1. Introduction
Selenium is an essential trace element which plays crucial roles in the health and development of several human disorders [
1]. Selenium supplementation is critical in the prevention and / or treatment of a variety of disorders ranging from Kashin-Beck disease [
2], hypothyroidism [
3,
4], atherosclerosis [
5], cardiovascular disease [
6], Alzheimer’s disease [
7], HIV and AIDS [
8] and more recent indications emerging for COVID-19 [9-11]. Furthermore, several selenium forms have been investigated in different cancer types for their potent anticancer activity including but not limited to lung, breast, prostate, colon cancer, cervical, bladder and pancreatic cancer [12-19].
During viral infections, several reactive oxygen species (ROS) could be produced, and these could be beneficial as well as harmful for a variety of cellular functions [
20,
21]. The viral replications can also be enhanced due to these ROS and because of the viral infection there is usually a demand for micronutrients in the host environment which could cause their deficiency. Selenium plays a vital role in antioxidant defense mechanisms influencing the redox signaling and redox homeostasis. The selenium deficiency, which is the main regulator of selenoprotein expression, has been associated with the pathogenicity of several viruses. In addition, several selenoproteins including glutathione peroxidases (GPX), thioredoxin reductases (TXNRD) seem to be playing important roles in various models of viral replication.
Selenium has also been implicated in systemic inflammatory response syndrome, sepsis, and septic shock [22-24] and can overcome adverse effects and reduce mortality rates in severe sepsis [
23]. In a sepsis related lung injury, selenium in combination with niacin caused synergistic activation of glutathione redox cycle, reduction of hydrogen peroxide level, and up-regulation of nuclear factor erythroid 2-related factor 2 (Nrf2) and improved the survival in the rats [
24].
In the recent years it has become clear that increased level of cytokines such as like IL-1, IL-2, IL-6, GMCSF, IFN-γ and TNF-α in COVID-19 patients can lead to tissue damage and can also lead to cell death and fever, as well as impact the vascular physiology and coagulation [
25]. Among the cytokines, the earliest report on Tocilizumab, an anti-IL-6 receptor for treatment of a patient with COVID-19 related lung disease [
26] opened possibilities for severe cases. Recent meta-analysis also revealed that IL-6 is a master regulator of COVID-19 severity biomarkers [
27]. In addition, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein S1 subunit activates TLR4 signaling to induce pro-inflammatory responses in murine and human macrophages. Therefore, TLR4 signaling in macrophages may be a potential target for regulating excessive inflammation in COVID-19 patients [
28].
Regional selenium deficiency might be related to an increased fatality rate of COVID-19 in China [
29]. To date most of the observation studies indicate that a lower serum selenium level was associated with worse outcomes [30-32]. The more severe selenium deficiency has also been proposed to correlate with COVID-19 disease progression and furthermore, the selenium deficiency is associated with overall organ system dysfunction and death [
33]. To our knowledge no randomized clinical trials have appropriately investigated the effect of selenium supplementation on COVID-19. However, a few studies on the effects of second most important trace element zinc supplementation in COVID-19 did not confirm its efficacy [
34].
For the current study, we planned to evaluate the effects of different selenium compounds on key players of cytokine storm that are critical to target in COVID-19 patients. Since lipopolysaccharide (LPS) induced expression profile of THP-1 cells was found to be like that of human PBMC-derived macrophages [
35], we induced inflammation with LPS in THP-1 macrophages to study the impact of selenium compounds on IL-6 and TNF-α. Our results strongly encourage follow-up research on selective selenium compounds on all the cytokines involved in cytokine storm for COVID-19 patients and potential for using selenium as an adjuvant therapy in long haulers of COVID-19 infection.
2. Materials and Methods
2.1. Selenium compounds
Selenous acid (SA), methylseleninic acid (MSeA), sodium selenite (Sel), selenomethionine (SM) were purchased from Sigma (St. Louis, MO) and used at 0.25 μM to 10 μM concentrations for treatments.
