Submitted:
14 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
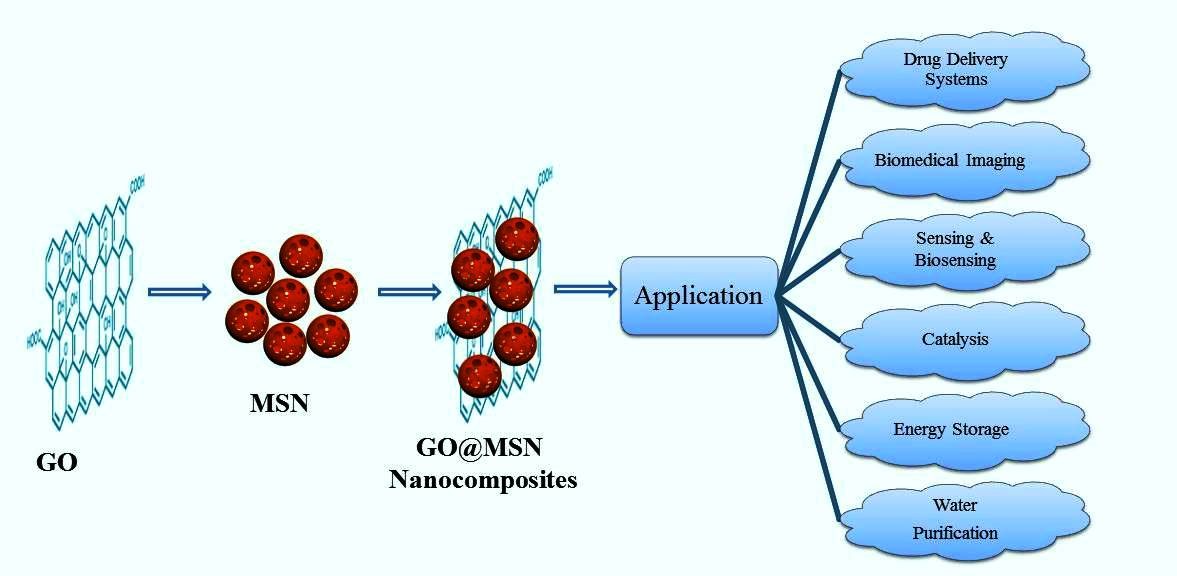
Keywords:
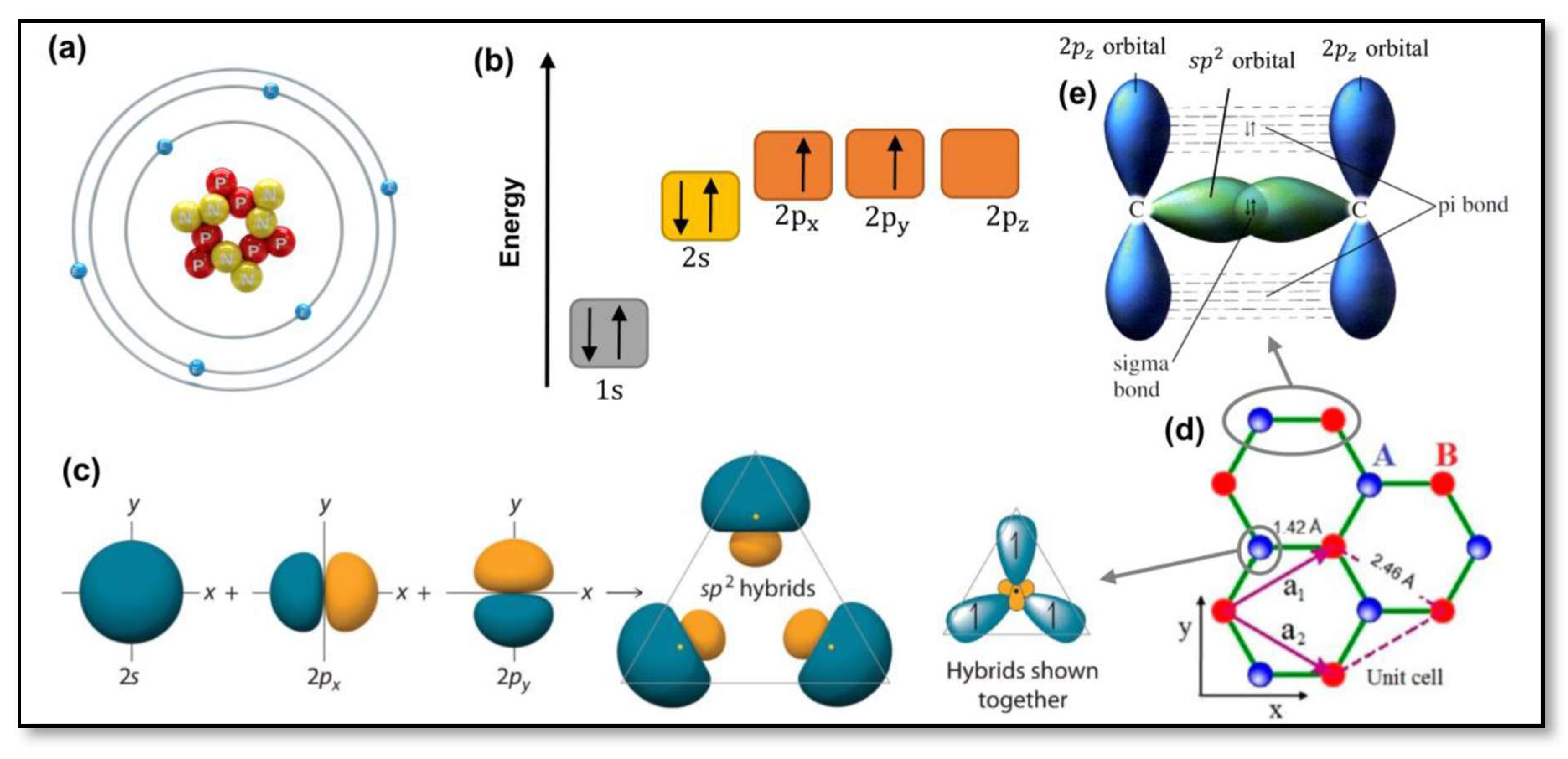
1. Introduction
2. Graphene oxide (GO)
2.1. Synthesis of graphene oxide (GO)
2.2. Unique properties of graphene
2.3. Applications of graphene in various fields
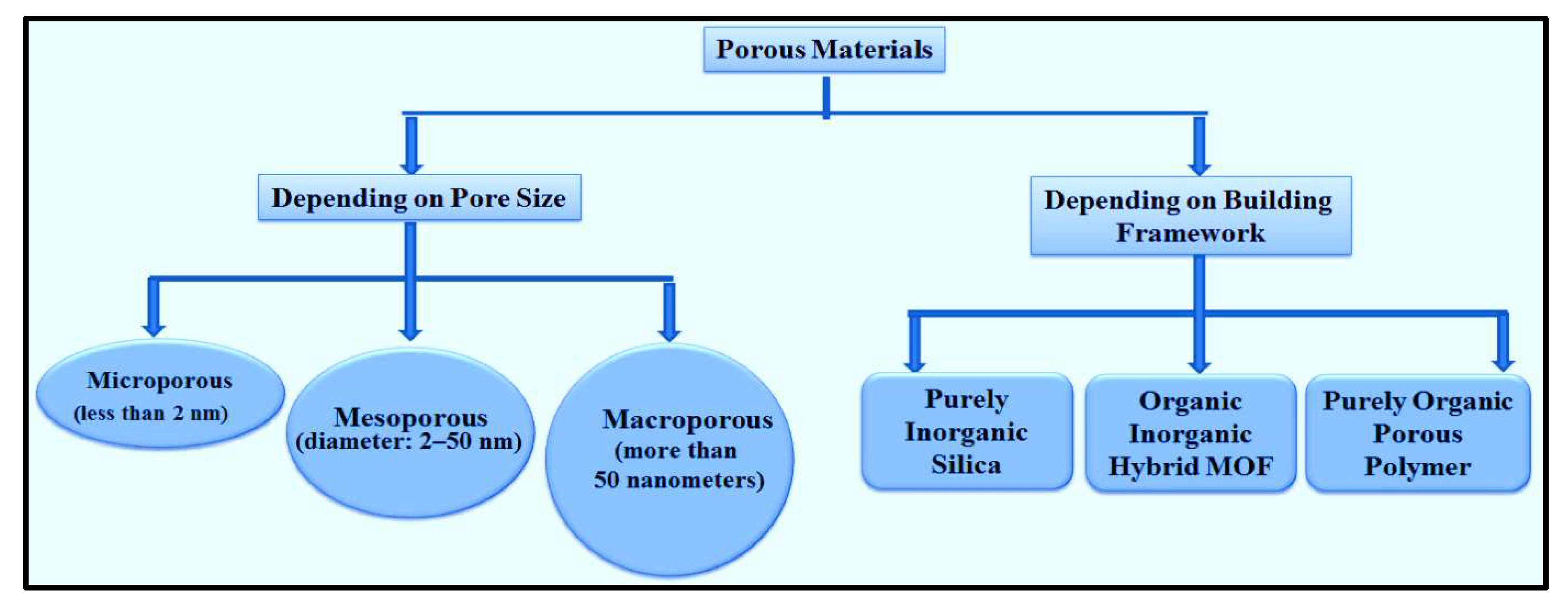
3. Mesoporous silica nanoparticles (MSN)
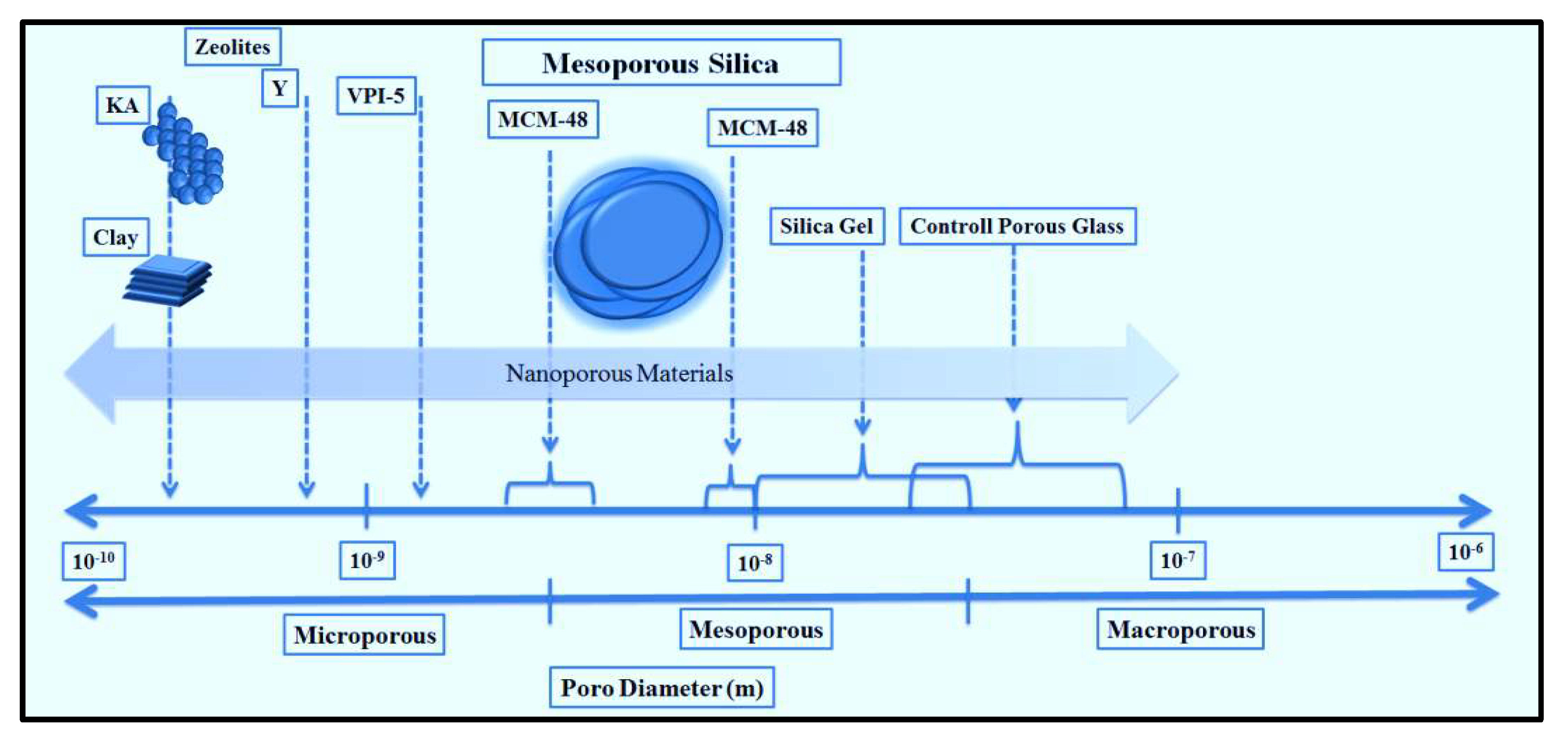
3.1. Synthesis methods of MSN
3.2. Properties of MSN
3.4. Applications of MSN
4. Bilayer Lipid Coated graphene@MSN nanocomposites
4.1. Fabrication of GO@MSN nanocomposites
4.2. Characterization of GO@MSN nanocomposites
| GO Synthesis Method |
MSN Synthesis Method | GO@MSN nanocomposites Method |
Drug Delivery | Drugs/Cargo | Outcome | Ref |
|---|---|---|---|---|---|---|
| Modified Hummers method | Sol-gel approach | - | Controlled Drug Delivery | Rizatriptan Benzoate | To effectively treat migraines, GO-MSN can transfer Rizatriptan Benzoate to the brain. | [63] |
| Modified Hummers method | Sol-gel method & Kim and Pinnavaia | Hydrothermal method | - | - | The supercapacitor, sensor, or a catalyst support | [67] |
| Modified Hummers method | Modified Stober method | - | - | Red blood cells and Human plasma proteins | Decreased hemolysis, Significantly reduced human blood plasma protein interaction with nanocomposites | [64] |
| Modified Hummers method | Sol-gel method | Gallery templated method | - | - | A broad range of porous graphene-based nanocomposites | [65] |
