1. Introduction
The cell wall is a protective structure, surrounding the cell membrane of some types of eukaryotic and prokaryotic cell including land plants and algae. In land plants structure, synthesis, and function of distinct types of cell and species have been identified extensively for decades [
1]. The fundamental roles of plant cell wall involve morphogenesis and growth, providing mechanical strength, defending against biotic and abiotic stresses, adaptation, and so on [
2]. The greater part of growing plant cell wall contains different types of polysaccharides: cellulose, hemicellulose and pectin and small number of structural proteins. Many experimental results indicate that the cell wall is formed by successive deposition of pectic substances, hemicelluloses-cellulose, and lignin during cell wall differentiation, with the cell wall assembly process itself being irreversible to form a dynamic network to bear the high intracellular turgor pressure. The polysaccharide in plant cell wall was found to essentially involve in many facets in growth and development such as cell expansion polarity and thickening of cell walls through the deposition of polysaccharides [
2].
Like land plants, macroalgae such as red, green and brown consist of polysaccharide-rich cell wall, approximately 4–76% of algal dry weight [
3]. Among them, brown algae (Phaeophyta) represent the largest biomass producing organism, containing more than 250 genera and 1,500–2,000 species, in marine ecosystem [
4] and shared different type of polysaccharides in common with plants (cellulose), animals (sulfated fucans) and some bacteria (alginates) [
1], [
5]. The main polysaccharides in brown algal cell wall are fucoidan and alginates which encompass up to 45% of algal dry weight, while cellulose only accounts for 1–8% of algal dry weight [
6]. Regarding the functionality of the main cell wall polysaccharide of brown algae, alginate fine structure likely contribute for cell wall rigidity, while sulfated polysaccharides: fucoidan probably act a main role in the osmotic regulation [
6]. Furthermore, preceding studies of brown algae found that the cell wall contributed to cell adhesion [
7], cell expansion [
8], cell development with cell differentiation in growing filaments [
9], polar axis fixation and cell fate [
10]. Although the cell wall was found to involve in growth and development, poor attention has been paid to date regarding the involvement of cell wall polysaccharide on growth.
Herburger
et al investigated that cell wall polysaccharide content in charophyte green algae changes during the growth. For instance, pectin content of
Zygnema and hemicelluloses content in
Klebsormidium increase with increasing cell age, [
11]. Furthermore, laminarans and fucoidans content of brown algal cell wall was found to be significantly higher in mature
Laminaria cichorioides in autumn compared to unripe algae suggesting that algal polysaccharide would play a key role in cell growth [
12].
Cladosiphon okamuranus, Okinawa Mozuku in Japanese, is one of the important edible brown algae in Japan and an excellent source of fucoidan among any brown algae.
Cladosiphon okamuranus is widely cultivated in Okinawa, Japan, from November to June, and its harvest season starts January and ends June depending on the location. Here, we analyzed the cell wall structure of immature thalli of
C. okamuranus (young thalli) which was harvested at beginning of the harvest season and mature thalli (old thalli) which was harvested at peak season or later. Our present study focuses on how cell wall polysaccharide contributes on growth in brown algae with special reference to
C. okamuranus which is identified as an abundant source of fucoidan among any brown algae spp.
2. Results
2.1. Physical Characteristic of Young and Old Thalli
Variation in physical characters were observed in young and old algae samples after harvesting. The young thalli feel stickier or slimier to the touch compared to the old thalli and old thalli is darker in color and feel harder than young thalli. To understand the differences of the strength between young and old thalli quantitatively, breaking strength were measured using tensile tester in both main axis and lateral branches in each sample. Results show that breaking strength of main axis and lateral branches of old thalli were 40 g and 36 g respectively, which were significantly higher than that of young thalli as presented in
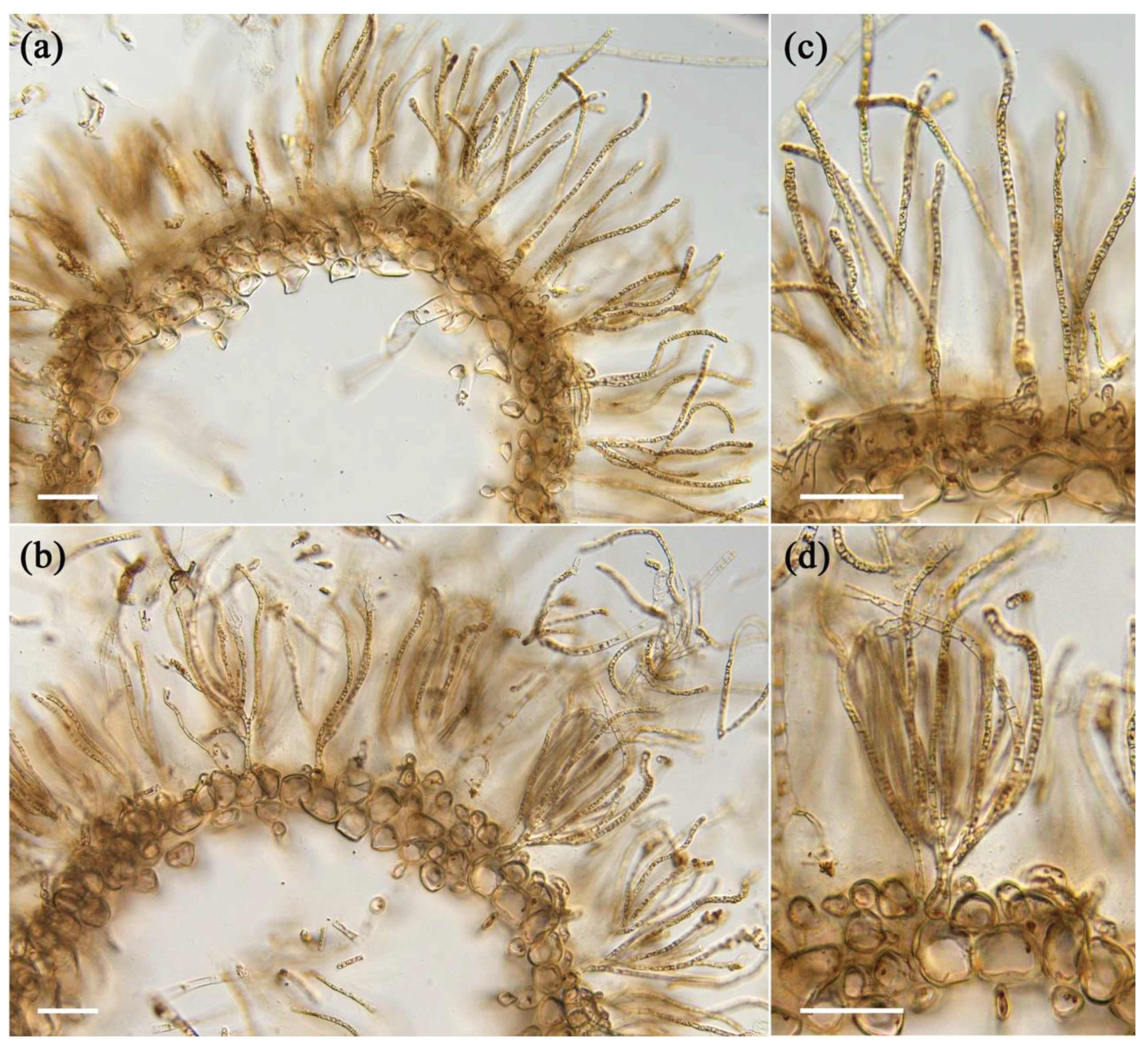
Figure 1b. The insides of the main axes were hollowed both in old and young thalli (
Figure 2). The main axes were composed of two elements, medullary cell layers and assimilatory filaments. In both thalli, a single cell layer was observed below the basal cell of assimilatory filaments (
Figure 2c,d) and the structure of the medullary layer was similar to each other. Regarding the assimilatory filaments, the number of branches from a single basal cell in the young thalli seemed to be more than the old one although the actual number was not counted in this study. Different physical properties of young and old thalli could reflect differences in the cell wall components that produce diverse physiochemical properties during growth. Hence, cell wall of each samples were prepared as alcohol insoluble residue (AIR) and fractionated.
