Submitted:
11 August 2023
Posted:
15 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. HBV genotypes and human populations
3. SNPs and HBV clinical outcomes
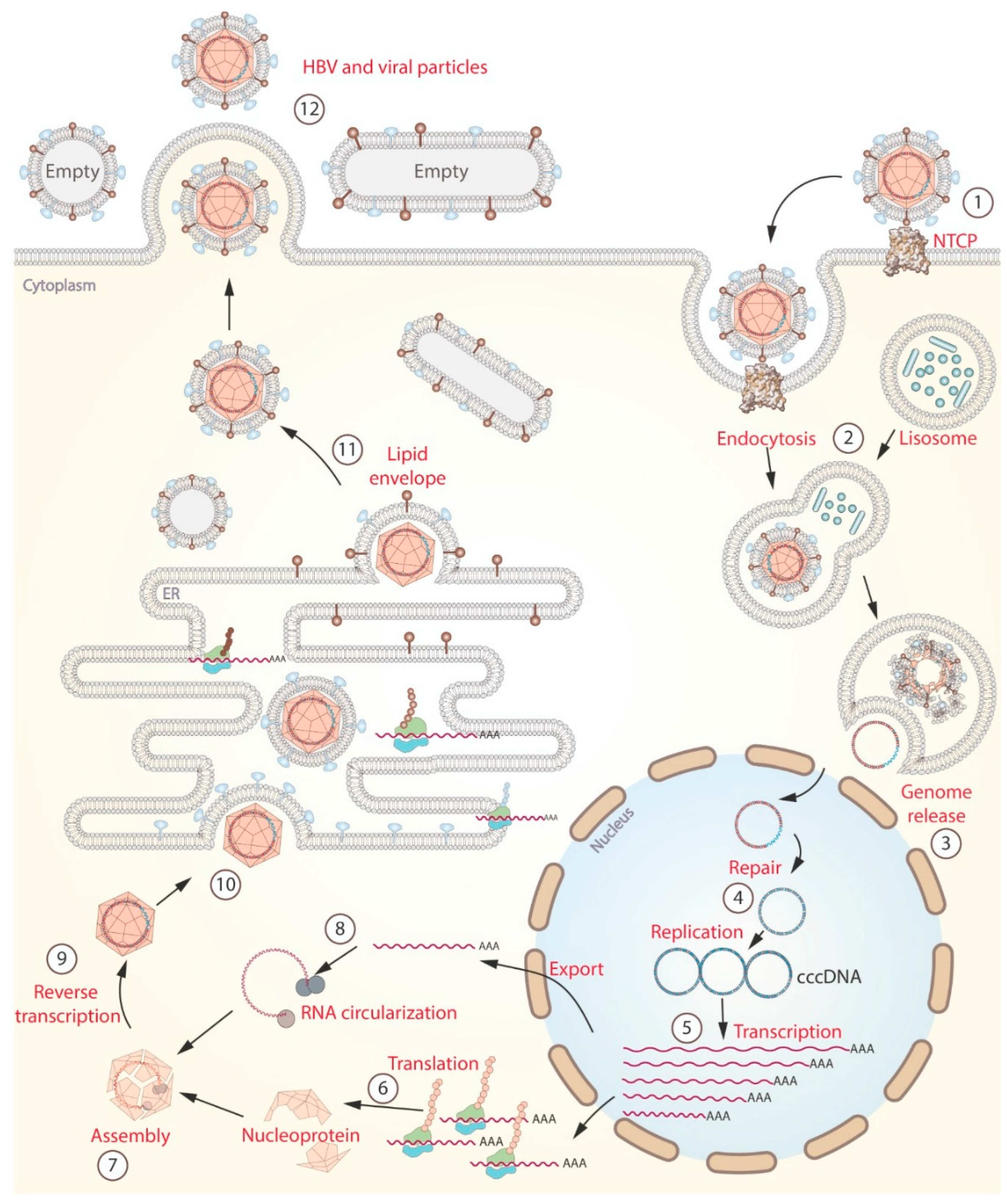
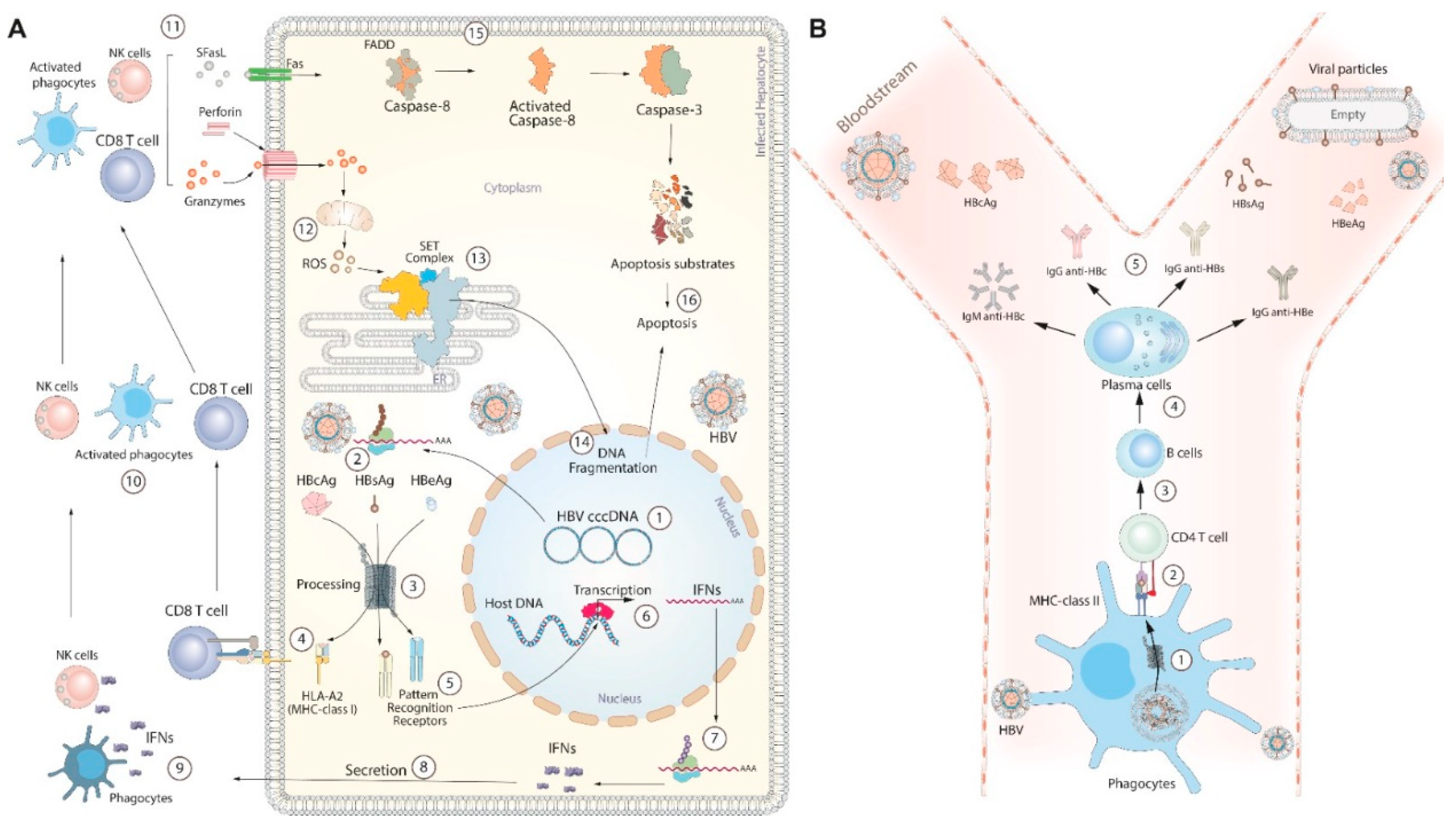
4. HBV life cycle and immune response
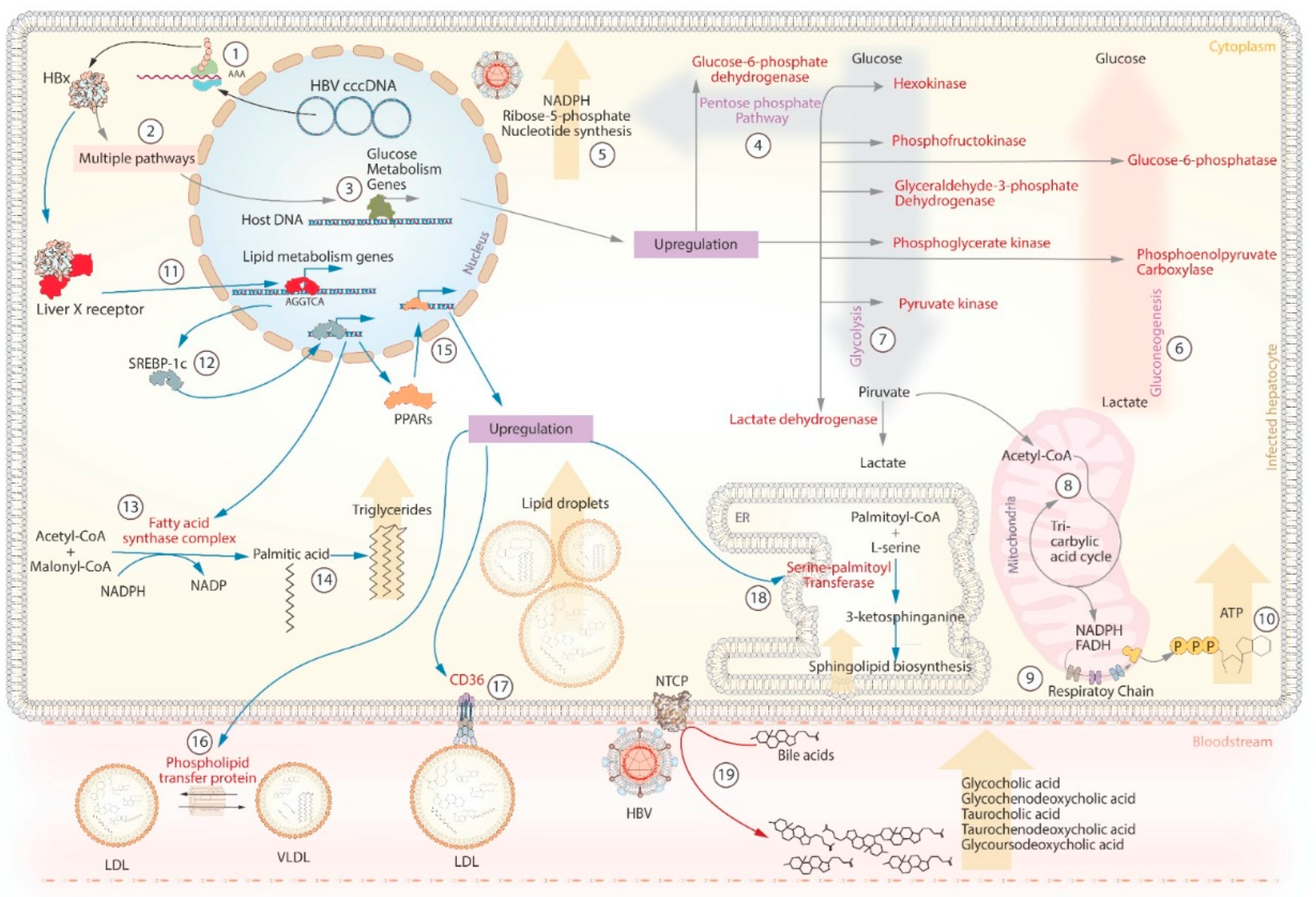
5. HBV and metabolism
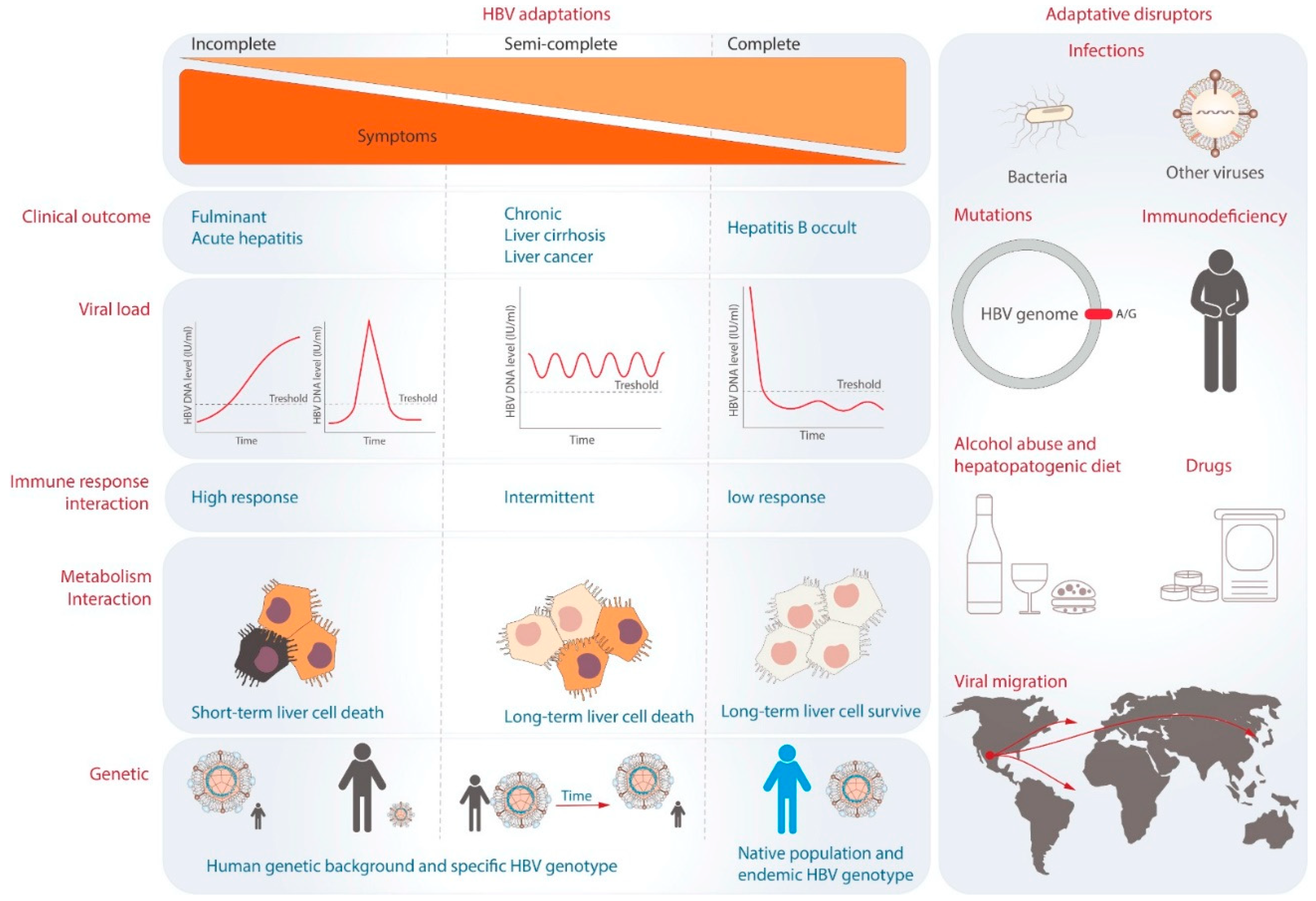
6. The theory of viral adaptation
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Hepatitis B, 2022; p. 4. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 13 June 2023).
- Jeng, W.J.; Papatheodoridis, G.V.; Lok, A.S.F. Hepatitis B. Lancet 2023, 401, 1039-1052. [CrossRef]
- Weisberg, I.S.; Brown, R.S., Jr.; Sigal, S.H. Hepatitis B and end-stage liver disease. Clin Liver Dis 2007, 11, pp. 893-916. [CrossRef]
- Schillie, S.; Vellozzi, C.; Reingold, A.; Harris, A.; Haber, P.; Ward, J.W.; Nelson, N.P. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018, 67, pp. 1-31. [CrossRef]
- Lee, W.M. Acute liver failure. N Engl J Med 1993, 329, pp. 1862-1872. [CrossRef]
- Petrosillo, N.; Ippolito, G.; Solforosi, L.; Varaldo, P.E.; Clementi, M.; Manzin, A. Molecular epidemiology of an outbreak of fulminant hepatitis B. J Clin Microbiol. 2000, 38, pp. 2975-2981. [CrossRef]
- Ichai, P.; Samuel, D. Management of Fulminant Hepatitis B. Curr Infect Dis Rep 2019, 21, 25. [CrossRef]
- Ostapowicz, G.; Fontana, R.J.; Schiodt, F.V.; Larson, A.; Davern, T.J.; Han, S.H.; McCashland, T.M.; Shakil, A.O.; Hay, J.E.; Hynan, L.; et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002, 137, pp. 947-954. [CrossRef]
- Pollicino, T.; Raimondo, G. Occult hepatitis B infection. J Hepatol 2014, 61, pp. 688-689. [CrossRef]
- Panduro, A.; Maldonado-Gonzalez, M.; Fierro, N.A.; Roman, S. Distribution of HBV genotypes F and H in Mexico and Central America. Antivir Ther 2013, 18, pp. 475-484. [CrossRef]
- Raimondo, G.; Filomia, R.; Maimone, S. Therapy of occult hepatitis B virus infection and prevention of reactivation. Intervirology 2014, 57, pp. 189-195. [CrossRef]
- Kafeero, H.M.; Ndagire, D.; Ocama, P.; Kato, C.D.; Wampande, E.; Walusansa, A.; Kajumbula, H.; Kateete, D.; Ssenku, J.E.; Sendagire, H. Mapping hepatitis B virus genotypes on the African continent from 1997 to 2021: a systematic review with meta-analysis. Sci Rep 2023, 13, 5723. [CrossRef]
- Okamoto, H.; Tsuda, F.; Sakugawa, H.; Sastrosoewignjo, R.I.; Imai, M.; Miyakawa, Y.; Mayumi, M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol 1988, 69 ( Pt 10), pp. 2575-2583. [CrossRef]