| Modified Hummers method | - | Oil-water biphase stratification approach | - | Silver nitrate | Excellent antibacterial effect against Rhodococuss, E.coli, and P.putida with low cytotoxicity |
[68] |
| Modified Hummers method | - | Oil-water biphase stratification approach | - | Polydopamine& doxorubicin hydrochloride |
To enhance the anticancer impact, use chemo-photothermal treatment. | [66] |
4.3. Miscellaneous application of GO@MSN nanocomposites
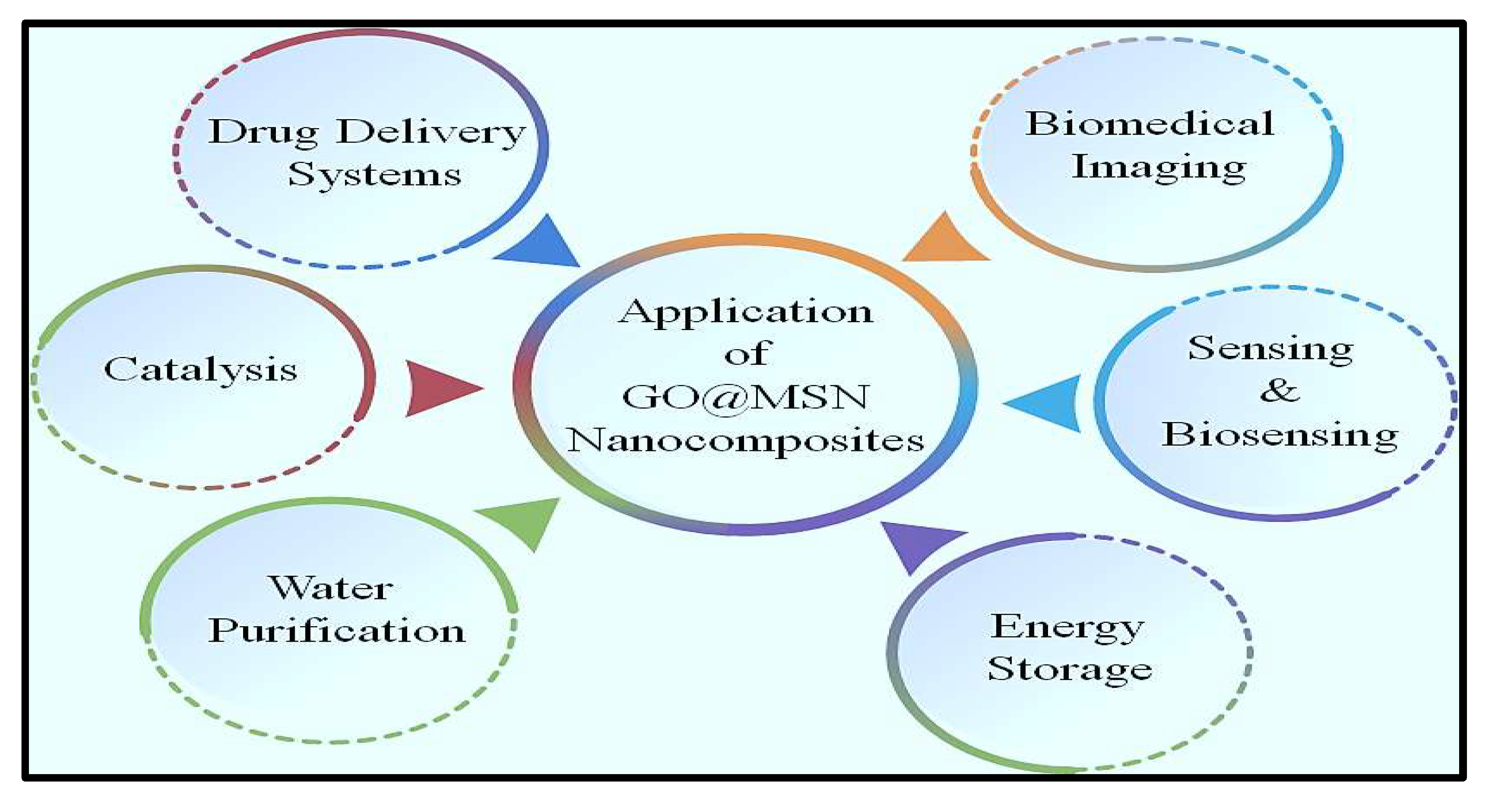
5. Challenges and limitations
7. Current research
8. Future perspectives
9. Conclusion
Acknowledgments
References
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Zobir, S.A.M., S. A. Rashid, and T. Tan, Recent development on the synthesis techniques and properties of graphene derivatives, in Synthesis, Technology and Applications of Carbon Nanomaterials. 2019, Elsevier. p. 77-107. [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: a review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Brisebois, P. and M. J.J.o.M.C.C. Siaj, Harvesting graphene oxide–years 1859 to 2019: a review of its structure, synthesis, properties and exfoliation. 2020, 8, 1517–1547. [Google Scholar]
- Dideikin, A.T. and A. Y.J.F.i.P. Vul', Graphene oxide and derivatives: the place in graphene family. 2019, 6, 149. [Google Scholar]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Feicht, P.; et al. Brodie's or Hummers’ method: oxidation conditions determine the structure of graphene oxide. 2019, 25, 8955-8959.