2.2. Yield of AIR
Yield of AIR prepared from young and old thalli were 2.8% and 5.5% based on wet weight basis respectively in 2018. The moisture content was 93.2% and 91.4% for young and old thalli, respectively in 2018. Similar trend was recorded for yield of AIR and moisture content of young and old thalli in 2019 as shown in
Table 1. These results suggested that once algal body grows from young to old, cell wall material content increase while moisture content decrease. Since we found many different characters such as color, strength, moisture and content of AIR between young and old thalli, our next steps were focused on detail structural analysis of cell wall to understand how these differences occur during growth of thalli. Therefore, AIR was fractionated in to 5 fractions and evaluated their chemical composition as the next steps to find whether growing cell wall involve in the chemical composition variation.
2.3. Yield and Composition of Different Cell Wall Fractions
Using sequential chemical extraction, AIR prepared from young and old thalli were fractionated into five fractions: hot water (HW), Amonium oxalate (AO), Hemicellulose (HC) -I, HC-II, and Cellulose (CL) and their yield displayed in
Table 2. Results show that HW and HC-I were the main fractions, yielded almost 80% of the total recovery cell wall from AIR and AO, HC-II and CL possessed rest of 20% in every samples. However, ratio between the 2 main fractions: HW and HC-I varied between young and old algal body. For instance, yield of HW and HC-I fractions in young thalli were 62.4% and 18.1% respectively while they were 49.8% and 30.8% respectively in old thalli in 2018. it showed the same tendency in 2019 as presented in
Table 2. These results imply that yield of HC-I increased, while yield of HW decreased, but total yield of HW and HC-I were almost constant during growth period. Significant variation was not shown in yield of other minor fractions: AO, HC-II and CL between old and young thalli as shown in
Figure 3.
Chemical composition of each cell wall fractions in young and old thalli harvested in 2018 presented in
Table 2. Approximately more than 50% of the dry weight of each fraction was sugar, with the highest Uronic Acid (UA) content recorded in AO fraction in young thalli. It is clear that higher sulfate contents (24.5%) showed in HW compared to all other fractions, and sulfate content of HC-I was 6.4% in young thalli. Protein was co-extracted together with a minor amount of polyphenol in every fraction, and it was highlighted that the highest content of protein and polyphenol is composed in HC-I fraction in young thalli. Composition analysis of all fractions obtained from old thalli appears to be consistent with the young thalli. In addition, chemical composition of each cell wall fractions in young and old thalli harvested in 2019 (
Table 3) showed the same tendency with the 2018 sample.
2.4. Sugar Composition Analysis
Since there were no more differences in the composition of the young and old thalli, sugar composition analysis were done to understand the structural differences in detail. Sugar composition of each cell wall fractions in young and old thalli harvested in 2018 presented in
Table 4. Our previous study found that HW and HC-I mainly contained fucoidan with different structure, while AO mostly composed of alginate and CL was almost cellulose [
13]. Similarly, result show that HW and HC-I contained mainly fucoidan and the molar ratio of Fuc: GlcA: SO
3- of HW and HC-I were 1.0: 0.3: 0.6~0.7 and 1.0: 0.3: 0.2~0.3 respectively with a trace amount of Gal, Glc and Xyl in both young and old thalli as shown in
Table 4. Xylose content of fucoidan in HC-I is higher than that of fucoidan in HW in both young and old thalli. Presence of fucoidan was confirmed in all fractions except CL for both young and old thalli. However, the molar ratio of Glc:Fuc was 55.9 in young and 26.7 in old, estimating that the amount of Fuc in old thalli was about twice as higher as that in young. Since Fuc is a constituent sugar of fucoidan, it is supposed that fucoidan in is more strongly bound to cellulose in the old thalli cell wall compared to young.
Approximately similar results were reported for sugar composition of each cell wall fractions in young and old thalli harvested in 2019 as shown in
Table 5. Since most of the compounds in cell wall from
C. okamuranus were extracted in HW and HC-I and there was no more variation in the chemical composition in the cell wall harvested in 2 consecutive years, further analysis was focused only for HW and HC-I in young and old thalli harvested in 2019.
