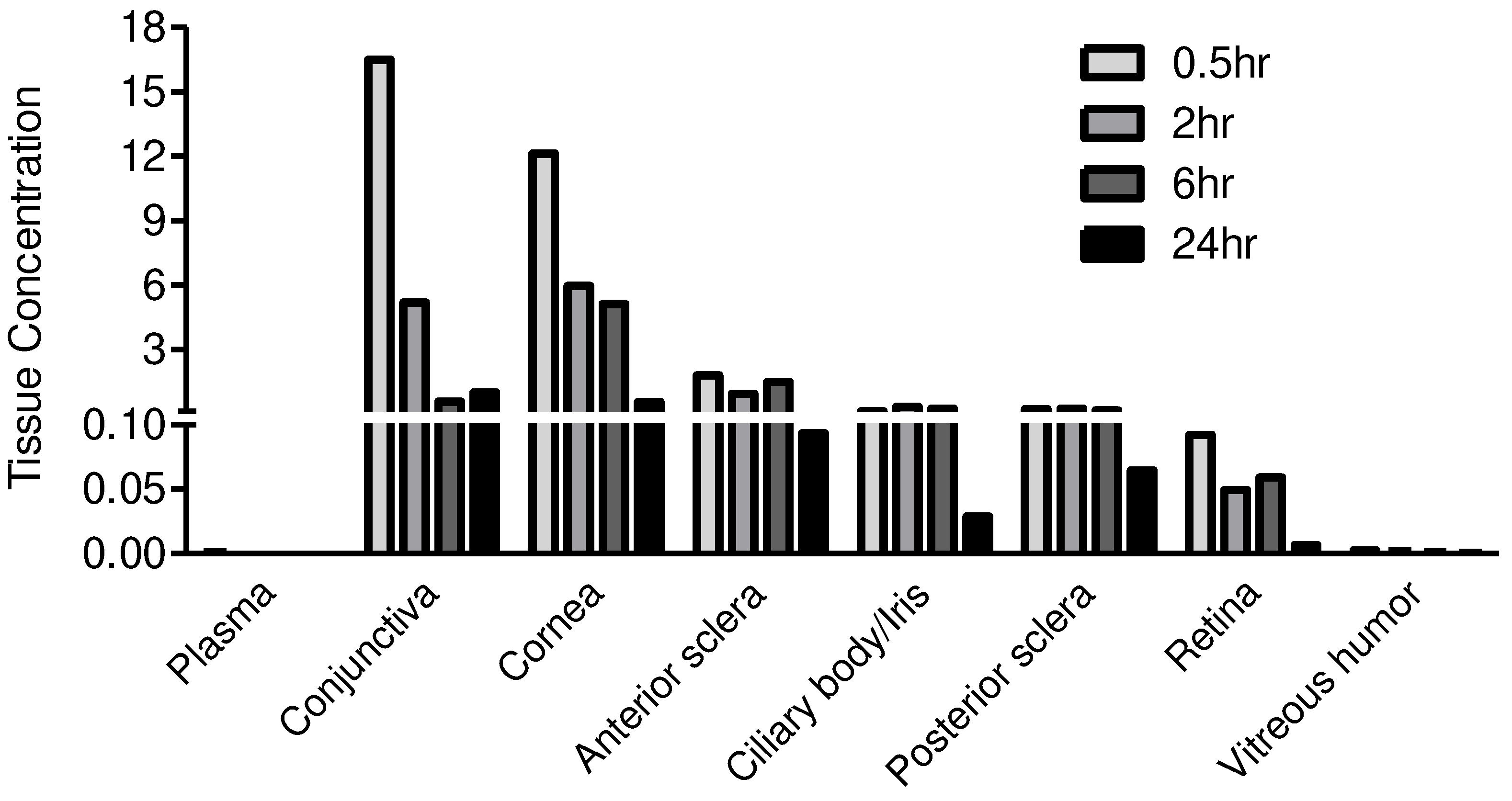
Conjunctiva | NF | 16.4919 | 5.1781 | 0.5660 | 0.9937 |
| Cornea | NF | 12.1249 | 5.9526 | 5.1255 | 0.5422 | |
| Anterior sclera | NF | 1.7856 | 0.9263 | 1.4785 | 0.0932 | |
| Ciliary body/Iris | NF | 0.1054 | 0.3049 | 0.2314 | 0.0289 | |
| Posterior sclera | NF | 0.2090 | 0.2395 | 0.1512 | 0.0645 | |
| Retina | NF | 0.0920 | 0.0491 | 0.0589 | 0.0068 | |
| Vitreous humor (μg/mL) | NF | 0.0026 | 0.0017 | 0.0012 | 0.0004 | |
| % Administered Dose |
Conjunctiva | 1.3929 | 0.4477 | 0.1288 | 0.1467 | |
| Cornea | 0.8622 | 0.4239 | 0.3709 | 0.0254 | ||
| Anterior sclera | 0.2982 | 0.1068 | 0.2139 | 0.0127 | ||
| Ciliary body/Iris | 0.0084 | 0.0174 | 0.0137 | 0.0021 | ||
| Posterior sclera | 0.0153 | 0.0323 | 0.0197 | 0.0088 | ||
| Retina | 0.0047 | 0.0036 | 0.0037 | 0.0004 | ||
| Vitreous humor | 0.0023 | 0.0012 | 0.0007 | 0.0004 | ||
