Submitted:
15 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media for Strain Activation
2.2. Antifungal Activity of Bacillus
2.3. Growth Curves of Bacillus Strains in Different Carbon Sources
2.4. Co-Cultures of Bacillus and Phytopathogen Fungi
2.5. Quantification of Plant Growth Regulators Produced by Bacillus subtilis subsp. spizizenii
2.6. Phosphate Solubilization in Liquid Medium
2.7. Growth Promotion Assays
2.8. Seed Germination Assay
2.9. Greenhouse Assays
2.10. Statistical Analysis
3. Results
3.1. Bacillus As a Biocontrol Agent
3.1.1. Antifungal Activity of Liquid Culture Supernatants of Bacillus subtilis subsp. spizizenii and Bacillus subtilis var. natto
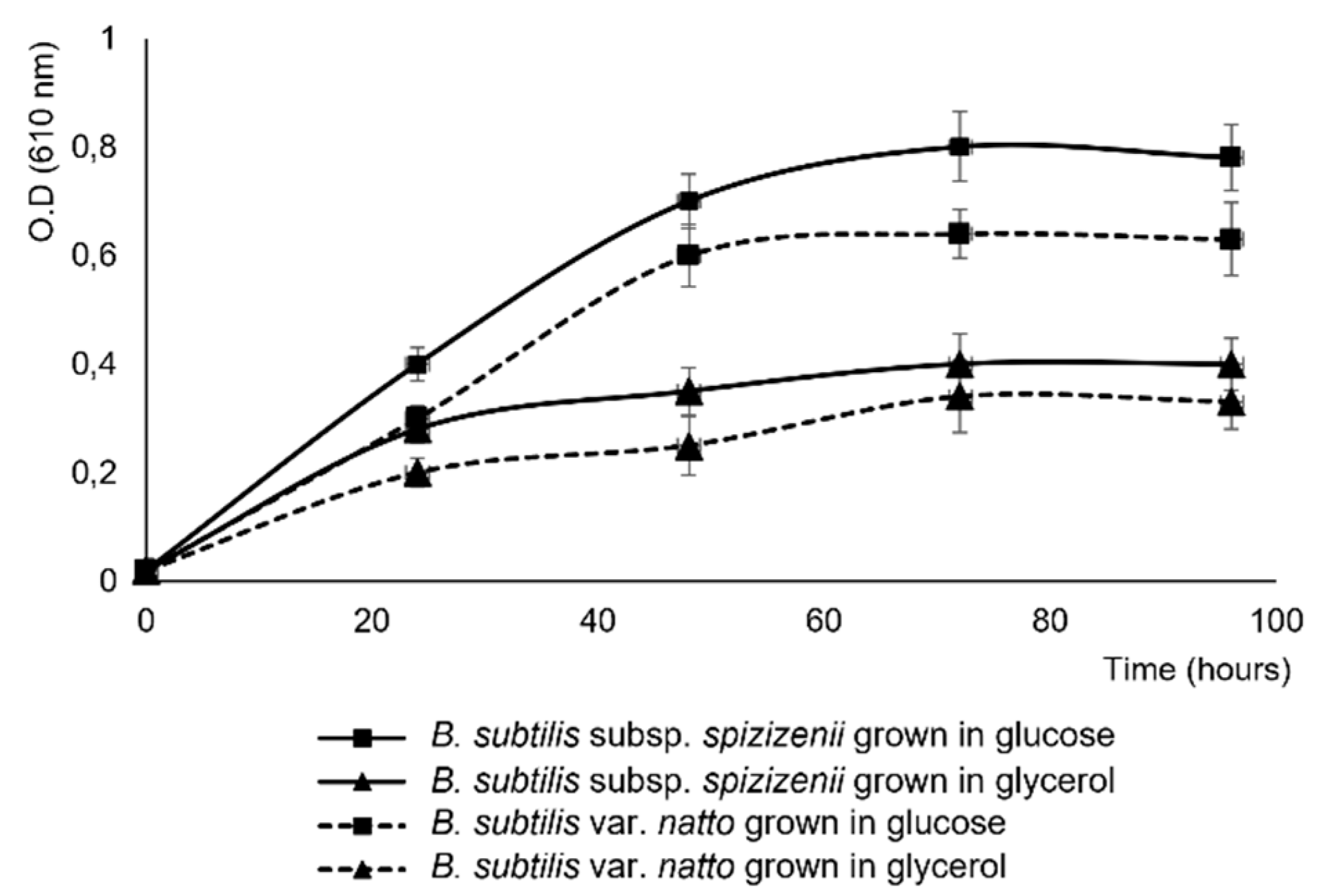
3.1.2. Growth and Antifungal Activity of Bacillus subtilis subsp. spizizenii and Bacillus subtilis var. natto in Simple Liquid Media
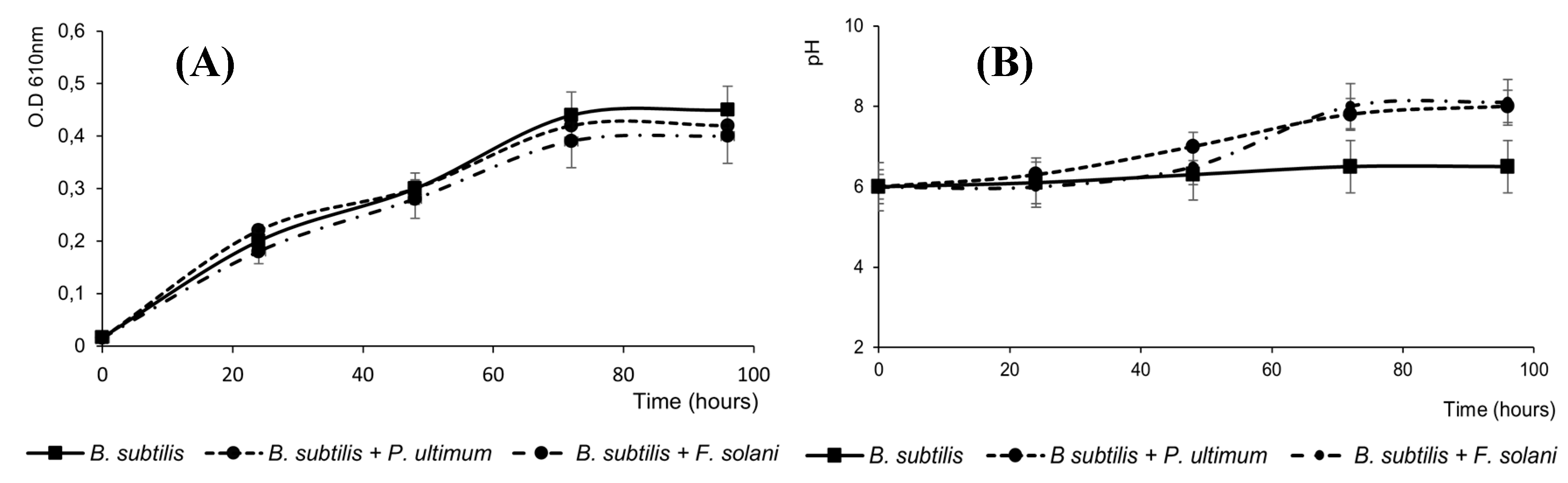
3.1.3. Effect of Co-Culture of Bacillus subtilis subsp. spizizenii with Phytopathogenic Fungi
3.2. Biofertilization Mechanisms
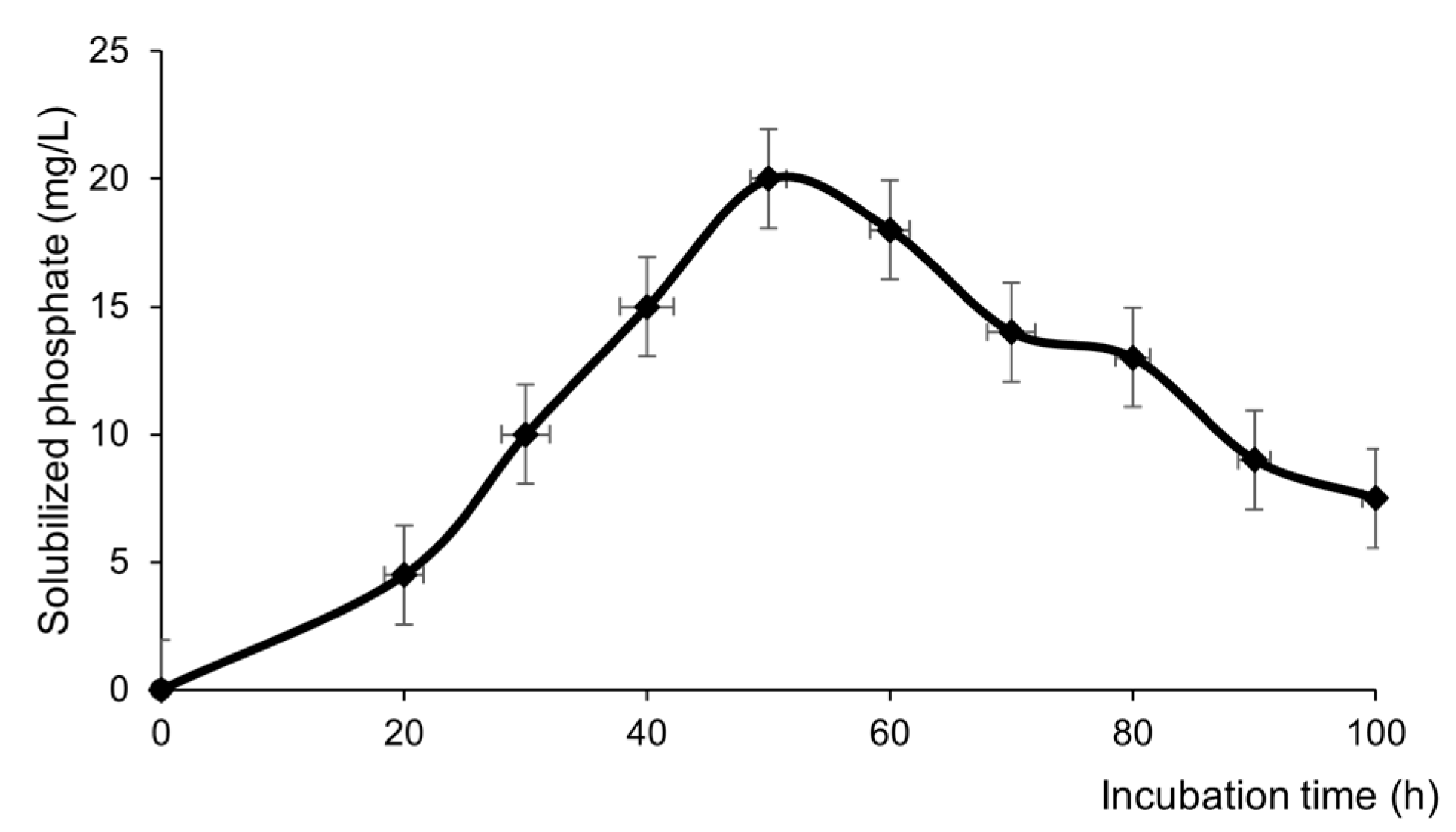
3.2.1. Quantitative Evaluation of Inorganic Phosphorus Solubilization by B. subtilis subsp. Spizizenii
