Introduction
The autopsy room is a sensitive and high-risk sections of the hospital unit. Globally, microbial profiles of autopsy rooms are not well documented. However, diverse microbial populations have been associated with autopsy procedures [
1]. This has increasingly contibuted to the occupational risks of the autopsy section of the hospital. Pollution of indoor air, including autopsy room can be attributed to bacteria and airborne fungi, especially filamentous fungi that grow in moist indoor environments [
2]. Fungi are a threat to public health, especially those implicated in diseases including species of
Aspergillus, Candida, and
Cryptococcus [
3,
4]. These fungi were recently described as WHO Critical Fungal Pathogens with high levels of resistance to antimicrobial agents [
5].
Fungal infections are on the rise, with an estimated 150 million severe cases resulting in 1.7 million annual death [
6]. Humans are exposed to pathogenic fungi via dispersed
Aspergillus spores in air or
Penicillium moulds in food [
7]. Pathogenic fungi pose a higher risk to immunocompromised patients with cancer, tuberculosis, diabetes, HIV/AIDS and secondary infections [
8,
9]. In Ghana, about 4% of the population suffer from severe fungal infections including chronic pulmonary and invasive aspergillosis, asthma and pneumocystis pneumonia, candidiasis and vulvovaginal annually [
10,
11]. Also, Cryptococcal meningitis,
Pneumocystis jirovecii pneumonia and histoplasmosis affect AIDS patients [
11]. This has contributed to fungi-associated public health-risks in Ghana, especially with fungal increasing resistance to antimicrobials.
In West African countries including Ghana, there is little or no information on the fungal profiles of autopsy units. In a referral hospital autopsy unit in Accra-Ghana, there was a case report of possible exposure of workers to microbial agents, especially airborne pathogenic fungi which could cause infections. This study, therefore, profiled the airborne fungi within a Ghanaian referral hospital autopsy unit and provide insights into their antimicrobial resistance.
Materials and Methods
Study Design and Sample collection
A referral hospital autopsy unit in Accra, Ghana was profiled for fungi. The autopsy (10 x 17 meters) has different compartments including the autopsy suites, staff offices, reception, secretariat and washrooms. There is an average of twenty working staff with equal and unrestricted access to the autopsy suites to perform autopsy. The autopsy rooms are disinfected with Denzal® germicide before and after autopsy. Ten air samples were collected (in duplicate with two per section) under aseptic condition using the standard open plate technique previously described [
12]. Briefly, sterile plates containing Saboraud Dextrose agar (SDA) were placed in different sections of the autopsy unit (offices, reception, washroom, secretariat, autopsy/postmortem room, dissection room). The plates were opened for 1-3 h during postmortem session, with sealed or unopened plate as controls. These plates were aseptically transported to the laboratory on ice for analysis at the Molecular Biology Unit, Department of Biochemistry, Cell and Molecular Biology, University of Ghana. The agar plates were incubated at 25 °C for 5-7 days.
Characterization of Fungal Isolates
Phenotypic and Morphological Characterization
Characteristic fungal colonies were isolated, successively sub-cultured and morphologically identified based on shape, form, elevation, margin and colour as displayed by the isolates on the SDA plates (S1). This was followed by lactophenol cotton blue (0.1 mg cotton blue, 40 mg phenol crystals, 80 ml Glycerol, 40 ml distilled water, 40 ml lactic acid) staining. Briefly, a small portion of the mycelium of the fungal culture was placed on a sterile slide with drops of lactophenol blue and carefully covered with a clean sterile coverslip. The stain was allowed to stand at room temperature for 3-5 min and examined under a light microscope at 40X magnification (S1).
