1. Introduction
Hepatocellular adenoma (HCA) and hepatocellular carcinoma (HCC) can occur in both canine and human patients. HCAs are rare, benign tumors that derive from proliferating hepatocytes, whereas HCCs are common, malignant tumors that can develop from HCAs [
1,
2,
3]. HCC accounts for 50-70% of hepatic tumors in dogs and is the sixth most common cancer in humans worldwide [
4,
5,
6,
7]. Canine HCC frequently occurs in patients from the age of ten years, with male sex as a predisposing factor [
8,
9]. Distinguishing HCA from HCC can be complicated in dogs, but correct tumor identification is crucial because the indicated treatment and prognosis differ between these two tumor types [
10,
11]. Therefore, there is a need for a minimally invasive diagnostic technique for differentiating HCA from HCC on a molecular basis for canine patients.
Noncoding RNAs (ncRNAs) are potentially implicated in hepatocellular tumorigenesis and may serve as a diagnostic marker for these tumors [
12,
13]. ncRNA categories encompass diverse transcripts, including miRNAs, long noncoding RNAs, and other RNA-like Y RNA fragments. Studies on miRNAs in human HCA are limited [
1,
14], and only one report on miRNAs involved in canine HCA [
15]. miRNA involvement in HCC growth has been the subject of in-depth research in dogs and humans [
15,
16,
17,
18,
19]. For example, our group has previously reported miR-1 dysregulation in canine HCC [
16]. However, none of the reports on ncRNAs in canine liver tumors have addressed Y RNA.
Y RNA is first reported in patients with systemic lupus erythematosus in 1981 [
20]. Despite being highly conserved molecules, Y RNAs exist in all vertebrate species, and the number of Y RNA transcripts varies between species [
21,
22]. Y RNAs are a type of regulatory RNA that have a sequence of 80-110 nucleotides. They are identified by a stem-loop structure formed by complementary 5' and 3' ends [
23,
24]. Y RNAs may follow the miRNA's biogenesis pathways due to having a stem-loop structure of both Y RNAs and miRNAs [
22]. Another study suggests that Y RNAs do not enter the miRNA biogenesis pathway and also do not bind to argonaut complex protein [
25]. Y RNAs are transcribed by the enzyme RNA polymerase III. These RNAs are bound to the polyuridine tail of the La protein, also known as small RNA binding exonucleolytic protection factor. This binding ensures nuclear retention and safeguards them from degradation [
26]. Additionally, Y RNAs are also bound to RO60, also known as SSA, which promotes nuclear export and makes them more stable [
27]. Y RNA-derived fragments (YRFs) are formed as a result of the partial breakdown of Y RNAs during apoptosis, which is carried out via the caspase-3-dependent pathway [
25]. YRFs have been detected in both normal and cancerous tissues [
28].
The dysregulation of Y RNAs may contribute to the development of tumors, affect cell growth, and promote inflammation [
29]. Y RNAs are crucial in initiating DNA replication, maintaining RNA stability, and responding to cell stress [
22,
30]. Y RNAs are responsible for cellular processes such as cell proliferation [
22]. Y RNAs and YRFs might be involved in signaling or a gene regulation function [
31,
32]. Y RNAs have not previously been investigated in human or canine HCC and HCA.
Y RNAs have been found in substantial amounts in plasma and serum from human patients [
33,
34], other biofluids [
35], and extracellular vesicles [
31]. Y RNAs have been established as reliable diagnostic biomarkers for a range of human cancers, including prostate and bladder cancers, melanoma, head, and neck squamous cell carcinoma (HNSCC), breast cancer, clear cell renal cell carcinoma, and lung cancer [
29,
33,
34,
36,
37,
38,
39]. Regarding evidence from dogs, our group has found decreased Y RNA-fragment expression in canine mammary gland tumors [
40].
Extracellular vehicles (EVs) are of potential interest for the quantification of Y RNA fragments and other ncRNAs. They are small structures released by cells to facilitate the transportation of vital components such as DNA, RNA, and proteins for effective intercellular communication [
41]. EV-derived ncRNAs have great significance for the early diagnosis of HCC due to their presence in circulation at an early stage of the disease, and they also have implications for any drug delivery system used in the treatment of HCC [
42,
43]. Recent studies have shown that EV-derived Y RNA is abundant in human brain tumors, melanoma, and small-cell lung cancer [
44,
45,
46]. However, EV-derived Y RNA has yet to be studied in either canine or human HCC or HCA.
Similar gene expression patterns, such as the significance of TGF-beta, seem to be evident in the development of HCC in dogs and humans [
47]. That is why exploring the role of Y RNA presents a promising avenue for gaining significant insights into the development of hepatic diseases.
Accordingly, in this study, we aimed to determine relative Y RNA expression levels in dogs with HCC and HCA using qRT-PCR analysis targeting tumor tissues, plasma, and plasma EVs from clinical samples, and HCC cell lines, to evaluate Y RNAs as diagnostic biomarkers for these two types of liver tumor in dogs.
2. Materials and Methods
2.1. Study Population (Clinical Samples)
The clinical samples evaluated in this study had been obtained from a population of 28 dogs (age range: 8-14 years) diagnosed histopathologically with HCA (n=15) or HCC (n=13) by a veterinary pathologist when undergoing surgery at the Kagoshima University Veterinary Teaching Hospital or an affiliated clinic, between September 2012 and December 2022. The owner of each dog gave informed consent for using samples in this research. Samples were also collected from nine healthy adult laboratory beagle dogs to include as healthy controls in the evaluation provided by Shin Nippon Biomedical Laboratories, Ltd. [
16].
Tumor tissue samples were collected at the time of surgery from the clinical patients, and biopsy samples were collected from the livers of healthy controls. Plasma samples were obtained from a subset of the study population (n=20; Healthy controls: n=6; HCA: n=5; HCC n=9). Full details of the HCA and HCC patients are summarized in
Table 1. Tissue samples were immersed in RNAlater immediately after collection and stored at -80°C for long-term preservation. Blood samples were collected in anticoagulant-treated tubes (Terumo Venoject tubes 3.2% sodium citrate) and centrifuged at 3000*g for 10 minutes to remove the cell debris. The plasma samples were separated and centrifuged again at 16000 ✕g at 4°C to remove the debris. The supernatant was transferred to new Eppendorf tubes and stored at -80°C as plasma samples.
2.2. Cell Lines and Cell Culture
In this study, we evaluated two HCC cell lines, 95-1044 (a fast-proliferating cell line) and AZACH (an intermediate-proliferating cell line) [
16,
48]. Cell lines were preserved using a CultureSure freezing medium and stored in liquid nitrogen (Wako Pure Chemical Industries, Ltd., Osaka, Japan). D-MEM medium (Sigma-Aldrich, St. Louis, Missouri), 5% fetal bovine serum (Thermo Fisher Scientific, Waltham, Massachusetts), 5% L-glutamine (Sigma-Aldrich), and 3.5 μg/mL spectinomycin (Sigma-Aldrich) were used to culture the cells. All cells were cultured in a humidified incubator with 5% CO
2 at 37°C. Cold phosphate-buffered saline (PBS) and 0.25% trypsin or 0.1% EDTA were applied for detaching the cells. Cells were counted using an automated cell counter (LUNAII, Logos).