- Gadipelli, S. and Z. X.J.P.i.M.S. Guo, Graphene-based materials: Synthesis and gas sorption, storage and separation. 2015, 69, 1–60. [Google Scholar]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Rhee, K.Y. Electronic and Thermal Properties of Graphene. Nanomaterials 2020, 10, 926. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef]
- Woo, Y.S. Transparent Conductive Electrodes Based on Graphene-Related Materials. Micromachines 2018, 10, 13. [Google Scholar] [CrossRef]
- Uz, M.; Jackson, K.; Donta, M.S.; Jung, J.; Lentner, M.T.; Hondred, J.A.; Claussen, J.C.; Mallapragada, S.K. Fabrication of High-resolution Graphene-based Flexible Electronics via Polymer Casting. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Jang, H.; Park, Y.J.; Chen, X.; Das, T.; Kim, M.-S.; Ahn, J.-H. Graphene-Based Flexible and Stretchable Electronics. Adv. Mater. 2016, 28, 4184–4202. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Kim, J.-H.; Ahn, J.-H. Graphene as a flexible electronic material: mechanical limitations by defect formation and efforts to overcome. Mater. Today 2015, 18, 336–344. [Google Scholar] [CrossRef]
- Im, M.J.; Hyeong, S.-K.; Lee, J.-H.; Kim, T.-W.; Lee, S.-K.; Jung, G.Y.; Bae, S. High uniformity and stability of graphene transparent conducting electrodes by dual-side doping. Appl. Surf. Sci. 2022, 605. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Yang, K.; Gu, P.; Lu, X.; Zhang, S.; Gao, Y.; Ren, N.; Dong, B.; Jiang, Y.; et al. Quantum Hall phase in graphene engineered by interfacial charge coupling. Nat. Nanotechnol. 2022, 17, 1272–1279. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Mondal, H.S.; Hossain, M.; Rahaman, E.; Amin, S.M.B.; Hossain, B.; Mahasin, M.H.; Mondal, P.K. Optoelectronics Based Dynamic Advancement of Graphene: Characteristics and Applications. Crystals 2018, 8, 171. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, L.; Hu, J.; Li, C.; Qi, J.; Hou, Y.; Wei, X. Advances in the Applications of Graphene-Based Nanocomposites in Clean Energy Materials. Crystals 2021, 11, 47. [Google Scholar] [CrossRef]
- Valorosi, F.; De Meo, E.; Blanco-Varela, T.; Martorana, B.; Veca, A.; Pugno, N.; Kinloch, I.A.; Anagnostopoulos, G.; Galiotis, C.; Bertocchi, F.; et al. Graphene and related materials in hierarchical fiber composites: Production techniques and key industrial benefits. Compos. Sci. Technol. 2019, 185, 107848. [Google Scholar] [CrossRef]
- Khort, A.; et al. High-performance selective NO2 gas sensor based on In2O3–graphene–Cu nanocomposites. 2023, 13, 7834. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-Based Sensors for Human Health Monitoring. Front. Chem. 2019, 7, 399. [Google Scholar] [CrossRef]
- Kulakova, I.I.; Lisichkin, G.V. Biosensors Based on Graphene Nanomaterials. Mosc. Univ. Chem. Bull. 2022, 77, 307–321. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427–e427. [Google Scholar] [CrossRef]
