1. Introduction
In the past two decades, quinolones, as veterinary drugs with antibacterial effects, have been widely used in animal husbandry and aquatic products industries, mainly including enrofloxacin (A), ciprofloxacin (B), ofloxacin (C), fleroxacin (D), sparfloxacin (E), enoxacin (F), gatifloxacin (G) and nadifloxacin(H), etc [
1,
2,
3,
4,
5]. If the quinolones in aquatic products and other animal foods exceeds the maximum residue limit (MRL), it will cause serious harm to consumers' health [
6]. In fact, the phenomenon of the quinolones exceeding their MRL in the foods is not uncommon. For example, according to relevant literature reports in 2020, enrofloxacin, ciprofloxacin, and ofloxacin were found to exceed their MRL in some aquatic products, eggs, and pork [
6,
7,
8,
9].To prevent this phenomenon from occurring, it is necessary to establish a rapid limit test method for the residues in these foods.
In China's national food standards, the MRLs of the eight quinolones (A, B, C, D, E, F, G, H) in these foods are specified as 100.0, 100.0, 2.0, 5.0, 5.0, 5.0, 5.0, 5.0 μg/kg, respectively, and these residues are usually determined by UPLC-MS/MS.Despite its salient advantages of high sensitivity and strong specificity in quantitative and qualitative analysis, the method (UPLC-MS/MS) still require complicated sample pretreatment, as well as hours or even days for the completion of the whole analysis process [
2,
3,
4,
5,
6].
Traditional thin-layer chromatography (TLC) is a fast and convenient method for separating chemical components from complex matrices.With the help of auxiliary means such as chemical color development and ultraviolet light irradiation, this method can only reflect the chemical structural characteristics of a certain functional group of the testing component, and its sensitivity is also relatively low [10–13], so it cannot be directly used for limit test of the residues in the food.
Analysis technology based on Raman spectroscopy is a new method that can be used for chemical composition rapid detection. The Raman spectroscopy can show the inelastic scattering phenomenon of compound molecules after being irradiated by laser, so the fingerprint structure information of the compounds can be reflected by the spectroscopy, and the spectroscopy is almost not affected by water and silica gel under experimental conditions. Although this method has high specificity, its sensitivity is relatively low. In order to meet the analysis of some trace components, the sensitivity can be increased by more than 10,000 times through surface enhanced Raman spectroscopy (SERS) [
10,
11,
12,
13]. In recent years, it was reported that components in complex matrix were separated rapidly by TLC, and the separated component was detected specifically by the SERS [
14,
15,
16,
17,
18,
19,
20].
The purpose of this study was to develop a new limit test method by combining the TLC with the SERS, abbreviating it as TLC-SERS. The eight quinolones residues in the foods can be rapidly sepseparated and specifically detectd by the TLC-SERS, so it will provide a new reference basis for the rapid analysis of the harmful residues in the food.
2. Materials and Methods
2.1. Materials
All reagents were analytical grade and were bought from Merck Drugs and Co, Ger-many in Germany. The reference substances of A (98%), B (82.1%), C (99.7%), D (99.2%), E (99.4%), F(91.5%), G (97.2%) and H (97%) were purchased from China Food and Drug Control Institute, and anhydrous ethanol (99.5%) was used to dissolve these quinolone compounds. There were ten batches of real samples of aquatic products which were supplied by five different manufacturers (China), and the ten real samples of other animal foods were supplied by the other manufacturers (China). Anhydrous sodium sulfate was used to remove protein from the food,and acetonitrile (99.5%) was used to extract the quinolone residues from the food. Dichloromethane (99.5%) and methanol (99.5%) were used as developing agents in the TLC. Silver nitrate and sodium citrate were used to prepare the SERS active substrates.
The TLC could be obtained by a thin-layer plate (Merck KGaA, Darmstadt, Germany) that is composed of high-performance silica gel and fluorescing additive F254. The plate is called GF254 thin-layer plate for short, the layer thickness is 0.2 ± 0.03 mm, the particle size is 8 ± 2 μm and the carrier is aluminum. The micro injector (10 μL) used for spotting on thin-layer plates was purchased from Zhenhai Glass Instrument Factory, Ningbo, China.
2.2. Apparatus and Conditions
SERS or Raman spectra of the quinolone components were obtained by use of a DXR™ xi Raman Imaging Microscope (Thermo Fisher Scientific, Waltham, MA, USA) with a laser excitation wavelength of 532 nm, a resolution of 5.0 cm−1, and a 10× long working distance microscope objective. The excitation power was 10 mW, the integration time was 0.5 s, and the number of scans was 20. The scan range was 3300–100 cm−1, with a 50 μm confocal pinhole DXR532 full range grating (400 line/mm). The detector was a TE-cooled electron-multiplying CCD (EMCCD). Point scanning was chosen as the scanning mode.
Ultraviolet analyzer (YOKO-2F, Wuhan YOKO Technology Ltd., in China) was used to mark principal spot on the TLC under 254 nm. Ultraviolet Visible spectrophotometer (T6, Beijing Puxi General Instrument Co., Ltd, in China) was used to detect the ultraviolet absorption spectrum of the SERS active substrates. Transmission Electron Microscope (HT 7700, Beijing Shengjiachen Ke & Trade Co., Ltd, in China) was used to characterize the particle appearance of the substrates. Nanoparticle size analyzer (Nicomp 380 ZLS, Shanghai of meijia Co., Ltd, in china)was used to measure the particle size and the particle Zeat potential.
Ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) was operated on a Dionex UltiMate 3000 ultra-performance liquid chromatography—TSQ quantum mass spectrometer system (ULTivo G6465A, Agilient.com.USA). The limit test results of the residues in real samples by the TLC-SERS were verified by the UPLC-MS/MS, and A ,B, C, D, E, F, G, and H were separated by gradient elution using a Kromasil C18 column (100 × 2.1 mm × 1.8 μm) with a mobile phase at a flow rate of 0.2 mL/min. The elution procedure was as follows: 0~3 min, 78%A, 20%B, 2%C; 3~6 min, 75%A, 20%B, 5%C; 6~8 min, 70%A, 20%B, 10%C; 8~13 min, 40%A, 20%B, 40%C; 13~13.1 min, 40%A, 20%B, 40%C; 13.1~16 min, 78%A, 20%B, 2%C; 16 min, 78%A, 20%B, 2%C ( A: 0.1% formic acid solution containing 5.0 mmol/L ammonium acetate; B: methanol; C:acetonitrile ).The column temperature was the same as the room temperature. ESI positive-ion in MRM mode was used to monitor the precursor ion→product ion transitions m/z 360→316 (A), 332→288 (B), 362→318 (C), 370→326 (D), 393→349 (E), 321→303 (F), 376.2→332.2 (G) and 361→343.2(H).
2.3. Solutions Preparation
The SERS active substrates : in order to obtain SERS of eight quinolones, it is needed that a SERS active substrates was prepared,it is also called as nanometer silver solution or surface enhancer. When 56 mg of silver nitrate was dissolved in 150 mL of water , a mixed solution was obtained by adding evenly 4 mL of 1% sodium citrate solution to the silver nitrate solution, and the nanometer silver solution was prepared by heating the mixed solution in a microwave oven until it boiled for 5 minutes, cooling it to room temperature. This solution was stored at 4 ℃ to ensure its stability.
Reference substance solution: for example, the preparation method of solution Enrofloxacin(A) is to dissolve an appropriate amount of reference substance A in anhydrous ethanol to a concentration of 1.00µg/mL. With the same method, the solutions of the other quinolones (B, C, D, E, F, G and H) were also prepared to the concentrations of 1.00µg/mL.
Mixed reference substances solution 1:in order to detect the degree of separation of the eight quinolones on the TLC, a mixture solution was prepareded by taking respectively 1.00 mL above reference ( A, B, C, D, E, F, G and H) solution into the same container. After the mixture solution was dryed in a water bath (85 °C), it was redissolved by the 1.00 mL anhydrous ethanol to obtain the mixed reference substance solution 1.
Mixed reference substances solution 2: according to the MRL of the quinolones in the food, the mixed reference substances solution was prepared by dissolving an appropriate amount of A, B, C, D, E, F, G and H together in the same portion of anhydrous ethanol. In the solution, the concentrations of various substance (A, B, C, D, E, F, G and H) were in order of 400.0, 400.0, 8.0, 20.0, 20.0, 20.0, 20.0 and 20.0 ng/mL.
The sample solutions: in order to accurately detect SERS of the quinolones in the food, it was need that 2.00 g the food and 10 g Na2SO4 powder and 10 mL of anhydrous ethanol were placed in the same centrifuge tube, the residues was extracted from the food substrate by sonicate method for 15 min. After centrifugation under 4000 rpm/min for 5 min, the supernatant that may contain the quinolones was obtained in the centrifuge tube. Passing the supernatant through the filter membranes (0.22 um), a filtrate was obtained. Concentrating the filtrate in a water bath (85 °C) to approximately 1 mL, transferring it to a chromatographic vial. Evaporating all the solvent in the vial in a water bath, the residues of the food was redissolved by 500.0 μL anhydrous ethanol to obtain the sample solution.
2.4. The TLC
TLC is a simple and fast separation technique. The eight quinolones could be preliminarily separated by the following methods: The 8.0 µL of the reference substance solutions and the mixture reference substance solution 1 were spotted on a GF254 thin-layer plate (10 cm × 10 cm) at a distance of 1 cm from the bottom, separately. When the plate were eluted to a distance of 8 cm by dichloromethane- methanol (5:1) in a glass container, it should be removed from the container, and the agent Rf on the plate should be naturally evaporated. Irradiating at 254 nm, the main spots on the thin layer chromatogram could be observed. Using fleroxacin (D) as object of reference, the relative Rf of A, B, C, D, E, F, G and H could be measured by these spots, respectively.
2.5. The TLC-SERS
In the study, we focused on developing a rapid and specific limit test method by the TLC-SERS to control the eight quinolone residues in the food. According to the above TLC, the 8.0 µL of the fleroxacin (D) reference solution and the mixed reference solution 2 were dropped onto the same thin layer plate separately. Under the ultraviolet rays irradiation at 254 nm, there was a very obvious spot on the thin layer chromatograph of the fleroxacin. Due to this fact that the concentration of the eight quinolone components in the mixed reference solution 2 was lower than the LOD of the TLC, so any spot could be not observed in the chromatogram of the mixed solution. Therefor, the eight components' position should be separately marked by their relative Rf using the fleroxacin as object of reference to obtain their SERS. In addition, the blank position which Rf was same as the eight quinolones should also be marked to identify the Raman signal of SERS active substrate itself. When an appropriate amount of the nanometer silver solution was added on these marked position, and silver on the position was irradiated by the laser at 532 nm, the SERS of the A, B, C, D, E, F, G, and H should be obtained respectively, but there should be no obvious Raman signal from the blank position.