2.5. Molecular Weight Distribution
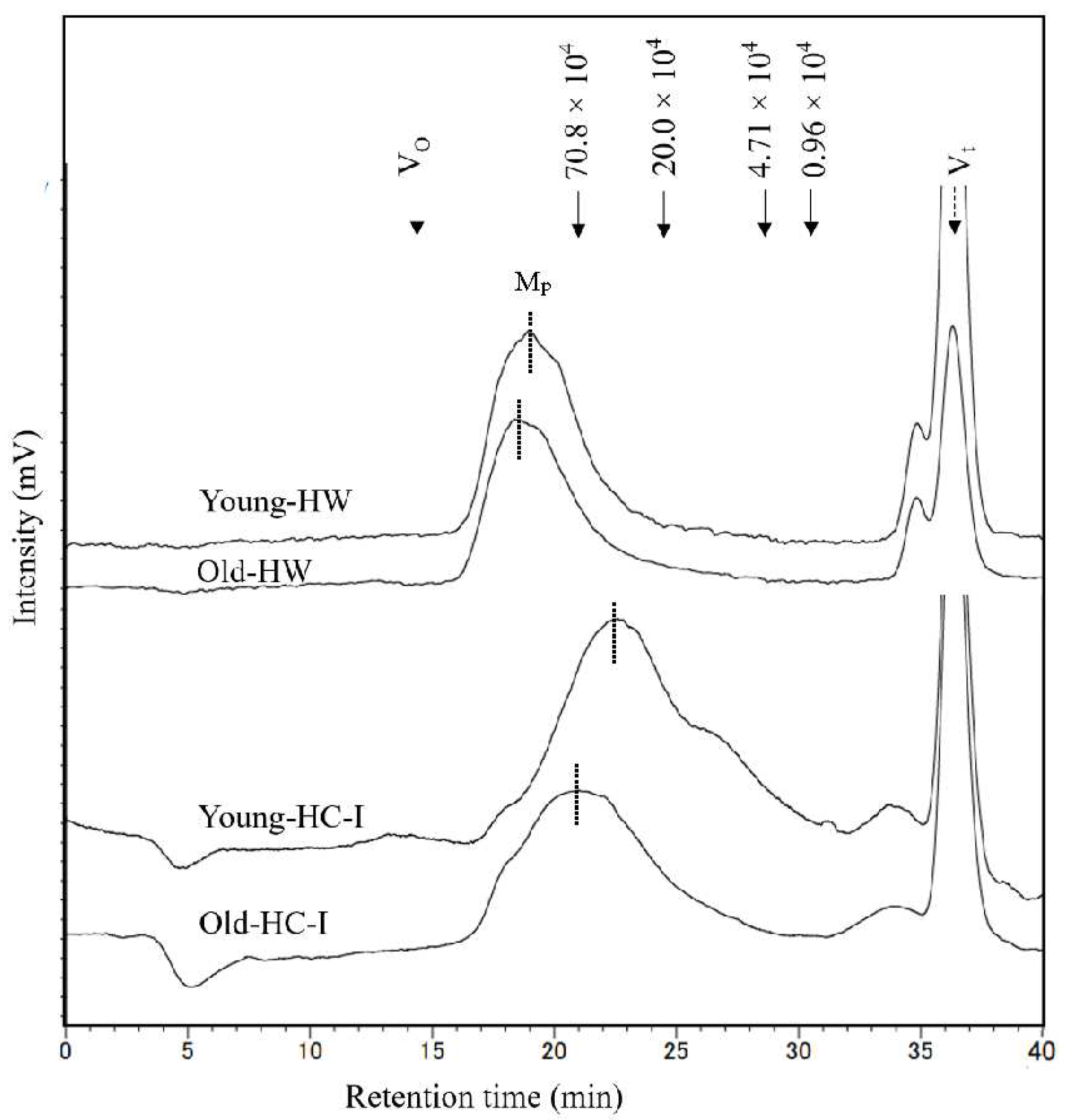
Molecular weight distribution of HW and HC-I in young and old thalli was determined by high-performance size-exclusion chromatography as shown in
Figure 4. Each chromatogram mainly comprised one major peak (Mp) which eluted later and broader in HC-I than HW. Molecular weight of HW (2.1 ×10
6) and HC-I (0.7×10
6) in old thalli was higher than that of HW (2.1 ×10
6) and HC-I (0.5 ×10
6) in young thalli. These results suggested that molecular weight of HW and HC-I increased with growing period. The peak at V
t was NaCl in the sample and eluting buffer while the small peak, occurred just before V
t was comparatively lower (<10%) than Mp in the chromatograms.
2.6. Anion Exchange Chromatography
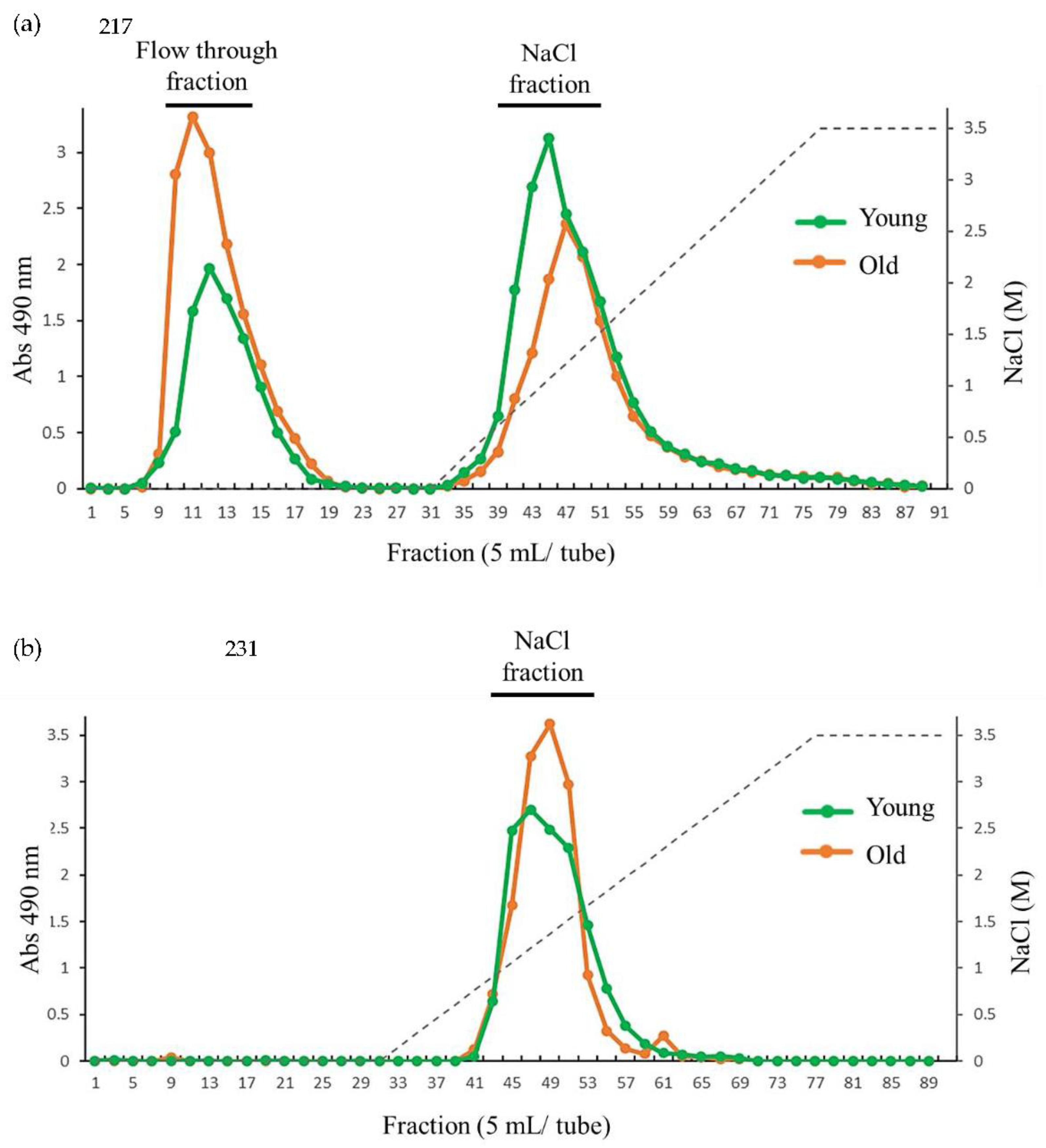
To identify further structural variation of cell wall during growth, HW and HC-I in young and old thalli were fractionated by anion exchange chromatography. The anion exchange chromatogram of HW from both young and old thalli showed mainly 2 peaks including peak at flow-through (HW-FT) and peak eluted with NaCl (HW-NaCl) as shown in
Figure 5a but, variation of the yield of each fraction between young and old thalli was reported. Yield of HW-FT in old thalli (28.2%) was higher than that of young thalli (14.4%) while yield of HW-NaCl in young thalli (76.4%) was higher than that of old thalli (67.2%). We expected that HW-FT have lower carboxyl and sulfated groups than HW-NaCl as it eluted as unbound polysaccharide in flow through fraction, but sugar composition analysis revealed that HW-FT, HW-NaCl were mainly composed of constitutes of fucoidan such as Fuc, GlcA and sulfate into the quite similar molar ratio. However, Glc content was higher in HW-FT than HW-NaCl in both young and old thalli (
Table 6).
Anion exchange chromatography of HC-I in young and old showed only one peak eluted with NaCl (HC-I-NaCl), whereas both elution profiles seem to be slightly similar (
Figure 5b). Sugar composition analysis of HC-I-NaCl in both young and old were predominant with the component of fucoidan composed of Fuc, GlcA and sulfate into the molar ratio of 1: 0.2~0.4: 0.5~0.6 as shown in
Table 6. These results suggested that purified HW and HC-I by anion exchange chromatography were basically fucoidan, but the yield of HW-FT and HW-NaCl fractions were vary in young and old.
3. Discussion
Many studies proved that polysaccharide in plant cell wall essentially involve in many facets in growth and development such as cell expansion polarity and thickening of cell walls through the deposition of polysaccharides [
2]. However, little is known about the involvement of polysaccharide to growth and development of algae. The present study provides new insights into the growth of brown algal cell wall by showing that cell the wall polysaccharide plays a leading role in the process of growth considering physicochemical characteristic of young and old
C. okamuranus. Firstly, we examined the physical characteristic and identified the several variations between young and old thalli. The young thalli were lighter in color and feel slimier to the touch compared to the old. It was found that molecular weight and sulfate content of the fucoidan might be the reason for variation in the slimness of thalli harvested at different time [
14]. Furthermore, breaking strength of main axis and lateral branches of old thalli were significantly higher than that of young thalli (
Figure 1b). In anatomical analysis, it was confirmed that the basic structures of the young and old thalli were almost similar except for the assimilatory filaments, suggesting that the generation of tension strength may be influenced by microscale factors such as the thickness and/or composition of the cell wall (