- Aghigh, A.; Alizadeh, V.; Wong, H.; Islam, S.; Amin, N.; Zaman, M. Recent advances in utilization of graphene for filtration and desalination of water: A review. Desalination 2015, 365, 389–397. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, M.; Li, C.; Shi, G. Graphene-Based Membranes for Molecular Separation. J. Phys. Chem. Lett. 2015, 6, 2806–2815. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Zeng, Z.; Zeng, Y.; Xie, T. Promising Graphene-Based Nanomaterials and Their Biomedical Applications and Potential Risks: A Comprehensive Review. ACS Biomater. Sci. Eng. 2021, 7, 5363–5396. [Google Scholar] [CrossRef] [PubMed]
- Shareena, T.P.D.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 1–34. [Google Scholar] [CrossRef]
- Priya Swetha, P.D.; Manisha, H.; Sudhakaraprasad, K. Graphene and Graphene-Based Materials in Biomedical Science. Part. Part. Syst. Charact. 2018, 35, 1800105. [Google Scholar] [CrossRef]
- Fu, Y.; Hansson, J.; Liu, Y.; Chen, S.; Zehri, A.; Samani, M.K.; Wang, N.; Ni, Y.; Zhang, Y.; Zhang, Z.-B.; et al. Graphene related materials for thermal management. 2D Mater. 2019, 7, 012001. [Google Scholar] [CrossRef]
- Lin, H.; Jian, Q.; Bai, X.; Li, D.; Huang, Z.; Huang, W.; Feng, S.; Cheng, Z. Recent advances in thermal conductivity and thermal applications of graphene and its derivatives nanofluids. Appl. Therm. Eng. 2023, 218. [Google Scholar] [CrossRef]