2.3. EV Isolation
The Total Exosome RNA and Protein Isolation Kit (Invitrogen, Thermo Fisher Scientific) was used to isolate EVs from plasma, following the manufacturer’s protocol. In brief, 300 μl plasma samples were mixed with a half volume of 1X PBS. 90 μl of exosome precipitation reagent was then added, and the resultant mixture was vortexed thoroughly and centrifuged at 10,000*g for 5 minutes. The supernatant was discarded, and the tube was centrifuged again at 1000✕g for 30 seconds to remove the residual reagent. Finally, the pellet was reconstituted in 150 μl 1X PBS and stored at -80°C for further analysis.
2.4. RNA Isolation of Clinical Samples and HCC Cell Lines
A mirVanaTM RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to extract total RNA from tissues and cells in accordance with the manufacturer’s protocol. A mirVana PARIS Kit (Thermo Fisher Scientific) was used to isolate total RNAs from plasma samples and EVs. Before extraction, 5 μl (5 femtomoles) of synthetic cel-miR-39 was added to every plasma and plasma EV sample for normalization. Briefly, each tissue sample or the relevant HCC cell preparation was mixed with the required amount of lysis buffer. A 300 µL aliquot of each plasma sample was mixed with an equivalent amount of 2x denaturation solution. A 1:10 ratio of a miRNA homogenate additive was added to the tissue or cell lysate, then kept on ice for 10 min. A 600 µL Acid-phenol: chloroform (Ambion®) was added to the tissue, cell lysate, or plasma, with subsequent thorough vortex-mixing and then centrifugation at 15000✕g for 5 min at room temperature. The supernatant was then collected carefully in an Eppendorf tube, to which a 1.25-fold amount of molecular-grade ethanol (99.9% in purity) was added (and the amount recorded), and the tube contents were filtered using centrifugation. In the final step, total RNA was obtained as sediment in the tube using an elution solution pre-heated to 95°C. The NanoDrop 2000c spectrophotometer was used to measure the concentration of total RNA (Thermo Fisher Scientific). To evaluate the quality and integrity of RNA, an Agilent 2100 Bioanalyzer was utilized (Agilent Technologies, Santa Clara, CA, USA). The cells and tissues had RNA Integrity Numbers ranging from 8.5 to 9.5.
2.5. ncRNAs Selection and qRT-PCR
Y RNA was selected based on a previously published NGS dataset (SRA: PRJNA716131) for canine mammary gland tumors [
40]. The qRT-PCR protocol was described previously [
49,
50,
51]. Briefly, 2 ng (for tissues and cell lines) or 1.25 µl (for plasma and plasma EVs) of total RNA were reverse transcribed to cDNA using the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific) with a T100 thermal cycler, following the manufacturer’s protocol. For qRT-PCR, a TaqMan First Advanced Master Mix Kit and a Quant Studio 3 real-time PCR system (Thermo Fisher Scientific) were applied. Each experiment was conducted two times to ensure accuracy. To evaluate the expression level, the 2
−ΔΔCT method was used. RNU6B was used as an internal control for tissues and plasma, miR-16 was for the plasma, and miR-186 was for EVs [
52]. The TaqMan primer sequences are as follows; 5´-GGCTGGTCCGAGTGCAGTGGTGCTTAC-3´ YRNA fragments (Ensembl ID: ENSCAFT00000034244.1).
2.6. Statistical Analysis
GraphPad Prism 9 (
https://www.graphpad.com/) was used for statistical analysis and graph visualization. A Mann–Whitney U test and a one-way analysis of variance (ANOVA) followed by the Kruskal-Wallis test were used to assess the qRT-PCR results where applicable. ROC curves and AUCs were plotted using Wilson/Brown method. A P-value <0.05 was considered statistically significant.
3. Results
3.1. Expression of Y RNA Using qRT-PCR
3.1.1. Relative Expression in the Clinical Tissue Samples
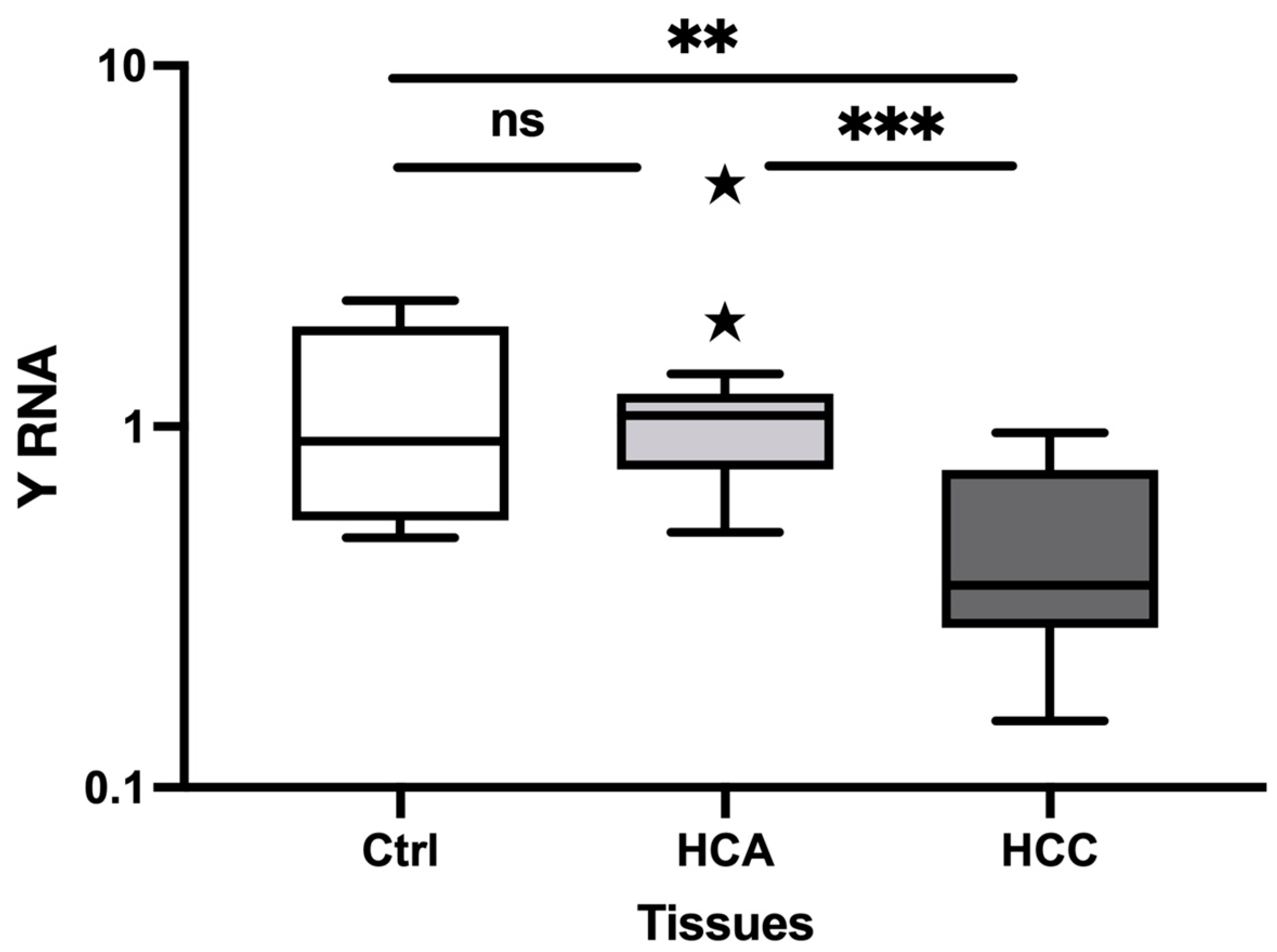
The relative expression of Y RNA was investigated in HCA and HCC tissue samples. YRNA was significantly decreased in HCC [fold change (FC) =0.43, P=0.008] versus healthy controls (
Figure 1A,B). In addition, Y RNA was preferentially decreased in HCC (FC=0.39, P=0.001) versus HCA. However, the Y RNA expression level did not significantly differ between healthy controls and HCA. Thus, the expression profile for Y RNA in HCC differed to those in healthy controls and HCA.