Figure 2). Consistency, preceding studies found that old algal tissue was stronger significantly than newly formed tissue, suggested to be due to the differences in tissue composition or structure [
15,
16,
17]. These biomechanical characteristics of the different tissues would corelate with prominent differences in moisture content, cortical layer thickness, cell wall thickness and amount of insoluble polysaccharide such as cellulose, holocellulose and fucoidan [
15]. According to the result of our study moisture content was lower in old thalli (91.4%) than young (93.2%), while yield of AIR was higher in old thalli (5.5%) than young (2.8%) (
Table 1), suggested that cell wall material content increase and moisture content decrease during growth. Similar trend was reported in moisture content and crude fucoidan content after March in
C. okamuranus and kelp species such as
L. digitata, L. hyperborea, Saccharina latissima and
Alaria esculenta [
14,
18]. Therefore, it can be proved that algal tissue is becoming more stronger in line with the deposition of cell wall materials and reducing of water content during the growth process.
Since increase in the cell wall material was observed with growth of thalli, variation in the cell wall composition needs to be analyzed to understand which polysaccharides mostly engage with the process of growth. Therefore, AIR was fractionated in to 5 fractions: HW, AO, HC-I, HC-II and CL. Results show that almost 80% of the total recovery cell wall from AIR was HW and HC-I and amount of HC-I increased, while HW decreased during growth (
Table 2 and
Table 3). Our previous study found that HW and HC-I mainly contained fucoidan composed of Fuc, GlcA, and sulfate in molar ratios of 1.0:0.3:0.9 and 1.0:0.2:0.3, respectively and their structure differed in terms of contents of sulfate and Xyl, MW and profile small angle x-ray scattering [
13]. Highly sulfated fucoidan in HW is typical fucoidan, suggested to be weakly held in cell wall matrix and may involve in osmotic regulation in brown algae [
19], while HC-I contained 1,4-linked Xyl and 1,4-linked Fuc as well as components of typical fucoidan, suggesting that these are likely to be 1,4-xylan and/or 1,4-fucan which may involve in reinforcing cell wall structure by cross-linking to cellulose as hemicellulose in terrestrial plants, by keeping CL microfibrils separated and controlling cell wall expansion [
20]. This hypothesis is further supported by the result of this study. The young thalli are rich in matrix polysaccharide: HW and the old thalli possessed a larger fibrillar polysaccharide: HC-I compared with young thalli, suggested that cell wall thickening in
Cladosiphon okamuranus during growth is characterized by increase in the HC-I content. Similar scenario was reported in land plants, but with different types of polysaccharide. For instance, pectic substances are mostly deposited at young stage, while contents of compositional sugars from cellulose and hemicelluloses increase with the maturation of the secondary wall [
21]. However, substantial variation was not found in yield of other minor fractions: AO, HC-II and CL between old and young. In contrast, variation in the cellulose amount have been shown to cause changes in both strength and rigidity of plant tissues [
22,
23]. Cellulose is the most abundant major polysaccharide representing up to 50% of terrestrial plants’ cell walls, but is only 5% in brown algae including young and old thalli in this study [
6].