2.2. THP-1 cell line, THP-1 macrophages, LPS challenge and selenium treatments
THP-1 cell line (ATCC, Manassas, VA) was cultured in RPMI 1640 (ATCC, Manassas) with 10% heat-inactivated FBS (Gemini Bio-Products, West Sacramento, CA) and 1% Penn-Strep (Invitrogen, Carlsbad, CA). These cells were maintained and treated at 37°C in a humidified incubator in the presence of 5% CO2. The cells were maintained in culture for only 4-5 passage numbers for a given experiment. Short Tandem Repeat (STR) DNA profiling was used to confirm the authenticity of the cell type. THP-1 cells were differentiated into macrophages (THP-1 macrophages) with 100 nM Phorbol 12-myristate 13-acetate (PMA; Sigma, St. Louis, MO) for 24 hours and next day the plate was rinsed twice in PBS and replenished with regular medium and the cells were given a rest for another 24 hours in regular medium. The THP-1 macrophages were then challenged with LPS (100 ng/ml) in absence and presence of selenium compounds for 24 hours. The spent media from the treated cells were collected and stored at -20°C until used for IL-6 and TNF-α measurements and the adherent cells were processed for MTT Assay.
2.3. Cytotoxicity (MTT Assay)
Selenium-treated THP-1 macrophages were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, St. Louis, MO) (500 μl of 5 mg/ml solution) for 3 h in dark at 37ºC. MTT solution was removed from the wells and was replaced by 500 μl of DMSO/well to dissolve the purple blue formazan particles and the plate was read at 570 nm with correction at 630 nm in Molecular Devices plate reader. MTT assay was performed in triplicate for each selenium compound.
2.4. Evaluating inflammatory response markers: IL-6 and TNF-α
The frozen spent medium from selenium-treated THP-1 macrophages was thawed and the secreted IL-6 and TNF-α levels were estimated by QuantikineTM ELISA Kits (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions.
2.5. Western Blotting
THP-1 cells were differentiated into macrophages with PMA as described above and MSeA (0, 5, 10 μM) was incubated in presence or absence of LPS for 30 min. The cells were collected, washed in cold PBS and lysed in RIPA buffer (Sigma, St. Louis, MO) containing protease inhibitors. Equal amounts of protein (50 μg) were subjected to SDS-PAGE and western blot was performed on the proteins transferred onto nitrocellulose membrane as described earlier [
36]. Primary antibodies against pIκBα, IκBα and Nrf2 (Cell Signaling, Danvers, MA) (1:1000 dilution) and β-actin (Proteintech, Chicago, IL) (1:10,000 dilution) were reacted separately with the blot. The HRP-conjugated anti-rabbit and anti-mouse secondary antibodies (Cell Signaling, Danvers, MA) were incubated at a dilution of 1:3000. Band expressions were developed using Pierce
TM ECL reagents (Thermo Scientific, Rockford, IL).
2.6. Statistical analysis
Descriptive statistics were generated for the continuous outcome variables (IL-6, TNF-α levels and MTT assay) for each selenium compound at various concentrations. The main comparisons were performed between the LPS alone treatment and each selenium compound at different concentrations. Dunnett’s tests were used on the pairwise comparisons between the LPS alone and other selenium groups. Due to the exploratory nature of this study the statistical significance level were not further adjusted for multiple testing. All analyses were performed using statistical programming language R version 4.3.1 (The R Foundation for Statistical Computing). All tests were two-sided and the statistical significance level used was 0.05.