3.1.2. Relative Expression in Plasma
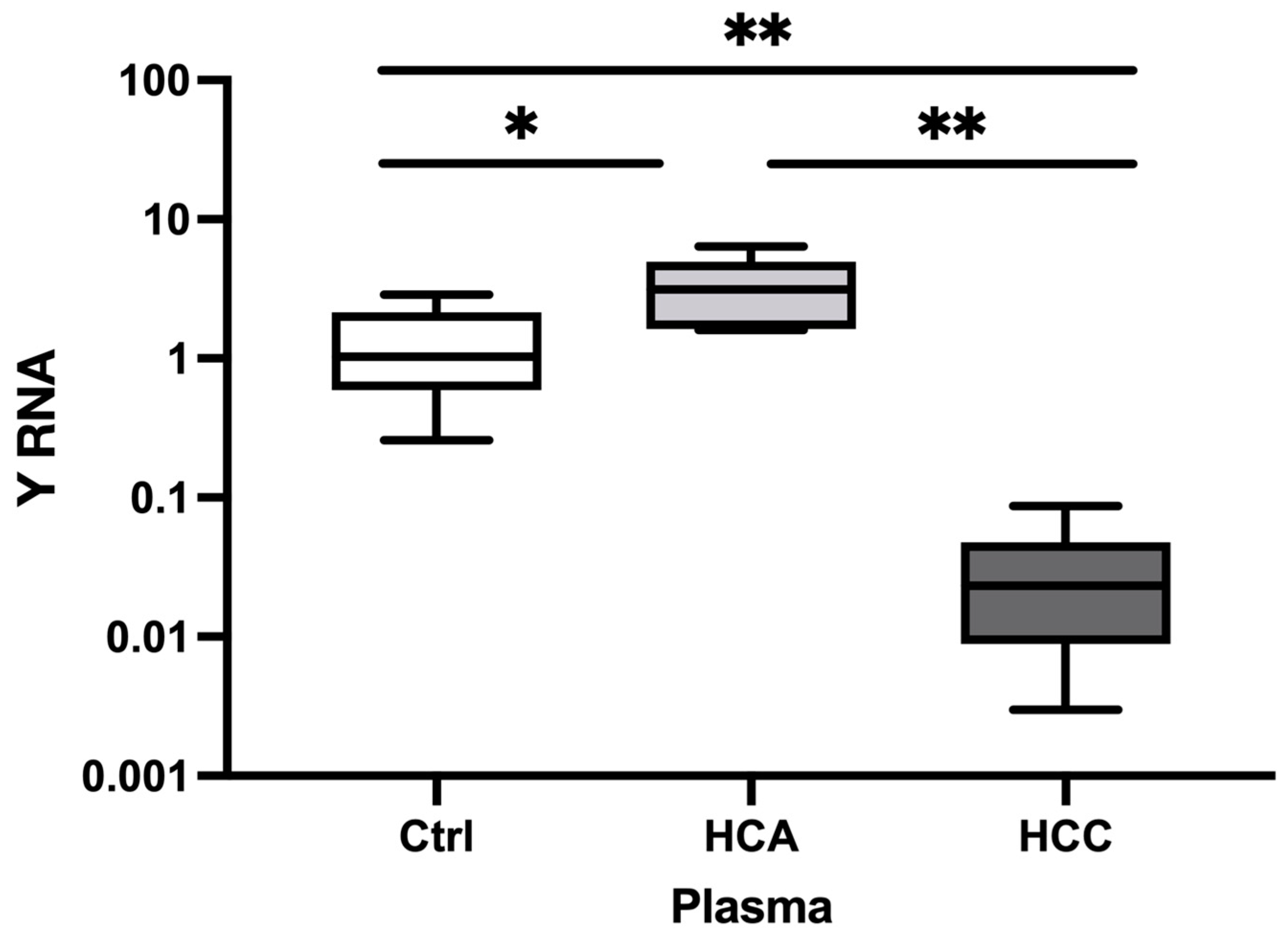
We evaluated the expression of the selected Y RNA in plasma samples. Y RNA was significantly decreased in HCC (FC=0.02, P=0.002) and significantly increased in HCA (FC=2.50, P=0.002) versus Healthy controls (
Figure 2). Furthermore, Y RNA expression was decreased in HCC (FC=0.009, P=0.030) versus HCA. Taken together, our findings indicate that Y RNA expression may differentiate HCC and HCA from Healthy controls and HCC from HCA.