When the sample solustion was dropped onto the thin layer plate , these positions should also be marked by the same method to obtain SERS of the residues in the food. Comparing their Raman shift and relative peaks intensity, if the SERS of the tested component is consistent with the that of the corresponding reference substance, we can determine that there are corresponding quinolones residues in the foods. Based on this, if the characteristic peaks of the sample solution is higher than that of the corresponding reference substance solution, we can determine that the residual quinolones in the food have exceed its MRL.
It must be emphasized here that the MRL of ofloxacin (C) in the food is only 2 µg/kg, which is equivalent to 8 µg/mL according to the preparation method of the sample solution, so the sensitivity of the above TLC-SERS could have some limitations, did not meeting the requirements for the limit test of the ofloxacin in the food. That was to say, the LOD of the ofloxacin may be higher than its MRL in the method. In order to make the LOD ≤8 µg/mL (2µg/kg), a in-situ enrichment of the ofloxacin must be performed with anhydrous ethanol at the marked position that may contain the ofloxacin on the on the thin layer plate to concentrate the compound, then an appropriate amount of nanometer silver solution should be added to the concentrated compound to obtain its SERS. Only through such TLC-SERS could the characteristic SERS of the trace ofloxacin be obtained.
4. Conclusions
In this study, a limited limit detection method (TLC-SERS) for the quinolones residues in aquatic products and other animal foods was established, which has high sensitivity, strong specificity, reliable accuracy, and good stability. In addition, compared to existing related methods, the method is indeed simpler and faster.
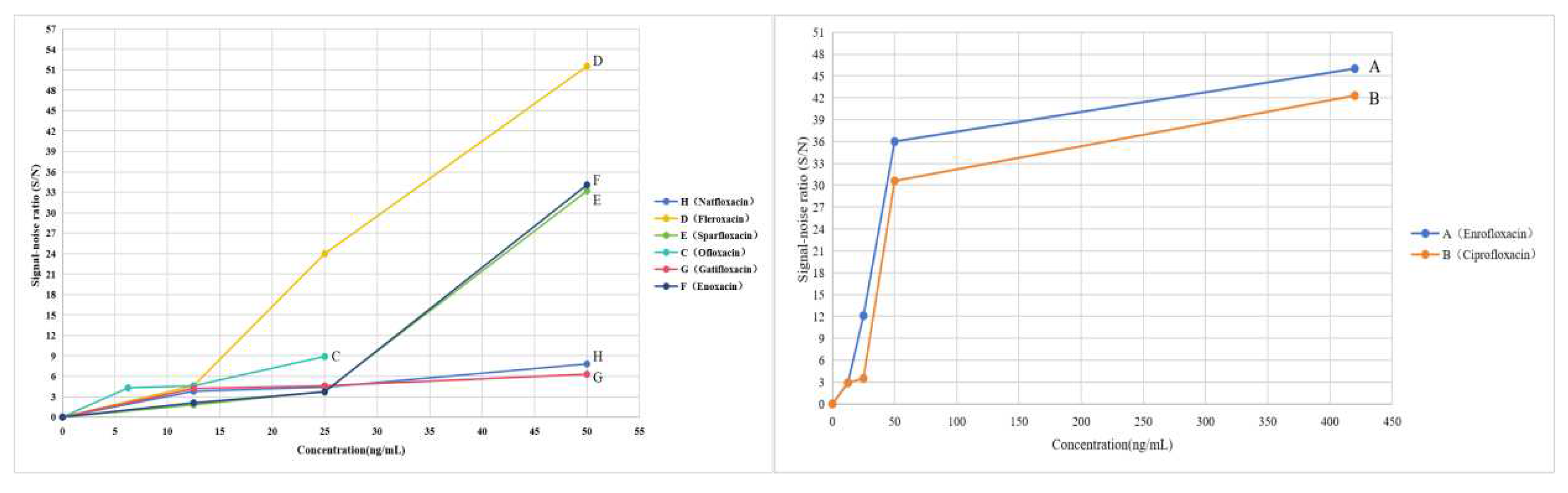
The results of this experiment showed that the SERS of the quinolones acquired by the TLC-SERS had a good correlation with the Raman spectra of the corresponding references substance powder; by comparing the relative intensity and the Raman shift of their characteristic peaks, the SERS of the different quinolones showed significant differences; by comparing the determination results of the three solutions (the reference substance solution, the simulated positive sample solution and the negative sample solution.), it is shown that the matrix components in the foods did not interfere with the limit test results of the residues; by measuring the relative height changes of the four characteristic peaks (γC=C, βCH2, βCH3, γC-N)of the same sample at different times, RSDs of the SERS for eight quinolones were obtained respectively at 1.9~2.2% (H), 2.6~3.3% (A), 2.2~3.5% (D), 3.5~3.7% (E), 4.1~4.9% (C), 3.6~3.9% (G), 3.2~3.5%(F) and 3.1~3.4%(B), indicating the RSD ≤4.9% for each quinolone compound; by measuring different concentrations of the quinolones reference substance solutions, the LOD of the eight components were obtained as 9.00 (H), 12.60 (A), 8.00 (D), 19.00 (E), 8.00 (C), 8.40 (G), 19.00 (F), and 12.60 (B) ng/mL, respectively; and the limit test results of the twenty real samples proved that except for enrofloxacin (A) and ciprofloxacin (B) found in two batches of samples, no quinolones residues were detected in the other samples, and only in one batch of samples, the content of enrofloxacin (A) exceeded its MRL, which was consistent with the results determined by the authoritative analysis method (UPLC–MS/MS).
In conclusion, this method can provide a new reference for the rapid limit test of harmful residues in foods.