DNA Extraction and Internal Transcribed Spacer PCR Amplification
Guanidine Hydrochloride (GHCl) DNA extraction method was used. Briefly, fungal cultures were suspended in sterile distilled water, centrifuged (5,600 rpm, 5 min) and transferred to a tube containing 450 µl of lysis solution (1 M Tris of pH 8, 0.5 M EDTA, 0.5 M NaCl, 10% SDS, distilled water, RNase A). The sample was bead beat with 50 mg of silica beads solution in a Disruptor Genie™ (Scientific Industries, US. Pat. 5707861) for 15 min, incubated (65 °C, 20 min) and centrifuged (2 min, 5,600 g). 400 µl of the supernatant was added to 150 µl of 5 M potassium acetate in a 2 ml microcentrifuge tube and cooled (1 h, -20 °C). This was further centrifuged (30 min, 5,600 g) and 400 µl of the supernatant added to 600 µl of 1 M GHCl. 700 µl of the mixture was transferred to a spin filter collection tube and centrifuged (2 min, 5,600 g). The flow-through discarded and 500 µl of wash buffer (1 M Tris of pH 8.0, 0.5 M EDTA, 5 M NaCl, absolute ethanol, distilled water) was added and centrifuged (2 min, 5,600 g). 500 µl of ice-cold ethanol was added and centrifuged (5,600 g, 2 min). This was incubated at 25 °C for 15 min and DNA was eluted with 200 µl of sterile distilled water. ITS regions within fungal isolates was PCR profiled using ITS-1 (5’ TCCGTAGGTGAACCTGCGG 3’), ITS -2 (5’ GCTGCGTTCTTCATCGATGC 3’), ITS-4 (5’ TCCTCCGCTTATTGATATGC 3’) and ITS-5 primers (5’ GGAATAAAAGTCGTAACAAGG 3’). A total reaction of 15 µl included 8.4 µl of OneTaq 2X, 0.4 µl of each primer, 3.8 µl of nuclease-free water and 2 µl of template DNA. The reaction was run in a thermocycler (Biometra-T professional TRIO Thermocycler, Sheffield, UK) at an initial denaturation (94 °C, 5 min), followed by 35 cycles of denaturation (94 °C, 45 s), annealing (50 °C, 1 min), final and a final extension (72 °C, 10 min). The PCR products were resolved on ethidium bromide stained 1.5% agarose gel electrophoresis (100 V for 35 min) and visualized by UV on an Amersham™ Imager 600 (S1). The amplicons purified with a QIAquick PCR kit before Sanger sequencing (Eurofins Genomics, India) and isolates identified using NCBI BLAST algorithm.
Antifungal and Disinfectant Resistance Profiling
Broth micro dilution was performed in increasing two-fold concentrations.10 ug/ml, 20 ug/ml and 40 ug/ml concentrations of fluconazole and itraconazole were tested against RPMI standardized fungi culture. The fungal strains were also tested against the recommended working concentration (0.1:6.4 v/v); also, double (0.2:6.4 v/v), triple (0.3:6.4 v/v) and quadruple (0.4:6.4 v/v) concentrations of Denzal® germicide routinely used for autopsy cleaning. To the test wells of a 96-well plate, 100 µl of the RPMI standardized inoculum was added to 100 µl of the antifungal or germicide, and the test was conducted in triplicates in two independent experiments. A control without the antifungal or germicide, containing 100 µl of the test strain and 100 µl of RPMI was included and incubated (25 °C, 24-48 h, 150 rpm). After incubation, resistance profile was determined by measuring absorbance at 600 nm using a Varioskan™ LUX multimode microplate reader. Data were interpreted according to CLSI standards for antifungal agents and resistance expressed as a percentage of the test to the controls [
13].
Statistical Analysis
Data were processed with MS Excel (version) and presented in graphs/tables using descriptive statistics (with SPSS 16.0 and GraphPad 6.0). The data were subjected to simple t-test and one-way ANOVA with significance level at p < 0.05.
Results and Discussion
Autopsy Air Harbors Diverse Fungi Isolates
Fifteen fungal isolates of four genera (Yeast,
Mucor, Aspergillus and
Penicillium) were obtained and identified from the ten air samples collected (
Figure 1). Yeast and
Mucor spp. were obtained from the autopsy suite, the offices and the reception;
Mucor spp.,
Aspergillus spp.and
Penicillium spp. from the secretariat and
Aspergillus spp. from the washroom (
Table 1)
. These fungal isolates have also been previously isolated in hospital indoor air [
14,
15,
16]. The Yeasts were majorly
Candida albicans which has been implicated in human infections, its presence in the autopsy room is alarming; however, it might be attributed to its opportunistic nature to survive outside the host in moist environments
. Aspergillus niger and
A. fumigatus found in the washroom and secretariat are opportunistic pathogens implicated in pneumonia and aspergillosis, especially in immunodeficient individuals [
17].