- Renteria, J.D.; Nika, D.L.; Balandin, A.A. Graphene Thermal Properties: Applications in Thermal Management and Energy Storage. Appl. Sci. 2014, 4, 525–547. [Google Scholar] [CrossRef]
- Hirani, R.A.K.; Asif, A.H.; Rafique, N.; Shi, L.; Zhang, S.; Wu, H.; Sun, H. Wastewater Remediation Technologies Using Macroscopic Graphene-Based Materials: A Perspective. Front. Nanotechnol. 2021, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chu, W.; Vipin, A.K.; Sun, L. Environmental Remediation Applications of Carbon Nanotubes and Graphene Oxide: Adsorption and Catalysis. Nanomaterials 2019, 9, 439. [Google Scholar] [CrossRef]
- Ollik, K.; Lieder, M. Review of the Application of Graphene-Based Coatings as Anticorrosion Layers. Coatings 2020, 10, 883. [Google Scholar] [CrossRef]
- McBain, J.W. The Sorption of Gases and Vapours by Solids. J. Phys. Chem. 1933, 37, 149–150. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium–Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q. (.; Millar, G.J. Advances in Mesoporous Molecular Sieve MCM-41. Ind. Eng. Chem. Res. 1996, 35, 2075–2090. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, B.; Xu, J.-Q.; Xiang, X.-C.; Ren, D.-F.; Yang, T.-Q.; Ma, S.-Y.; Zhang, K.; Chen, Q.-M. A non-surfactant self-templating strategy for mesoporous silica nanospheres: beyond the Stöber method. Nanoscale 2020, 12, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, P.P., M.J.J.o.C. Jaroniec, and I. Science, Renaissance of Stöber method for synthesis of colloidal particles: New developments and opportunities. 2021, 584, 838-865.