- Norder, H.; Hammas, B.; Lofdahl, S.; Courouce, A.M.; Magnius, L.O. Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J Gen Virol 1992, 73 ( Pt 5), pp. 1201-1208. [CrossRef]
- Stuyver, L.; De Gendt, S.; Van Geyt, C.; Zoulim, F.; Fried, M.; Schinazi, R.F.; Rossau, R. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J Gen Virol 2000, 81, pp. 67-74. [CrossRef]
- Arauz-Ruiz, P.; Norder, H.; Robertson, B.H.; Magnius, L.O. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol 2002, 83, pp. 2059-2073. [CrossRef]
- Hannoun, C.; Norder, H.; Lindh, M. An aberrant genotype revealed in recombinant hepatitis B virus strains from Vietnam. J Gen Virol 2000, 81, pp. 2267-2272. [CrossRef]
- Olinger, C.M.; Jutavijittum, P.; Hubschen, J.M.; Yousukh, A.; Samountry, B.; Thammavong, T.; Toriyama, K.; Muller, C.P. Possible new hepatitis B virus genotype, southeast Asia. Emerg Infect Dis 2008, 14, pp. 1777-1780. [CrossRef]
- Tatematsu, K.; Tanaka, Y.; Kurbanov, F.; Sugauchi, F.; Mano, S.; Maeshiro, T.; Nakayoshi, T.; Wakuta, M.; Miyakawa, Y.; Mizokami, M. A genetic variant of hepatitis B virus divergent from known human and ape genotypes isolated from a Japanese patient and provisionally assigned to new genotype J. J Virol 2009, 83, pp. 10538-10547. [CrossRef]
- Velkov, S.; Ott, J.J.; Protzer, U.; Michler, T. The Global Hepatitis B Virus Genotype Distribution Approximated from Available Genotyping Data. Genes 2018, 9. [CrossRef]
- Osiowy, C.; Coffin, C.; Andonov, A. Review of Laboratory Tests used in Monitoring Hepatitis B Response to Pegylated Interferon and Nucleos(t)ide Analog Therapy. Curr Treat Options Infect Dis 2016, 8, pp. 177-193. [CrossRef]
- Alvarado-Mora, M.V.; Pinho, J.R. Distribution of HBV genotypes in Latin America. Antivir Ther 2013, 18, pp. 459-465. [CrossRef]
- Roman, S.; Panduro, A. HBV endemicity in Mexico is associated with HBV genotypes H and G. World J Gastroenterol 2013, 19, pp. 5446-5453. [CrossRef]
- Shah, A.A.; Bodewes, R.; Reijnen, L.; Boelsums, T.; Weller, C.M.; Fanoy, E.B.; Veldhuijzen, I.K. Outbreaks of mumps genotype G viruses in the Netherlands between October 2019 and March 2020: clusters associated with multiple introductions. BMC Infect Dis 2021, 21, 1035. [CrossRef]
- Jose-Abrego, A.; Roman, S.; Laguna-Meraz, S.; Rebello-Pinho, J.R.; Justo Arevalo, S.; Panduro, A. Tracing the evolutionary history of hepatitis B virus genotype H endemic to Mexico. Front Microbiol 2023, 14, 1180931. [CrossRef]