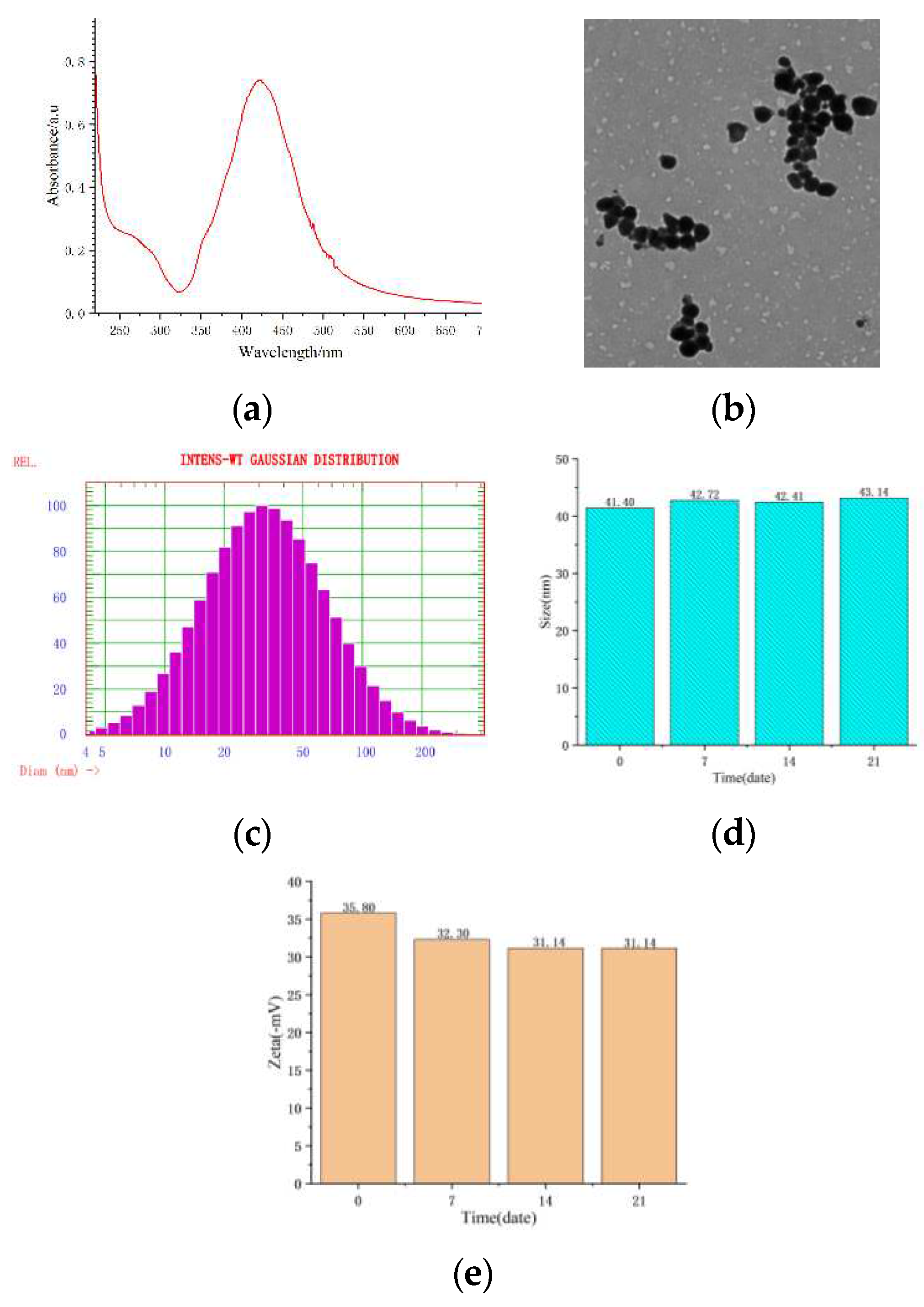
Figure 1.
Special nature of the SERS active substrates. a: the ultraviolet absorption spectrum; b: the appearance; c-d: the particle size ; e: the zeta potential.
Figure 1.
Special nature of the SERS active substrates. a: the ultraviolet absorption spectrum; b: the appearance; c-d: the particle size ; e: the zeta potential.