- Rizzi, F.; Castaldo, R.; Latronico, T.; Lasala, P.; Gentile, G.; Lavorgna, M.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Depalo, N.; et al. High Surface Area Mesoporous Silica Nanoparticles with Tunable Size in the Sub-Micrometer Regime: Insights on the Size and Porosity Control Mechanisms. Molecules 2021, 26, 4247. [Google Scholar] [CrossRef]
- Niroumand, U.; Firouzabadi, N.; Goshtasbi, G.; Hassani, B.; Ghasemiyeh, P.; Mohammadi-Samani, S. The effect of size, morphology and surface properties of mesoporous silica nanoparticles on pharmacokinetic aspects and potential toxicity concerns. Front. Mater. 2023, 10. [Google Scholar] [CrossRef]
- Niedermayer, S.; et al. Multifunctional polymer-capped mesoporous silica nanoparticles for pH-responsive targeted drug delivery. 2015, 7, 7953-7964.
- Kolimi, P.; Narala, S.; Youssef, A.A.A.; Nyavanandi, D.; Dudhipala, N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023, 7, 70–89. [Google Scholar] [CrossRef]
- Mohamed, F.; Oo, M.K.; Chatterjee, B.; Alallam, B. Biocompatible Supramolecular Mesoporous Silica Nanoparticles as the Next-Generation Drug Delivery System. Front. Pharmacol. 2022, 13, 886981. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Culita, D.C.; Atkinson, I. Thermal stability enhancement of mesoporous SBA-15 silica through nanoconfinement of ceria nanoparticles. Microporous Mesoporous Mater. 2020, 306, 110484. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, H.; Zhang, Z.; Wen, B.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Applications and Biocompatibility of Mesoporous Silica Nanocarriers in the Field of Medicine. Front. Pharmacol. 2022, 13, 829796. [Google Scholar] [CrossRef]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; A, P.; Agrawal, M.; Alexander, A. Biomedical applications of mesoporous silica nanoparticles as a drug delivery carrier. J. Drug Deliv. Sci. Technol. 2022, 76. [Google Scholar] [CrossRef]
- ivojević, K.; et al. Advanced mesoporous silica nanocarriers in cancer theranostics and gene editing applications. 2021, 337, 193-211.
- Carvalho, A.M.; Cordeiro, R.A.; Faneca, H. Silica-Based Gene Delivery Systems: From Design to Therapeutic Applications. Pharmaceutics 2020, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. WIREs Nanomed. Nanobiotechnology 2019, 11, e1573. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. and T.J.J.F.i.M. Webster, Mesoporous silica based nanostructures for bone tissue regeneration. 2021, 8, 692309.
- Abbasi, M.; Gholizadeh, R.; Kasaee, S.R.; Vaez, A.; Chelliapan, S.; Al-Qaim, F.F.; Deyab, I.F.; Shafiee, M.; Zareshahrabadi, Z.; Amani, A.M.; et al. An intriguing approach toward antibacterial activity of green synthesized Rutin-templated mesoporous silica nanoparticles decorated with nanosilver. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Quirós, S.; Vallet-Regí, M.; Palacios, A.; Anguita, J.; Prados-Rosales, R.C.; González, B.; Luque-Garcia, J.L. Mesoporous Silica Nanoparticles as a Potential Platform for Vaccine Development against Tuberculosis. Pharmaceutics 2020, 12, 1218. [Google Scholar] [CrossRef]
- Deshmukh, P.K.; Lakade, S.H.; Jaiswal, U.R.; Harde, M.T.; More, M.P. One step synthesis approach of mesoporous silica packed with graphene oxide nanosheet: Characterisation and drug release aspects. Mater. Technol. 2021, 37, 1677–1690. [Google Scholar] [CrossRef]
- Fonseca, L.C.; de Araújo, M.M.; de Moraes, A.C.M.; da Silva, D.S.; Ferreira, A.G.; Franqui, L.S.; Martinez, D.S.T.; Alves, O.L. Nanocomposites based on graphene oxide and mesoporous silica nanoparticles: Preparation, characterization and nanobiointeractions with red blood cells and human plasma proteins. Appl. Surf. Sci. 2018, 437, 110–121. [Google Scholar] [CrossRef]
- Wei, L.; Lu, W.; Wei, H.; Chen, C.; Hou, Z. Porous sandwich-like silica/graphene nanocomposites obtained via templating of porous silica with CTAB in the gallery region of graphene oxide. Microporous Mesoporous Mater. 2017, 241, 58–65. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Zhang, F.; Wang, X.; Liu, X.; Zhang, Y. Polydopamine doped reduced graphene oxide/mesoporous silica nanosheets for chemo-photothermal and enhanced photothermal therapy. Mater. Sci. Eng. C 2019, 96, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Dalagan, J.Q. and E.P.J.B.o.M.S. Enriquez, One-step synthesis of mesoporous silica–graphene composites by simultaneous hydrothermal coupling and reduction of graphene oxide. 2014, 37, 589–595.