Further structural analysis of HW and HC-I were conducted related to young and old thalli. Sugar composition analysis showed that HW and HC-I in young and old thalli contained mainly fucoidan composed of Fuc, GlcA, and sulfate in molar ratios of 1.0: 0.3: 0.6~0.7 and 1.0: 0.3: 0.2~0.3 respectively with a trace amount of Gal, Glc and Xyl. Although there were variations in the sulfate and xylose content between HW and HC-I, there were no significant differences observed in sugar composition and sulfate content between young and old thalli. However, fucoidan appears to be more strongly bound to cellulose in the old thalli cell wall compared to young according to the Glc:Fuc molar ratio in CL fraction (
Table 4 and
Table 5). We believe that large quantity of fucoidan in HC-I in old thalli might be bound strongly to the cellulose. Moreover, HW and HC-I in young and old thalli were further characterized by the determination of their molecular weight which shows larger in old thalli than young. Zvyagintseva et al., also found that molecular weight of fucoidan in 2 years old
L. cichorioides was significantly higher (20-30 kDa) than 0.8 years old sample (8-10 kDa) [
12]. It can be suggested that high molecule weight of old thalli appears to be due to an increase in its degree of polymerization during growth [
24]. Further fractionation was done for HW and HC-I in both young and old thalli by anion exchange chromatography to explore their purity and heterogeneity based on their charge density. These results further indicated that purified HW contains main 2 fractions: HW-FT and HW-NaCl, but the yield of HW-NaCl fractions in young thalli were higher than that of old, while HC-I eluted only HC-I-NaCl and both young and old thalli were appeared to be similar (
Table 6). These results confirmed that there were many structural variation in the main fractions: HW and HC-I which may related with localization of young and old thalli by involving the process of growth.
4. Materials and Methods
4.1. Algal Sample
Cladosiphon okamuranus, cultivated in Chinen, Nanjo City, Okinawa Prefecture were harvested in about 40 days (young thalli) and about 90 days (old thalli) after planting in the sea in 2018 and 2019, respectively.
4.2. Measurement of Moisture Content
About 0.1 g of sample was taken after harvesting and moisture content was measured using a moisture content analyzer (MOC-63U SHIMADZU, Japan)
4.3. Measurement of Tensile Strength
The main axis was defined as the thickest branch growing from the base of the thalli and the lateral branches were defined as branches departing from the main axis as shown in
Figure 1a. The tensile strength of each main axis and lateral branches were determined using a tensile tester (STB1225S, A&D Co., Ltd., Tokyo, Japan). About 10 mm of thalli was fixed between two clamps and stretched by raising the upper clamp at a speed of 20 mm/min until the thalli broke.
4.4. Light Microscopy
The fixed samples with 100% ethanol were used for making cross sections without washing. The samples were observed by a light microscope (BX53F2, OLYMPUS, Japan) equipped with a digital camera (WRAYCUM NOA2000, WRAYMER, Japan).
4.5. Preparation of Alcohol Insoluble Residue (AIR)
Algal sample (50 g) was ground using a blender in 4 volumes of ethanol (200 mL) followed by centrifugation at 8,000 × g at 25°C for 15 min, then precipitate was sequentially treated with 80% ethanol, 100% ethanol, and methanol:chloroform (1:1, v:v) followed by acetone. Suction filtration was performed and residue was dried at room temperature and used as AIR [
13].
4.6. Fractionation of Cell Wall Polysaccharides
Fractionation procedure was based on the different solubilities of the polysaccharides from brown seaweeds as described in our previous study [
14]. Briefly, AIR was sequentially treated with hot water (HW), 0.25% ammonium oxalate (AO), 4% KOH, and 24% KOH to produce 4 fractions: HW, AO, hemicellulose-I (HC-I), HC-II, and final residue was collected as cellulose (CL). After neutralizing HC-I and HC-II using acetic acid, AO, HC-I, and HC-II were dialyzed, lyophilized, and used for analysis. The CL was washed with water after neutralizing with acetic acid, and then lyophilized.
4.7. Chemical Composition Analysis
Total sugar and UA were determined by phenol–sulfuric acid method using Fuc as a standard [
25] and
m-hydroxybiphenyl method using GlcA as a standard [
26], respectively. Total polyphenols were quantified using the Folin–Ciocalteu method using gallic acid as a standard [
27]. Protein content was measured using bicinchoninic acid assay (BCA) following the manufacturer’s instructions of a BCA Protein Assay Kit (Takara Bio Inc., Shiga, Japan). The calibration curve was prepared using bovine serum albumin.
To estimate SO
3−, sample was hydrolyzed in 2 M trifluoroacetic acid at 121°C for 1 h, hydrolysate was subjected to high-performance liquid chromatography with an AS4A-SC column (4 mm × 250 mm, Dionex Co., Tokyo, Japan). The column was eluted at 1.5 mL/min at room temperature with a buffer containing 1.7 mM NaHCO
3 and 1.8 mM Na
2CO
3 [
28]. The SO
3− content in the sample was calculated from a calibration curve using Na
2SO
4 as a standard [
13].
4.8. Sugar Composition Analysis
To analyze sugar composition, sample were hydrolyzed as used for SO
3− estimation, while the AIR and CL was treated with ice-cold 72% (w/w) H
2SO
4 at 4°C for 1 h with sonication, followed by hydrolysis with 2 N H
2SO
4 at 121°C for 1 h [
29,
30]. Monosaccharide in the hydrolysate was analyzed by high-performance anion-exchange chromatography coupled with a pulsed amperometric detector (HPAEC-PAD) using a Carbo Pac PA1 column (4 mm × 250 mm, Dionex Co.). The column was eluted at a flow rate of 1 mL/min at 35°C with 14 mM NaOH for neutral sugar, followed by a linear gradient program of 0–250 mM CH
3COONa in 100 mM NaOH for UA.