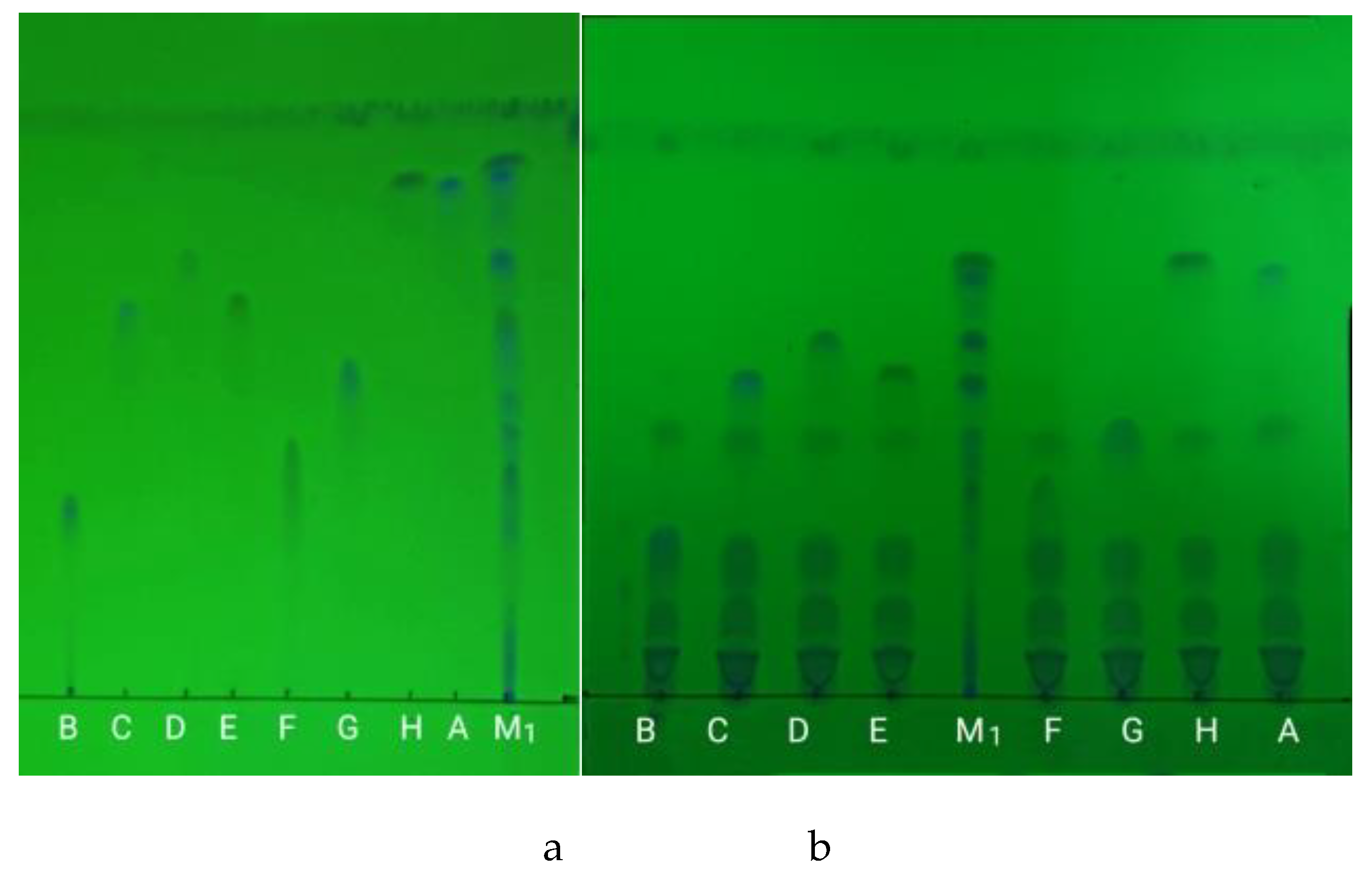
Figure 2.
The thin-layer chromatogram of the eight quinolones. A ,B ,C, D, E, F, G, H and M1 represents the eight quinolones reference substance solution and the mixed reference solution 1, respectively.
Figure 2.
The thin-layer chromatogram of the eight quinolones. A ,B ,C, D, E, F, G, H and M1 represents the eight quinolones reference substance solution and the mixed reference solution 1, respectively.
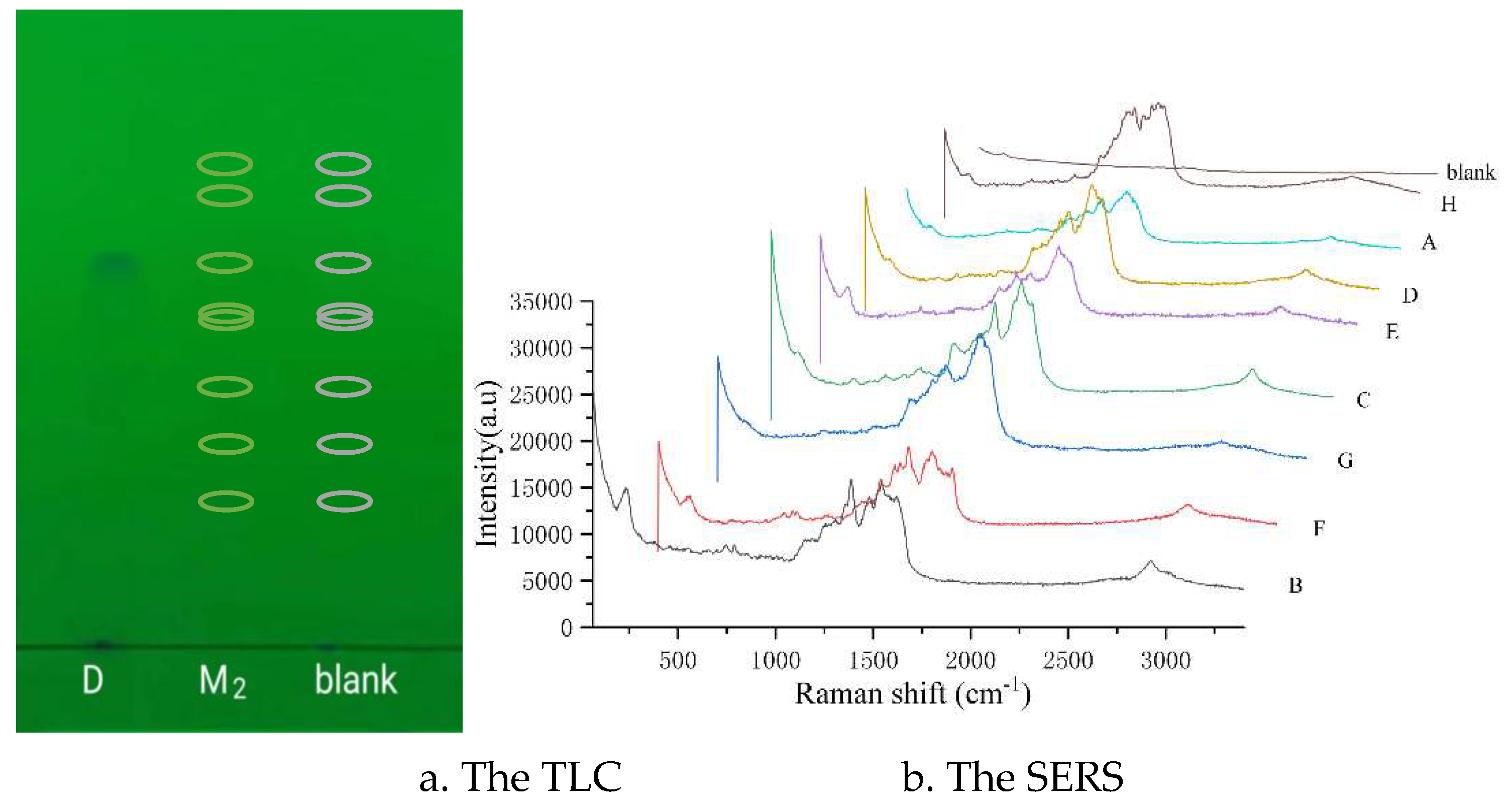
Figure 3.
