Submitted:
15 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experiments
2.1. Materials and Chemicals
2.2. Preparation of Biochar Composite Microspheres
2.3. Adsorption Tests
2.4. Adsorption Kinetics
2.5. Analytical Methods
3. Results and Discussion
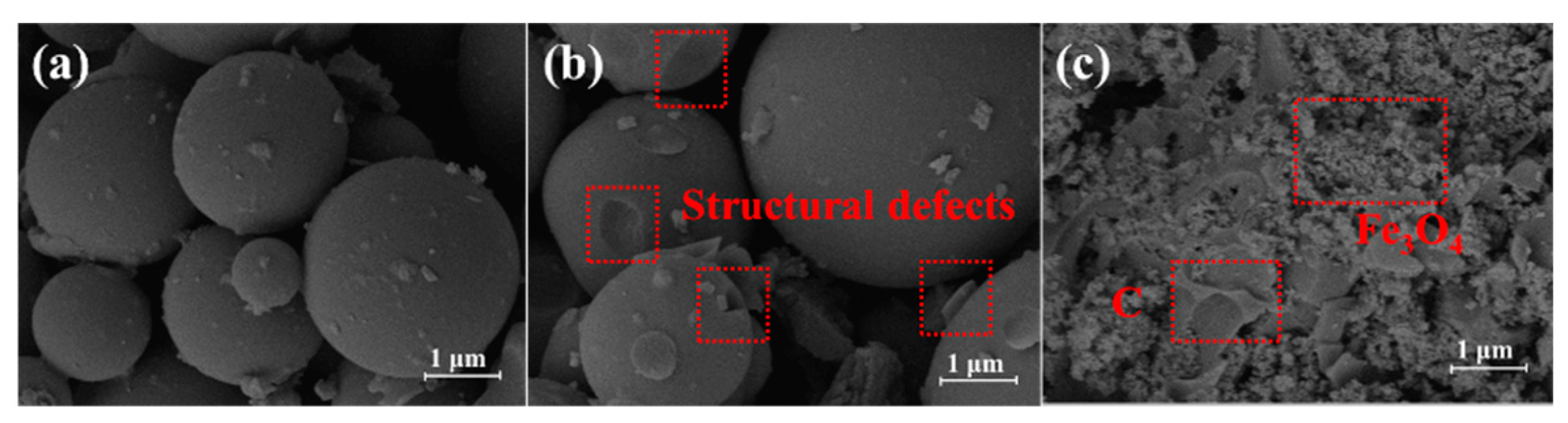
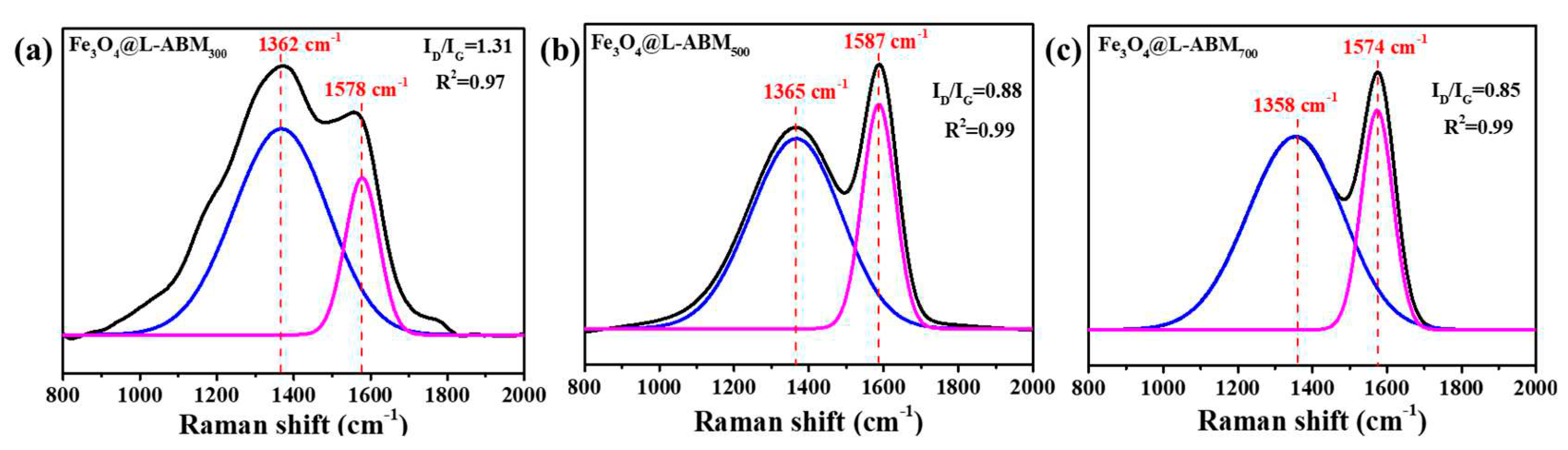
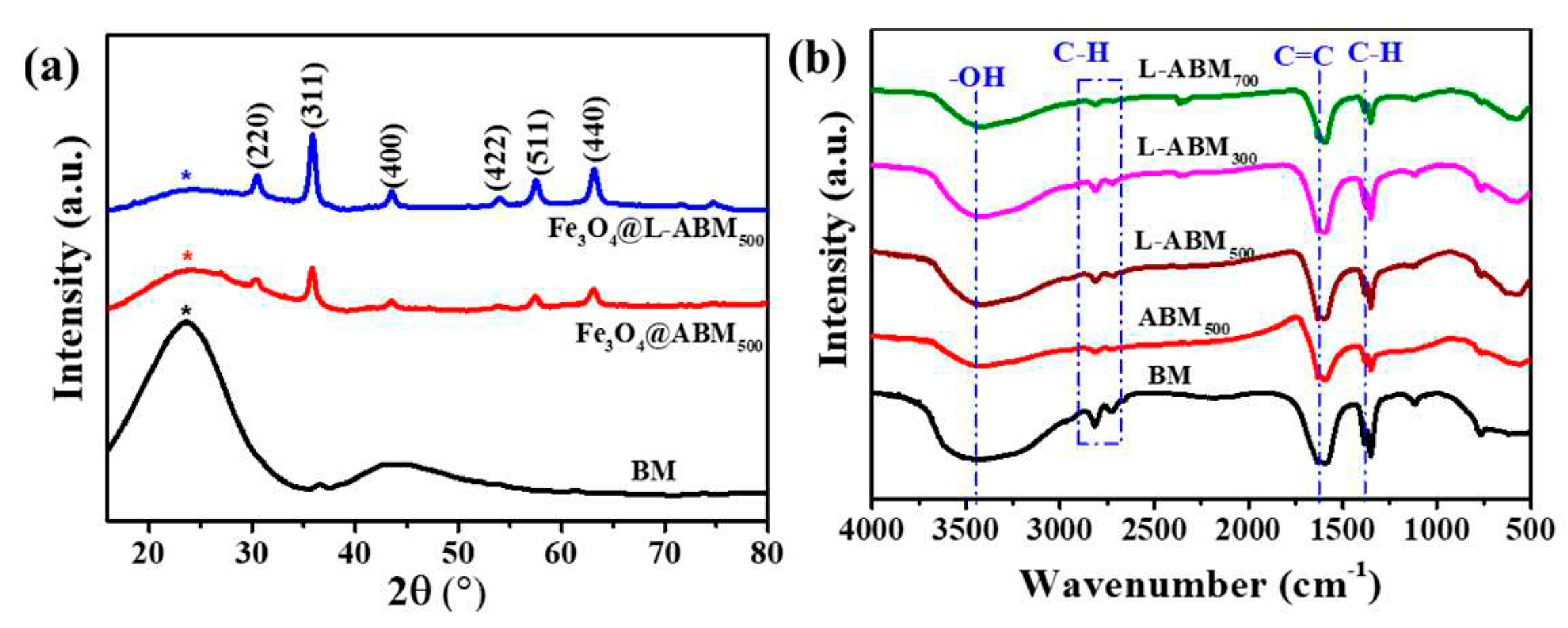
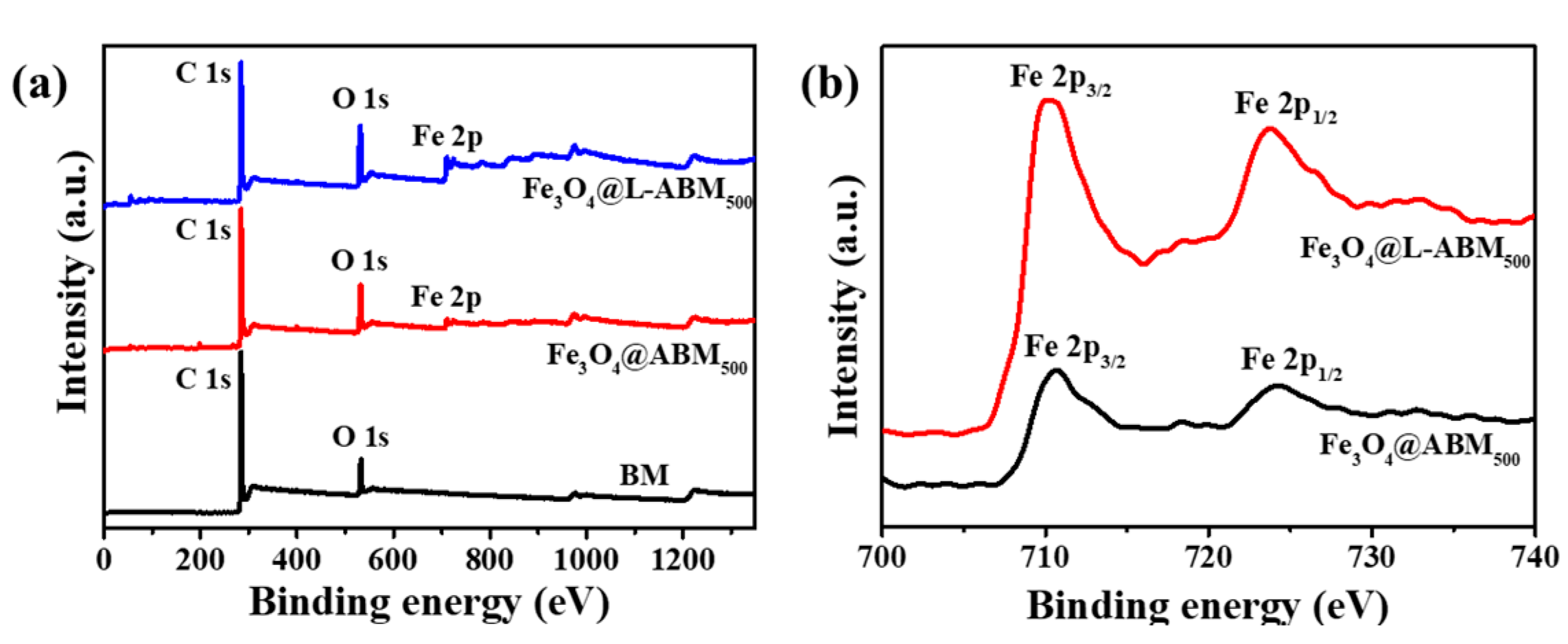