4.9. Determination of Molecular Weight (MW)
The MW of HW and HC-I were determined by size-exclusion chromatography (LC-6A; Shimadzu Co., Kyoto, Japan) equipped with a TSKgel G5000 PWXL column (7.8 mm × 300 mm, Tosoh Co., Tokyo, Japan) and a refractive index detector RID-10A [
31]. The column was eluted by 0.2 M NaCl at a flow rate of 0.3 mL/min at 40°C. Pullulan P-10 (MW = 0.96 × 10
4), P-50 (4.71 × 10
4), P-200 (20.0 × 10
4), and P-800 (70.8 × 10
4) (Showa Denko Co., Tokyo, Japan) were used as standards.
4.10. Anion Exchange Chromatography
For further analysis of HW and HC-I extracted from young and old thalli, they were applied onto anion exchange chromatography. 50 mg of each fraction was dissolved in 20 mM Tris-HCl (pH 7.4) and applied onto a DEAE–Sephacel column (2.5 × 10 cm, GE Healthcare, Uppsala, Sweden) equilibrated with the same buffer. The column was washed with 20 mM Tris-HCl (pH 7.4) and then eluted with a linear gradient of 0–3.5 M NaCl in 20 mM Tris-HCl (pH 7.4), followed with 3.5 M NaCl in the same buffer.
4.11. Statistical Analysis
Data were expressed as mean ± standard deviation of three determinations. Statistical comparison was performed via a one-way analysis of variance followed by Tukey’s test. Probability values of less than 0.05 (p < 0.05) were considered as significant.
5. Conclusions
In conclusion, differences in physical and chemical composition in young and old thalli of C. okamuranus were observed related to their growth. The old thalli were stronger, darker in color and feel less slimy to the touch compared to the young thalli. Presence of high amount of cell wall material in old thalli reinforce us to fractionate cell wall into different fraction. Almost 80% of the total recovery cell wall from both young and old thalli was HW and HC-I contained mainly fucoidan composed of Fuc, GlcA, and sulfate in molar ratios of 1.0: 0.3: 0.6~0.7 and 1.0: 0.3: 0.2~0.3, respectively. Further analysis showed that fucoidan in HW was a highly sulfated matrix polysaccharide abundance in young thalli, while fucoidan in HC-I was rich in old thalli and appears to involve in reinforcing cell wall structure by cross-linking to cellulose as hemicellulose in terrestrial plants. We found that HW and HC-I are particularly involved in the growth and stronger thalli of C. okamuranus appears to be due to the deposition of HC-I and reducing of water content during the growth process.
Author Contributions
Conceptualization, T.K.; methodology, T.K.; formal analysis, Y.M. and M.G.G.A.; investigation, Y.M., K.S. and A.T.; resources, M.I., Y.N. and Y.S.; writing—original draft preparation, Y.M. and M.G.G.A.; writing—review and editing, T.K.; visualization, T.K.; supervision, T.K.; project administration, T.K.; funding acquisition, M.I. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
A part of this study was financially supported by JSPS KAKENHI Grant No. 17K07944 and 20H03118.
Acknowledgments
We sincerely thank the Akihisa Hayashi at Chinen Fisheries Cooperative Association for kindly providing of algal samples. We also thank University of the Ryukyus, Center for Research Advancement and Collaboration for offering their facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AIR, alcohol insoluble residue; UA, Uronic Acid; Fuc, Fucose; Gal, Galactose; Glc, Glucose; Man, Mannose; Xyl,Xylose; GlcA, Glucuronic Acid, GalA, Galacturonic Acid; GulA, Guluronic Acid; ManA, Mannuronic Acid
References
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and diversity of plant cell walls: from algae to flowering plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Srinorasing, T.; Chirasuwan, N.; Tamtin, M.; Bunnag, B. The potential of polysaccharide extracts from Caulerpa lentillifera waste. Int. J. Biol. Macromol. 2020, 2020 161, 1021–1028. [Google Scholar] [CrossRef]
- Silberfeld, T.; Leigh, J. W.; Verbruggen, H.; Cruaud, C.; de Reviers, B.; Rousseau, F. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the ‘brown algal crown radiation. Mol. Phylogenet. Evol. 2010, 2010 56, 659–674. [Google Scholar] [CrossRef]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J. M.; Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010, 188, 82–97. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 1203. [Google Scholar] [CrossRef]
- Salgado, L.T.; Cinelli, L.P.; Viana, N.B.; Tomazetto de Carvalho, R.; de Souza Mourao, P.A.; Teixeira, V.L.; Farina, M.; Filho, A.G.M.A. Avanadium bromoperoxidase catalyzes the formation of high-molecular-weight complexes between brown algal phenolic substances and alginates1. J. Phycol. 2009, 45, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Siméon, A.; Kridi, S.; Kloareg, B.; Hervé, C. Presence of exogenous sulfate is mandatory for tip growth in the brown alga Ectocarpus subulatus. Front. Plant Sci. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- le Bail, A.; Billoud, B.; le Panse, S.; Chenivesse, S.; Charrier, B. etoile regulates developmental patterning in the filamentous brown alga Ectocarpus siliculosus. Plant Cell 2011, 23, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Taylor, A.; Brownlee, C. Cell fate determination by the cell wall in early fucus development. science.org, 1421. [Google Scholar] [CrossRef]
- Herburger, K.; Ryan, L. M.; Popper, Z. A.; Holzinger, A. Localisation and substrate specificities of transglycanases in charophyte algae relate to development and morphology. Journal of cell science 2018, 131. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Chizhov, A.O.; Krupnova, T.N.; Sundukova, E.V.; Isakov, V.V. Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Bio. Ecol. [CrossRef]
- Awanthi, M.G.G.; Umosa, M.; Yuguchi, Y.; Oku, H.; Kitahara, K.; Ito, M.; Tanaka, A.; Konishi, T. Fractionation and characterization of cell wall polysaccharides from the brown alga Cladosiphon okamuranus, Carbohydr. Res. 2023, 523, 108722. [Google Scholar] [CrossRef]
- Tsuji, M.; Sudou, Y.; Enoki, M.; Tako, M.; Konishi, T. Variation in the contents and structure of fucoidan from cultivated Cladosiphon okamuranus; Tokida. Bull. Appl. Glycosci. 2013, 3, 248–252. [Google Scholar] [CrossRef]
- Starko, S.; Mansfield, S. D.; Martone, P. T. Cell wall chemistry and tissue structure underlie shifts in material properties of a perennial kelp. Eur. J. Phycol. 2018, 53, 307–317. [Google Scholar] [CrossRef]
- Krumhansl, K. A.; . Demes, K. W; Carrington, E.; Harley, C. D. G. Divergent growth strategies between red algae and kelps influence biomechanical properties. Am. J. Bot. 2015, 102, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Martone, P. T. Kelp versus coralline: Cellular basis for mechanical strength in the wave-swept seaweed Calliarthron (Corallinaceae, Rhodophyta). J. Phycol. 2007, 43, 882–891. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K. D. , Stanley, M. S.; Green, D. H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydrate Polymers. [CrossRef]
- Albersheim, P.; Darvill, A.; Roberts, K.; Sederoff, R.; Staehelin, A. Plant cell walls. Garland Science. New York, 2009, pp. 365–407.