- Bottecchia, M.; Madejon, A.; Sheldon, J.; Garcia-Samaniego, J.; Barreiro, P.; Soriano, V. Hepatitis B virus genotype A2 harbours an L217R polymorphism which may account for a lower response to adefovir. J Antimicrob Chemother 2008, 62, pp. 626-627. [CrossRef]
- Chan, H.L.; Hui, A.Y.; Wong, M.L.; Tse, A.M.; Hung, L.C.; Wong, V.W.; Sung, J.J. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut 2004, 53, pp. 1494-1498. [CrossRef]
- Gounder, P.P.; Bulkow, L.R.; Snowball, M.; Negus, S.; Spradling, P.R.; McMahon, B.J. Hepatocellular Carcinoma Risk in Alaska Native Children and Young Adults with Hepatitis B Virus: Retrospective Cohort Analysis. J Pediatr 2016, 178, pp. 206-213. [CrossRef]
- Shi, Y.H. Correlation between hepatitis B virus genotypes and clinical outcomes. Jpn J Infect Dis 2012, 65, pp. 476-482. [CrossRef]
- Jose-Abrego, A.; Roman, S.; Rebello-Pinho, J.R.; Gomes-Gouvea, M; Panduro, A. High Frequency of Antiviral Resistance Mutations in HBV Genotypes A2 and H: Multidrug Resistance Strains in Mexico. J Clin Transl Hepatol 2023, pp. 1-12. [CrossRef]
- Roca, T.P.; Villar, L.M.; Nogueira Lima, F.S.; Vasconcelos, M.P.A.; Borzacov, L.M.P.; Silva, E.C.E.; Lago, B.V.D.; Silva, M.; Botelho Souza, L.F.; Salcedo, J.M.V.; et al. Genomic Variability of Hepatitis B Virus Circulating in Brazilian Western Amazon. Viruses 2022, 14. [CrossRef]
- di Filippo Villa, D.; Cortes-Mancera, F.; Payares, E.; Montes, N.; de la Hoz, F.; Arbelaez, M.P.; Correa, G.; Navas, M.C. Hepatitis D virus and hepatitis B virus infection in Amerindian communities of the Amazonas state, Colombia. Virol J 2015, 12, 172. [CrossRef]
- Roman, S.; Panduro, A.; Aguilar-Gutierrez, Y.; Maldonado, M.; Vazquez-Vandyck, M.; Martinez-Lopez, E.; Ruiz-Madrigal, B.; Hernandez-Nazara, Z. A low steady HBsAg seroprevalence is associated with a low incidence of HBV-related liver cirrhosis and hepatocellular carcinoma in Mexico: a systematic review. Hepatol Int 2009, 3, pp. 343-355. [CrossRef]
- Oba, U.; Koga, Y.; Hoshina, T.; Suminoe, A.; Abe, K.; Hayashida, M.; Taguchi, T.; Hara, T. An adolescent female having hepatocellular carcinoma associated with hepatitis B virus genotype H with a deletion mutation in the pre-S2 region. J Infect Chemother 2015, 21, pp. 302-304. [CrossRef]
- Zhang, X.Q.; Hong, X.J.; Bai, X.J. Susceptibility to active decompensated cirrhosis is associated with polymorphisms of intercellular adhesion molecule-1 (ICAM-1) in chronic HBV carriers. J Viral Hepat 2008, 15, pp. 173-178. [CrossRef]
- Sun, Y.; Lu, Y.; Xie, L.; Deng, Y.; Li, S.; Qin, X. Interferon gamma polymorphisms and hepatitis B virus-related liver cirrhosis risk in a Chinese population. Cancer Cell Int 2015, 15, 35. [CrossRef]
