Submitted:
15 August 2023
Posted:
16 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Lipids in milk fat globule membrane
3. Characterization of Lipids in Milk Fat Globule Membrane
4. Phospholipids
4.1. The promoting effect phospholipid supplementation on development
4.2. Phospholipids have a regulating effect on gut health
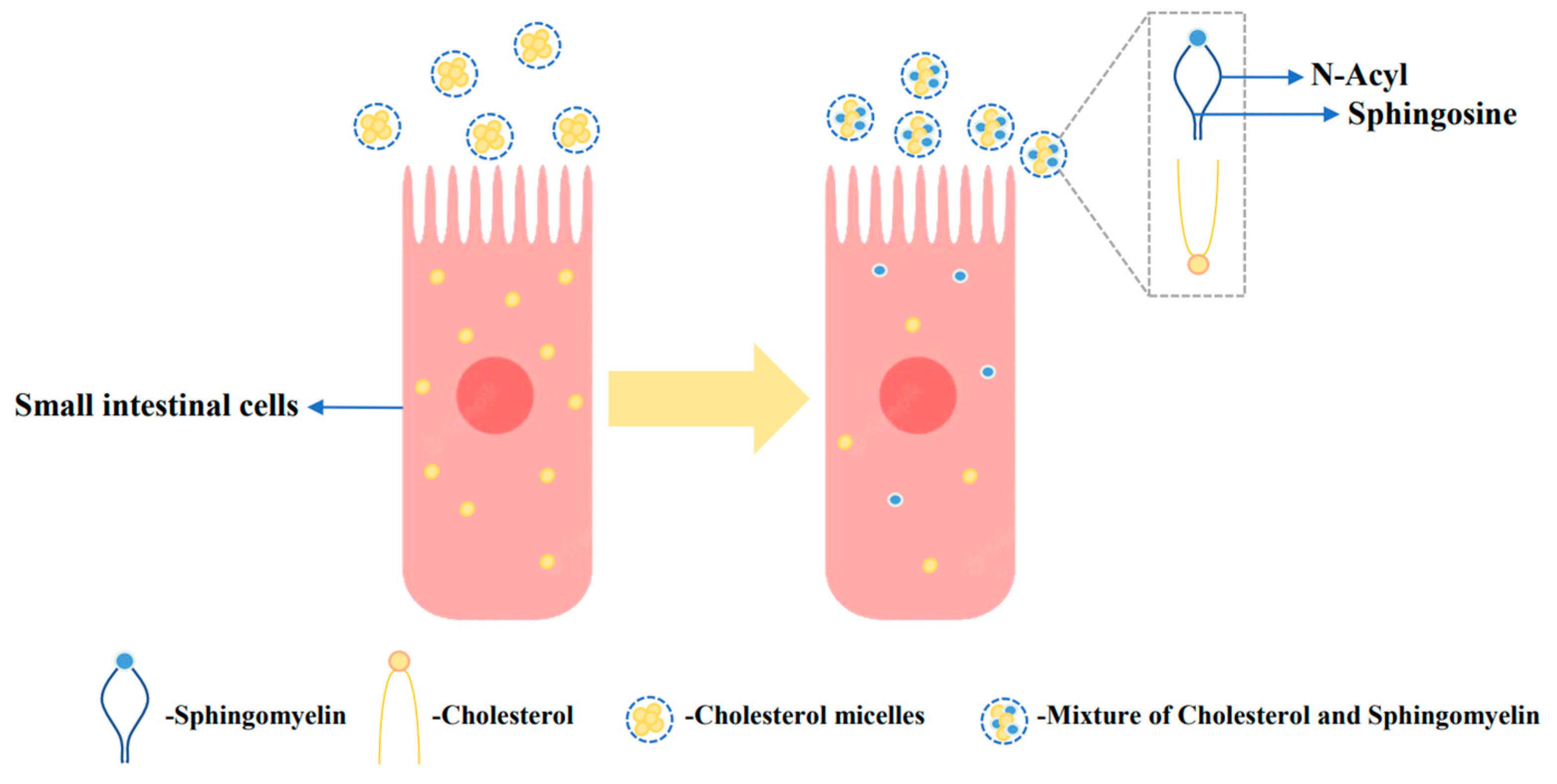
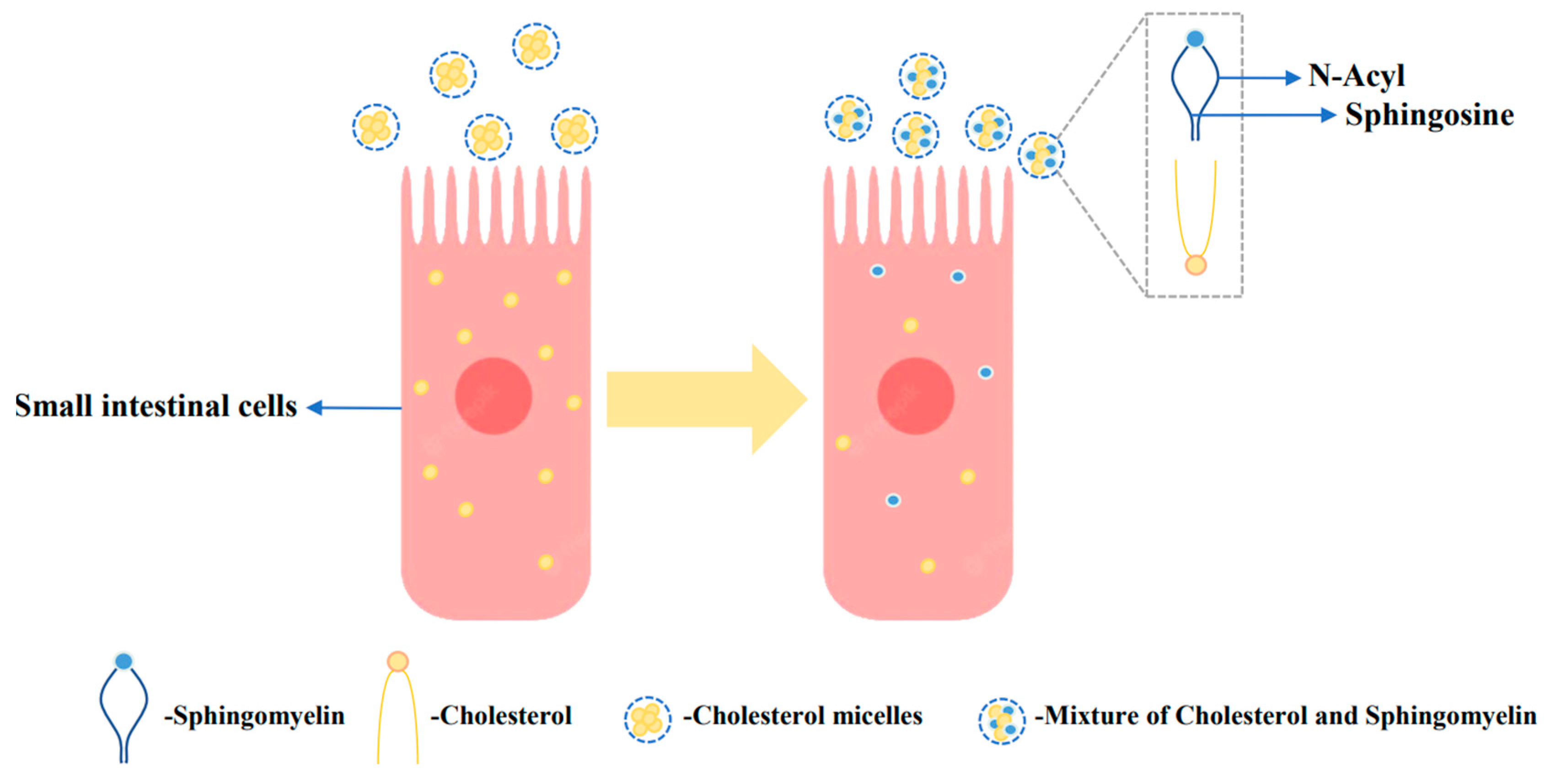
4.3. Phospholipids regulate cholesterol metabolism
4.4. Anticancer effects of dietary phospholipids
5. Gangliosides
5.1. Gangliosides promote brain development
5.2 Inhibitory effect of gangliosides on intestinal pathogenic microorganisms
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jukkola, A.; Rojas, O.J. Milk fat globules and associated membranes: Colloidal properties and processing effects. Adv. Colloid Interface Sci. 2017, 245, 92–101. [Google Scholar] [CrossRef]
- Wei, W.; Jin, Q.; Wang, X. Human milk fat substitutes: Past achievements and current trends. Prog. Lipid Res. 2019, 74, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jiménez-Flores, R.; Everett, D.W. Bovine Milk Fat Globule Membrane Proteins Are Affected By Centrifugal Washing Processes. J. Agric. Food Chem. 2013, 61, 8403–8411. [Google Scholar] [CrossRef] [PubMed]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Yano, M.; Haramizu, S.; Ota, N.; Minegishi, Y.; Shimotoyodome, A. Continuous Supplementation of Milk Fat Globule Membrane with Habitual Exercise from a Young Age Improves Motor Coordination and Skeletal Muscle Function in Aged Mice. J. Nutr. Sci. Vitaminol. 2019, 65, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiao, X.; Gao, Z.; Jiang, L.; Mu, Z. Advancement on Milk Fat Globule Membrane: Separation, Identification, and Functional Properties. Front. Nutr. 2022, 8, 807284. [Google Scholar] [CrossRef]
- Holzmüller, W.; Kulozik, U. Isolation of milk fat globule membrane (MFGM) material by coagulation and diafiltration of buttermilk. Int. Dairy J. 2016, 63, 88–91. [Google Scholar] [CrossRef]
- Lopez, C.; Cauty, C.; Guyomarc'H, F. Unraveling the Complexity of Milk Fat Globules to Tailor Bioinspired Emulsions Providing Health Benefits: The Key Role Played by the Biological Membrane. Eur. J. Lipid Sci. Technol. 2018, 121. [Google Scholar] [CrossRef]
- Anderson, J.W.; Jones, A.E.; Riddell Mason, S. Ten Different Dietary Fibers Have Significantly Different Effects on Serum and Liver Lipids of Cholesterol-Fed Rats. J. Nutr. 2002, 124, 78–83. [Google Scholar] [CrossRef]