3. Results
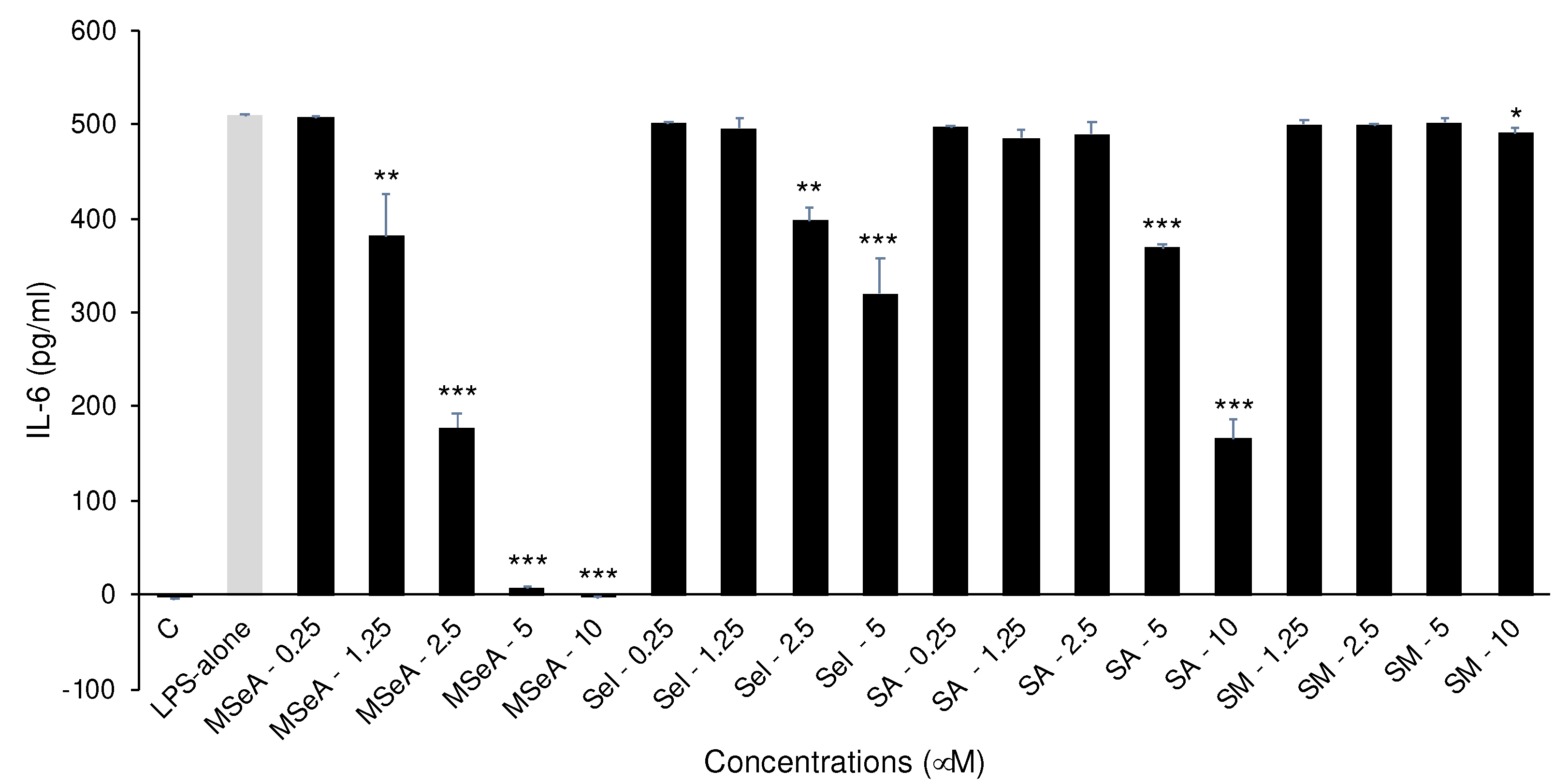
3.1. MSeA, Sel and SA effectively reduce IL-6 levels
The LPS-induced IL-6 levels secreted from THP-1 macrophages were reduced about 25% by 1.25 μM of MSeA followed by a dose-dependent significant decrease by 65%, 98% and 100% from 2.5 μM - 10 μM (p < 0.001) (
Figure 1). Whereas Sel showed significant reduction in IL-6 levels at 2.5 μM (22%, p < 0.01) and 5 μM (37%, p <0.001) and SA was able to decrease IL-6 levels at 5 μM (28%) and 10 μM (67%, p < 0.001). However, SM did not impact IL-6 levels at these doses except only 3% at 10 μM (p <0.05) (
Figure 1).