- Yu, S.K.; Kwon, O.S.; Jung, H.S.; Bae, K.S.; Kwon, K.A.; Kim, Y.K.; Kim, Y.S.; Kim, J.H. Influence of transforming growth factor-beta1 gene polymorphism at codon 10 on the development of cirrhosis in chronic hepatitis B virus carriers. J Korean Med Sci 2010, 25, pp. 564-569. [CrossRef]
- Yan, X.H.; Wu, J.L.; Yu, R.; Ma, X.H.; Li, Q.F.; Xie, R.F. Associations between gene polymorphisms of signal transducer and activator of transcription 3 and the susceptibility to hepatitis B virus related liver cirrhosis. Zhonghua Yu Fang Yi Xue Za Zhi 2022, 56, pp. 185-191. [CrossRef]
- Jiang, D.K.; Ma, X.P.; Wu, X.; Peng, L.; Yin, J.; Dan, Y.; Huang, H.X.; Ding, D.L.; Zhang, L.Y.; Shi, Z.; et al. Genetic variations in STAT4, C2, HLA-DRB1 and HLA-DQ associated with risk of hepatitis B virus-related liver cirrhosis. Sci Rep 2015, 5, 16278. [CrossRef]
- Liu, W.; Ma, N.; Zhao, D.; Gao, X.; Zhang, X.; Yang, L.; Liu, D. Correlation between the DEPDC5 rs1012068 polymorphism and the risk of HBV-related hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2019, 43, pp. 446-450. [CrossRef]
- Mai, H.; Xie, H.; Hou, J.; Chen, H.; Zhou, B.; Hou, J.; Jiang, D. A Genetic Variant of PPP1CB Influences Risk of Hepatitis B Virus-Related Hepatocellular Carcinoma in Han Chinese: A Pathway Based Analysis. J Hepatocell Carcinoma 2021, 8, pp. 1055-1064. [CrossRef]
- Zeisel, M.B.; Guerrieri, F.; Levrero, M. Host Epigenetic Alterations and Hepatitis B Virus-Associated Hepatocellular Carcinoma. J Clin Med 2021, 10. [CrossRef]
- [43] Jiang, J.H.; Gao, Q.; Shen, X.Z.; Yu, Y.; Gu, F.M.; Yan, J.; Pan, J.F.; Jin, F.; Fan, J.; Zhou, J.; et al. An X-chromosomal association study identifies a susceptibility locus at Xq22.1 for hepatitis B virus-related hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2013, 37, 586-595. [CrossRef]
- Tsai, T.Y.; Huang, M.T.; Sung, P.S.; Peng, C.Y.; Tao, M.H.; Yang, H.I.; Chang, W.C.; Yang, A.S.; Yu, C.M.; Lin, Y.P.; et al. SIGLEC-3 (CD33) serves as an immune checkpoint receptor for HBV infection. J Clin Invest 2021, 131. [CrossRef]
- Yoon, Y.J.; Chang, H.Y.; Ahn, S.H.; Kim, J.K.; Park, Y.K.; Kang, D.R.; Park, J.Y.; Myoung, S.M.; Kim, D.Y.; Chon, C.Y.; et al. MDM2 and p53 polymorphisms are associated with the development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Carcinogenesis 2008, 29, pp. 1192-1196. [CrossRef]