- Hellhammer, J.; Waladkhani, A.; Hero, T.; Buss, C. Effects of milk phospholipid on memory and psychological stress response. Br. Food J. 2010, 112, 1124–1137. [Google Scholar] [CrossRef]
- Palmano, K.P.; MacGibbon, A.K.H.; Gunn, C.A.; Schollum, L.M. In Vitro and In Vivo Anti-inflammatory Activity of Bovine Milkfat Globule (MFGM)-derived Complex Lipid Fractions. Nutrients 2020, 12, 2089. [Google Scholar] [CrossRef]
- Murthy, A.V.R.; Guyomarc'H, F.; Paboeuf, G.; Vié, V.; Lopez, C. Cholesterol strongly affects the organization of lipid monolayers studied as models of the milk fat globule membrane: Condensing effect and change in the lipid domain morphology. Biochim. et Biophys. Acta (BBA) - Biomembr. 2015, 1848, 2308–2316. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Pryce, J.; Rochfort, S. Comprehensive Characterization of Bovine Milk Lipids: Phospholipids, Sphingolipids, Glycolipids, and Ceramides. J. Agric. Food Chem. 2020, 68, 6726–6738. [Google Scholar] [CrossRef]
- Calvo, M.V.; Martín-Hernández, M.C.; García-Serrano, A.; Castro-Gómez, M.P.; Alonso-Miravalles, L.; García-Martín, R.; Megino-Tello, J.; Alonso, L.; Fontecha, J. Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. J. Food Compos. Anal. 2019, 86, 103386. [Google Scholar] [CrossRef]
- Ferreiro, T.; Gayoso, L.; Rodríguez-Otero, J. Milk phospholipids: Organic milk and milk rich in conjugated linoleic acid compared with conventional milk. J. Dairy Sci. 2015, 98, 9–14. [Google Scholar] [CrossRef]
- Raza, G.S.; Herzig, K.-H.; Leppäluoto, J. Invited review: Milk fat globule membrane—A possible panacea for neurodevelopment, infections, cardiometabolic diseases, and frailty. J. Dairy Sci. 2021, 104, 7345–7363. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, G.; Xiang, J.; Zou, X.; Jin, Q.; Wang, X. Lipid composition and structural characteristics of bovine, caprine and human milk fat globules. Int. Dairy J. 2016, 56, 64–73. [Google Scholar] [CrossRef]
- Beare-Rogers, J.L.; Dieffenbacher, A.; Holm, J.V. Lexicon of lipid nutrition (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 685–744. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef]
- Wang, L.; Shimizu, Y.; Kaneko, S.; Hanaka, S.; Abe, T.; Shimasaki, H.; Hisaki, H.; Nakajima, H. Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatr. Int. 2000, 42, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- Lopez, C.; Madec, M.-N.; Jimenez-Flores, R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem. 2010, 120, 22–33. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. FUNCTIONS OF LIPID RAFTS IN BIOLOGICAL MEMBRANES. Annu. Rev. Cell Dev. Biol. 1998, 14, 111–136. [Google Scholar] [CrossRef]