| Test Article | JV-DE1 | |||||
|---|---|---|---|---|---|---|
| Dosing site | Periorbital skin, OD | |||||
| Animal No. | 101 | 101 | 102 | 103 | 104 | |
| Time (h) | 0 | 0.5 | 3 | 6 | 24 | |
| Dose Level (μg/eye) | 0 | 179.1 | 160.6 | 162.2 | 159.3 | |
| Drug in Plasma (μg/mL) | BLQ | 0.0001 | 0.0002 | 0.0003 | BLQ | |
| Drug in OD Tissues (μg/g) |
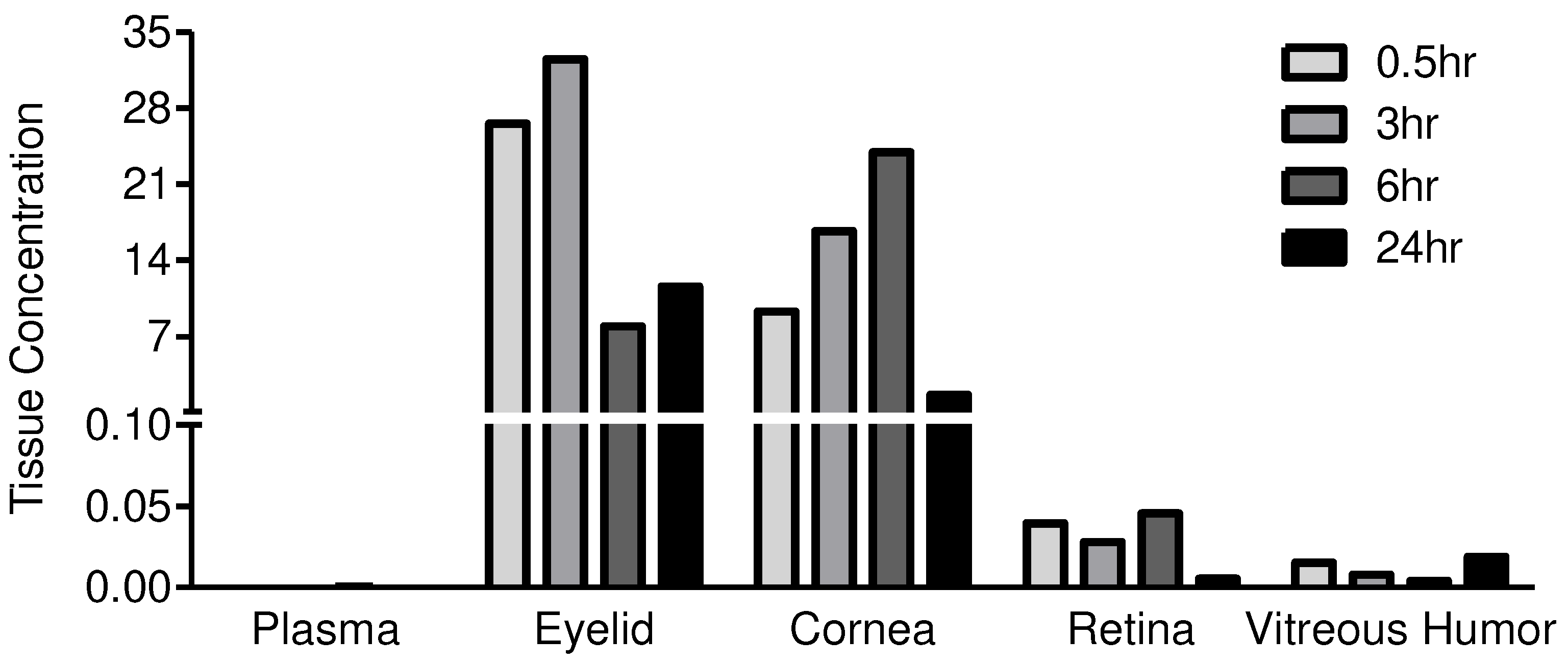
Eyelid/Periorbital skin | NF | 26.5354 | 32.4547 | 7.9456 | 11.5978 |
| Cornea | NF | 9.2993 | 16.6887 | 23.9295 | 1.6213 | |
| Retina | NF | 0.0396 | 0.0279 | 0.0455 | 0.0055 | |
| Vitreous humor (μg/ml) | NF | 0.0152 | 0.0079 | 0.0039 | 0.0190 | |
| % Administered Dose |
Eyelid/Periorbital skin | 0.6370 | 1.4956 | 0.3477 | 0.7501 | |
| Cornea | 0.1921 | 0.3430 | 0.5900 | 0.0326 | ||
| Retina | 0.0004 | 0.0004 | 0.0005 | 0.0002 | ||
| Vitreous humor | 0.0047 | 0.0029 | 0.0031 | 0.0018 | ||