- Mardian, Y.; Yano, Y.; Wasityastuti, W.; Ratnasari, N.; Liang, Y.; Putri, W.A.; Triyono, T.; Hayashi, Y. Genetic polymorphisms of HLA-DP and isolated anti-HBc are important subsets of occult hepatitis B infection in Indonesian blood donors: a case-control study. Virol J 2017, 14, 201. [CrossRef]
- Wang, T.; Shen, C.; Chen, L.; Liu, S.; Ji, Y. Association of human leukocyte antigen polymorphisms with occult hepatitis B virus infection in a Shaanxi Han population. J Gene Med 2017, 19. [CrossRef]
- Wang, Y.; Han, S.B. Hepatitis B Reactivation: A Review of Clinical Guidelines. J Clin Gastroenterol 2021, 55, pp. 393-399. [CrossRef]
- Zhu, Y.; Li, H.; Wang, X.; Zheng, X.; Huang, Y.; Chen, J.; Meng, Z.; Gao, Y.; Qian, Z.; Liu, F.; et al. Hepatitis B Virus Reactivation Increased the Risk of Developing Hepatic Failure and Mortality in Cirrhosis With Acute Exacerbation. Front Microbiol 2022, 13, 910549. [CrossRef]
- Matsuda, H.; Hiramatsu, K.; Akazawa, Y.; Nosaka, T.; Saito, Y.; Ozaki, Y.; Hayama, R.; Takahashi, K.; Naito, T.; Ofuji, K.; et al. Genetic polymorphism and decreased expression of HLA class II DP genes are associated with HBV reactivation in patients treated with immunomodulatory agents. J Med Virol 2018, 90, pp. 712-720. [CrossRef]
- Hsiao, L.T.; Wang, H.Y.; Yang, C.F.; Chiou, T.J.; Gau, J.P.; Yu, Y.B.; Liu, H.L.; Chang, W.C.; Chen, P.M.; Tzeng, C.H.; et al. Human Cytokine Genetic Variants Associated With HBsAg Reverse Seroconversion in Rituximab-Treated Non-Hodgkin Lymphoma Patients. Medicine 2016, 95, e3064. [CrossRef]
- Lee, H.W.; Park, H.J.; Jin, B.; Dezhbord, M.; Kim, D.Y.; Han, K.H.; Ryu, W.S.; Kim, S.; Ahn, S.H. Effect of S267F variant of NTCP on the patients with chronic hepatitis B. Sci Rep 2017, 7, 17634. [CrossRef]
- Wu, J.F.; Chen, C.H.; Ni, Y.H.; Lin, Y.T.; Chen, H.L.; Hsu, H.Y.; Chang, M.H. Toll-like receptor and hepatitis B virus clearance in chronic infected patients: a long-term prospective cohort study in Taiwan. J Infect Dis 2012, 206, pp. 662-668. [CrossRef]
- Yao, Y.; Shen, Y.; Shao, H.; Liu, Y.; Ji, Y.; Du, G.; Ye, X.; Huang, P.; Chen, H. Polymorphisms of RIG-I-like receptor influence HBV clearance in Chinese Han population. J Med Virol 2021, 93, pp. 4957-4965. [CrossRef]
- Shen, Z.; Yang, H.; Yang, S.; Wang, W.; Cui, X.; Zhou, X.; Liu, W.; Pan, S.; Liu, Y.; Zhang, J.; et al. Hepatitis B virus persistence in mice reveals IL-21 and IL-33 as regulators of viral clearance. Nat Commun 2017, 8, 2119. [CrossRef]
- Wang, L.; Zou, Z.Q.; Wang, K. Clinical Relevance of HLA Gene Variants in HBV Infection. J Immunol Res 2016, 2016, 9069375. [CrossRef]