3.1. Characterizations
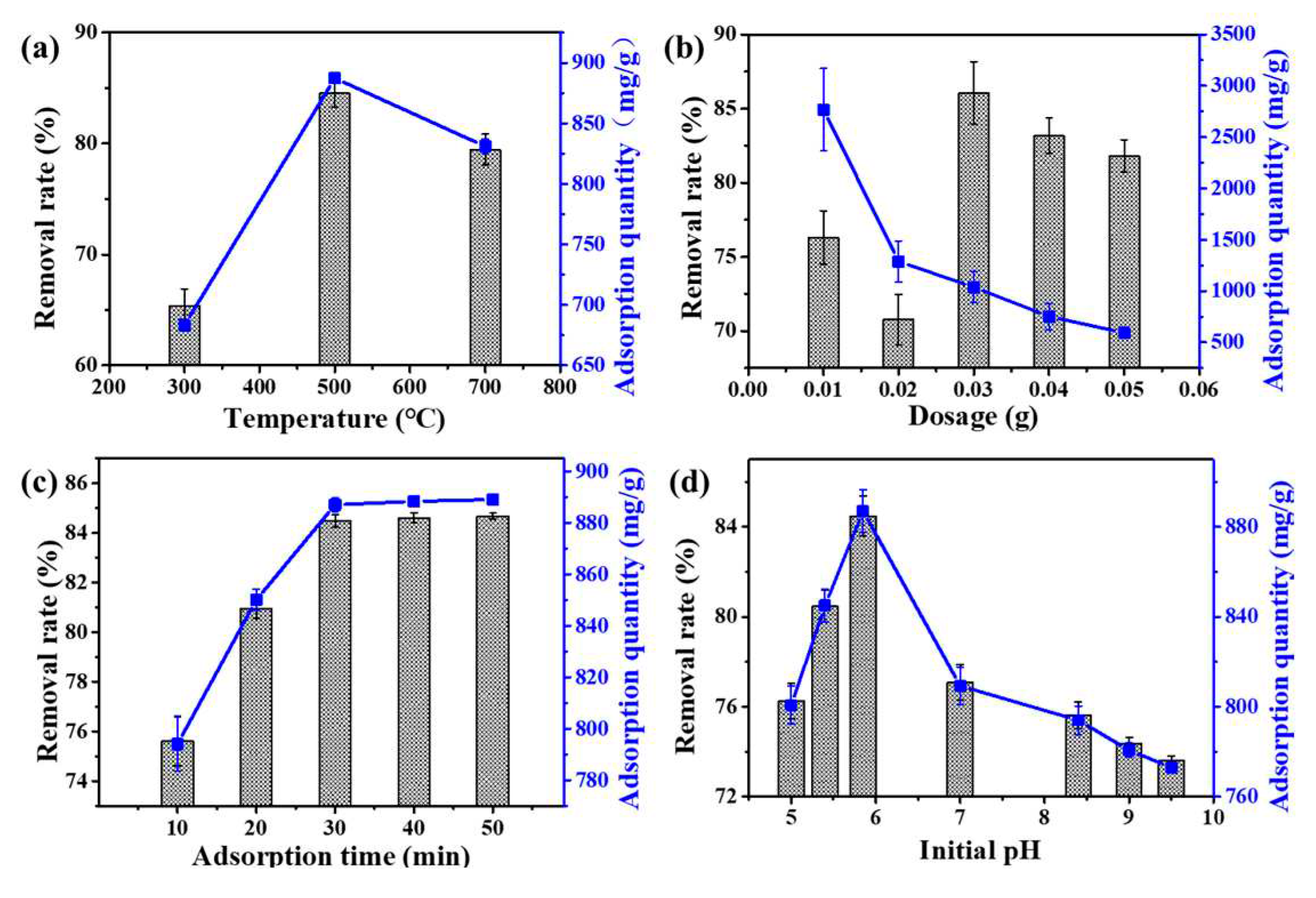
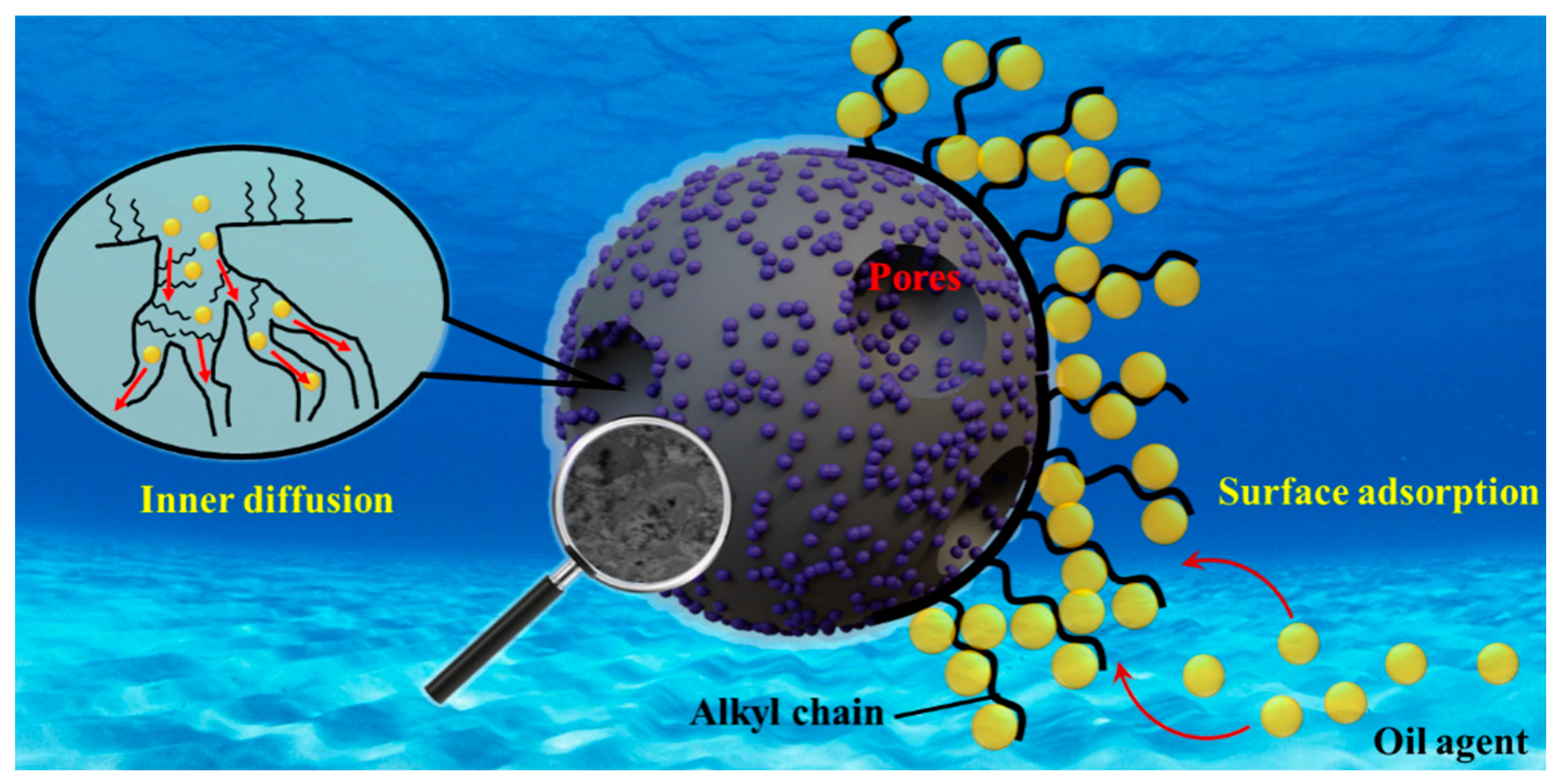
3.2. Adsorption Process Analysis
3.3. Adsorption Kinetics
4. Conclusion
Conflicts of Interest
Acknowledgments
References
- Jue, H. , Pan D., Chuanyu G.; et al. Emulsified oily wastewater treatment via fertilizer drawn forward osmosis using a corrugated thin film composite membrane. Journal of Membrane Science 2023, 685, 121926. [Google Scholar]
- Hong, C.; Anqi, Z.; Yifan, Z.; et al. Carbonaceous nanofibrous membranes with enhanced superhydrophilicity and underwater superoleophobicity for effective purification of emulsified oily wastewater. Chemical Engineering Journal 2023, 468, 143602. [Google Scholar]
- Mahsa, K.H.; Lei, L.; Parisa, K.H.; et al. ; et al. Performance evaluation of a pilot-scale membrane filtration system for oily wastewater treatment: CFD modeling and scale-up design. Journal of Water Process Engineering 2023, 52, 103570. [Google Scholar]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: a review. Bioresource technology 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Jechan, L.; Seonho, L.; Young-Kwon, P. Reduction of odor-causing compounds in wastewater using biochar: A review. Bioresource Technology 2023, 385, 129419. [Google Scholar]
- Jamiu OEniola Banu, S.; et al. Fabrication of engineered biochar-iron oxide from date palm frond for the effective removal of cationic dye from wastewater. Journal of Water Process Engineering 2023, 54, 104046. [Google Scholar]
- Haichuan, J.; Yanping, S.; Ping, G.; et al. Effect of synthetic fibers on the mechanical performance of asphalt mixture: A review. Journal of Traffic and Transportation Engineering (English Edition), 3: (3).