- Lopez, C.; Cheng, K.; Perez, J. Thermotropic phase behavior of milk sphingomyelin and role of cholesterol in the formation of the liquid ordered phase examined using SR-XRD and DSC. Chem. Phys. Lipids 2018, 215, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Et-Thakafy, O.; Guyomarc’h, F.; Lopez, C. Lipid domains in the milk fat globule membrane: Dynamics investigated in situ in milk in relation to temperature and time. Food Chem. 2017, 220, 352–361. [Google Scholar] [CrossRef]
- Zou, X.-Q.; Guo, Z.; Huang, J.-H.; Jin, Q.-Z.; Cheong, L.-Z.; Wang, X.-G.; Xu, X.-B. Human Milk Fat Globules from Different Stages of Lactation: A Lipid Composition Analysis and Microstructure Characterization. J. Agric. Food Chem. 2012, 60, 7158–7167. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Guo, Z.; Jin, Q.; Huang, J.; Cheong, L.; Xu, X.; Wang, X. Composition and microstructure of colostrum and mature bovine milk fat globule membrane. Food Chem. 2015, 185, 362–370. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Selvalatchmanan, J.; Torta, F.; Bendt, A.K.; Wenk, M.R.; Murray, K.; Wlodek, M.E.; Geddes, D.T. Healthy Breastfeeding Infants Consume Different Quantities of Milk Fat Globule Membrane Lipids. Nutrients 2021, 13, 2951. [Google Scholar] [CrossRef]
- Ali, A.H.; Wei, W.; Abed, S.M.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Jin, Q.; Wang, X. Impact of technological processes on buffalo and bovine milk fat crystallization behavior and milk fat globule membrane phospholipids profile. Lwt 2018, 90, 424–432. [Google Scholar] [CrossRef]
- Brink, L.R.; Herren, A.W.; McMillen, S.; Fraser, K.; Agnew, M.; Roy, N.; Lönnerdal, B. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J. Dairy Sci. 2020, 103, 3002–3016. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of Dietary Sphingomyelin on Central Nervous System Myelination in Developing Rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef]
- Babin, F.; Sarda, P.; Limasset, B.; Descomps, B.; Rieu, D.; Mendy, F.; de Paulet, A.C. Nervonic acid in red blood cell sphingomyelin in premature infants: An index of myelin maturation? Lipids 1993, 28, 627–630. [Google Scholar] [CrossRef]
- Deoni, S.; Dean, D.; Joelson, S.; O'Regan, J.; Schneider, N. Early nutrition influences developmental myelination and cognition in infants and young children. NeuroImage 2018, 178, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.-J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar] [CrossRef]