- Hu, H.H.; Liu, J.; Lin, Y.L.; Luo, W.S.; Chu, Y.J.; Chang, C.L.; Jen, C.L.; Lee, M.H.; Lu, S.N.; Wang, L.Y.; et al. The rs2296651 (S267F) variant on NTCP (SLC10A1) is inversely associated with chronic hepatitis B and progression to cirrhosis and hepatocellular carcinoma in patients with chronic hepatitis B. Gut 2016, 65, pp. 1514-1521. [CrossRef]
- Hu, P.; Liu, J.; Zhang D. Association of NTCP Gene Polymorphisms and Spontaneous Clearance of Hepatitis B Virus in Asia: A Meta-Analysis. Hepat Mon 2019; 19: pp. 2-8. [CrossRef]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1, e00049. [CrossRef]
- Yang, W.; Summers, J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor. J Virol 1999, 73, 9710-9717. [CrossRef]
- Xia, Y.; Guo, H. Hepatitis B virus cccDNA: Formation, regulation and therapeutic potential. Antiviral Res 2020, 180, 104824. [CrossRef]
- Kim, J.H.; Kang, S.; Kim, J.; Ahn, B.Y. Hepatitis B virus core protein stimulates the proteasome-mediated degradation of viral X protein. J Virol 2003, 77, pp. 7166-7173. [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009, 22, pp. 240-273, Table of Contents. [CrossRef]
- Li, Q.; Sun, B.; Zhuo, Y.; Jiang, Z.; Li, R.; Lin, C.; Jin, Y.; Gao, Y.; Wang, D. Interferon and interferon-stimulated genes in HBV treatment. Front Immunol 2022, 13, 1034968. [CrossRef]
- Liu, Q.; Zheng, Y.; Yu, Y.; Tan, Q.; Huang, X. Identification of HLA-A*0201-restricted CD8+ T-cell epitope C(6)(4)(-)(7)(2) from hepatitis B virus core protein. Int Immunopharmacol 2012, 13, pp. 141-147. [CrossRef]
- Lee, J.Y.; Chae, D.W.; Kim, S.M.; Nam, E.S.; Jang, M.K.; Lee, J.H.; Kim, H.Y.; Yoo, J.Y. Expression of FasL and perforin/granzyme B mRNA in chronic hepatitis B virus infection. J Viral Hepat 2004, 11, pp. 130-135. [CrossRef]
- Schreiber, S.; Honz, M.; Mamozai, W.; Kurktschiev, P.; Schiemann, M.; Witter, K.; Moore, E.; Zielinski, C.; Sette, A.; Protzer, U.; et al. Characterization of a library of 20 HBV-specific MHC class II-restricted T cell receptors. Mol Ther Methods Clin Dev 2021, 23, pp. 476-489. [CrossRef]
- [68] Asao, H. Interleukin-21 in Viral Infections. Int J Mol Sci 2021, 22. [CrossRef]
- [69] Wang, S.; Wang, J.; Kumar, V.; Karnell, J.L.; Naiman, B.; Gross, P.S.; Rahman, S.; Zerrouki, K.; Hanna, R.; Morehouse, C.; et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun 2018, 9, 1758. [CrossRef]
- Schillie, S.; Murphy, T.V.; Sawyer, M.; Ly, K.; Hughes, E.; Jiles, R.; de Perio, M.A.; Reilly, M.; Byrd, K.; Ward, J.W.; et al. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013, 62, pp. 1-19.