- Anderson FV, S.; Jonas, S.; Renata, V.; et al. Recent advances in surface modification using polydopamine for the development of photocatalytic membranes for oily wastewater treatment. Journal of Water Process Engineering 2023, 53, 103743. [Google Scholar]
- Juan, M.; Weiwei, C.; Junjie, Q.; et al. Co-pressing and co-sintering preparation of cost-effective and high-performance asymmetric ceramic membrane for oily wastewater treatment. Separation and Purification Technology 2023, 323, 124373. [Google Scholar]
- Muhammad, A.J.; Forat, Y.A.; Ali, D.S.; et al. Studying the effect of reactor design on the electrocoagulation treatment performance of oily wastewater. Heliyon 2023, 9, e17794. [Google Scholar]
- Zhai, S.; Li, M.; Wang, D.; et al. Cyano and acylamino group modification for tannery sludge bio-char: Enhancement of adsorption universality for dye pollutants. Journal of Environmental Chemical Engineering 2021, 1, 9. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Peng, H.; et al. Cost-effective resource utilization for waste biomass: A simple preparation method of photo-thermal biochar cakes (BCs) toward dye wastewater treatment with solar energy. Environmental Research 2021, 194, 110720. [Google Scholar] [CrossRef] [PubMed]
- Mcmulan, G.; Meehan, C.; Conneely, A.; et al. Microbial decolourisation and degradation of textile dyes. Applied microbiology and biotechnology 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Donkadokul, N.Y.; Kol, A.K.; Naz, I.; et al. A review on advanced physico-chemical and biological textile dye wastewater treatment techniques. Reviews in environmental science and bio/technology 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Turesky, R.J.; Gross, G.A.; Stillwell, W.G.; et al. Species differences in metabolism of heterocyclic aromatic amines, human exposure, and biomonitoring. Environmental health perspectives 1994, 102 (Suppl. 6), 47–51. [Google Scholar] [PubMed]
- Liang, J.; Bing, C.; Baiyu, Z.; Xudong, Y. Modeling marine oily wastewater treatment by a probabilistic agent-based approach. Marine Pollution Bulletin 2018, 127, 217–224. [Google Scholar]
- Navarrathn, C.M.; Bombuwala, D.N.; Keeton, C.; et al. Biochar adsorbents with enhanced hydrophobicity for oil spill removal. ACS applied materials & interfaces 2020, 12, 9248–9260. [Google Scholar]