3.2.2. Synthesis of Plant Growth Regulators by Bacillus subtilis subsp. Spizizenii
3.3. Effects of Bacillus subtilis subsp. Spizizenii Inoculation As Planktonic Form or Biofilm on Three Varieties of Lactuca sativa
3.3.1. Effects on Seed Germination
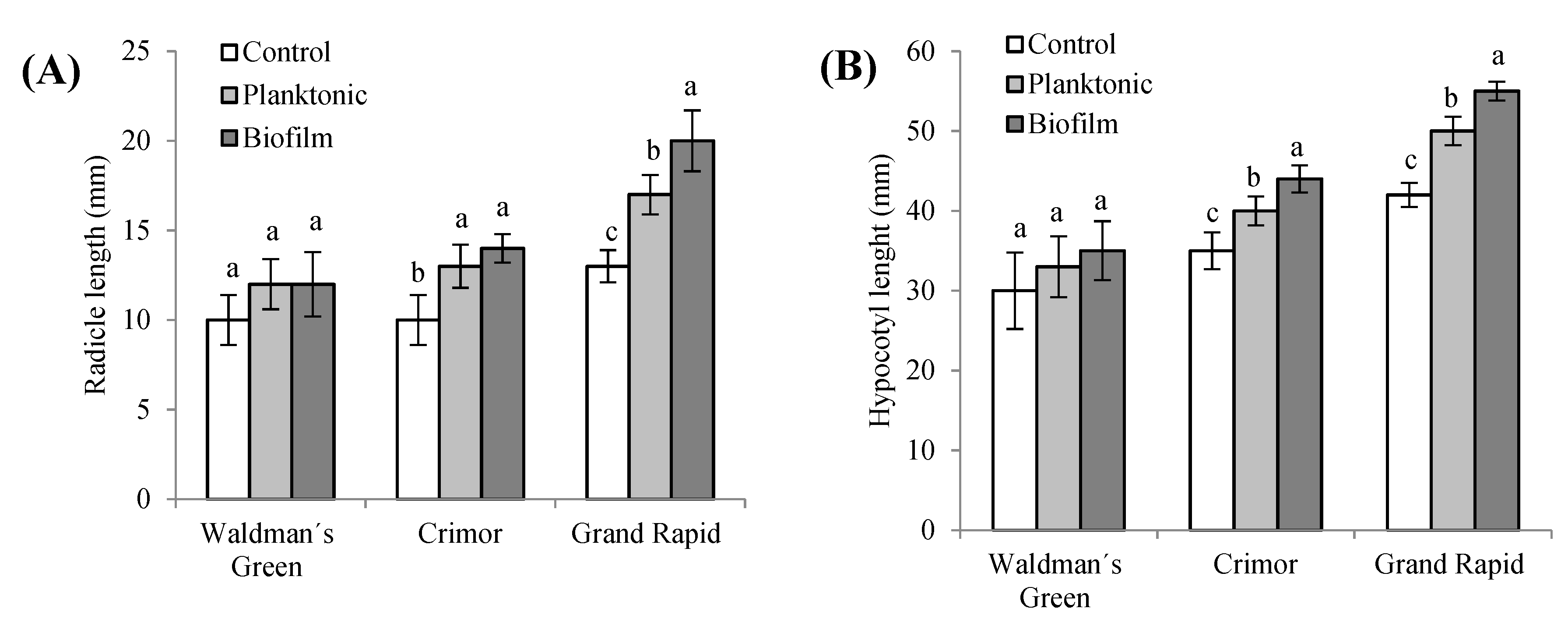
3.3.2. Effects on Seedlings
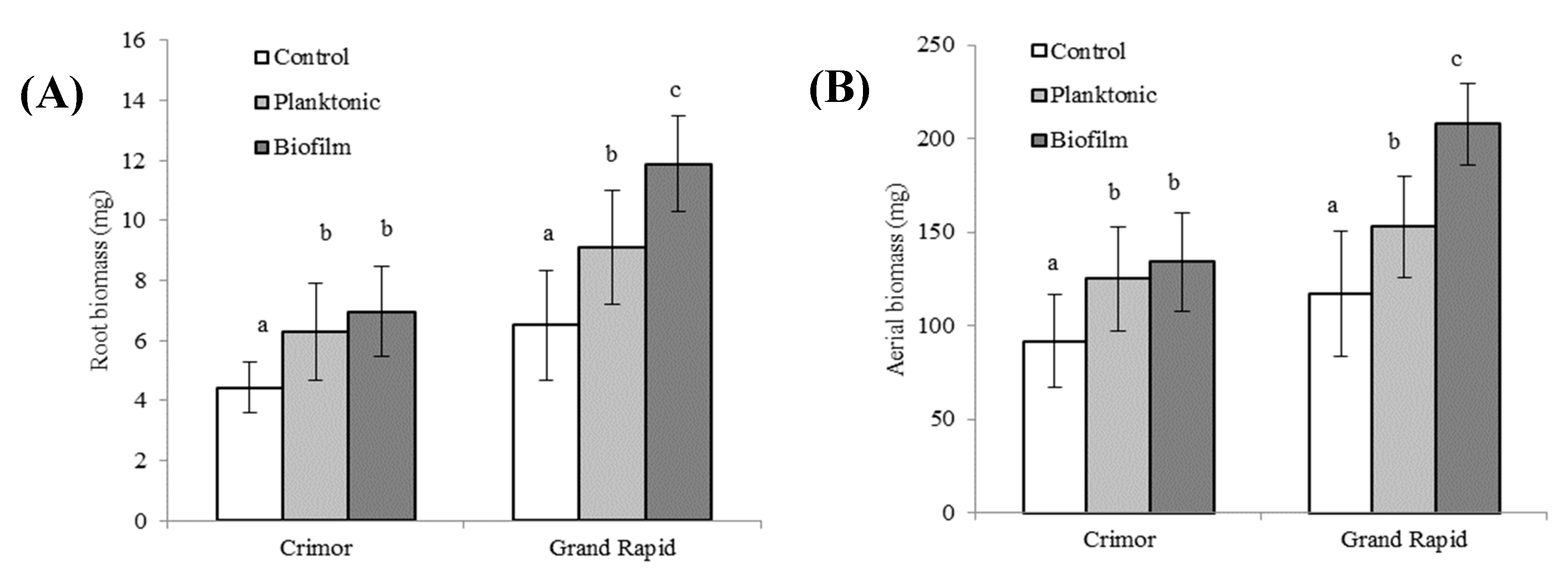
3.3.3. Effect on the Growth of 25-Day-Old Plants of the Crimor and Grand Rapid Varieties
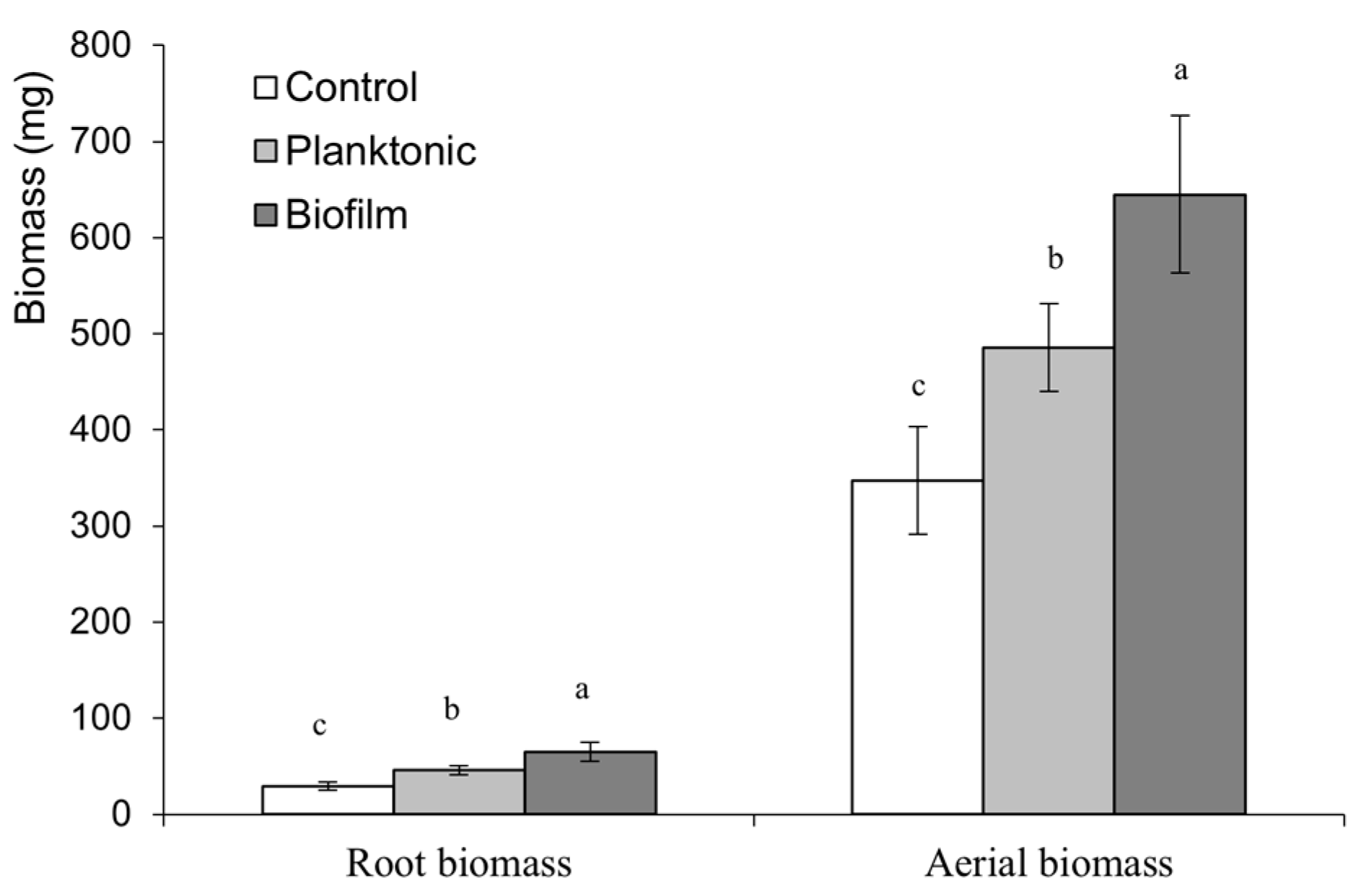
3.3.4. Effect on the Growth of Plants of the Grand Rapid Variety at Harvest Time
3.4. Bacterial Endophytism
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giuffré, L.; Ratto, S.; Marbán, L.; Schonwald, J.; Romaniuk R. Riesgo por metales pesados en horticultura urbana. Cienc. suelo, Argentina. 2005, 23, 1, 101-106.
- Crispo, M.; Dobson, M.C.; Blevins, R.S; Meredith, W.; Lake, J.A. Heavy metals and metalloids concentrations across UK urban horticultural soils and factors influencing their bioavailability to food crops. Environ. pollut. 2001, 288, 117960. [CrossRef]
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, E.A.; Elsiddig; H.; Haberl, R.; Harper, J.; House, M.; Jafari, O.; Masera, C.; Mbow, N.H.; Ravindranath, C.W.; Rice, C.; Robledo Abad, A.; Romanovskaya, F.; Sperling, F.; Tubiello, J. Agriculture, Forestry and Other Land Use (AFOLU). In: Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Edenhofer, O., R. Pichs-Madruga, Y. Sokona, E. Farahani, S. Kadner, K. Seyboth, A. Adler, I. Baum, S. Brunner, P. Eickemeier, B. Kriemann, J. Savolainen, S. Schlömer, C. von Stechow, T. Zwickel and J.C. Minx (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA. 2014.