3.2. MSeA drastically reduces TNF-α levels
The LPS-induced TNF-α levels secreted from THP-1 macrophages were inhibited by 11%, 48% and 75% at 2.5 μM, 5 μM and 10 μM (p < 0.001) respectively (
Figure 2). By contrast Sel slightly (2%) but significantly inhibited TNF-α levels at 2.5 μM and 5 μM (p < 0.05) but SA and SM did not impact the TNF-α levels secreted at the doses tested.
3.3. Moderate effect of selenium compounds on cytotoxicity
As reported in literature, LPS alone (100 ng/ml) caused cell death in THP-1 macrophages in our set up (data not shown). To evaluate the cytotoxicity of selenium compounds in these LPS treated THP-1 macrophages, an MTT assay was performed following 24 h treatments. MSeA at all doses was able to rescue the macrophage cell death caused by LPS (
Figure 3). However, significant cytotoxicity was observed in SM (10 μM, p < 0.01) treated cells as compared to LPS alone.
3.4. MSeA influences Nrf2 and IκBα levels
Following a 30 min treatment of THP-1 macrophages, MSeA strongly increased Nrf2 levels in absence LPS and moderatly increase in presence of LPS (
Figure 4). Further, the pIκBα levels were diminished by MSeA in absence of LPS and moderately decreased in presence of LPS. On the other hand, the native IκBα levels were elevated in absence of LPS and reduced by LPS however, MSeA did not change these levels in presence or absence of LPS. (
Figure 4). Since LPS is inducing phosphorylation, the native IκBα will be degraded as is evident from the results in presence of LPS. This in turn will allow NF-κB to go to the nucleus. Without LPS however, IκBα is intact and therefore not degraded.
4. Discussion
Several reviews in recent literature have cited the potential role of selenium in SARS-CoV-2 infection [9-11, 37]. However, a recent study showed that daily intravenous selenium (1mg Sel) in a cohort of COVID-19 patients with severe ARDS for a total of two weeks elevated their selenoprotein P and GPX3 levels [
38]. Further, the selenoprotein P levels were inversely proportional to the levels of inflammatory cytokines CRP, IL-6 and IL-1β. A caveat in the study was that the patients had also received different combinations of artificial nutrition which contained varied amounts of selenium and zinc thus making it more challenging to infer if the impact on the patients was solely due to selenium. Others have proposed evaluating a series of selenium-based compositions (patents pending) to fight against COVID-19 [
39] and it would be worth investigating if any of these selenium forms influence components of the cytokine storm.
Since SARS-CoV-2 spike protein S1 subunit has been shown to activate TLR4 signaling, which induces pro-inflammatory response in murine and human macrophages. Thus, TLR4 signaling in macrophages could be a potential target for regulating excessive inflammation in COVID-19 patients [
28]. In our current study we used LPS as the TLR4 agonist in THP-1 cells after being differentiated by PMA treatment. Using these relevant THP-1 macrophages the potential impact of selenium on the release of IL-6 and TNF-α was measured following stimulation with LPS in presence and absence of several selenium compounds. We chose four prominently researched selenium compounds MSeA, Sel, SA and SM. MSeA has been proposed to accumulate in SARS-CoV-2 infected cells and inactivate the M
pro of SARS-CoV-2 in these cells by modifying the Cys145 residue of the protease [
9]. Sel is an acceptable inorganic selenium form showing promising effects on several viral infections in human studies [
40]. SA was proposed as a potent selenium compound for treatment of moderately ill, severely ill, and critically ill COVID-19 patients (NCT04869579). SM was recently evaluated in an herbal combination against human beta coronavirus
in vitro and
in vivo [
41]. In our study, MSeA, Sel and SA showed reduction in IL-6 levels. While, MSeA and to a minor extent Sel decreased TNF-α levels in the THP-1 macrophages in the presence of LPS. It was clear from these experiments that the impact of selenium compounds varied based on the form of selenium selected. However, the impact of selenium was independent of any cytotoxicity in THP-1 macrophages.