- Shi, Y.X.; Huang, C.J.; Yang, Z.G. Impact of hepatitis B virus infection on hepatic metabolic signaling pathway. World J Gastroenterol 2016, 22, pp. 8161-8167. [CrossRef]
- Hu, J.J.; Song, W.; Zhang, S.D.; Shen, X.H.; Qiu, X.M.; Wu, H.Z.; Gong, P.H.; Lu, S.; Zhao, Z.J.; He, M.L.; et al. HBx-upregulated lncRNA UCA1 promotes cell growth and tumorigenesis by recruiting EZH2 and repressing p27Kip1/CDK2 signaling. Sci Rep 2016, 6, 23521. [CrossRef]
- Li, Z.; Li, X.; Wu, S.; Xue, M.; Chen, W. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci 2014, 105, 951-955. [CrossRef]
- Lamontagne, R.J.; Casciano, J.C.; Bouchard, M.J. A broad investigation of the HBV-mediated changes to primary hepatocyte physiology reveals HBV significantly alters metabolic pathways. Metabolism 2018, 83, pp. 50-59. [CrossRef]
- Sadrolodabaee, L.; Low, T. K.; Feng, H.; Chen, W. N. Role of HBV Replication in Host Cell Metabolism: A Proteomics Analysis. Curr Proteomics 2013, 10, pp. 29-37. [CrossRef]
- Shin, H.J.; Park, Y.H.; Kim, S.U.; Moon, H.B.; Park, D.S.; Han, Y.H.; Lee, C.H.; Lee, D.S.; Song, I.S.; Lee, D.H.; et al. Hepatitis B virus X protein regulates hepatic glucose homeostasis via activation of inducible nitric oxide synthase. The Journal of biological chemistry 2011, 286, pp. 29872-29881. [CrossRef]
- Borchani-Chabchoub, I.; Mokdad-Gargouri, R.; Gargouri, A. Glucose dependent [correction of dependant] negative translational control of the heterologous expression of the preS2 HBV antigen in yeast. Gene 2003, 311, pp. 165-170. [CrossRef]
- Liu, B.; Fang, M.; He, Z.; Cui, D.; Jia, S.; Lin, X.; Xu, X.; Zhou, T.; Liu, W. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation. Cell Death Dis 2015, 6, e1980. [CrossRef]
- Kim, K.; Kim, K.H.; Kim, H.H.; Cheong, J. Hepatitis B virus X protein induces lipogenic transcription factor SREBP1 and fatty acid synthase through the activation of nuclear receptor LXRalpha. Biochem J 2008, 416, pp. 219-230. [CrossRef]
- Willy, P.J.; Umesono, K.; Ong, E.S.; Evans, R.M.; Heyman, R.A.; Mangelsdorf, D.J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev 1995, 9, pp. 1033-1045. [CrossRef]
- DeBose-Boyd, R.A.; Ye, J. SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond. Trends Biochem Sci 2018, 43, pp. 358-368. [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int J Mol Sci 2020, 21. [CrossRef]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 2009, 50 Suppl, pp. 138-143. [CrossRef]
- Marechal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARgamma Pathway in Metabolic Disorders. Int J Mol Sci 2018, 19. [CrossRef]
- Park, J.H.; Iwamoto, M.; Yun, J.H.; Uchikubo-Kamo, T.; Son, D.; Jin, Z.; Yoshida, H.; Ohki, M.; Ishimoto, N.; Mizutani, K.; et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 2022, 606, pp. 1027-1031. [CrossRef]
- Wang, X.; Xie, G.; Zhao, A.; Zheng, X.; Huang, F.; Wang, Y.; Yao, C.; Jia, W.; Liu, P. Serum Bile Acids Are Associated with Pathological Progression of Hepatitis B-Induced Cirrhosis. J Proteome Res 2016, 15, 1126-1134. [CrossRef]
- Lauber, C.; Seitz, S.; Mattei, S.; Suh, A.; Beck, J.; Herstein, J.; Borold, J.; Salzburger, W.; Kaderali, L.; Briggs, J.A.G.; et al. Deciphering the Origin and Evolution of Hepatitis B Viruses by Means of a Family of Non-enveloped Fish Viruses. Cell Host Microbe 2017, 22, pp. 387-399 e386. [CrossRef]
- Kocher, A.; Papac, L.; Barquera, R.; Key, F.M.; Spyrou, M.A.; Hubler, R.; Rohrlach, A.B.; Aron, F.; Stahl, R.; Wissgott, A.; et al. Ten millennia of hepatitis B virus evolution. Science 2021, 374, pp. 182-188. [CrossRef]
- Larsen, C.S. The past 12,000 years of behavior, adaptation, population, and evolution shaped who we are today. Proc Natl Acad Sci U S A 2023, 120, e2209613120. [CrossRef]
- Roman, S.; Jose-Abrego, A.; Fierro, N.A.; Escobedo-Melendez, G.; Ojeda-Granados, C.; Martinez-Lopez, E.; Panduro, A. Hepatitis B virus infection in Latin America: a genomic medicine approach. World J Gastroenterol 2014, 20, pp. 7181-7196. [CrossRef]
- Peacock, T.P.; Penrice-Randal, R.; Hiscox, J.A.; Barclay, W.S. SARS-CoV-2 one year on: evidence for ongoing viral adaptation. J Gen Virol 2021, 102. [CrossRef]
- Liang, T.J. Hepatitis B: the virus and disease. Hepatology 2009, 49, pp. 13-21. [CrossRef]
- Tu, T.; Budzinska, M.A.; Shackel, N.A.; Urban, S. HBV DNA Integration: Molecular Mechanisms and Clinical Implications. Viruses 2017, 9. [CrossRef]
- Roman, S.; Tanaka, Y.; Khan, A.; Kurbanov, F.; Kato, H.; Mizokami, M.; Panduro, A. Occult hepatitis B in the genotype H-infected Nahuas and Huichol native Mexican population. J Med Virol 2010, 82, pp. 1527-1536. [CrossRef]
- Jose-Abrego, A.; Roman, S.; Laguna-Meraz, S.; Rebello-Pinho, J.R.; Justo Arevalo, S.; Panduro, A. Tracing the evolutionary history of hepatitis B virus genotype H endemic to Mexico. Front Microbiol 2023, 14, 1180931. [CrossRef]
- Jaramillo, C.M.; de La Hoz, F.; Porras, A.; di Filippo, D.; Choconta-Piraquive, L.A.; Payares, E.; Montes, N.; Navas, M.C. Characterization of hepatitis B virus in Amerindian children and mothers from Amazonas State, Colombia. PloS one 2017.