The TLC and the SERS of eight quinolones as their concentration at the MRL. According to the thin-layer chromatogram , D represents the fleroxacin solution, and the spot was from the fleroxacin; M2 represents the mixed reference substance solution 2, and there were eight quinolones (H, A, D, E, C, F, G, F and B) in the marked positions on the chromatogram ,respectively; blank represents the anhydrous ethanol , there were no quinolones in the marked position on the chromatogram. According to the SERS diagram, A, B, C, D, E, F, G, and H represents the different quinolones in the mixture solution 2 respectively; the blank meaning as above.
Figure 3.
The TLC and the SERS of eight quinolones as their concentration at the MRL. According to the thin-layer chromatogram , D represents the fleroxacin solution, and the spot was from the fleroxacin; M2 represents the mixed reference substance solution 2, and there were eight quinolones (H, A, D, E, C, F, G, F and B) in the marked positions on the chromatogram ,respectively; blank represents the anhydrous ethanol , there were no quinolones in the marked position on the chromatogram. According to the SERS diagram, A, B, C, D, E, F, G, and H represents the different quinolones in the mixture solution 2 respectively; the blank meaning as above.
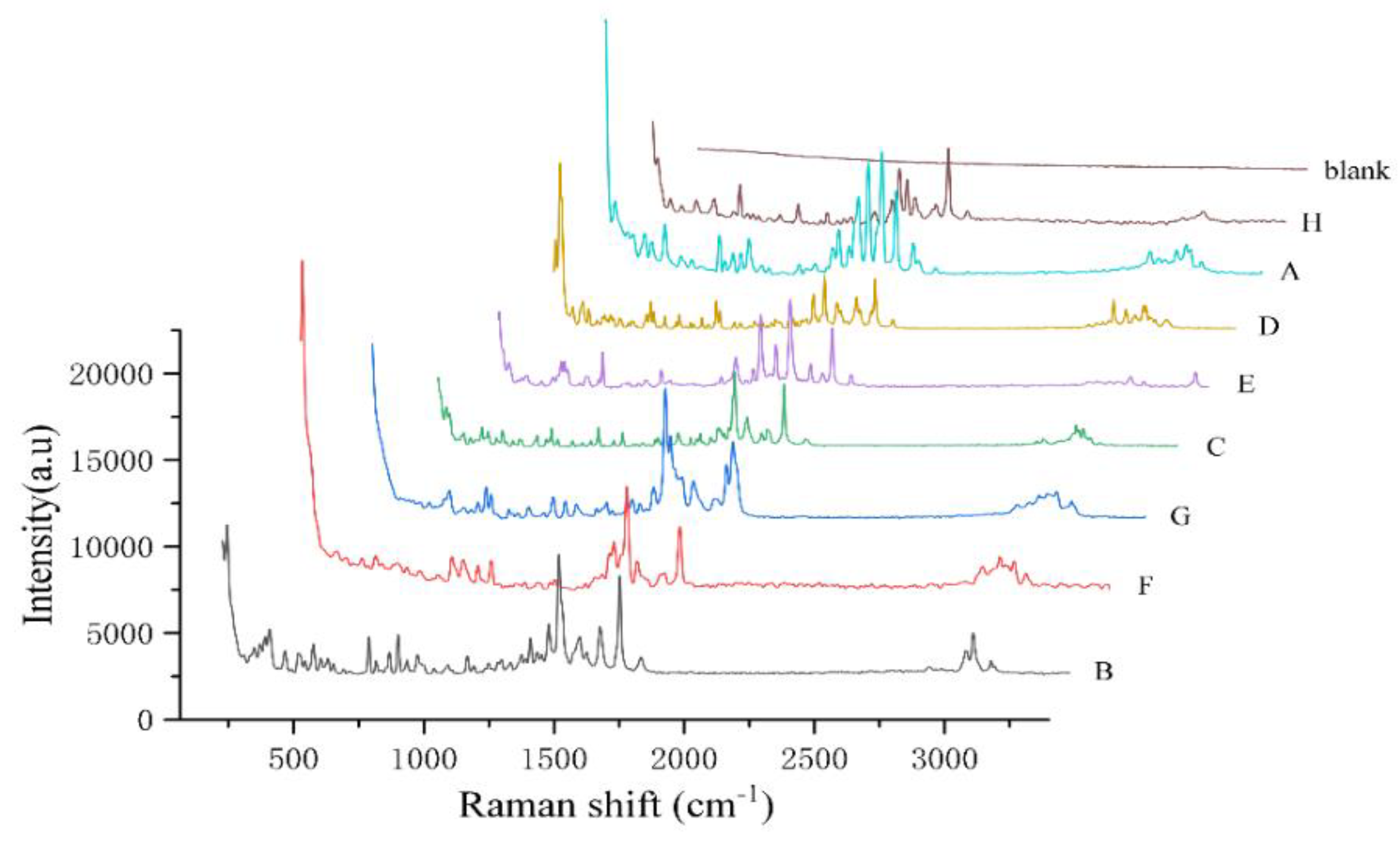
Figure 4.