- Schubert, M.; Contreras, C.; Franz, N.; Hellhammer, J. Milk-based phospholipids increase morning cortisol availability and improve memory in chronically stressed men. Nutr. Res. 2011, 31, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Radlowski, E.C.; Conrad, M.S.; Li, Y.; Dilger, R.N.; Johnson, R.W. Early Supplementation of Phospholipids and Gangliosides Affects Brain and Cognitive Development in Neonatal Piglets. J. Nutr. 2014, 144, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; MacGibbon, A.; Fong, B.; Zhang, R.; Liu, K.; Rowan, A.; McJarrow, P. Long-Term Supplementation with Beta Serum Concentrate (BSC), a Complex of Milk Lipids, during Post-Natal Brain Development Improves Memory in Rats. Nutrients 2015, 7, 4526–4541. [Google Scholar] [CrossRef]
- Haramizu, S.; Mori, T.; Yano, M.; Ota, N.; Hashizume, K.; Otsuka, A.; Hase, T.; Shimotoyodome, A. Habitual exercise plus dietary supplementation with milk fat globule membrane improves muscle function deficits via neuromuscular development in senescence-accelerated mice. SpringerPlus 2014, 3, 1–17. [Google Scholar] [CrossRef]
- Kim, H.; Suzuki, T.; Kim, M.; Kojima, N.; Ota, N.; Shimotoyodome, A.; Hase, T.; Hosoi, E.; Yoshida, H. Effects of Exercise and Milk Fat Globule Membrane (MFGM) Supplementation on Body Composition, Physical Function, and Hematological Parameters in Community-Dwelling Frail Japanese Women: A Randomized Double Blind, Placebo-Controlled, Follow-Up Trial. PLOS ONE 2015, 10, e0116256–e0116256. [Google Scholar] [CrossRef]
- Herrmann, F.; Nieto-Ruiz, A.; Sepúlveda-Valbuena, N.; Miranda, M.T.; Diéguez, E.; Jiménez, J.; De-Castellar, R.; García-Ricobaraza, M.; García-Santos, J.A.; Bermúdez, M.G.; et al. Infant formula enriched with milk fat globule membrane, long-chain polyunsaturated fatty acids, synbiotics, gangliosides, nucleotides and sialic acid reduces infections during the first 18 months of life: The COGNIS study. J. Funct. Foods 2021, 83, 104529. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Singh, P.; Liu, Y.; Medina-Morales, E.; Yakah, W.; Freedman, S.D.; Martin, C.R. Breast Milk Lipids and Fatty Acids in Regulating Neonatal Intestinal Development and Protecting against Intestinal Injury. Nutrients 2020, 12, 534. [Google Scholar] [CrossRef]
- Billeaud, C.; Puccio, G.; Saliba, E.; Guillois, B.; Vaysse, C.; Pecquet, S.; Steenhout, P. Safety and Tolerance Evaluation of Milk Fat Globule Membrane-Enriched Infant Formulas: A Randomized Controlled Multicenter Non-Inferiority Trial in Healthy Term Infants. Clin. Med. Insights: Pediatr. 2014, 8, 51–60. [Google Scholar] [CrossRef]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficacy of an MFGM-enriched Complementary Food in Diarrhea, Anemia, and Micronutrient Status in Infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Marciniak, A.; Jiménez-Flores, R. Milk fat globule membrane phospholipids modify adhesion of Lactobacillus to mucus-producing Caco-2/Goblet cells by altering the cell envelope. Food Res. Int. 2021, 146, 110471. [Google Scholar] [CrossRef]