Aspergillus spp. have genetic architecture to survive in different environments, including harsh conditions such as in the presence of antimicrobials and extremely high temperature [
18].
Mucor, a fungus commonly distributed in the autopsy unit have been implicated in rare but severe fungal infection, mucormycosis [
19].
Mucor spp. also increases the severity of secondary infections with immunocompromised patients [
20].
Penicillium spp. are associated with keratitis, pneumonia and UTIs [
21,
22].
Autopsy Rooms Fungal Strains are Highly Resistant to Antimicrobials
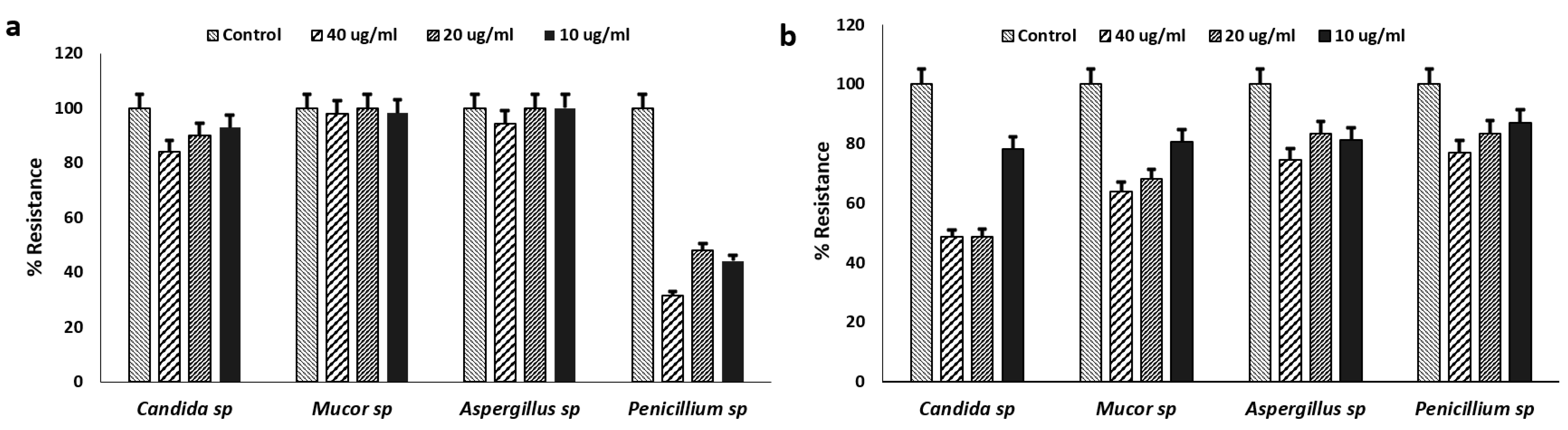
Relative to the control (strains without antifungal agents), fungal isolates survived 10 µg/ml, 20 µg/ml and 40 µg/ml fluconazole and itraconazole (
Figure 1a). Yeast and species of Mucor and Aspergillus showed 80-100% levels of resistance to fluconazole, and 50-100% to Itraconazole. This is similar to previous studies that indicated highly resistant Candida spp. to these antifungals, with clinical C. auris showing approximately 90% resistance to fluconazole [
23,
24]. Penicillium spp. showed relative low levels of resistance (30-50%) to fluconazole as compared to Itraconazole at all concentrations (
Figure 1b). Aspergillus spp. and Penicillium spp. have been reported to have intrinsic resistance to azole (itraconazole and fluconazole) antifungals, especially in European and Asian countries similar to the observation in this study [
25,
26]. In addition, resistance of Mucor spp. to itraconazole as observed in this study, has also been associated with septicemia [
27]. This also suggests that there is possibility for microbial spread within the hospital environment where the autopsy unit is situated, raising concerns of risks to public health.