- Lima, L.; Ongaratto, F.; Fernandes, M.; Cardoso, A.; Lage, J.; Silva, L.; Reis, R.; Malheiros, E. Response of pasture nitrogen fertiliation on greenhouse gas emission and net protein contribution of nellore Young Bulls. Animals. 2022, 12, 3173. [CrossRef]
- Sammauria, S.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial inoculants: potential tool for sustainability of agricultural production systems. Arch. Microbiol. 2020, 202, 677–693. [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 6, 1583–1594. [CrossRef]
- Pérez, J.; Carmona, S.; Zamudio, E.; Rivera, N.; Calva G. Bioremediation of soils from oil spill impacted sites using biosurfactants producing, native, free-living nitrogen fixing bacteria. Rev. Int. de Contam. Amb. 2017, 33, 105-114. [CrossRef]
- Gomez Ramirez, L.F.; Uribe Velez, D. Phosphorus solubilizing and mineralizing Bacillus spp. Contribute to rice growth promotion using soil amended with rice Straw. Current Microbiol. 2021, 78, 932-943. [CrossRef]
- Das, P.P.; Singh, K.R.B.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.H.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 1, 113821. [CrossRef]
- Suzuki, H.; Park, S. Okubo, K.; Kitamura, J.; Ueguchi-Tanaka, M.; Iuchi, S,; Katoh, E.; Kobayashi, M.; Yamaguchi, I.; Matsuoka, M.; Asami, T.; Nakajima, M. Differential expression and affinities of Arabidopsis giberellins receptors can explain variation in phenotypes of multiple knock-out mutants. The Plant Journal. 2009, 60, 48-55. [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Frontiers of Sustainable Food Systems. 2021, 4. [CrossRef]
- Sarti, G.; Miyazaki, S. Actividad antifúngica de extractos crudos de Bacillus subtilis contra fitopatógenos de soja (Glycine max) y efecto de su coinoculación con Bradyrhizobium japonicum. Agrociencia. 2013, 47, 373-383.
- Mekonnen, H.; Kibret, M. The roles of plant growth promoting rhizobacteria in sustainable vegetable production in Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 15. [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular Microbiology. 2005, 56, 4, 845-857. [CrossRef]
- Miao, S.; Liang, J.; Xu, Y.; Yu, G.; Shao, M. Bacillaene, Sharp objects consist in the arsenal of antibiotics produced by Bacillus. Cell. Physio. 2022. [CrossRef]
- Sarti, G.; Miguez Cristóbal, J.; Curá, A. Optimización de las condiciones de cultivo para el desarrollo de una biopelícula bacteriana y su aplicación como biofertilizante en Solanum lycopersicum L.var. Río grande. Rev. Protección Veg. Cuba. 2019, 34, 2224-4697.
- Reichard, C.; Parsek, M. Confocal laser scanning microscopy for analysis of Pseudomonas aeruginosa biofilm architecture and matrix localization. Front. Microbiol. 2019, 10, 677. [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thumheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends in Microbiol. 2020, 28, 8, 668-681. [CrossRef]
- Billings, N.; Ramirez Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Pseudomonas aeruginosa Biofilms. PLoS Pathog. 2013, 9, 8, e1003526. [CrossRef]
- Daboor, S.; John, R.; Rohde, A.; Zhenyu, C. Disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate lyase enhances pathogen eradication by antibiotics. J. Cyst. Fibros. 2020, 30, 4-11. [CrossRef]
- Viteri, M.L.; Ghezán, G.; Iglesias, D. Tomate y lechuga: producción comercialización y consumo. Estudio socioeconómico de los sistemas: INTA, Balcarce, Argentina. 2013, 14, 185-190.
- Gerhardt, P.; Murray, R.; Wood, W.; Krieg, N. Methods of general and molecular Bacteriology. American Society for Microbiology. Washington DC. 1994.
- Dobrev, P.; Kaminek, M. Fast and Efficient Separation of Cytokinins from Auxin and Abscisic Acid and Their Purification. J. Chromatogr. A. 2002, 950, 21-29. [CrossRef]
- Nautiyal, S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms C. FEMS Microbiol. Lett. 1999, 170, 265-270. [CrossRef]
- Kitson, R.; Mellon, M.Colorimetric Determination of Phosphorus as molybdivanadophosphoric acid. Industrial and Engineering. Chem. Anal. Edit. 1994, 16, 6, 379-383. [CrossRef]
- Frioni, L. Ecología Microbiana del Suelo. Dpto. de Publ. y Ed. de la Univ. de la Rep., Montevideo Uruguay. 1990, pp 90-94.
- Marti, L. Efecto de la salinidad y de la temperatura en la germinación de semillas de Linonium mansaltrarum. Tesis doctoral, Universidad Politécnica Superior de Valencia. España, Gandía. 2010.
- Araya, E.; Gómez, L.; Hidalgo, N.; Valverde, R. Efecto de la luz y del ácido giberélico sobre la germinación in vitro de (Alunus Acuminata). Agron. Costarricense. 2000, 24, 1, 75-80.