3.1.3. Relative Expression in Plasma EVs
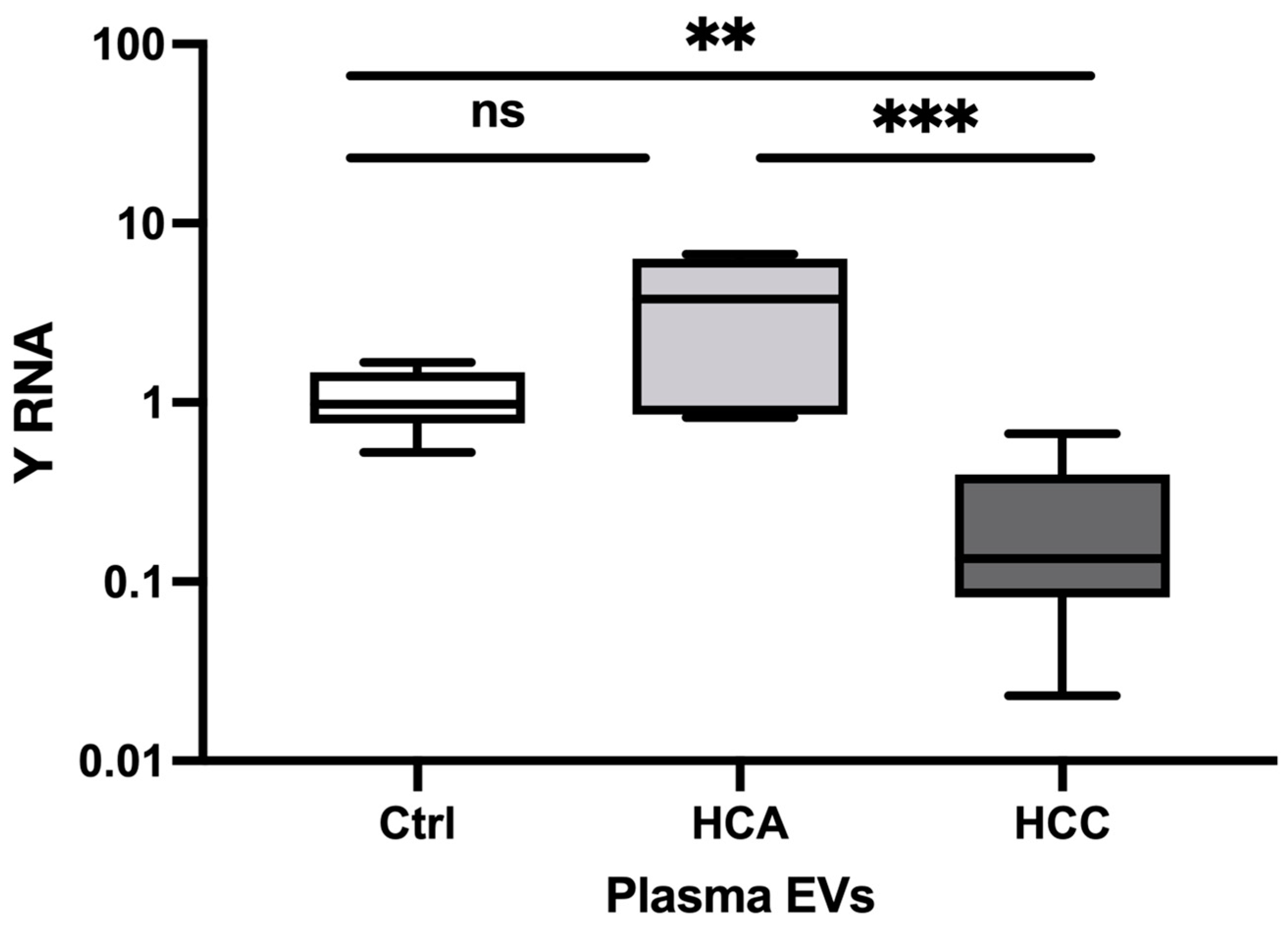
In plasma, the relative Y RNA expression was significantly decreased in HCC (FC=0.21, P=0.001) versus Healthy controls (FC=0.21, P=0.001), and HCA (FC=0.06, P=0.001) [
Figure 3]. However, Y RNA expression did not significantly differ between HCA and Healthy controls. Thus, Y RNA expression could distinguish HCC and HCA from Healthy controls. The Y RNA expression profile in plasma was consistent with that in clinical tumor tissue samples.
3.1.4. Relative Expression in Canine HCC Cell Lines
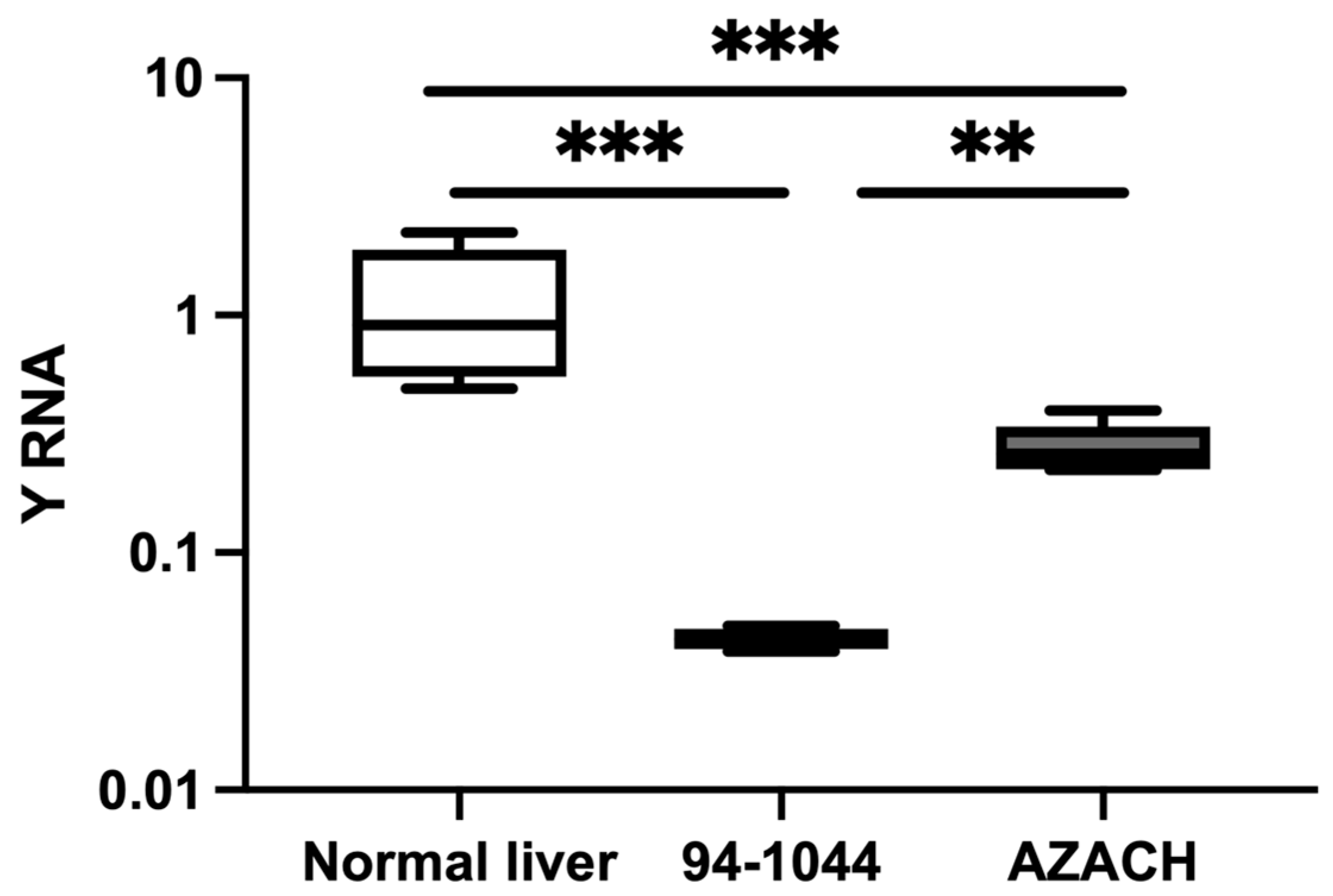
The relative expression of Y RNA was evaluated in a fast-proliferative 95-1044 and intermediate-proliferating AZACH cell lines. Y RNA was significantly decreased in 95-1044 (FC=0.03, P=0.0002) and AZACH (FC=0.24, P=0.0007) cells versus normal liver tissue (
Figure 4). In addition, Y RNA was significantly decreased in 95-1044 cells (FC=0.15, P=0.004) versus AZACH cells. Our results thus suggest that Y RNA expression is substantially decreased in fast-proliferative HCC cell lines, which is consistent with the results for clinical tumor tissue samples.