- Wang, L.; Wang, K.; Zou, Z.Q. Crosstalk between innate and adaptive immunity in hepatitis B virus infection. World J Hepatol 2015, 7, 2980-2991. [CrossRef]
- Yamada, N.; Shigefuku, R.; Sugiyama, R.; Kobayashi, M.; Ikeda, H.; Takahashi, H.; Okuse, C.; Suzuki, M.; Itoh, F.; Yotsuyanagi, H.; et al. Acute hepatitis B of genotype H resulting in persistent infection. World J Gastroenterol 2014, 20, pp. 3044-3049. [CrossRef]
- Centers for disease control and prevention, 2020. The age-adjusted death rate for hepatitis C and Hepatitis B during 2020. Available online: https://www.cdc.gov/hepatitis/policy/npr/2022/reduce-reported-hepatitis-b-deaths.htm#:~:text=The%20age%2Dadjusted%20hepatitis%20B,the%20target%20rate%20of%200.42 (accessed on 4 July 2023).
- Centers for disease control and prevention, 2012. Viral Hepatitis and Liver Cancer. Available online: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/viral-hep-liver-cancer.pdf (accessed on 4 July 2023).
- World Health Organization, 2017. Guidelines on hepatitis B and C testing. Available online: https://www.who.int/publications/i/item/9789241549981(accessed on 4 July 2023).
- Forni, D.; Cagliani, R.; Pontremoli, C.; Pozzoli, U.; Vertemara, J.; De Gioia, L.; Clerici, M.; Sironi, M. Evolutionary Analysis Provides Insight Into the Origin and Adaptation of HCV. Front Microbiol 2018, 9, 854. [CrossRef]
| Clinical outcome and associated genes | Population | SNP database | OR | 95% CI | Reference |
|---|---|---|---|---|---|
| Cirrhosis | |||||
| Intercellular adhesion molecule-1 (ICAM-1) | China | rs1799969 (A) | 4.197 | 2.550-7.074 | [35] |
| Interferon gamma (IFN-γ) | China | rs2430561 (A) + rs1861494 (A) | 1.485 | 1.065-2.070 | [36] |
| Transforming growth factor (TGF)-beta1 | Korea | rs1982073 (LL) | 3.408 | 1.279-9.085 | [37] |
| Signal transducer and activator of transcription 3 (STAT3) | China | rs4796793 (GG) | 2.17 | 1.11-4.23 | [38] |
| Signal transducer and activator of transcription 4 (STAT4) | China | rs7574865 (TG) | 1.17 | 1.03–1.34 | [39] |
| Complement component 2 (C2) | China | rs9267673 (TC) | 1.37 | 1.15–1.63 | [39] |
| Human leukocyte antigen (HLA)-DRB1 | China | rs2647073 (CA) | 1.63 | 1.29–2.06 | [39] |
| Human leukocyte antigen (HLA)-DRB1 | China | rs3997872 (AT) | 1.86 | 1.32–2.62 | [39] |
| Human leukocyte antigen (HLA)-DQ | China | rs9275319 (GA) | 1.32 | 1.06–1.64 | [39] |
| Hepatocellular carcinoma | |||||
| Protein Phosphatase 1 Catalytic Subunit Beta (PPP1CB) | China | rs13025377 (AA) | 1.54 | 1.22-1.95 | [41] |
| Mouse double minute 2 homolog (MDM2) | Korea | rs2279744 (G) | 4.27 | 2.23-8.20 | [45] |
| Tumor protein 53 (p53) | Korea | rs1042522 (Pro/Pro) | 3.59 | 1.77-7.31 | [45] |
| MDM2 + p53 | Korea | rs2279744 (G/G) + rs1042522 (Pro/Pro) | 20.78 | 5.25-82.36 | [45] |
| DEP Domain Containing 5 (DEPDC5) | China | rs1012068 (CC) | 2.397 | 1.251-4.595 | [40] |
| X-chromosome long arm band 22.1 (Xq22.1) | China | rs5945919 (AG) | 2.22 | 1.15-4.30 | [43] |
| CD33 molecule (SIGLEC3 or CD33) | Taiwan | rs12459419 (C) | 1.256 | 1.027-1.535 | [44] |
| Occult hepatitis B | |||||
| Human leukocyte antigen (HLA)-DPA1 | Indonesia | rs3077 (CC) | 6.12 | 1.30-28.85 | [46] |
| Human leukocyte antigen (HLA)-DPA1 | Indonesia | rs3077 (T) + rs3135021(G) + rs9277535 (A) | 4.9 | 1.12-21.52 | [46] |
| Human leukocyte antigen (HLA)-DQB1 | China | NA | 2.15 | 1.118-4.161 | [47] |
| Human leukocyte antigen (HLA)-HLA-C*07:01 | China | NA | 2.146 | 1.070-4.306 | [47] |
| Human leukocyte antigen (HLA)-B*44:03 | China | NA | 4.693 | 1.822-12.086 | [47] |
| Human leukocyte antigen (HLA)-DRB1*07:01 | China | NA | 1.919 | 1.188-3.101 | [47] |
| Human leukocyte antigen (HLA)-DQB1*02:02 | China | NA | 2.012 | 1.303-3.107 | [47] |
| HBV reactivation | |||||
| Human leukocyte antigen (HLA)-DPB2 | Japan | rs872956 (AA) | 8.277 | 1.540-51.550 | [50] |
| Interleukin 13 (IL-13) | Taiwan | rs1295686 (AA) | 4.683 | 1.030-19.156 | [51] |
| Clearance or protection | |||||
| Sodium taurocholate cotransporting polypeptide (NTCP) | Korea | rs2296651(CT) | 0.455 | 0.220-0.942 | [52] |
| Toll-like receptor (TLR5) | Taiwan | rs5744174 (T) | 1.32 | 1.03-1.69 | [53] |
| Interferon-induced helicase C domain-containing protein 1 (IFIH1) | China | rs2111485 (G) | 0.47 | 0.25-0.87 | [54] |
| DExD/H-box helicase 58 (DDX58) | China | rs3824456 (C) or rs2074160 (A) | 0.69 | 0.49-0.97 | [54] |
| Sodium taurocholate cotransporting polypeptide (NTCP) | Taiwan | rs2296651(AA) | 0.13 | 0.05-0.34 | [57] |
| NA: Not available; letters in parentheses indicate allele or genotype; OR: Odds ratio; 95% CI: 95% confidence interval | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