- Cichosz, G.; Czeczot, H.; Bielecka, M. The anticarcinogenic potential of milk fat. J. Neurol. Sci. 2020, 27, 512–518. [Google Scholar] [CrossRef]
- Bruno, R.S.; Pokala, A.; Torres-Gonzalez, M.; Blesso, C.N. Cardiometabolic health benefits of dairy-milk polar lipids. Nutr. Rev. 2021, 79, 16–35. [Google Scholar] [CrossRef]
- Noh, S.K.; Koo, S.I. Milk Sphingomyelin Is More Effective than Egg Sphingomyelin in Inhibiting Intestinal Absorption of Cholesterol and Fat in Rats. J. Nutr. 2004, 134, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Maulik, P.R.; Shipley, G.G. N-Palmitoyl Sphingomyelin Bilayers: Structure and Interactions with Cholesterol and Dipalmitoylphosphatidylcholine. Biochemistry 1996, 35, 8025–8034. [Google Scholar] [CrossRef] [PubMed]
- Conway, V.; Couture, P.; Richard, C.; Gauthier, S.; Pouliot, Y.; Lamarche, B. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, E.R.; Wang, D.Q.-H.; Donovan, J.M.; Carey, M.C. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology 2002, 122, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Smedman, A.; Lindmark-Månsson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Risérus, U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: a randomized study. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Drai, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M.; et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: towards a gut sphingomyelin-cholesterol interplay. Gut 2019, 69, 487–501. [Google Scholar] [CrossRef]
- Schmelz, E.M.; Sullards, M.C.; Dillehay, D.L.; Merrill, A.H. Colonic Cell Proliferation and Aberrant Crypt Foci Formation Are Inhibited by Dairy Glycosphingolipids in 1,2-Dimethylhydrazine-Treated CF1 Mice. J. Nutr. 2000, 130, 522–527. [Google Scholar] [CrossRef]
- Vesper, H.; Schmelz, E.-M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H. Sphingolipids in Food and the Emerging Importance of Sphingolipids to Nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef]
- Zanabria, R.; Tellez, A.M.; Griffiths, M.; Corredig, M. Milk fat globule membrane isolate induces apoptosis in HT-29 human colon cancer cells. Food Funct. 2012, 4, 222–230. [Google Scholar] [CrossRef]