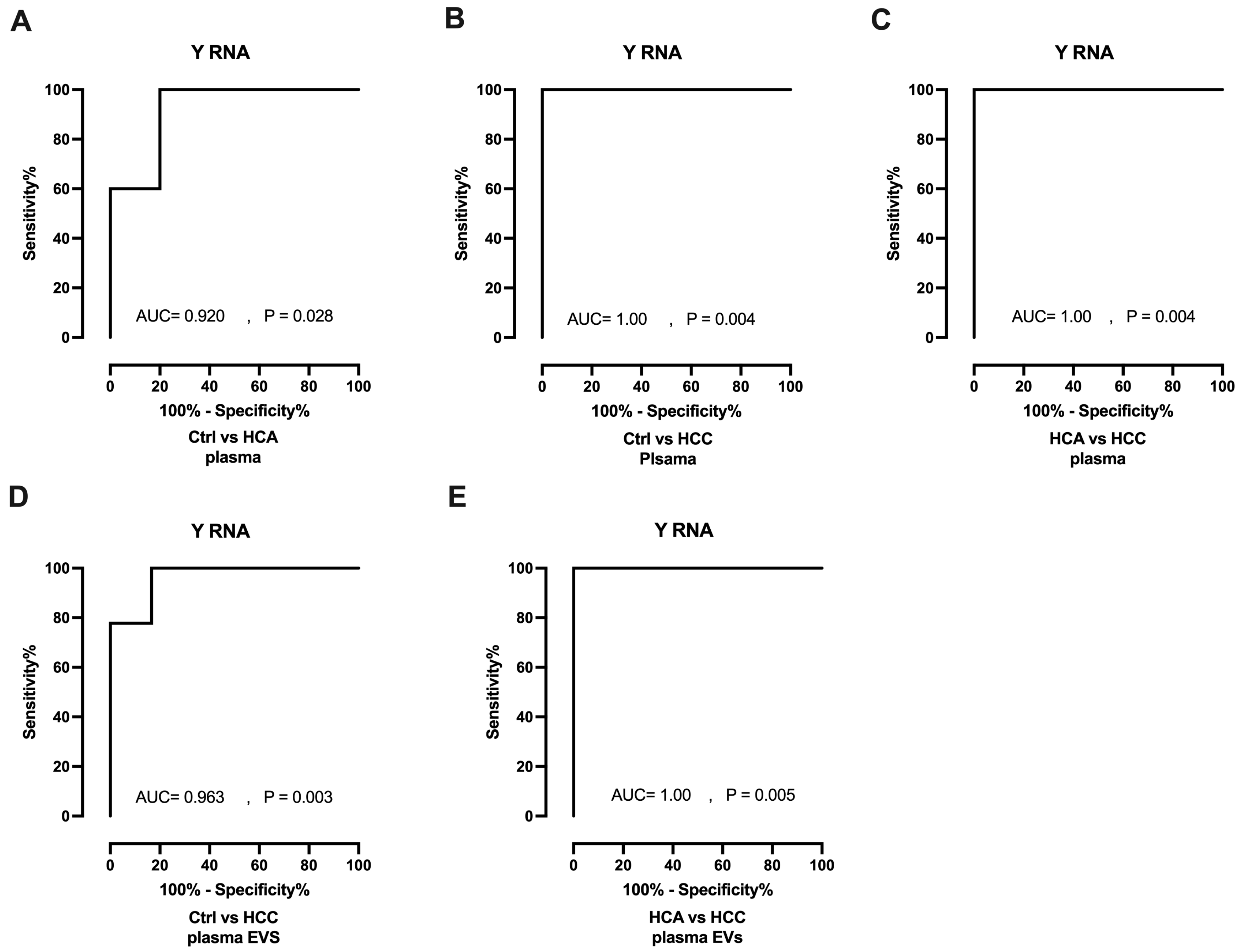
3.2. Diagnostic Value of Y RNA
Receiver operating characteristics (ROC) curves and areas under the curve (AUCs) were generated to investigate the diagnostic value of Y RNA. In plasma analyses, Y RNA yielded AUCs of 0.920 (P=0.028) and 1.00 (P=0.004) for HCA and HCC, respectively, when evaluated against Healthy controls (
Figure 5A,B). Y RNA also differentiated HCC (AUC=1.00, P=0.004) from HCA in plasma samples (
Figure 5C). In plasma EV analyses, Y RNA significantly distinguished HCC (AUC=0.963, P=0.003) from Healthy controls (
Figure 5D).and from HCA (AUC=1.00, P=0.005;
Figure 5E); however, it could not distinguish HCA from Healthy controls (AUC=0.833, P=0.088;
Figure S1). In summary, Y RNA could discriminate HCC and HCA from Healthy controls and HCC from HCA in plasma and plasma EVs.
4. Discussion
ncRNAs play a pivotal role in HCC development and progression, and evidence exists to support their utility as diagnostic and prognostic biomarkers for this disease [
53,
54,
55]. Among ncRNAs, miRNAs have showed similar expression patterns in extensive studies on human and canine HCC [
15,
16,
17,
18,
19]. miRNAs are less studied in human HCA [
1,
14], and have featured in only one study in canine HCA, in which dysregulation was found [
15]. In contrast, Y RNA-derived fragments have not previously been studied in human or canine HCC or HCA, and here we report original findings (to our knowledge) on Y RNA expression in these two types of liver tumors in canine patients.
In key findings, Y RNA expression was significantly decreased in canine HCC tumor tissue versus healthy controls, and HCA tumor tissue, and the same pattern was noted in plasma EV samples. In plasma samples, Y RNA was significantly decreased in canine HCC and significantly increased in HCA versus Healthy controls. We also investigated Y RNA in two canine HCC cell lines and found it was significantly decreased in fast-proliferating 95-1044 cells and intermediate proliferating AZACH cells versus normal liver tissue. ROC analyses revealed that Y RNA could distinguish HCC from the Healthy controls and HCA patients in plasma and plasma EV analyses.