Raman spectra of the eight quinolones powder. (H, A, D, E, C, G, F, and B represents Nadifloxacin, Enrofloxacin, Fleroxacin, Sparfloxacin, Ofloxacin, Gatifloxacin, Enoxacin, and Ciprofloxacin, respectively.).
Figure 4.
Raman spectra of the eight quinolones powder. (H, A, D, E, C, G, F, and B represents Nadifloxacin, Enrofloxacin, Fleroxacin, Sparfloxacin, Ofloxacin, Gatifloxacin, Enoxacin, and Ciprofloxacin, respectively.).
Figure 5.
LOD of the eight quinolones by TLC-SERS. H, A, D, E, C, G, F, and B represents Nadifloxacin, Enrofloxacin, Fleroxacin, Sparfloxacin, Ofloxacin, Gatifloxacin, Enoxacin, and Ciprofloxacin, respectively.
Figure 5.
LOD of the eight quinolones by TLC-SERS. H, A, D, E, C, G, F, and B represents Nadifloxacin, Enrofloxacin, Fleroxacin, Sparfloxacin, Ofloxacin, Gatifloxacin, Enoxacin, and Ciprofloxacin, respectively.
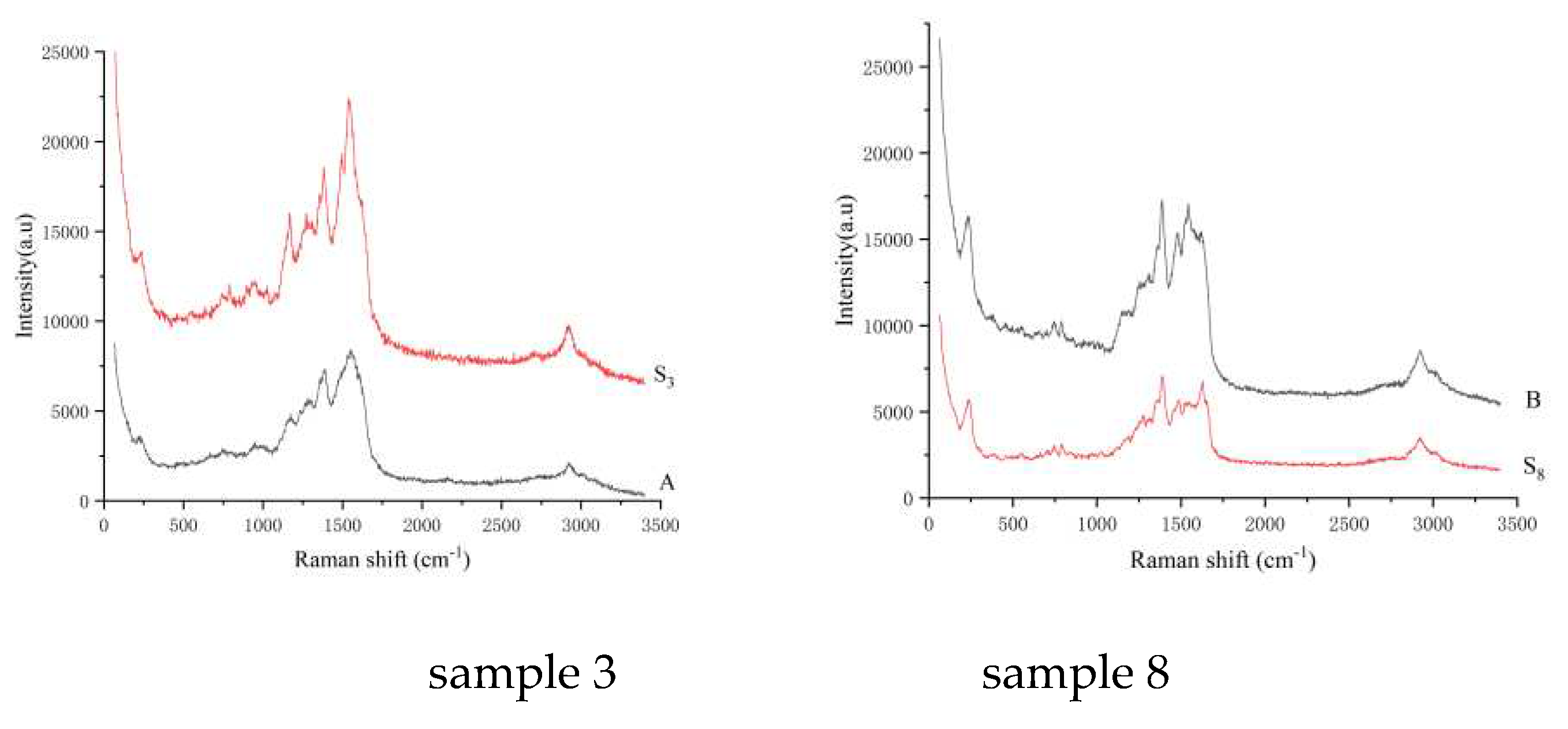
Figure 6.