- Castro-Gómez, M.; Rodriguez-Alcalá, L.; Calvo, M.; Romero, J.; Mendiola, J.; Ibañez, E.; Fontecha, J. Total milk fat extraction and quantification of polar and neutral lipids of cow, goat, and ewe milk by using a pressurized liquid system and chromatographic techniques. J. Dairy Sci. 2014, 97, 6719–6728. [Google Scholar] [CrossRef]
- Castro-Gómez, M.; Rodriguez-Alcalá, L.; Calvo, M.; Romero, J.; Mendiola, J.; Ibañez, E.; Fontecha, J. Total milk fat extraction and quantification of polar and neutral lipids of cow, goat, and ewe milk by using a pressurized liquid system and chromatographic techniques. J. Dairy Sci. 2014, 97, 6719–6728. [Google Scholar] [CrossRef]
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Krägeloh, C.U.; Breier, B.H.; Davison, M.; Fong, B.; Norris, C.; McJarrow, P.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; E Rice, G.; Mitchell, M.D. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr. Res. 2013, 33, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Guillermo, R.B.; Yang, P.; Vickers, M.H.; McJarrow, P.; Guan, J. Supplementation with complex milk lipids during brain development promotes neuroplasticity without altering myelination or vascular density. Food Nutr. Res. 2015, 59, 25765. [Google Scholar] [CrossRef]
- Rohrhofer, J.; Zwirzitz, B.; Selberherr, E.; Untersmayr, E. The Impact of Dietary Sphingolipids on Intestinal Microbiota and Gastrointestinal Immune Homeostasis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Otnaess, A.B.; Laegreid, A.; Ertresvåg, K. Inhibition of enterotoxin from Escherichia coli and Vibrio cholerae by gangliosides from human milk. Infect. Immun. 1983, 40, 563–569. [Google Scholar] [CrossRef]
- Idota, T.; Kawakami, H.; Nakajima, I. Growth-promoting Effects ofN-Acetylneuraminic Acid-containing Substances on Bifidobacteria. Biosci. Biotechnol. Biochem. 1994, 58, 1720–1722. [Google Scholar] [CrossRef]
- Rueda, R.; Sabatel, J.L.; Maldonado, J.; Molina-Font, J.A.; Gil, A. Addition of gangliosides to an adapted milk formula modifies levels of fecal Escherichia coli in preterm newborn infants. J. Pediatr. 1998, 133, 90–94. [Google Scholar] [CrossRef]
- Idota, T.; Kawakami, H.; Murakami, Y.; Sugawara, M. Inhibition of Cholera Toxin by Human Milk Fractions and Sialyllactose. Biosci. Biotechnol. Biochem. 1995, 59, 417–419. [Google Scholar] [CrossRef]