Altered Y RNA and YRFs expression levels are potentially implicated in carcinogenesis, and there is evidence that they act as diagnostic and prognostic biomarkers for several cancers [
29,
36,
56]. Oncologists focussing on the human prostate have found that RNY1, RNY3, RNY4, and RNY5 are downregulated in prostate adenocarcinoma versus normal tissue and benign prostate hyperplasia [
36]. These Y RNAs (RNY1, RNY3, RNY4, and RNY5) are reportedly similarly downregulated in human bladder cancer versus normal urothelial bladder tissue and act as a prognostic indicator for this condition [
37]. RNY3P1, RNY4P1, and RNY4P25 show significantly higher expression in stage 0 human melanoma than at more advanced stages [
33]. YRNA1 and YRNA5 are downregulated in human HNSCC, for which YRNA1 is regarded as a potential biomarker [
29]. Deep sequencing and bioinformatics analysis-based study has reported that dysregulated Y RNAs are also abundant in serum of human breast cancer patients [
34]. YRNA-RNY1 is downregulated in human lung cancer patients compared to normal patients, whereas YRNA-RNY1 is found to be upregulated in lung cancer patients suffering from tuberculosis compared to normal controls [
38]. In clear cell renal cell carcinoma, hY3 and hY4 show altered expression compared to normal renal tissue [
39]. hY1 and hY3 RNA are highly abundant and upregulated in colon cancer patients than in healthy controls [
57]. A set of Y RNAs ( hY1, hY3, and hY4) are shown an increase in human cervix cancer [
57]. We are currently compiling evidence that Y RNA is substantially decreased in metastasized canine mammary gland tumors versus those classified as benign mixed tumors [
40]. A recent study revealed that hY4 RNA fragments are upregulated in human small-cell lung cancer-derived EVs and it inhibits tumor development by inhibiting MAPK/NF-
kB signaling [
44]. Deep sequencing-based studies have shown that EV-derived Y RNAs are abundant in human melanoma [
45]. Y RNAs are also found to be abundant in human brain tumors-derived EVs [
46]. RNY4 fragments are highly abundant in non-Hodgkin lymphoma-derived EVs [
58]. hY5 RNA is shown to be enriched in blood cancer-derived EVs (K562 cells, and myelogenous leukemia) [
59]. Overall, the findings in this study are consistent with several reports on human cancer and canine MGT, indicating that Y RNA expression is decreased in malignant tumors (such as canine HCC) relative to benign tumors (such as canine HCA) and healthy controls.
A recent study revealed that canine HCA transforms into HCC, which means recurrence may occur [
60]. Therefore, this study demonstrated that Y RNA has a high potential for distinguishing canine HCA from HCC. We believe these findings provided insights into comprehending the knowledge of differential diagnoses among hepatic diseases.
The functional roles of Y RNA in canine HCC and HCA and its participation in the relevant underlying molecular mechanisms have yet to be fully elucidated. Here, we have demonstrated the aberrant expression of this ncRNA in canine HCC and HCA patients. We posit that Y RNA might be involved in cancer malignancy through its downregulated expression in HCC. Y RNA could be a biomarker distinguishing malignant tumors (HCC) from benign tumors (HCA) and tumor-free patients. However, this study still has some limitations. First, we need to validate Y RNA in a large cohort sample to strengthen our findings further. Second, the roles of Y RNA in canine HCC development need to be investigated in a bio-functional study.
Pet dogs have great potential utility for comparative oncology clinical trials, partly because they maintain an intact immune system and experience natural co-evolution of the tumor microenvironment [
61]. Humans and dogs are known to develop cancer through aberrations occurring for the same genes [
62]. Therefore, this study has great potential to enhance our understanding of the expression of Y RNA in hepatic diseases.
5. Conclusions
To our knowledge, this is the first report on Y RNA in canine HCC and HCA. This ncRNA has distinctive characteristics and differentiates malignant tumors (HCC) from benign tumors (HCA). Notably, its expression pattern is consistent across clinical samples and cell lines. Y RNA shows great potential as a biomarker for differentiating HCC from HCA. Our findings provide significant insights into how Y RNA contributes to the progression of hepatic disease in dogs. Further research is required to fully elucidate the role of Y RNA in the development and progression of canine HCC and HCA.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, N.U., M.N.H. and N.M.; methodology, N.U., M.N.H., M.A. and N.M.; validation, N.U., M.N.H., M.A. and N.M..; formal analysis,., M.N.H. and N.M.; investigation, N.U. and M.N.H.; resources, N.U., N.M.; writing—original draft preparation, N.U., M.N.H. and N.M.; writing—review and editing, N.U., M.N.H. and N.M.; supervision, M.N.H. and N.M.; project administration, M.N.H. and N.M.; funding acquisition, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant nos. 20K21375).
Institutional Review Board Statement
This study was approved by the ethics committee of the Kagoshima University Veterinary Teaching Hospital (Approval No. KVH220001) and was conducted in accordance with the regulations of this committee and Kagoshima University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are provided in the article.
Acknowledgments
The authors thank Nobuhiro Nozaki and Ms. Ayako Masuda for assisting with experiments, and Dr. Henry Smith (Co-chair of the Veterinary Special Interest Group in the European Medical Writers Association), of the Joint Faculty of Veterinary Medicine, Kagoshima University, for his help with the English editing of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, J.; Sadot, E.; Vigidal, J.A.; Klimstra, D.S.; Balachandran, V.P.; Kingham, T.P.; Allen, P.J.; D'Angelica, M.I.; DeMatteo, R.P.; Jarnagin, W.R.; et al. Characterization of hepatocellular adenoma and carcinoma using microRNA profiling and targeted gene sequencing. PLoS ONE 2018, 13, e0200776. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.S.; Couto, C.G.; Ayl, R.D.; Shank, K.A. Treatment of tumor-bearing dogs with actinomycin D. J. Vet. Intern. Med. 1994, 8, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Oo, T.; Sasaki, N.; Ikenaka, Y.; Ichise, T.; Nagata, N.; Yokoyama, N.; Sasaoka, K.; Morishita, K.; Nakamura, K.; Takiguchi, M. Serum steroid profiling of hepatocellular carcinoma associated with hyperadrenocorticism in dogs: A preliminary study. Front. Vet. Sci. 2022, 9, 1014792. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H.; Johnson, G.F. Canine hepatocellular carcinoma. Vet. Pathol. 1981, 18, 427–438. [Google Scholar] [CrossRef] [PubMed]
- van Sprundel, R.G.; van den Ingh, T.S.; Guscetti, F.; Kershaw, O.; Kanemoto, H.; van Gils, H.M.; Rothuizen, J.; Roskams, T.; Spee, B. Classification of primary hepatic tumours in the dog. Vet. J. 2013, 197, 596–606. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H. Canine hepatic neoplasms: a clinicopathologic study. Vet. Pathol. 1980, 17, 553–564. [Google Scholar] [CrossRef]
- Selmic, L.E. Hepatobiliary Neoplasia. Vet. Clin. North. Am. Small Anim. Pract. 2017, 47, 725–735. [Google Scholar] [CrossRef]