- Liu, R.; Wang, X.; Ye, J.; Xue, X.; Zhang, F.; Zhang, H.; Hou, X.; Liu, X.; Zhang, Y. Enhanced antibacterial activity of silver-decorated sandwich-like mesoporous silica/reduced graphene oxide nanosheets through photothermal effect. Nanotechnology 2018, 29, 105704. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Mukheem, A.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Shahabuddin, S.; Saidur, R.; Akbar, N.; Sridewi, N. Fabrication and Characterization of an Electrospun PHA/Graphene Silver Nanocomposite Scaffold for Antibacterial Applications. Materials 2018, 11, 1673. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Datar, A.; Jeong, C.; Deng, X.; Chung, Y.G.; Lin, L.-C. Surface Area Determination of Porous Materials Using the Brunauer–Emmett–Teller (BET) Method: Limitations and Improvements. J. Phys. Chem. C 2019, 123, 20195–20209. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Yin, H.; Hou, Y. Graphene-based nanocomposites for energy storage and conversion in lithium batteries, supercapacitors and fuel cells. J. Mater. Chem. A 2013, 2, 15–32. [Google Scholar] [CrossRef]
- Moghaddam, S.P.H., R. Mohammadpour, and H.J.J.o.C.R. Ghandehari, In vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. 2019, 311, 1–15.
- Seré, S.; De Roo, B.; Vervaele, M.; Van Gool, S.; Jacobs, S.; Seo, J.W.; Locquet, J.-P. Altering the Biodegradation of Mesoporous Silica Nanoparticles by Means of Experimental Parameters and Surface Functionalization. J. Nanomater. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional Mesoporous Silica-Coated Graphene Nanosheet Used for Chemo-Photothermal Synergistic Targeted Therapy of Glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef]
- Okamoto, Y.; Tsuzuki, K.; Iwasa, S.; Ishikawa, R.; Sandhu, A.; Tero, R. Fabrication of Supported Lipid Bilayer on Graphene Oxide. J. Physics: Conf. Ser. 2012, 352, 012017. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, ume 11, 1927–1945. [Google Scholar] [CrossRef]
- Kwon, S.; Singh, R.K.; A Perez, R.; Neel, E.A.A.; Kim, H.-W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano 2010, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The Shape Effect of Mesoporous Silica Nanoparticles on Biodistribution, Clearance, and Biocompatibility in Vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef]
- Passaretti, P.J.F.i.M.B. , Graphene oxide and biomolecules for the production of functional 3D graphene-based materials. 2022, 9, 774097.
- Tene, T.; Usca, G.T.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Gomez, C.V. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Tan, L.K.S.; Lee, W.L.; Ming, L.C.; How, C.W.; Foo, J.B.; Kifli, N.; Goh, B.H.; Ong, Y.S. Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids? Cancers 2021, 13, 5346. [Google Scholar] [CrossRef]
- Dumontel, B.; Conejo-Rodríguez, V.; Vallet-Regí, M.; Manzano, M. Natural Biopolymers as Smart Coating Materials of Mesoporous Silica Nanoparticles for Drug Delivery. Pharmaceutics 2023, 15, 447. [Google Scholar] [CrossRef]
- Rahmatolahzadeh, R.; Hamadanian, M.; Ma’mani, L.; Shafiee, A. Aspartic acid functionalized PEGylated MSN@GO hybrid as an effective and sustainable nano-system for in-vitro drug delivery. Adv. Med Sci. 2018, 63, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: an update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering. Biomol. Concepts 2020, 11, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.K.; Mishra, D.K.; Rosling, A.; Rosenholm, J.M. Therapeutic Potential of Polymer-Coated Mesoporous Silica Nanoparticles. Appl. Sci. 2019, 10, 289. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Sha, L.; Wang, D.; Shi, W.; Zhao, Q.; Wang, S. Thermosensitive Lipid Bilayer-Coated Mesoporous Carbon Nanoparticles for Synergistic Thermochemotherapy of Tumor. ACS Appl. Mater. Interfaces 2018, 10, 19386–19397. [Google Scholar] [CrossRef]
- Dennahy, I.S.; Han, Z.; MacCuaig, W.M.; Chalfant, H.M.; Condacse, A.; Hagood, J.M.; Claros-Sorto, J.C.; Razaq, W.; Holter-Chakrabarty, J.; Squires, R.; et al. Nanotheranostics for Image-Guided Cancer Treatment. Pharmaceutics 2022, 14, 917. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