Previously, we had observed changes in cytokine profile of healthy men being supplemented with selenized yeast. The inflammatory cytokines such as IL-6, IL-8, MCP-1, IL-12p70, IFN-γ and TGF-β were reduced in the selenized-yeast group as compared to placebo group [
42]. However, the major form of selenium in selenized-yeast is SM which only had a minor impact on IL-6 in our current
in vitro study. An earlier report showed that Sel (2 - 8 µM) limited the gene expression of pro-inflammatory cytokines IL-1β, TNF-α and IL-6 within 6 hours under inflammatory conditions induced by
Staphylococcus aureus. Further, these observations along with suppression of phosphorylation of IκBα and p65 at 4 μM and 8 μM Sel substitution [
43] would in part indicate suppression of the inflammatory response by NF-κB pathway. Similarly, in our study we observed a decline in phosphorylation of IκBα levels in MSeA-treated THP-1 macrophages following LPS induction. A recent review and meta-analysis [
44] suggested that oral and intravenous selenium supplementation influences IL-6 and TNF-α levels.
On the contrary, the effects of selenium on NF-kB are controversial depending on the dose and cell type (murine vs human). The most cited report of selenium on NF-κB was performed in RAW macrophages by first creating a selenium deficiency which resulted in the activation of NF-κB in presence of LPS and was reduced by addition 250 nM Sel [
45]. While results from a recent study in THP-1 macrophages showed that 50 nM Sel facilitates LPS-induced NF-κB activation [
46].
Our preliminary study also revealed that MSeA increases Nrf2 levels and decreases pIκBα levels in the absence of LPS in THP-1 macrophages. Previously, an organic form of selenium has been shown to induce Nrf2 levels in the lungs [
47]. In part, induction of moderate Nrf2 might inhibit IL-6 secretion in THP-1 macrophages [
48].
Alternatively, selenium levels in populations could be improved by supplementing soil by selenium enriched fertilizers that kept low mortality rates due to COVID-19 in Finland [
49]. The nationwide supplementation of fertilizers with sodium selenate is shown to be effective and safe in increasing the selenium intake of the entire population. Future studies in a larger population would be valuable. Improving the selenium status by nutritional measures or supplementation may be helpful in reducing the devastation caused by COVID-19 especially in areas affected by selenium deficiency worldwide [
50]. Moreover, it is important to consider selenium as a cofactor to reach more effective immune response to COVID vaccination [
51].
In summary, our results indicate that MSeA reduces IL-6 and TNF-α levels in THP-1 macrophages. COVID-19 patients might benefit by suppressing their cytokine storm with selenium such as MSeA when used alongside the standard therapy. It is also worth exploring the use of selenium in building a better defense in COVID-19 long haulers.
Supplementary Materials
Not applicable
Author Contributions
Conceptualization, I.S. and R.S.; methodology, I.S.; validation, I.S. and R.S.; formal analysis, I.S., J.Z. and R.S.; investigation, I.S.; writing—original draft preparation, R.S.; writing—review and editing, I.S., J.Z. and R.S.; visualization, I.S. and R.S.; supervision, R.S.; project administration, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Research data are clearly depicted in the graphs and are also available to the readers upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxidants Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, H.; Yang, Z.; Bao, M.; Lin, X.; Han, J.; Qu, C. Progress of Selenium Deficiency in the Pathogenesis of Arthropathies and Selenium Supplement for Their Treatment. Biol. Trace Element Res. 2021, 200, 4238–4249. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat. Rev. Endocrinol. 2011, 8, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low Population Selenium Status Is Associated With Increased Prevalence of Thyroid Disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017, 9, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Mejia, S.B.