- Lapsley, J.M.; Wavreille, V.; Barry, S.; Dornbusch, J.A.; Chen, C.; Leeper, H.; Bertran, J.; Scavelli, D.; Liptak, J.M.; Wood, C.; et al. Risk factors and outcome in dogs with recurrent massive hepatocellular carcinoma: A Veterinary Society of Surgical Oncology case-control study. Vet. Comp. Oncol. 2022, 20, 697–709. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Jeannot, E.; Nhieu, J.T.; Scoazec, J.Y.; Guettier, C.; Rebouissou, S.; Bacq, Y.; Leteurtre, E.; Paradis, V.; Michalak, S.; et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006, 43, 515–524. [Google Scholar] [CrossRef]
- Bioulac-Sage, P.; Laumonier, H.; Couchy, G.; Le Bail, B.; Sa Cunha, A.; Rullier, A.; Laurent, C.; Blanc, J.F.; Cubel, G.; Trillaud, H.; et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 2009, 50, 481–489. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Patel, T. Noncoding RNA as therapeutic targets for hepatocellular carcinoma. Semin. Liver Dis. 2015, 35, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ladeiro, Y.; Couchy, G.; Balabaud, C.; Bioulac-Sage, P.; Pelletier, L.; Rebouissou, S.; Zucman-Rossi, J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology 2008, 47, 1955–1963. [Google Scholar] [CrossRef]
- Chiu, L.Y.; Kishnani, P.S.; Chuang, T.P.; Tang, C.Y.; Liu, C.Y.; Bali, D.; Koeberl, D.; Austin, S.; Boyette, K.; Weinstein, D.A.; et al. Identification of differentially expressed microRNAs in human hepatocellular adenoma associated with type I glycogen storage disease: a potential utility as biomarkers. J. Gastroenterol. 2014, 49, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, K.; Verzijl, T.; Grinwis, G.C.; Favier, R.P.; Penning, L.C.; Burgener, I.A.; van der Laan, L.J.; Fieten, H.; Spee, B. Use of Serum MicroRNAs as Biomarker for Hepatobiliary Diseases in Dogs. J. Vet. Intern. Med. 2016, 30, 1816–1823. [Google Scholar] [CrossRef]
- Lai, Y.C.; Ushio, N.; Rahman, M.M.; Katanoda, Y.; Ogihara, K.; Naya, Y.; Moriyama, A.; Iwanaga, T.; Saitoh, Y.; Sogawa, T.; et al. Aberrant expression of microRNAs and the miR-1/MET pathway in canine hepatocellular carcinoma. Vet. Comp. Oncol. 2018, 16, 288–296. [Google Scholar] [CrossRef]
- Dirksen, K.; Verzijl, T.; van den Ingh, T.S.; Vernooij, J.C.; van der Laan, L.J.; Burgener, I.A.; Spee, B.; Fieten, H. Hepatocyte-derived microRNAs as sensitive serum biomarkers of hepatocellular injury in Labrador retrievers. Vet. J. 2016, 211, 75–81. [Google Scholar] [CrossRef]
- Ratnasari, N.; Lestari, P.; Renovaldi, D.; Raditya Ningsih, J.; Qoriansas, N.; Wardana, T.; Hakim, S.; Signa Aini Gumilas, N.; Indrarti, F.; Triwikatmani, C.; et al. Potential plasma biomarkers: miRNA-29c, miRNA-21, and miRNA-155 in clinical progression of Hepatocellular Carcinoma patients. PLoS One 2022, 17, e0263298. [Google Scholar] [CrossRef]
- Zhu, Q.; Gong, L.; Wang, J.; Tu, Q.; Yao, L.; Zhang, J.R.; Han, X.J.; Zhu, S.J.; Wang, S.M.; Li, Y.H.; et al. miR-10b exerts oncogenic activity in human hepatocellular carcinoma cells by targeting expression of CUB and sushi multiple domains 1 (CSMD1). BMC Cancer 2016, 16, 806. [Google Scholar] [CrossRef]
- Lerner, M.R.; Boyle, J.A.; Hardin, J.A.; Steitz, J.A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science 1981, 211, 400–402. [Google Scholar] [CrossRef]
- Boccitto, M.; Wolin, S.L. Ro60 and Y RNAs: structure, functions, and roles in autoimmunity. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.P.; Krude, T. Functional roles of non-coding Y RNAs. Int. J. Biochem. Cell Biol. 2015, 66, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Pruijn, G.J.; Wingens, P.A.; Peters, S.L.; Thijssen, J.P.; van Venrooij, W.J. Ro RNP associated Y RNAs are highly conserved among mammals. Biochim. Biophys. Acta 1993, 1216, 395–401. [Google Scholar] [CrossRef]
- Happel, C.; Ganguly, A.; Tagle, D.A. Extracellular RNAs as potential biomarkers for cancer. J. Cancer Metastasis Treat. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.E.; Hall, A.E.; Csorba, T.; Turnbull, C.; Dalmay, T. Biogenesis of Y RNA-derived small RNAs is independent of the microRNA pathway. FEBS Lett. 2012, 586, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Kenan, D.; Martin, B.J.; Keene, J.D. Genomic structure and amino acid sequence domains of the human La autoantigen. J. Biol. Chem. 1988, 263, 18043–18051. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, S.L.; Harley, J.B.; Keene, J.D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc. Natl. Acad. Sci. USA 1988, 85, 9479–9483. [Google Scholar] [CrossRef]
- Yamazaki, F.; Kim, H.H.; Lau, P.; Hwang, C.K.; Iuvone, P.M.; Klein, D.; Clokie, S.J. pY RNA1-s2: a highly retina-enriched small RNA that selectively binds to Matrin 3 (Matr3). PLoS ONE 2014, 9, e88217. [Google Scholar] [CrossRef]
- Guglas, K.; Kolenda, T.; Stasiak, M.; Kopczyńska, M.; Teresiak, A.; Ibbs, M.; Bliźniak, R.; Lamperska, K. YRNAs: New Insights and Potential Novel Approach in Head and Neck Squamous Cell Carcinoma. Cells 2020, 9. [Google Scholar] [CrossRef]
- Gardiner, T.J.; Christov, C.P.; Langley, A.R.; Krude, T. A conserved motif of vertebrate Y RNAs essential for chromosomal DNA replication. Rna 2009, 15, 1375–1385. [Google Scholar] [CrossRef]
- Dhahbi, J.M.; Spindler, S.R.; Atamna, H.; Boffelli, D.; Mote, P.; Martin, D.I. 5'-YRNA fragments derived by processing of transcripts from specific YRNA genes and pseudogenes are abundant in human serum and plasma. Physiol. Genomics 2013, 45, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.; Kowalski, M.P.; Langley, A.R.; Rodriguez, R.; Balasubramanian, S.; Hsu, S.T.; Krude, T. Nucleotide contributions to the structural integrity and DNA replication initiation activity of noncoding y RNA. Biochemistry 2014, 53, 5848–5863. [Google Scholar] [CrossRef]
- Solé, C.; Tramonti, D.; Schramm, M.; Goicoechea, I.; Armesto, M.; Hernandez, L.I.; Manterola, L.; Fernandez-Mercado, M.; Mujika, K.; Tuneu, A.; et al. The Circulating Transcriptome as a Source of Biomarkers for Melanoma. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Dhahbi, J.M.; Spindler, S.R.; Atamna, H.; Boffelli, D.; Martin, D.I. Deep Sequencing of Serum Small RNAs Identifies Patterns of 5' tRNA Half and YRNA Fragment Expression Associated with Breast Cancer. Biomark. Cancer 2014, 6, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, Y.; Niehoff, E.M.; Stahl, A.F.; Zhao, C.; Kristiansen, G.; Müller, S.C.; Ellinger, J. YRNA expression in prostate cancer patients: diagnostic and prognostic implications. World J. Urol. 2018, 36, 1073–1078. [Google Scholar] [CrossRef]
- Tolkach, Y.; Stahl, A.F.; Niehoff, E.M.; Zhao, C.; Kristiansen, G.; Müller, S.C.; Ellinger, J. YRNA expression predicts survival in bladder cancer patients. BMC Cancer 2017, 17, 749. [Google Scholar] [CrossRef]
- Gu, W.; Shi, J.; Liu, H.; Zhang, X.; Zhou, J.J.; Li, M.; Zhou, D.; Li, R.; Lv, J.; Wen, G.; et al. Peripheral blood non-canonical small non-coding RNAs as novel biomarkers in lung cancer. Mol. Cancer 2020, 19, 159. [Google Scholar] [CrossRef]
- Nientiedt, M.; Schmidt, D.; Kristiansen, G.; Müller, S.C.; Ellinger, J. YRNA Expression Profiles are Altered in Clear Cell Renal Cell Carcinoma. Eur. Urol. Focus. 2018, 4, 260–266. [Google Scholar] [CrossRef]
- Hasan M.N.; Rahman M.M.; Husna A.A.; Chen H.W.; Nozaki N.; Yamato O.; Miura N. YRNA and tRNA fragments can differentiate benign from malignant canine mammary gland tumors. Gene 2023, GENEJOURNAL-D-23-01591 (submitted; under review).