SERS of the sample by the TLC-SERS. S3: sample 3 from the aquatic products, A: enrofloxacin; S8: sample 8 from the other animal foods, B: ciprofloxacin.
Figure 6.
SERS of the sample by the TLC-SERS. S3: sample 3 from the aquatic products, A: enrofloxacin; S8: sample 8 from the other animal foods, B: ciprofloxacin.
Table 1.
The Assignments of the characteristic peaks of the eight quinolones.
Table 1.
The Assignments of the characteristic peaks of the eight quinolones.
| Chemical Structure |
Raman Shift (cm−1)
of the Podwer/Relative Peak Intensity |
Raman Shift (cm−1)
by the TLC-SERS/
Relative Peak Intensity |
Assignments |
Relative
Rf
|
Nadifloxacin(H)

|
2964~2861 ( 2 peaks)
1721~1147 ( 9 peaks)
1721/0.28
1621/1.33
1363/1.00
1324/0.46
1147/0.16 |
2991~2846 (1 peak)
1610~1157 ( 8 peaks)
1610/1.01
1587/1.03
1398/1.00
1257/0.57
1157/0.32 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:
νC=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
1.22 |
Enrofloxacin(A)

|
3094~2833 ( 5 peaks)
1743~1129 ( 9 peaks)
1743/0.08
1629/0.29
1400/1.00
1350/0.71
1129/0.12 |
2995~2823 ( 1 peak)
1600~1173 ( 4 peaks)
1600/0.94
1552/1.14
1389/1.00
1281/0.77
1173/0.65 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:ν
C=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
1.16 |
Fleroxacin(D)

|
3057~2800 ( 6 peaks)
1791~1147 ( 7 peaks)
1791/0.21
1634/0.94
1387/1.00
1332/0.65
1147/0.21 |
3037~2806 (1 peak)
1595~1255 ( 5 peaks)
1595/1.12
1535/1.32
1379/1.00
1298/0.77
1255/0.64 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:
νC=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
1.00 |
Sparfloxacin(E)

|
3095~2835 ( 6 peaks)
1721~1179 ( 9 peaks)
1721/0.16
1632/0.68
1435/1.00
1296/0.83
1179/0.34 |
2997~2823 (1 peak)
1621~1176 (5 peaks)
1621/1.21
1549/1.48
1373/1.00
1279/1.03
1176/0.71 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:
νC=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
0.90 |
Ofloxacin(C)

|
3002~2763 (6 peaks)
1720~1147 (9 peaks)
1720/0.16
1632/0.85
1401/1.00
1329/0.29
1147/0.23 |
3003~2810 (1 peak)
1612~1151 (5 peaks)
1612/0.98
1552/1.19
1394/1.00
1300/0.68
1151/0.57 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:
νC=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
0.89 |
Gatifloxacin(G)

|
3081~2846 (6 peaks)
1620~1185 (9 peaks)
no signal
1620/0.93
1350/1.00
1328/1.57
1185/0.24 |
3026~2806 (1 peak)
1562~1151 (6 peaks)
no signal
1562/1.33
1357/1.00
1286/0.85
1151/0.65 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:
νC=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
0.74 |
Enoxacin(F)

|
3055~2878 (4 peaks)
1674~1239 (7 peaks)
1674/0.07
1627/0.63
1409/1.00
1355/0.48
1239/0.10 |
3032~2823 (1 peak)
1649~1161 ( 7 peaks)
1649/0.74
1540/0.93
1414/1.00
1265/0.60
1161/0.34 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:
νC=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
0.56 |
Ciprofloxacin(B)

|
3089~2990 (3 peaks)
1711~1162 (8 peaks)
1711/0.16
1628/0.82
1389/1.00
1277/0.31
1162/0.14 |
2995~2823 (1 peak)
1621~1151 (8 peaks)
1621/0.85
1543/0.98
1386/1.00
1307/0.64
1151/0.47 |
common peak: ν=CH,ν-CH2,ν-CH3
characteristic peak:
νC=O
νC=C from phenyl rings
νC=C from phenyl rings
βCH2, βCH3
νC-N
|
0.44 |
Table 2.
Comparison between the LOD and the MRL of the eight quinolones residues in the food.
Table 2.
Comparison between the LOD and the MRL of the eight quinolones residues in the food.
| Quinolones |
MRL
μg/kg |
MRL
ng/mL |
concentration range (ng/mL) |
LOD
(ng/mL) |
| Nadifloxacin (H) |
5.0 |
20.0 |
12.5~50.0 |
9.0 |
| Enrofloxacin (A) |
100.0 |
400.0 |
12.5~420.0 |
12.6 |
| Fleroxacin (D) |
5.0 |
20.0 |
12.5~50.0 |
8.0 |
| Sparfloxacin (E) |
5.0 |
20.0 |
12.5~50.0 |
19.0 |
| Ofloxacin (C) |
2.0 |
8.0 |
8.0~32.0 |
8.0 |
| Gatifloxacin (G) |
5.0 |
20.0 |
12.5~50.0 |
8.4 |
| Enoxacin (F) |
5.0 |
20.0 |
12.5~50.0 |
19.0 |
| Ciprofloxacin (B) |
100.0 |
400.0 |
12.5~420.0 |
12.6 |