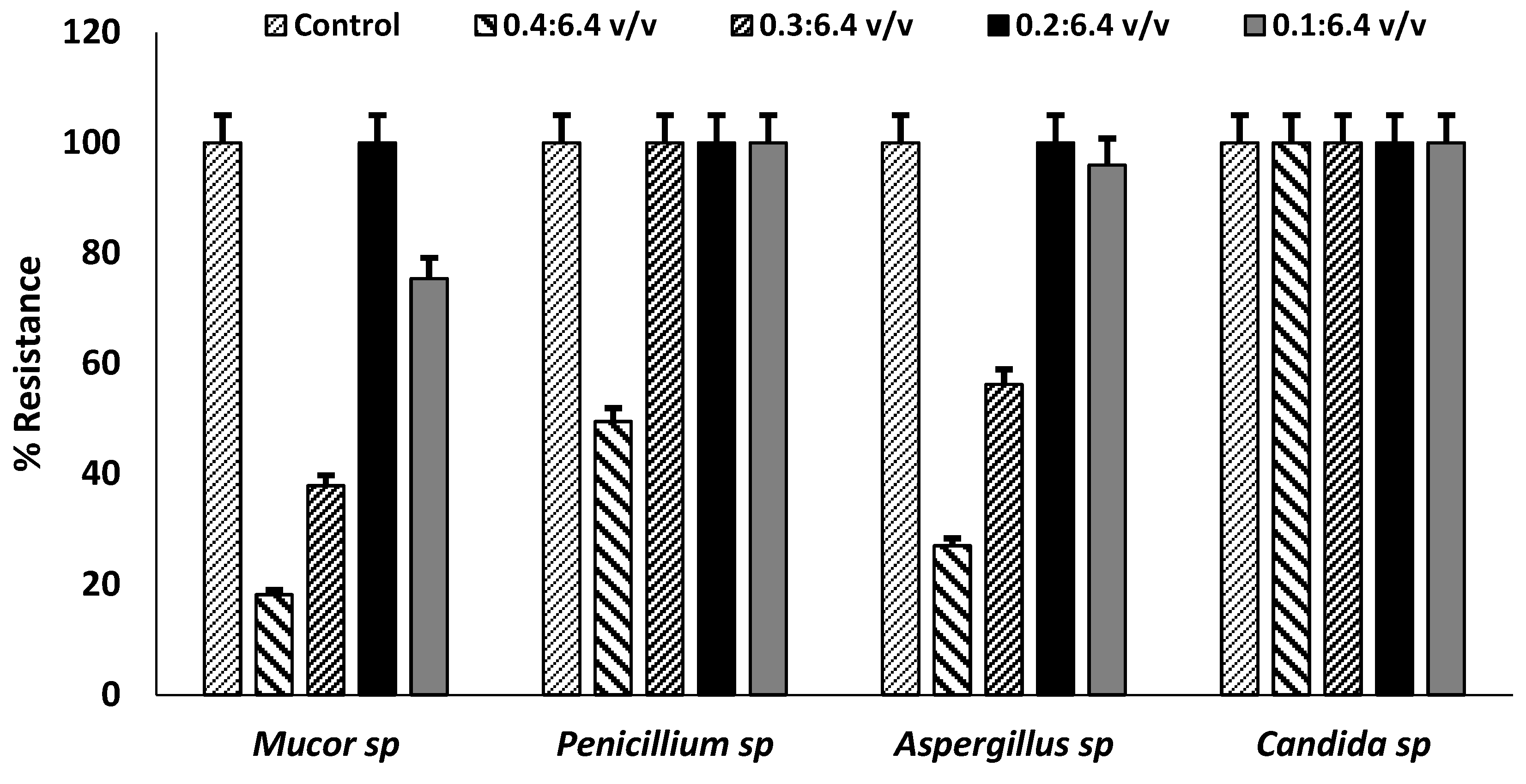
The isolated fungal strains were also tested against the germicide used for routine cleaning of the autopsy unit at 0.1/6.4 v/v, 0.2/6.4 v/v, 0.3/6.4 v/v and 0.4/6.4 v/v (
Figure 2)
. Relative to the controls, the recommended standard working Denzal® germicide concentration (0.4/6.4 v/v) showed a relative effectiveness against Mucor spp. and Aspergillus spp. with reduction in microbial growth; however, Candida spp. and Penicillium spp. were resistant. All the fungal strains showed 40-100% levels of resistance to the Denzal® germicide at all concentrations. This is of high occupational risks as germicide is a cresylic creosote with 27% w/v phenols with high fungicidal activities. The germicide was ineffective against the fungal strains at recommended working concentration and increased concentrations. This suggests increased risk of exposure of the autopsy staff to these pathogenic fungi, as the cleaning and disinfection regimen of the unit is likely unable to inhibit spores generated during autopsy, those resident on fomites and dispersed into the air.