- Yoshinaka, Y.; Soga, S.; Ota, N.; Yokoyama, K.; Yamada, Y.; Kimura, M. Light rhythmic exercise with dietary milk fat globule membrane improves physical fitness in an elderly Japanese population: a double-blind randomized placebo-controlled trial. Biosci. Biotechnol. Biochem. 2018, 82, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.R.; Jimenez-Flores, R.; Ward, R.E.; Cambell, J.; Young, M.J.; Nemere, I.; Hintze, K.J. Dietary Milk Fat Globule Membrane Reduces the Incidence of Aberrant Crypt Foci in Fischer-344 Rats. J. Agric. Food Chem. 2010, 58, 2157–2163. [Google Scholar] [CrossRef]
| Lipid | Participants | Dose | Time | Results | Reference | |
|---|---|---|---|---|---|---|
| Sphingomyelin | Low-birth-weight preterm babies | 20% of total phospholipids in milk | 18 months | Supplementation of sphingomyelin in milk has a positive effect on the neurobehavioral development of low-birth-weight preterm infants. | [32] | |
| Sphingomyelin | Wistar Rat | 810 mg/100g Sphingomyelin/diet | 28 days | Sphingomyelin contributes to myelination in developing rats. | [33] | |
| Sphingomyelin and Phosphatidylcholine | Children aged 0-5 | 62mg/L 85mg/L | 90 days | Sphingomyelin and phosphatidylcholine have significant effects on neural and cognitive development. | [35] | |
| Phospholipids | Healthy preschool children aged 2.5 to 6 years | 250mg/100mL | 6 months | High phospholipid concentration in milk is beneficial to children's behavior regulation, and the frequency of fever is significantly reduced. | [36] | |
| Phospholipids | Infants aged ≤14 days | 647mg/L | 4 months | Diarrhea, vomiting, ear infections, conjunctivitis, and eczema were significantly reduced in infants fed the milk fat globule membrane phospholipid formula. | [44] | |
| Sphingomyelin | Male Sprague-Dawley Rat | 19.5±1.4% dose | 7 weeks | Compared with egg-origin sphingomyelin, milk-origin sphingomyelin had a stronger effect in inhibiting the absorption of fat and cholesterol in the rat intestinal tract. | [49] | |
| Phospholipids | Menopausal women | 0.3, 0.5g/day | 4 weeks | Phospholipids may reduce specific interactions involved in cholesterol absorption in the gut. | [54] | |
| Phospholipids | Overweight men and women | 40g/day | 8 weeks | Milk-derived phospholipids significantly reduce fasting and postprandial plasma cholesterol concentrations. Milk fat enclosed by MFGM does not impair lipoprotein profiles. | [53] | |
| Phospholipids | Men and women with serum low-density lipoprotein cholesterol (LDL-C) <5.0 mmol/L | 187.5mg/day | 8 weeks | The intake of phospholipids reduces cholesterol levels in the body, mainly by inhibiting the absorption of cholesterol in the gut. | [51] | |
| Sphingomyelin and Phosphatidylserine | Healthy men with an average age of 41.5 years | 13.5g/day- | 3 weeks | High doses of phospholipids can dampen the activity and reactivity of the hypothalamic-pituitary-adrenal axis (HPAA) and make subject a blunted psychological stress response. | [11] | |