- Wen, Q.; Chen, Y.; Rao, X.; et al. Preparation of magnesium Ferrite-Doped magnetic biochar using potassium ferrate and seawater mineral at low temperature for removal of cationic pollutants. Bioresource Technology 2022, 350, 126860. [Google Scholar] [CrossRef]
- Meng, Z.; Huang, S.; Xu, T.; et al. Competitive adsorption, immobilization, and desorption risks of Cd, Ni, and Cu in saturated-unsaturated soils by biochar under combined aging. J Hazard Mater 2022, 434, 128903. [Google Scholar] [CrossRef]
- Ferreira, C.I.A.; Calisto, V.; Otero, M.; et al. Comparative adsorption evaluation of biochars from paper mill sludge with commercial activated carbon for the removal of fish anaesthetics from water in Recirculating Aquaculture Systems. Aquacultural Engineering 2016, 74, 76–83. [Google Scholar] [CrossRef]
- Zhu, H.; Li, L.; Chen, W.; et al. Controllable synthesis of coral-like hierarchical porous magnesium hydroxide with various surface area and pore volume for lead and cadmium ion adsorption. J Hazard Mater 2021, 416, 125922. [Google Scholar] [CrossRef]
- Xiao, C.; Song, Q.; Shen, Q.; et al. Understanding on interlaminar nano-reinforcement induced mechanical performance improvement of carbon/carbon composites after silicon infiltration. Composites Part B: Engineering 2022, 239, 109946. [Google Scholar] [CrossRef]
- Alberto, E. Regazzoni. Adsorption kinetics at solid/aqueous solution interfaces: On the boundaries of the pseudo-second order rate equation. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2020, 585, 124093. [Google Scholar]
- Rohollah, E. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chemical Engineering Journal 2020, 392, 123705. [Google Scholar]
- Seongbin, G.; Nahyeon, A.; Chonghyo, J.; et al. pyAPEP: An all-in-one software package for the automated preparation of adsorption process simulations. Computer Physics Communications 2023, 291, 108830. [Google Scholar]
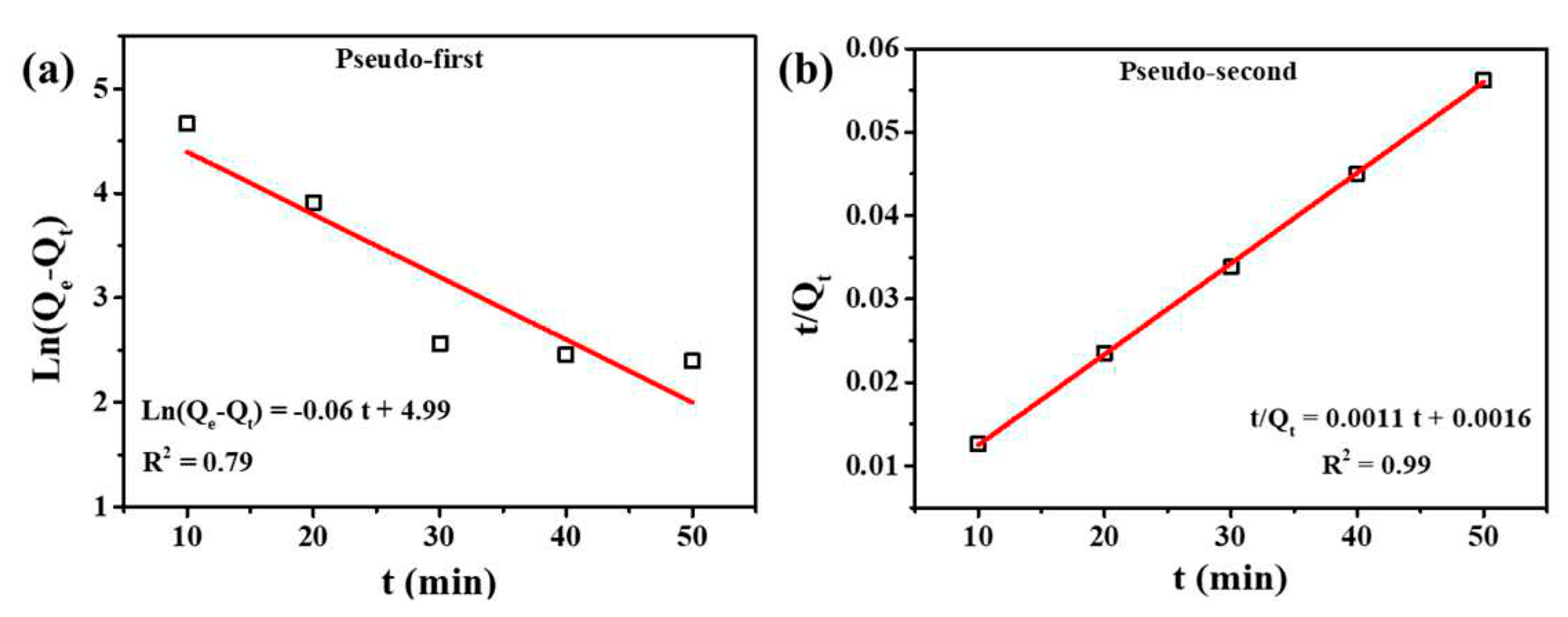
| Model | Fitting parameters | Values |
|---|---|---|
| Pseudo-first order equation | K1 (min-1) | 0.06 |
| Qe (mg/g) | 146.94 | |
| R2 | 0.79 | |
| Pseudo-second order equation | K2 (min-1) | 7.56*10^-4 |
| Qe (mg/g) | 909.11 | |
| R2 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