- Kim, J.H.; Kim, J.S.; Wi, S.G.; Mun, S.P.; Chung, B.Y. The cell wall characterization at immature and mature stages of Arabidopsis thaliana L. researchgate.net.
- Burgert, I.; x Fratzl, I. Plants control the properties and actuation of their organs through the orientation of cellulose fibrils in their cell walls. Integr. Comp. Biol. 2009, 49, 69–79. [Google Scholar] [CrossRef]
- Turner S., R.; Somerville, C. R. Collapsed xylem phenotype of arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell, 9. [CrossRef]
- Revilla, G. Zarra, I. Changes in the molecular weight distribution of the hemicellulosic polysaccharides from rice coleoptiles growing under different conditions. J. Exp. Bot. 1987, 38, 1818–1825. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K. A.; Hamilton, J. K.; Rebers, P. A. Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboehansen, G. New method for quantitative of uranic acids determination. Anal. Biochem. 1973, 89, 484–489. [Google Scholar] [CrossRef]
- Hillis W., E.; Swain, T. The phenolic constituents of Prunus domestica. II.—The analysis of tissues of the Victoria plum tree. J. Sci. Food Agric. 1959, 10, 135–144. [Google Scholar] [CrossRef]
- Konishi, T.; Nakata, I.; Miyagi, Y.; Tako, M. Extraction of β-1,3 xylan from green seaweed, Caulerpa lentillifera,” J. Appl. Glycosci. 2012, 59, 161–163. [Google Scholar] [CrossRef]
- Kato, Y.; Matsukura, J. Carbohydrate composition of major leaf vegetables : Carbohydrate composition of vegetable food (Part I). Bull. Fac. Educ. Hirosaki,.
- Peng, L.; Hocart, C. H.; Redmond, J. W.; Williamson, R. E. Fractionation of carbohydrates in Arabidopsis root cell walls shows that three radial swelling loci are specifically involved in cellulose production. Planta 2000 2113 2000, 211, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, R.; Konishi, T.; Uechi, S.; Tako, M. Structural study of fucoidan from the brown seaweed Hizikia fusiformis. Food Sci. Technol. Res. 2008, 14, 176–182. [Google Scholar] [CrossRef]
Figure 1.
(a) Schematic view of Cladosiphon okamuranus and its main axis and lateral branches. (b) Hardness (Breaking strength) of the main axis and lateral branches of young and old Cladosiphon okamuranus. Different letters indicate a significant difference (P<0.01; Tukey-Kramer test) among samples (n = 20).
Figure 1.
(a) Schematic view of Cladosiphon okamuranus and its main axis and lateral branches. (b) Hardness (Breaking strength) of the main axis and lateral branches of young and old Cladosiphon okamuranus. Different letters indicate a significant difference (P<0.01; Tukey-Kramer test) among samples (n = 20).
Figure 2.
Cross sections of Young and Old Cladosiphon okamuranus. (a,b) The main axes become hollow both in old (a) and young (b) thalli. Anatomically, there are no differences in the number of medullary layers and in the cell shape of medullary cells. (c,d) Magnified images of (a) and (b), respectively. The numbers of branches of assimilatory filaments from a basal cell seem to be different between old (c) and young (d) thalli. Scales; 100μm.
Figure 2.
Cross sections of Young and Old Cladosiphon okamuranus. (a,b) The main axes become hollow both in old (a) and young (b) thalli. Anatomically, there are no differences in the number of medullary layers and in the cell shape of medullary cells. (c,d) Magnified images of (a) and (b), respectively. The numbers of branches of assimilatory filaments from a basal cell seem to be different between old (c) and young (d) thalli. Scales; 100μm.
Figure 3.
Yield of different polysaccharide fractions extracted from young and old thalli of C. okamuranus: HW, Hot water; AO, Ammonium oxalate; HC-I, Hemicellulose-I; HC-II, Hemicellulose-II; CL, Cellulose.
Figure 3.
Yield of different polysaccharide fractions extracted from young and old thalli of C. okamuranus: HW, Hot water; AO, Ammonium oxalate; HC-I, Hemicellulose-I; HC-II, Hemicellulose-II; CL, Cellulose.
Figure 4.
The molecular weight distribution of young-HW, old-HW, young-HC-I and old HC-I harvested in 2019. Mp is the main peak at each chromatogram marked by dash line. Solid arrows indicate the elution positions of size standards of pullulan with molecular weight of highest peak. Dashed arrows indicate the void volume (Vo) and total column volume (Vt).
Figure 4.
The molecular weight distribution of young-HW, old-HW, young-HC-I and old HC-I harvested in 2019. Mp is the main peak at each chromatogram marked by dash line. Solid arrows indicate the elution positions of size standards of pullulan with molecular weight of highest peak. Dashed arrows indicate the void volume (Vo) and total column volume (Vt).
Figure 5.
Anion exchange chromatography of (a) HW and (b) HC-I harvested in 2019.
Figure 5.
Anion exchange chromatography of (a) HW and (b) HC-I harvested in 2019.
Table 1.
Moisture content and yield of AIR in young and old C. okamuranus harvested in 2018 and 2019.
Table 1.
Moisture content and yield of AIR in young and old C. okamuranus harvested in 2018 and 2019.
| |
Harvested Year |
Yield of AIR* |
Moisture Content * |
| Young |
2018 |
2.8 |
93.2 |
| 2019 |
3.2 |
92.9 |
| Old |
2018 |
5.5 |
91.4 |
| 2019 |
5.0 |
91.9 |
Table 2.
Yield and chemical composition of cell wall fractions from C. okamuranus harvested in 2018.
Table 2.