| .Test Article | JV-MD2 (Docosahexaenoic acid, DHA) | |||||
|---|---|---|---|---|---|---|
| Dosing site | Periorbital skin, OS | |||||
| Animal No. | 101 | 101 | 102 | 103 | 104 | |
| Time (h) | 0 | 0.5 | 3 | 6 | 24 | |
| Dose Level (μg/eye) | 0 | 6568 | 6768 | 7018 | 6747 | |
| Drug in Plasma (µg/mL) | 2 | 2 | 3 | 2 | 1 | |
| Drug in OS Tissues (µg/g) |
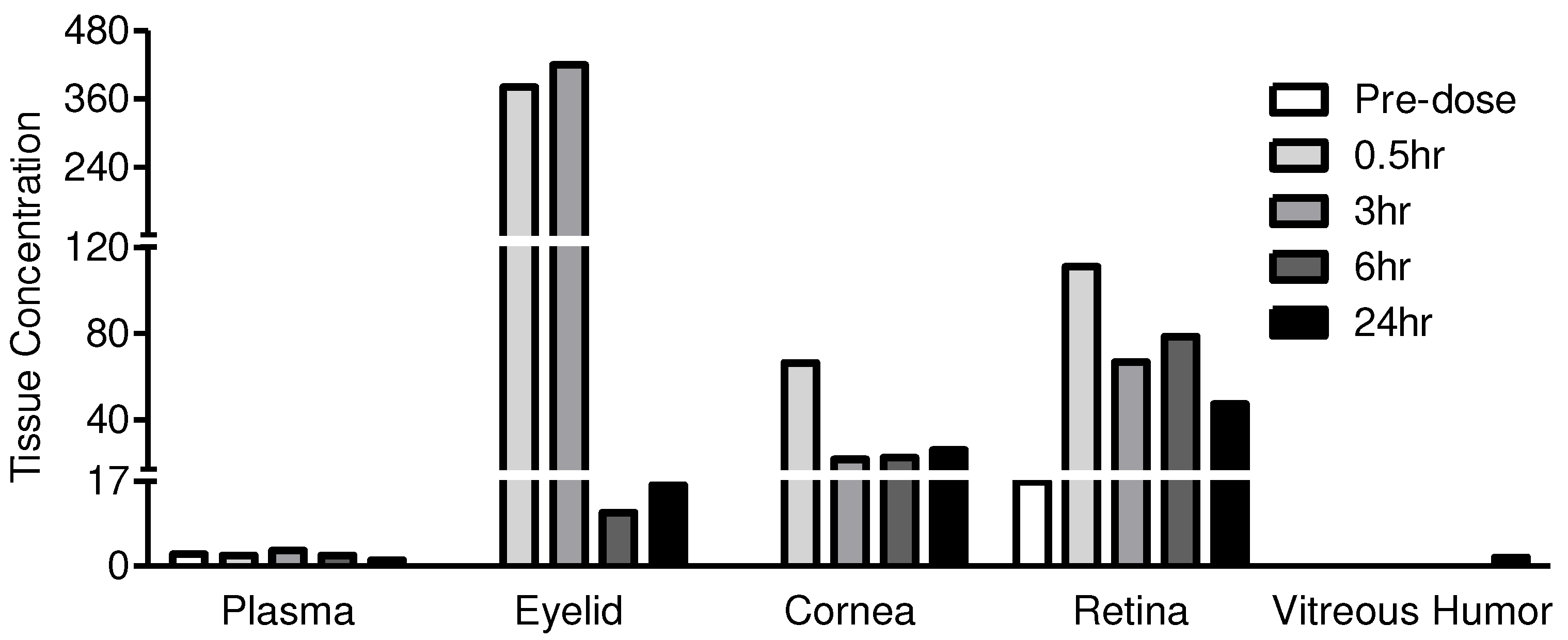
Eyelid/Periorbital skin | BLQ* | 381 | 420 | 11 | 16 |
| Cornea | BLQ* | 66 | 22 | 22 | 26 | |
| Retina | 17* | 111 | 67 | 79 | 47 | |
| Vitreous humor | BLQ* | 0 | 0 | 0 | 2 | |
| % Administered Dose |
Eyelid/Periorbital skin | 0.4288 | 0.3844 | 0.0149 | 0.0197 | |
| Cornea | 0.0283 | 0.0109 | 0.0149 | 0.0173 | ||
| Retina** | 0.0343 | 0.0264 | 0.0228 | 0.0175 | ||
| Vitreous humor | 0.0000 | 0.0000 | 0.0000 | 0.0236 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