Figure 2.
Resistance Profiles of Fungal Isolates to Denzal® germicide. High levels of percentage resistance at different recommended and two-fold concentrations with significance at p<0.05 by one-way ANOVA. Error bars represent percentage error of the mean of three replicates of each value.
Figure 2.
Resistance Profiles of Fungal Isolates to Denzal® germicide. High levels of percentage resistance at different recommended and two-fold concentrations with significance at p<0.05 by one-way ANOVA. Error bars represent percentage error of the mean of three replicates of each value.
Conclusions
This is the first study to provide baseline data on fungal profiles of a Ghanaian hospital referral autopsy unit. The fungal isolates dispersed into autopsy indoor air are of clinical relevance with increasing occupational risks. Species of Yeast, Penicillium, Mucor and Aspergillus have been implicated in diverse human infections, and could pose a threat to autopsy workers and visitors. The high levels of antimicrobial resistance displayed by the isolated fungi is a threat to public health; necessitating a need for consistent surveillance of autopsy unit with proper cleaning and disinfection practices.
Author Contributions
MO, KA, LM and AI conceptualized. MO and AI designed the study. AI, MO, and FG obtained, processed, analyzed and interpreted the data. AI and MO prepared the first draft of the manuscript. AI and LM revised the draft for important intellectual content. All the authors approved the final draft of the manuscript for submission.
Funding
Maame Oforiwaa and Francis Gyapong was supported by funds from a World Bank African Centre of Excellence grant (ACE02-WACCBIP) and a DELTAS Africa grant (DEL-15-007) to Gordon Awandare of the West African Centre for Cell Biology of Infectious Pathogens (WACCBIP).
Ethics Statement
No human or animal samples were obtained or analyzed. This is a part of a bigger study profiling different sections of Ghanaian hospital environments as approved by Ghana Health Service (GHS-ERC01/02/19).
Acknowledgements
The authors would like to thank the staff and workers of the referral autopsy unit. Members of Mosi Lab and AMR Research Group (led by Dr. Abiola Isawumi), especially Molly Kukua Abban and Eunice Ampadubea Ayerakwa for technical supports.
Conflicts of Interest
The authors declare no conflict of interests.
References
- de Oliveira Cardoso, T. A., Cynamon-Cohen, S., de Azevedo, B., & Chein, D. (2019). Biosafety in autopsy room: a systematic review. Revista de Salud Pública, 21(6).
- Heseltine, E., & Rosen, J. (Eds.). (2009). WHO guidelines for indoor air quality: dampness and mould.
- Fapohunda, S. O., Moore, G. G., Ganiyu, O. T., & Beltz, S. B. (2012). Toxigenic Aspergillus flavus and other fungi of public health concern in food and organic matter in southwest Nigeria. Mycology, 3(3), 210-219.
- Mayer, F. L., Wilson, D., & Hube, B. (2013). Candida albicans pathogenicity mechanisms. Virulence, 4(2), 119-128.
- World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action. Geneva; 2022.