Yield and chemical composition of cell wall fractions from C. okamuranus harvested in 2018.
| |
Fraction |
Yielda |
Total sugar |
UAb |
SO3−
|
Protein |
Polyphenol |
| |
HW |
62.4 |
49.4 |
24.5 |
24.5 |
8.9 |
2.9 |
| |
AO |
12.5 |
59.5 |
34.3 |
3.5 |
6.6 |
1.7 |
| Young |
HC-I |
18.1 |
49.9 |
22.3 |
6.4 |
31.5 |
8.4 |
| |
HC-II |
2.5 |
92.3 |
7.8 |
1.9 |
8.1 |
5.6 |
| |
CL |
5.1 |
100.0 |
tr |
tr |
tr |
tr |
| |
HW |
49.8 |
56.2 |
22.9 |
21.7 |
14.5 |
3.9 |
| |
AO |
10.6 |
64.8 |
30.5 |
6.1 |
8.0 |
2.1 |
| Old |
HC-I |
30.6 |
62.9 |
21.1 |
9.9 |
22.9 |
7.3 |
| |
HC-II |
2.9 |
80.1 |
13.7 |
5.9 |
10.0 |
2.4 |
| |
CL |
5.5 |
100.0 |
tr |
tr |
tr |
tr |
Table 3.
Yield and chemical composition of cell wall fractions from C. okamuranus harvested in 2019.
Table 3.
Yield and chemical composition of cell wall fractions from C. okamuranus harvested in 2019.
| |
Fraction |
Yielda |
Total Sugar |
UAb |
SO3−
|
Protein |
Polyphenol |
| |
HW |
66.7 |
55.3 |
25.6 |
15.7 |
15.0 |
4.3 |
| |
AO |
8.9 |
59.2 |
30.7 |
3.8 |
7.4 |
2.4 |
| Young |
HC-I |
18.7 |
67.4 |
21.8 |
6.7 |
21.9 |
6.8 |
| |
HC-II |
2.5 |
114.0 |
9.8 |
2.9 |
5.1 |
1.2 |
| |
CL |
4.1 |
100.0 |
tr |
tr |
tr |
tr |
| |
HW |
58.5 |
61.6 |
23.7 |
20.6 |
12.1 |
4.5 |
| |
AO |
9.6 |
61.6 |
31.4 |
10.4 |
8.4 |
2.6 |
| Old |
HC-I |
26.7 |
65.3 |
21.2 |
8.5 |
18.9 |
5.3 |
| |
HC-II |
2.4 |
112.1 |
11.5 |
11.5 |
6.8 |
2.5 |
| |
CL |
4.7 |
100.0 |
tr |
tr |
tr |
tr |
Table 4.
Sugar compositional analysis of cell wall fractions from C. okamuranus harvested in 2018.
Table 4.
Sugar compositional analysis of cell wall fractions from C. okamuranus harvested in 2018.
| |
Fraction |
Neutral Sugar |
UA |
SO3− |
| |
Fuc |
Gal |
Glc |
Man |
Xyl |
GlcA |
GalA/GulA |
ManA |
| |
HW |
1.0 |
tr |
tr |
|
tr |
0.3 |
tr |
|
0.7 |
| |
AO |
1.0 |
0.1 |
tr |
tr |
0.1 |
tr |
0.3 |
0.3 |
0.1 |
| Young |
HC-I |
1.0 |
tr |
0.1 |
tr |
0.2 |
0.3 |
tr |
|
0.2 |
| |
HC-II |
1.0 |
|
10.8 |
0.4 |
0.6 |
0.9 |
|
|
0.6 |
| |
CL |
1.0 |
|
55.9 |
|
3.7 |
|
|
|
|
| |
HW |
1.0 |
tr |
tr |
|
tr |
0.3 |
tr |
|
0.6 |
| |
AO |
1.0 |
tr |
|
|
tr |
tr |
0.3 |
0.1 |
0.2 |
| Old |
HC-I |
1.0 |
tr |
tr |
|
0.1 |
0.3 |
tr |
|
0.3 |
| |
HC-II |
1.0 |
|
1.3 |
0.1 |
0.3 |
0.4 |
|
|
0.4 |
| |
CL |
1.0 |
|
26.7 |
|
0.8 |
|
|
|
|
Table 5.
Sugar compositional analysis of cell wall fractions from C. okamuranus harvested in 2019.
Table 5.
Sugar compositional analysis of cell wall fractions from C. okamuranus harvested in 2019.
| |
Fraction |
Neutral Sugar |
UA |
SO3− |
| |
Fuc |
Gal |
Glc |
Man |
Xyl |
GlcA |
GalA/GulA |
ManA |
| |
HW |
1.0 |
tr |
0.1 |
|
|
0.3 |
tr |
|
0.5 |
| |
AO |
1.0 |
tr |
tr |
0.1 |
0.1 |
tr |
0.2 |
0.2 |
0.1 |
| Young |
HC-I |
1.0 |
tr |
tr |
tr |
tr |
0.3 |
tr |
|
0.2 |
| |
HC-II |
1.0 |
|
12.7 |
3.1 |
3.1 |
2.0 |
|
|
1.4 |
| |
CL |
1.0 |
0.8 |
71.5 |
|
|
|
|
|
|
| |
HW |
1.0 |
tr |
0.1 |
|
tr |
0.3 |
tr |
|
0.7 |
| |
AO |
1.0 |
tr |
tr |
|
tr |
tr |
0.2 |
0.2 |
0.4 |
| Old |
HC-I |
1.0 |
tr |
0.1 |
|
0.1 |
0.3 |
tr |
|
0.5 |
| |
HC-II |
1.0 |
|
4.3 |
0.1 |
0.6 |
0.7 |
|
|
1.7 |
| |
CL |
1.0 |
|
18.9 |
|
1.2 |
|
|
|
|
Table 6.
Sugar compositional analysis of flow through (FT) and NaCl fractions obtained by anion exchange chromatography of young and old HW and HC-I from C. okamuranus harvested in 2019.
Table 6.
Sugar compositional analysis of flow through (FT) and NaCl fractions obtained by anion exchange chromatography of young and old HW and HC-I from C. okamuranus harvested in 2019.
| Fraction |
Fuc |
Gal |
Glc |
Man |
Xyl |
GlcA |
SO3−
|
| |
HW-FT |
1.0 |
tr |
0.6 |
|
|
0.3 |
0.4 |
| Young |
HW-NaCl |
1.0 |
tr |
tr |
tr |
tr |
0.2 |
0.4 |
| |
HC-I-NaCl |
1.0 |
0.1 |
0.1 |
0.1 |
tr |
0.4 |
0.5 |
| |
HW-FT |
1.0 |
- |
0.3 |
|
|
0.3 |
0.4 |
| Old |
HW-NaCl |
1.0 |
tr |
tr |
tr |
tr |
0.2 |
0.3 |
| |
HC-I-NaCl |
1.0 |
tr |
tr |
0.1 |
tr |
0.2 |
0.6 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).