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Yang, N.; Li, S.; Li, G.; Zhang, S.; Tang, X.; Ni, S.; Jian, X.; Xu, C.; Zhu, J.; Lu, M. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget 2017, 8, 3683–3695. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tran, B.V.; Wang, J.J.; Liang, I.Y.; You, S.; Zhu, Y.; Agopian, V.G.; Tseng, H.R.; Yang, J.D. The Role of Extracellular Vesicles in Disease Progression and Detection of Hepatocellular Carcinoma. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Sun, Y.; Ni, Y.; Qin, F.; Li, X.; Wang, T.; Guo, M.; Sun, G. Selective sorting and secretion of hY4 RNA fragments into extracellular vesicles mediated by methylated YBX1 to promote lung cancer progression. J. Exp. Clin. Cancer Res. 2022, 41, 136. [Google Scholar] [CrossRef]
- Lunavat, T.R.; Cheng, L.; Kim, D.K.; Bhadury, J.; Jang, S.C.; Lässer, C.; Sharples, R.A.; López, M.D.; Nilsson, J.; Gho, Y.S.; et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells--Evidence of unique microRNA cargos. RNA Biol. 2015, 12, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, C.; Adnani, L.; Choi, D.; Rak, J. Extracellular Vesicles as Conduits of Non-Coding RNA Emission and Intercellular Transfer in Brain Tumors. Noncoding RNA 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Grabarević, Z.; Corić, M.; Seiwerth, S.; Dzaja, P.; Artuković, B.; Kurilj, A.G.; Beck, A.; Hohsteter, M.; Sostarić-Zuckermann, I.C.; Brcić, L.; et al. Comparative analysis of hepatocellular carcinoma in men and dogs. Coll. Antropol. 2009, 33, 811–814. [Google Scholar] [PubMed]
- Fujimoto, A.; Neo, S.; Ishizuka, C.; Kato, T.; Segawa, K.; Kawarai, S.; Ogihara, K.; Hisasue, M.; Tsuchiya, R. Identification of cell surface antigen expression in canine hepatocellular carcinoma cell lines. J. Vet. Med. Sci. 2013, 75, 831–835. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lai, Y.C.; Husna, A.A.; Chen, H.W.; Tanaka, Y.; Kawaguchi, H.; Hatai, H.; Miyoshi, N.; Nakagawa, T.; Fukushima, R.; et al. Aberrantly expressed snoRNA, snRNA, piRNA and tRFs in canine melanoma. Vet. Comp. Oncol. 2020, 18, 353–361. [Google Scholar] [CrossRef]
- Hino, Y.; Rahman, M.M.; Lai, Y.C.; Husna, A.A.; Chen, H.W.; Hasan, M.N.; Nakagawa, T.; Miura, N. Hypoxic miRNAs expression are different between primary and metastatic melanoma cells. Gene 2021, 782, 145552. [Google Scholar] [CrossRef]
- Husna, A.A.; Rahman, M.M.; Chen, H.W.; Hasan, M.N.; Nakagawa, T.; Miura, N. Long non-coding RNA and transfer RNA-derived small fragments in exosomes are potential biomarkers for canine oral melanoma. Vet. Comp. Oncol. 2022. [Google Scholar] [CrossRef]
- Husna, A.A.; Rahman, M.M.; Lai, Y.C.; Chen, H.W.; Hasan, M.N.; Nakagawa, T.; Miura, N. Identification of melanoma-specific exosomal miRNAs as the potential biomarker for canine oral melanoma. Pigment. Cell Melanoma Res. 2021, 34, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Pea, A.; Jamieson, N.B.; Braconi, C. Biology and Clinical Application of Regulatory RNAs in Hepatocellular Carcinoma. Hepatology 2021, 73 Suppl 1, 38–48. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, F.; Ma, C.; Cheng, Q. Involvement of microRNAs and their potential diagnostic, therapeutic, and prognostic role in hepatocellular carcinoma. J. Clin. Lab. Anal. 2022, 36, e24673. [Google Scholar] [CrossRef]
- Xue, C.; Gu, X.; Bao, Z.; Su, Y.; Lu, J.; Li, L. The Mechanism Underlying the ncRNA Dysregulation Pattern in Hepatocellular Carcinoma and Its Tumor Microenvironment. Front. Immunol. 2022, 13, 847728. [Google Scholar] [CrossRef]
- Guglas, K.; Kołodziejczak, I.; Kolenda, T.; Kopczyńska, M.; Teresiak, A.; Sobocińska, J.; Bliźniak, R.; Lamperska, K. YRNAs and YRNA-Derived Fragments as New Players in Cancer Research and Their Potential Role in Diagnostics. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Christov, C.P.; Trivier, E.; Krude, T. Noncoding human Y RNAs are overexpressed in tumours and required for cell proliferation. Br. J. Cancer 2008, 98, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Lovisa, F.; Di Battista, P.; Gaffo, E.; Damanti, C.C.; Garbin, A.; Gallingani, I.; Carraro, E.; Pillon, M.; Biffi, A.; Bortoluzzi, S.; et al. RNY4 in Circulating Exosomes of Patients With Pediatric Anaplastic Large Cell Lymphoma: An Active Player? Front. Oncol. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortty, S.K.; Prakash, A.; Nechooshtan, G.; Hearn, S.; Gingeras, T.R. Extracellular vesicle-mediated transfer of processed and functional RNY5 RNA. Rna 2015, 21, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Jornet-Rius, O.; Agulla, B.; López, M.C.; Viñeta, C.; García-Ferrer, A.; Serrano, B.; Marco, A.; Palomares, A.; Novellas, R.; Espada, Y.; et al. Needle tract seeding and malignant transformation of hepatocellular adenoma into well-differentiated hepatocellular carcinoma in a dog. Vet. Clin. Pathol. 2023. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Mazcko, C.N. Improving human cancer therapy through the evaluation of pet dogs. Nat. Rev. Cancer 2020, 20, 727–742. [Google Scholar] [CrossRef]
- Rogers, N. Canine clues: Dog genomes explored in effort to bring human cancer to heel. Nat. Med. 2015, 21, 1374–1375. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).