- Kloepper, J.; Zablotowicz, R. Tipping, E.; Lifshitz, R. Plant growth promotion mediated by bacterial rhizosphere colonizer. D.L. Keister y P.B. Cregan (eds.) The rhizosphere and plant growth. Kluwer. Dordrecht. The Netherlands. 1991, 315-326.
- Walker, R.; Powell, A.; Seddon, B. Bacillus isolates from the spermosphere of peas and dwarf French beans with antifungal activity against Botrytis cinerea and Pythium species. J. Applied Microbiol. 1998, 84,791-801. [CrossRef]
- Sullivan, E. Molecular genetics of biosurfactants production. Curr. Opin. Biotech. 1998, 9, 263-269. [CrossRef]
- Hultberg, M.; Alsberg, T.; Khalil, S.; Alsanius, B. Suppression of disease in tomato infected by Pythium ultimum with a biosurfactant produced by Pseudomonas koreensis. Biocontrol. 2010, 55, 3, 435-444. [CrossRef]
- Ambrico, A.; Trupo, M. Efficacy of cell free supernatant from Bacillus subtilis ET-1, an Iturin a producer strain, on biocontrol of green and gray mold. Postharvest Biol. Technology. 2017, 134, 5-10. [CrossRef]
- Cornea, C.; Grebenisan, I.; Mateescu, R.; Campeanu, E. Isolation and characterization of new Bacillus spp. Strains useful as biocontrol agents of plants pathogens. Roumanian Biotechnol. Letters.2003, 8, 1, 1115-1122.
- Mayak, S.; Tirosh, T.; Glick, B. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and pepper. Plant Science. 2004, 166, 525-530. [CrossRef]
- Turan, M.; Melek, E.; Ertan, Y.; Adem, G.; Kenan, K.; Recep, K.; Atilla, D. Plant growthpromoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Turk. J. Agricul. For. 2014, 38, 327-333. [CrossRef]
- Valero, N. Potencial biofertilizante de bacterias diazotróficas y solubilizadoras de fosfatos asociadas al cultivo de arroz (Oryza sativa L.) Tesis de Maestría. Universidad Nacional de Colombia. 2003.
- Ahmed, A.; Hasnain, S. Auxin-producing Bacillus sp.: Auxin quantification and effect on the growth of Solanum tuberosum. Pure Appl Chem. 2010, 82, 1, 313-319. [CrossRef]
- Vikram, A. Efficacy of phosphate solubilizing bacteria isolated from vertisols on growth and yield parameters of sorghum. Res. J. Microbiol. 2007, 2, 550-559.
- Ortíz-Castro, R.; Contreras-Cornejo, H. Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009. [CrossRef]
- Barreto, D.; Valero, N.; Muñoz, A.; Peralta, A. Efecto de microorganismos rizosféricos sobre germinación y crecimiento temprano de Anacardium excelsum. Zonas Áridas 2007 11, 1, 240-250.
- Galelli, M.; Sarti, G.; Miyazaki, S. Lactuca sativa biofertilization using biofilm from Bacillus with PGPR activity. J. Appl. Hortic. 2015, 17,2, 186-191.
- Hobley, L. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649-669. [CrossRef]
- González, F.; Fuentes, N. Mecanismo de acción de cinco microorganismos promotores de crecimiento vegetal. Rev. Col. de Ciencias Agr. 2016, 34,1, 17-22.
- Ogata, K.; Arellano, C.; Zúñiga, D. Efecto de diferentes bacterias aisladas de rizósfera de Caesalpina spinosa en la germinación de diferentes especies vegetales culivados. Zonas Áridas 2008, 12, 1, 137-153.
- Dakora, F.; Phillips, D. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil. 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Díaz Vargas, P.; Ferrera-Cerrato, R.; Almaraz-Suárez, J.; Alcántar, G. Inoculación de bacterias promotoras de crecimiento en lechuga. Terra. 2001, 4, 231–238. [Google Scholar]
- Varela, S.; Arana, V. Latencia y germinación de semillas. Tratamientos pregerminativos. INTA Bariloche, Silvicultura en Vivero. Cuadernillo Nº 3. 2011, 3-10.
- Pereira, J.; Cavalcante, V.; Baldani, J.; Dobereiner, J. Sorghum and rice inoculation with Azospirillum sp. and Herbaspirillum seropedicae in field. Plant Soil. 1988, 110, 269-274. [CrossRef]
- Wulff, E.; van Vuurde, L.; Hockenhull, J. The ability of the biological control agent Bacillus subtilis, strain BB, to colonise vegetable brassicas endophytically following seed inoculation. Plant and Soil. 2003, 255, 463-474. [CrossRef]
- Kloepper, W.; Wei, G.; Tuzun, S. Rhizosphere population dynamics and internal colonisation of cucumber by plant growth-promoting rhizobacteria which induce systemic resistance to Colletotrichum orbiculare. In Biological Control of Plant Diseases. Ed. E C Tjamos, G C Papavizas and R J Cook. 1992; pp. 185-191. [CrossRef]
- Lamb, T.; Tonkyn, D.; Kluepfel, D. Movement of Pseudomonas aerofaciens from the rhizosphere to aerial plant tissue. Can. J. Microbiol. 1996, 42, 112-1120.