- Bongomin, F., Gago, S., Oladele, R. O., & Denning, D. W. (2017). Global and multi-national prevalence of fungal diseases—estimate precision. Journal of fungi, 3(4), 57. [CrossRef]
- Smedbold, H. T., Ahlen, C., Unimed, S., Nilsen, A. R. M., Norbäck, D., & Hilt, B. R. (2002). Relationships between indoor environments and nasal inflammation in nursing personnel. Archives of Environmental Health: An International Journal, 57(2), 155-161. [CrossRef]
- Rali, P., Veer, M., Gupta, N., Singh, A. C., & Bhanot, N. (2016). Opportunistic pulmonary infections in immunocompromised hosts. Critical Care Nursing Quarterly, 39(2), 161-175. [CrossRef]
- Perlroth, J., Choi, B., & Spellberg, B. (2007). Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Medical mycology, 45(4), 321-346. [CrossRef]
- Sham, N. M., Ahmad, N. I., Pahrol, M. A., & Leong, Y. H. (2021). Fungus and mycotoxins studies in hospital environment: A scoping review. Building and Environment, 193, 107626. [CrossRef]
- Ocansey, B. K., Pesewu, G. A., Codjoe, F. S., Osei-Djarbeng, S., Feglo, P. K., & Denning, D. W. (2019). Estimated burden of serious fungal infections in Ghana. Journal of Fungi, 5(2), 38. [CrossRef]
- Abiola, I., Abass, A., Duodu, S., & Mosi, L. (2018). Characterization of culturable airborne bacteria and antibiotic susceptibility profiles of indoor and immediate-outdoor environments of a research institute in Ghana. AAS open research, 1. [CrossRef]
- Clinical and Laboratory Standards Institute. (2020). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M61-Ed2, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- Araujo, R., Cabral, J. P., & Rodrigues, A. G. (2008). Air filtration systems and restrictive access conditions improve indoor air quality in clinical units: Penicillium as a general indicator of hospital indoor fungal levels. American journal of infection control, 36(2), 129-134. [CrossRef]
- Eddouzi, J., Lohberger, A., Vogne, C., Manai, M., & Sanglard, D. (2013). Identification and antifungal susceptibility of a large collection of yeast strains isolated in Tunisian hospitals. Medical mycology, 51(7), 737-746. [CrossRef]
- Vallabhaneni, S., Benedict, K., Derado, G., & Mody, R. K. (2017). Trends in hospitalizations related to invasive aspergillosis and mucormycosis in the United States, 2000–2013. In Open forum infectious diseases (Vol. 4, No. 1, p. ofw268). US: Oxford University Press. [CrossRef]
- Meersseman, W., Lagrou, K., Maertens, J., & Wijngaerden, E. V. (2007). Invasive aspergillosis in the intensive care unit. Clinical infectious diseases, 45(2), 205-216. [CrossRef]
- D’Accolti, M., Soffritti, I., Bini, F., Mazziga, E., Mazzacane, S., & Caselli, E. (2022). Pathogen control in the built environment: a probiotic-based system as a remedy for the spread of antibiotic resistance. Microorganisms, 10(2), 225. [CrossRef]
- Reid, G., Lynch III, J. P., Fishbein, M. C., & Clark, N. M. (2020, February). Mucormycosis. In Seminars in respiratory and critical care medicine (Vol. 41, No. 01, pp. 099-114). Thieme Medical Publishers.
- Ajmal, S., Mahmood, M., Abu Saleh, O., Larson, J., & Sohail, M. R. (2018). Invasive fungal infections associated with prior respiratory viral infections in immunocompromised hosts. Infection, 46, 555-558. [CrossRef]
- Deshpande, S. D., & Koppikar, G. V. (1999). A study of mycotic keratitis in Mumbai. Indian journal of pathology & microbiology, 42(1), 81-87.
- Egbuta, M. A., Mwanza, M., & Babalola, O. O. (2017). Health risks associated with exposure to filamentous fungi. International journal of environmental research and public health, 14(7), 719.
- Botelho, T. K. R., Danielli, L. J., Seide, M., Borges, P. P., & Cruz, A. B. (2022). Distribution and antifungal susceptibility of Candida species isolated from clinical samples in southern Brazil. Brazilian Journal of Pharmaceutical Sciences, 58. [CrossRef]
- Rybak, J. M., Muñoz, J. F., Barker, K. S., Parker, J. E., Esquivel, B. D., Berkow, E. L., ... & Rogers, P. D. (2020). Mutations in TAC1B: a novel genetic determinant of clinical fluconazole resistance in Candida auris. MBio, 11(3), 10-1128. [CrossRef]
- Gonçalves, S. S., Souza, A. C. R., Chowdhary, A., Meis, J. F., & Colombo, A. L. (2016). Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses, 59(4), 198-219.
- Van Rhijn, N., Bromley, M., Richardson, M., & Bowyer, P. (2021). CYP51 paralogue structure is associated with intrinsic azole resistance in fungi. MBio, 12(5), 10-1128. [CrossRef]
- Caetano, L. A., Faria, T., Springer, J., Loeffler, J., & Viegas, C. (2019). Antifungal-resistant Mucorales in different indoor environments. Mycology, 10(2), 75-83. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).