| Phospholipids, Sphingolipids and Exercise | 15-week-old male SAMP1 and ICR rat | 356 ± 9 mg/day diet (contain 16.6% phospholipids) | 28 weeks | Milk fat globule membrane combined with exercise can improves muscle function deficits. | [40] | |
| Phospholipids and exercise | Seniors aged 71-75 | 1g tablet contain 16% phospholipid milk fat globule membrane per day | 8 weeks | Participants taking globular membrane tablets performed better in tapping and stepping. | [69] | |
| Phospholipids and exercise | Older women aged 82-84 | 1g milk fat globule membrane tablet per day | 12 weeks | Exercise and phospholipid supplementation may improve frailty in older adults. | [41] | |
| Ganglioside | -- | 2mL breast milk | -- | Gangliosides in breast milk protect infants from diarrhea caused by enterotoxins. | [65] | |
| Ganglioside | Preterm infants | 1.43mg/100 kcal | 30 days | Formulas supplemented with gangliosides can promote the growth of bifidobacteria, thereby inhibiting the growth of E. coli and other potentially pathogenic microorganisms in the gut of premature infants. | [67] | |
| Phospholipids andGangliosides | Piglets | 0.8 or 2.5% Lacprodan PL-20 | 26 days | Supplementation with gangliosides and phospholipids improved spatial learning in piglets and affected brain development. | [38] | |
| Ganglioside | Infants aged 2 to 8 weeks | 11~12 μg/mL | 16 weeks | Formula with increased ganglioside content in the diet is beneficial for cognitive development in healthy infants aged 0-6 months. | [61] | |
| Ganglioside | Wistar Rat | 0.2%, 1.0%CML | 80 days | Dietary gangliosides benefit cognitive development in infants. | [60] | |
| Sphingomyelin and glycosphingolipids | Female CF1 Rat | 0.025或0.1 g/100 g | 4 weeks | Milk-derived sphingolipids and glycosphingolipids can suppress colon cancer in female mice at an early stage, reducing the appearance of aberrant crypt foci. | [55] | |
| Sphingomyelin | Male Fischer-344 Rat | 0.11% w/w | 13 weeks | Diets containing sphingomyelin are protective against colon cancer in Fischer-344 rats. | [70] | |
| Polar lipids (%) | Bovine | Goat | Human |
|---|---|---|---|
| PI | 8.97 ± 0.06 | 9.37 ± 0.06 | 7.85 ± 0.07 |
| PC | 33.12 ± 0.21 | 31.64 ± 0.21 | 24.39 ± 0.12 |
| PS | 9.07 ± 0.07 | 14.03 ± 0.05 | 13.12 ± 0.03 |
| PE | 23.42 ± 0.13 | 19.92 ± 0.10 | 25.33 ± 0.14 |
| SM | 25.40 ± 0.19 | 25.04 ± 0.17 | 29.28 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