- Jacobs, M.; Bugbee, W.; Gabrielson, D. Enumeration, location, and characterization of endophytic bacteria within sugar beet roots. Can. J. Bot. 1985, 63, 1262–1265. [Google Scholar] [CrossRef]
| Inhibition halo diameter (mm) | |||||
|---|---|---|---|---|---|
| Bacteria | Fungus | Incubation time (h) | |||
| 24 | 48 | 72 | 96 | ||
| B. subtilis subsp. spizizenii | F. solani | ND | 6,7 ± 1,8 b | 9,3 ± 1,5 b | 15,9 ± 2,1 a |
| P. ultimum | ND | 6,8± 2,2 b | 8,4 ± 1,8 b | 16,2 ± 1,4 a | |
| B. subtilis var. natto | F. solani | ND | 3,1± 1,8 a | 4,2 ± 2,3 a | 5,8 ± 2,1 a |
| P. ultimum | ND | 2,9± 1,1 a | 3,8 ± 1,0 a | 4,6 ± 1,3 a | |
| Inhibition halo diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Culture medium | Bacteria | Fungus | Incubation time (h) | ||||
| 24 | 48 | 72 | 96 | ||||
| Glycerol 1% | B. subtilis subsp. spizizenii | F. solani | ND | ND | 10,2 ± 0,7 a | 15,6 ± 1,2 b | |
| P. ultimum | ND | ND | 12,3 ± 1,1 a | 16,0 ± 0,8 b | |||
| B. subtilis var. natto | F. solani | ND | ND | 5,1 ± 1,4 a | 5,3 ± 1,7 a | ||
| P. ultimum | ND | ND | 6,2 ± 0,9 a | 5,9 ± 1,2 a | |||
| Glucose 1% | B. subtilis subsp. spizizenii | F. solani | ND | ND | 11,2 ± 0,8 a | 14,1 ± 1,1 b | |
| P. ultimum | ND | ND | 13,3 ± 1,1 a | 13,9 ± 2,1 a | |||
| B. subtilis var. natto | F. solani | ND | ND | 4,3 ± 1,8 a | 3,5 ± 1,3 a | ||
| P. ultimum | ND | ND | 5,2 ± 2,3 a | 4,3 ± 1,2 a | |||
| Inhibition halo diameter (mm) | |||||
|---|---|---|---|---|---|
| Culture | Fungus | Incubation time (h) | |||
| 24 | 48 | 72 | 96 | ||
| B. subtilis subsp. Spizizenii | F. solani | ND | ND | 12,2 ± 0,9 a | 15,2 ± 1,2 b |
| P. ultimum | ND | ND | 10,0 ± 1,1 a | 15,4 ±1,7 b | |
| Coculture of B. subtilis subsp. Spizizenii + F. solani | F. solani | ND | ND | 11,2 ± 1,5 a | 11,3 ± 1,1 a |
| P. ultimum | ND | ND | 13,8 ± 1,2 a | 14,1 ± 0,8 a | |
| Coculture of B. subtilis subsp. Spizizenii + P. ultimum | F. solani | ND | ND | 10,9 ± 1,8 a | 14,9 ± 2,1 b |
| P. ultimum | ND | ND | 9,8 ± 1,9 a | 14,7 ± 1,5 b | |
| IAA | tZ | tZR | ABA |
|---|---|---|---|
| 0.38 ± 0.14 | 0.14 ± 0.05 | ND | 0.29 ± 0.05 |
| Germination (%) at 4 days | Germination (%) at 7 days | |||||
|---|---|---|---|---|---|---|
| L. sativa varieties | Control | Planktonic inoculum | Biofilm | Control | Planktonic inoculum | Biofilm |
| Waldman´s Green | 97,3 ± 0,8 a | 96,2 ± 1,4 a | ND | 97,3 ± 0,9 a | 96,2 ± 1,4 a | 96,4 ± 1,1 a |
| Crimor | 80,2 ± 1,3 a | 90,1 ± 2,0 b | ND | 80,2 ± 1,3 a | 90,1 ± 2,0 b | 94,2 ± 2,3 b |
| Grand Rapid | 96,3 ± 1,1 a | 97,1 ± 1,5 a | ND | 96,3 ± 1,4 a | 97,1 ± 0,8 a | 97,3 ± 0,9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