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.; Drobyshev, E.; Bjørklund, G.; Dubrovskii, Y.; Lysiuk, R.; Rayman, M.P. Selenium, selenoprotein P, and Alzheimer's disease: is there a link? Free. Radic. Biol. Med. 2018, 127, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Al-Quraishy, S.; A Dkhil, M.; Wunderlich, F.; Sies, H. Dietary Selenium in Adjuvant Therapy of Viral and Bacterial Infections. Adv. Nutr. Int. Rev. J. 2015, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020, 37, 101715–101715. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19—A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M.K. Role of Selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 23, 280. [Google Scholar] [CrossRef]
- Ip, C.; Dong, Y. Methylselenocysteine modulates proliferation and apoptosis biomarkers in premalignant lesions of the rat mammary gland. Anticancer Res. 2001, 21. [Google Scholar]
- Unni, E.; Koul, D.; Yung, W.-K.A.; Sinha, R. Se-methylselenocysteine inhibits phosphatidylinositol 3-kinase activity of mouse mammary epithelial tumor cells in vitro. Breast Cancer Res. 2005, 7, R699–R707. [Google Scholar] [CrossRef]
- Das, A.; Bortner, J.; Desai, D.; Amin, S.; El-Bayoumy, K. The selenium analog of the chemopreventive compound S,S′-(1,4-phenylenebis[1,2-ethanediyl])bisisothiourea is a remarkable inducer of apoptosis and inhibitor of cell growth in human non-small cell lung cancer. Chem. Interactions 2009, 180, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bonorden, M.J.; Li, G.-X.; Lee, H.-J.; Hu, H.; Zhang, Y.; Liao, J.D.; Cleary, M.P.; Lü, J. Methyl-Selenium Compounds Inhibit Prostate Carcinogenesis in the Transgenic Adenocarcinoma of Mouse Prostate Model with Survival Benefit. Cancer Prev. Res. 2009, 2, 484–495. [Google Scholar] [CrossRef]
- Sinha, I.; Allen, J.E.; Pinto, J.T.; Sinha, R. Methylseleninic acid elevates REDD1 and inhibits prostate cancer cell growth despite AKT activation and mTOR dysregulation in hypoxia. Cancer Med. 2014, 3, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Li, Q.; Reszka, E.; Wieczorek, E.; Tarhonska, K.; Wang, T. Therapeutic Potential of Selenium and Selenium Compounds in Cervical Cancer. Cancer Control. 2021, 28. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Cantor, K.P.; Silverman, D.T.; Malats, N. Selenium and Bladder Cancer Risk: a Meta-analysis. Cancer Epidemiology Biomarkers Prev. 2010, 19, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Wooten, D.J.; Sinha, I.; Sinha, R. Selenium Induces Pancreatic Cancer Cell Death Alone and in Combination with Gemcitabine. Biomedicines 2022, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Harthill, M. Review: Micronutrient Selenium Deficiency Influences Evolution of Some Viral Infectious Diseases. Biol. Trace Element Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [PubMed]
- Angstwurm, M.W.A.; Schottdorf, J.; Schopohl, J.; Gaertner, R. Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit. Care Med. 1999, 27, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Angstwurm, M.W.A.; Engelmann, L.; Zimmermann, T.; Lehmann, C.; Spes, C.H.; Abel, P.; Strauß, R.; Meier-Hellmann, A.; Insel, R.; Radke, J.; et al. Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock*. Crit. Care Med. 2007, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.Y.; Suh, G.J.; Kim, K.S.; Jung, Y.S.; Kim, S.H.; Kim, J.S.; You, K.M. Niacin and Selenium Attenuate Sepsis-Induced Lung Injury by Up-Regulating Nuclear Factor Erythroid 2–Related Factor 2 Signaling*. Crit. Care Med. 2016, 44, e370–e382. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.-M.; Albiges, L.; Chaput, N.; Saada, V.; Pommeret, F.; Griscelli, F.; Balleyguier, C.; Besse, B.; Marabelle, A.; Netzer, F.; et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. 2020, 31, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.; Brown, S.J.; Mohamed, A.E.; Fuller, H.R. A meta-summary and bioinformatic analysis identified interleukin 6 as a master regulator of COVID-19 severity biomarkers. Cytokine 2022, 159, 156011–156011. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, e06187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Zhang, A.-R.; Lu, Q.-B.; Zhang, X.-A.; Zhang, Z.-J.; Guan, X.-G.; Che, T.-L.; Yang, Y.; Li, H.; Liu, W.; et al. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect. Dis. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Fakhrolmobasheri, M.; Mazaheri-Tehrani, S.; Kieliszek, M.; Zeinalian, M.; Abbasi, M.; Karimi, F.; Mozafari, A.M. COVID-19 and Selenium Deficiency: a Systematic Review. Biol. Trace Element Res. 2021, 200, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selenium Deficiency in COVID-19—A Possible Long-Lasting Toxic Relationship. Nutrients 2022, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Balboni, E.; Zagnoli, F.; Filippini, T.; Fairweather-Tait, S.J.; Vinceti, M. Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J. Trace Elements Med. Biol. 2022, 71, 126956–126956. [Google Scholar] [CrossRef] [PubMed]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 2007, 8, 1–17. [Google Scholar] [CrossRef]
- Sinha, I.; Goel, R.; Bitzer, Z.T.; Trushin, N.; Liao, J.; Sinha, R. Evaluating electronic cigarette cytotoxicity and inflammatory responses in vitro. Tob. Induc. Dis. 2022, 20, 1–13. [Google Scholar] [CrossRef]
- Bermano, G.; Méplan, C.; Mercer, D.K.; Hesketh, J.E. Selenium and viral infection: are there lessons for COVID-19? Br. J. Nutr. 2020, 125, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Notz, Q.; Herrmann, J.; Schlesinger, T.; Helmer, P.; Sudowe, S.; Sun, Q.; Hackler, J.; Roeder, D.; Lotz, C.; Meybohm, P.; et al. Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS. Nutrients 2021, 13, 2113. [Google Scholar] [CrossRef]
- Alshammari, M.K.; Fatima, W.; Alraya, R.A.; Alzahrani, A.K.; Kamal, M.; Alshammari, R.S.; Alshammari, S.A.; Alharbi, L.M.; Alsubaie, N.S.; Alosaimi, R.B.; et al. Selenium and COVID-19: A spotlight on the clinical trials, inventive compositions, and patent literature. J. Infect. Public Heal. 2022, 15, 1225–1233. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]
- Dound, Y.A.; Sehgal, R. Preclinical Efficacy and Safety Studies of Formulation SSV-003, a Potent Anti-Viral Herbal Formulation. J. Exp. Pharmacol. 2021, ume 13, 913–921. [Google Scholar] [CrossRef]
- Sinha, I.; Karagoz, K.; Fogle, R.L.; Hollenbeak, C.S.; Zea, A.H.; Arga, K.Y.; Stanley, A.E.; Hawkes, W.C.; Sinha, R. “Omics” of Selenium Biology: A Prospective Study of Plasma Proteome Network Before and After Selenized-Yeast Supplementation in Healthy Men. OMICS: A J. Integr. Biol. 2016, 20, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bi, C.; Wang, Y.; Sun, J.; Meng, X.; Li, J. Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways. BMC Veter- Res. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Khalili, A.; Roodi, P.B.; Saeedy, S.A.G.; Najafi, S.; Mohammadian, M.K.; Djafarian, K. Selenium supplementation decreases CRP and IL-6 and increases TNF-alpha: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elements Med. Biol. 2023, 79, 127199. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Zamamiri-Davis, F.; Stewart, J.B.; Thompson, J.T.; Sordillo, L.M.; Reddy, C.C. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: role of nuclear factor-κB in up-regulation. Biochem. J. 2002, 366, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, T.; Weidenbach, L.M.; Adolf, J.; Schwarz, M.; Schädel, P.; Gollowitzer, A.; Werz, O.; Koeberle, A.; Kipp, A.P.; Koeberle, S.C. The Trace Element Selenium Is Important for Redox Signaling in Phorbol Ester-Differentiated THP-1 Macrophages. Int. J. Mol. Sci. 2021, 22, 11060. [Google Scholar] [CrossRef] [PubMed]
- Emmert, S.W.; El-Bayoumy, K.; Das, A.; Sun, Y.-W.; Amin, S.; Desai, D.; Aliaga, C.; Richie, J.P. Induction of lung glutathione and glutamylcysteine ligase by 1,4-phenylenebis(methylene)selenocyanate and its glutathione conjugate: Role of nuclear factor-erythroid 2-related factor 2. Free. Radic. Biol. Med. 2012, 52, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.-R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. The relationship between nutrition and the immune system. Front. Nutr. 2022, 9, 1082500. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).